Abstract
Age-related increases in oxidative stress impair endothelium-dependent vasodilatation in humans, leading to the speculation that endothelial dysfunction contributes to impaired muscle blood flow and vascular control during exercise in older adults. We directly tested this hypothesis in 14 young (22 ± 1 years) and 14 healthy older men and women (65 ± 2 years). We measured forearm blood flow (FBF; Doppler ultrasound) and calculated vascular conductance (FVC) responses to single muscle contractions at 10, 20 and 40% maximum voluntary contraction (MVC) before and during ascorbic acid (AA) infusion, and we also determined the effects of AA on muscle blood flow during mild (10% MVC) continuous rhythmic handgrip exercise. For single contractions, the peak rapid hyperaemic responses to all contraction intensities were impaired ∼45% in the older adults (all P < 0.05), and AA infusion did not impact the responses in either age group. For the rhythmic exercise trial, FBF (∼28%) and FVC (∼31%) were lower (P= 0.06 and 0.05) in older versus young adults after 5 min of steady-state exercise with saline. Subsequently, AA was infused via brachial artery catheter for 10 min during continued exercise. AA administration did not significantly influence FBF or FVC in young adults (1–3%; P= 0.24–0.59), whereas FBF increased 34 ± 7% in older adults at end-exercise, and this was due to an increase in FVC (32 ± 7%; both P < 0.05). This increase in FBF and FVC during exercise in older adults was associated with improvements in vasodilator responses to acetylcholine (ACh; endothelium dependent) but not sodium nitroprusside (SNP; endothelium independent). AA had no effect on ACh or SNP responses in the young. We conclude that acute AA administration does not impact the observed age-related impairment in the rapid hyperaemic response to brief muscle contractions in humans; however, it does significantly increase muscle blood flow during continuous dynamic exercise in older adults, and this is probably due (in part) to an improvement in endothelium-dependent vasodilatation.
Blood flow and oxygen delivery increase to contracting muscle, a complex response involving mechanical factors, the sympathetic nervous system and local metabolic and endothelium-derived substances that influence vascular tone (Saltin et al. 1998). With respect to the latter, a variety of local endothelium-dependent vasodilators are recognized to increase during exercise and partially control blood flow and vascular tone in contracting human skeletal muscle (Clifford & Hellsten, 2004; Saltin, 2007). For example, local inhibition of nitric oxide and vasodilating prostaglandins during rhythmic handgrip exercise independently reduces forearm hyperaemia in young healthy humans (Schrage et al. 2004), and combined inhibition of these substances has been documented to reduce muscle blood flow during knee extensor exercise (Boushel et al. 2002; Mortensen et al. 2007). Additionally, recent work by Hillig and colleagues demonstrated redundancy between nitric oxide and the cytochrome P450 pathway, leading to the speculation that an endothelium-derived hyperpolarizing factor increases during exercise and is also involved in local vascular control (Hillig et al. 2003). Together, these observations indicate a significant role of the endothelium in regulating exercise hyperaemia in healthy humans.
Human ageing is associated with a progressive decline in endothelial function that predisposes older adults to increased risk for thrombosis and atherosclerotic vascular disease, as well as ischaemic heart and cerebrovascular disease (Luscher et al. 1993b; Wu & Thiagarajan, 1996; Shimokawa, 1999). Ageing is also associated with reductions in exercise capacity (Holloszy & Kohrt, 1995) and this may be due in part to impaired blood flow and oxygen delivery to contracting muscles (Lawrenson et al. 2003; Poole et al. 2003; Proctor et al. 2003). Collectively, these findings have lead to the hypothesis that impaired endothelial vasodilator function contributes to exercise intolerance with age and other disease states involving endothelial dysfunction via impaired vascular control during exercise (Drexler & Hornig, 1996; Proctor & Parker, 2006). Consonant with this concept, recent work by Schrage and colleagues indicates that the normal contributions of endothelium-derived nitric oxide and vasodilating prostaglandins to exercise hyperaemia are significantly reduced with age in older healthy humans (Schrage et al. 2007). Although the mechanisms underlying these changes are unclear, age-related increases in oxidative stress are suggested to impair endothelium-dependent vasodilatation in humans, and accumulating data indicate that acute antioxidant administration (e.g. ascorbic acid) can reverse endothelial dysfunction as evidenced by the restoration of acetylcholine- and flow-mediated vasodilatation in older adults (Taddei et al. 2001; Eskurza et al. 2004). Despite these observations, it is presently unknown whether acutely improving endothelial function augments muscle blood flow via local vasodilatation during dynamic exercise in older humans.
To date, the majority of studies designed to understand muscle blood flow and vascular control in exercising young and older adults have been performed during steady-state exercise. However, in recent years, particular attention has been given to the rapid hyperaemic response following a single muscle contraction, as this allows for the determination of contraction-induced vasodilatation without the impeding effects of subsequent contractions on vascular tone (Hamann et al. 2004; Tschakovsky et al. 2004; Kirby et al. 2007). Additionally, the mechanisms influencing vascular tone at exercise onset appear to differ from those involved in the control during steady-state exercise (Clifford & Hellsten, 2004). Upon the release of a single contraction, the typical rapid hyperaemic response peaks within 3–6 cardiac cycles and the magnitude is graded with contraction intensity (Tschakovsky et al. 2004; Kirby et al. 2007). Importantly, this response is independent of sympathetic neural influences (Corcondilas et al. 1964; Buckwalter & Clifford, 1999), and during small muscle mass exercise (e.g. isolated handgrip), can also occur without significant changes in heart rate and arterial pressure (Tschakovsky et al. 2004; Kirby et al. 2007; Carlson et al. 2008). Thus, under specific experimental conditions, this rapid hyperaemic response reflects a local vasodilator response within the vascular bed of the contracting muscle. With respect to ageing, we recently documented that skeletal muscle contraction-induced rapid vasodilatation is significantly impaired in the forearm vasculature of older healthy adults (Carlson et al. 2008). It is currently unknown whether impaired endothelial vasodilator function is mechanistically linked with this blunted rapid vasodilatory pattern following a single brief muscle contraction. Further, whether improving endothelium-dependent vasodilatation restores the blunted rapid hyperaemia in older adults has not been investigated.
Accordingly, the purpose of the present study was to directly test the hypothesis that acute improvements in endothelium-dependent vasodilator function via brachial artery infusion of ascorbic acid augments blood flow to contracting muscle of older adults via local vasodilatation. To do so, we determined the effects of ascorbic acid in young and older adults on (1) forearm haemodynamic responses to single, brief muscle contractions, (2) forearm haemodynamics during dynamic (continuous) contractions of the forearm muscles, and (3) the vasodilator responses to intra-arterial infusions of endothelium-dependent (acetylcholine) and endothelium-independent (sodium nitroprusside) agonists.
Methods
Subjects
With Institutional Review Board approval and after written informed consent, a total of 14 young and 14 older healthy adult men and women participated in the present study. All subjects were normotensive and free from overt cardiovascular disease as assessed from casual blood pressure measurements and a medical history. Older subjects were further evaluated for clinical evidence of cardiopulmonary disease with a physical examination and resting and maximal exercise electrocardiograms. All subjects were sedentary to moderately active, non-smokers, not taking any medications including antioxidants, and studies were performed after a minimum of a 4 h fast. Subjects provided written, informed consent after all potential risks and procedures were explained. This study was approved by the Human Research Committee of Colorado State University and was performed according to the Declaration of Helsinki.
Arterial catheterization
A 20 gauge, 7.6 cm catheter was placed in the brachial artery of the non-dominant arm under aseptic conditions after local anaesthesia (2% lidocaine (lignocaine)) for local administration of study drugs. The catheter was connected to a 3-port connector as well as a pressure transducer for mean arterial pressure (MAP) measurement and continuously flushed at 3 ml h−1 with heparinized saline (Dinenno et al. 2003; Dinenno & Joyner, 2004; Kirby et al. 2008). The two side ports were used for infusions of vasoactive drugs.
Blood samples
Measures of total cholesterol, low- and high-density lipoproteins (LDL and HDL), and triglycerides were performed via conventional methods by the clinical laboratory of the Poudre Valley Hospital (Fort Collins, CO, USA). Oxidized LDL was measured via standard ELISA assay (Mercodia, Inc., Uppsala, Sweden) as a marker of circulating oxidative stress via the General Clinical Research Center of the Milton S. Hershey Medical Center (Hershey, PA, USA).
Body composition and forearm volume
Body composition was determined by dual-energy X-ray absorptiometry (DEXA; DPX-IQ, Lunar Radiation). Total forearm volume and fat-free mass (FFM) were calculated from regional analysis of the experimental forearm (from the proximal to distal radioulnar joint) from whole-body DEXA scans with Lunar software version 4.7e for normalization of individual drug doses (Dinenno et al. 2002; Carlson et al. 2008). Body mass index (BMI) was calculated as body weight (kg) divided by height (m) squared.
Forearm blood flow and vascular conductance
A 4 MHz pulsed Doppler probe (Model 500V, Multigon Industries, Mt Vernon, NY, USA) was used to measure brachial artery mean blood velocity (MBV) with the probe securely fixed to the skin over the brachial artery proximal to the catheter insertion site, as previously described by our laboratory (Dinenno & Joyner, 2003; Kirby et al. 2007; Carlson et al. 2008). The probe insonation angle relative to the skin was 45 deg. A linear 12 MHz echo Doppler ultrasound probe (GE Vingmed Ultrasound Vivid7, Horten, Norway) was placed in a holder securely fixed to the skin immediately proximal to the velocity probe to measure brachial artery diameter. For the single contraction trials, brachial artery diameter was measured in triplicate prior to any contractions, as we and others have shown that brachial diameter does not change in response to this stimulus (Tschakovsky et al. 2004; Carlson et al. 2008). For the rhythmic handgrip exercise trials, brachial diameter was measured in triplicate at rest and at each minute of exercise. For the pharmacological tests, brachial diameter was measured in triplicate at rest and after 5 min of drug infusion (see below for details). Forearm blood flow was calculated as:
where the FBF is in ml min−1, the MBV is in cm s−1, the brachial diameter is in cm, and 60 is used to convert from ml s−1 to ml min−1. Forearm vascular conductance (FVC) was calculated as (FBF/MAP) × 100, and expressed as ml min−1 (100 mmHg)−1.
Single dynamic forearm contractions
Maximum voluntary contraction (MVC) was determined for the experimental arm as the average of three maximal squeezes of a handgrip dynamometer (Stoelting, Chicago, IL, USA) that were within 3% of each other. Brief, dynamic forearm contractions were performed at 10, 20 and 40% of the subject's MVC using a handgrip pulley system attached to weights corresponding to each workload. The weight was lifted 4–5 cm over the pulley for a single, 1 s dynamic contraction as previously described (Carlson et al. 2008). These mild-to-moderate contraction intensities were chosen to limit the contribution of systemic haemodynamics to forearm vasodilator responses and to eliminate reflex increases in sympathetic nervous system activity, and thus isolate the local effects of muscle contraction on vascular tone (Carlson et al. 2008). Two minutes of relaxation were given between each contraction to allow continuous measures of forearm haemodynamics post-contraction, as well as ample time for haemodynamics to return to baseline values (Tschakovsky et al. 2004; Kirby et al. 2007; Carlson et al. 2008). Workload intensity was randomized and counterbalanced across subjects to eliminate any order effect and trials were performed in triplicate to calculate an average response for each subject.
Rhythmic handgrip exercise
Using the same pulley system, subjects performed rhythmic, dynamic handgrip exercise at 10% MVC with a duty cycle of 1 s contraction–2 s relaxation (20 contractions per minute) using audio and visual signals to ensure the correct timing (Dinenno & Joyner, 2003; Kirby et al. 2008). Similar to the rationale provided above, this mild intensity rhythmic handgrip exercise was chosen to (a) limit the contribution of systemic haemodynamics to forearm hyperaemic responses, and (b) eliminate reflex activation of the sympathetic nervous system (Seals & Victor, 1991; Carlson et al. 2008). Further, mild contractions can be performed for a significant amount of time without evoking progressive increases in heart rate and arterial pressure (Schrage et al. 2004, 2007). Thus, our experimental model aims to isolate the local effects of muscle contractions on forearm hyperaemia without engaging potentially confounding systemic influences on vascular tone.
Vasoactive drug administration
Endothelium-dependent vasodilatation was determined by intra-arterial infusion of acetylcholine (ACh; Miochol-E, Novartis Inc.) at 16 μg (100 ml forearm volume)−1 min−1 for 5 min and endothelium-independent vasodilatation was assessed via intra-arterial infusion of sodium nitroprusside (SNP; Nitropress, Hospira Inc.) at 4 μg (100 ml forearm volume)−1 min−1 for 5 min (DeSouza et al. 2000, 2002). As a method of acutely improving endothelium-dependent vasodilatation (Taddei et al. 2000, 2001), the potent antioxidant ascorbic acid (vitamin C, American Regent Inc.) was infused at 8 mg (100 ml forearm volume)−1 min−1 for 10 min during handgrip exercise as a loading dose (see ‘Experimental protocol’ below), and at 40% of this loading dose for maintenance infusion throughout the remainder of the experiment.
Experimental protocol
Subjects were studied in the supine position with the experimental arm extended 90 deg laterally at heart level. The experimental time line is depicted in Fig. 1. Two minutes of resting data were acquired prior to all experimental trials. To establish endothelium-dependent and -independent vasodilator responsiveness, ACh and SNP (respectively) were individually infused via brachial artery catheter for 5 min. The order of ACh and SNP was counterbalanced across subjects and 15 min of rest was allowed following each drug infusion. Next, subjects performed single brief forearm contractions at 10, 20 and 40% MVC for 1 s with 2 min of rest in between contractions. After 10 min of rest, rhythmic handgrip exercise was performed at 10% MVC with saline for 5 min to achieve steady-state haemodynamics, and ascorbic acid was then infused during continued exercise for a further 10 min, giving a total time of 15 min of handgrip exercise (Trial 1). The dose of ascorbic acid was reduced to 40% of the original dose for the remainder of the experiment. Following 15 min of rest, ACh and SNP infusions were repeated during maintenance ascorbic acid administration to determine the influence of ascorbic acid on endothelium-dependent and -independent vasodilatation. Single forearm contractions were again performed to determine the impact of ascorbic acid on contraction-induced rapid vasodilatation. Lastly, a second trial (Trial 2; n= 13 for young, n= 14 for older) of rhythmic handgrip exercise was performed for 5 min to determine forearm haemodynamics during the transition from rest to steady-state exercise with ascorbic acid already present and to confirm that any changes in forearm haemodynamics during Trial 1 were not simply due to a ‘time effect’ of continued exercise.
Figure 1. Experimental time line.
Following placement of the brachial artery catheter and general setup, subjects received a 5 min intra-arterial infusion of either acetylcholine (ACh) or sodium nitroprusside (SNP) to assess endothelium-dependent or endothelium-independent vasodilatation, respectively. Next, single 1 s dynamic forearm muscle contractions were performed at 10, 20 and 40% MVC in triplicate and in random order. Noted as Trial 1, rhythmic handgrip (HG) exercise was performed at 10% MVC for 5 min with saline to achieve ‘steady-state’ haemodynamics, and the next 10 min consisted of continued exercise with concurrent ascorbic acid infusion to give a total of 15 min. The dose of ascorbic acid was then reduced to 40% of the original dose and infused for the remainder of the experiment. During maintenance ascorbic acid administration, endothelium-dependent and -independent vasodilatation was again tested, followed by single contractions, and then a second 5 min rhythmic handgrip exercise trial (Trial 2). See text for further details.
Data acquisition and analysis
Data were collected and stored on computer at 250 Hz and analysed off-line with signal-processing software (WinDaq, DATAQ Instruments, Akron, OH, USA). Mean arterial pressure (MAP) was determined from the arterial pressure waveform. Baseline FBF, heart rate (HR) and MAP represent an average of the last minute of the resting time period prior to all exercise trials and pharmacological vasodilatory tests. The data presented for the ACh and SNP trials represent the final 30 s of drug infusion. For the single contraction trials, the post-contraction data represent the first unimpeded cardiac cycle immediately after release of the contraction, and this beat-by-beat analysis was performed for a total of 30 cardiac cycles (Tschakovsky et al. 2004; Kirby et al. 2007; Carlson et al. 2008). For the rhythmic exercise trials, the minute-by-minute hyperaemic (FBF) and vasodilatory values (FVC) represent the last 30 s of that minute at rest and during exercise. The percentage change in FBF during drug infusions and exercise was calculated as:
Changes in FVC were calculated in a similar fashion.
Statistics
All values are reported as means ±s.e.m. Comparison of subject characteristics and the haemodynamic values at specific time points between groups for the ACh, SNP and exercise conditions were made with unpaired t tests, and the within group values for each hyperaemic condition with paired t tests. Specific hypothesis testing within exercise trials was performed to assess mean group differences between young and older adults using two-way repeated measures analysis of variance. Post hoc analysis was performed using Tukey's test when significance was observed. Significance was set at P < 0.05.
Results
Subject characteristics
The mean age difference between the young and older adults was 43 years. There were no significant age-group differences in BMI, whole-body FFM, forearm volume, forearm FFM, MVC, or HDL cholesterol. Older individuals had a greater body fat percentage and total and LDL cholesterol (P < 0.05; Table 1), although these values were within normal levels. Baseline haemodynamics for all trials were not different between young and older adults.
Table 1.
Subject characteristics and baseline haemodynamics
| Variable | Young | Older |
|---|---|---|
| Male : female | 9 : 5 | 9 : 5 |
| Age (years) | 22 ± 1 | 65 ± 2* |
| Body mass index (kg m−2) | 24.5 ± 0.8 | 24.1 ± 0.8 |
| Body fat (%) | 19.5 ± 2.62 | 29.04 ± 2.45* |
| Whole-body FFM (kg) | 58.68 ± 3.76 | 48.59 ± 3.48 |
| Forearm FFM (g) | 842 ± 75 | 700 ± 36 |
| Forearm volume (ml) | 968 ± 75 | 830 ± 68 |
| MVC (kg) | 44 ± 3 | 36 ± 3 |
| 10% MVC (kg) | 4.4 ± 0.3 | 3.6 ± 0.3 |
| Total cholesterol (mmol l−1) | 4.0 ± 0.3 | 5.1 ± 0.4* |
| LDL cholesterol (mmol l−1) | 2.6 ± 0.2 | 3.4 ± 0.3* |
| HDL cholesterol (mmol l−1) | 1.1 ± 0.1 | 1.2 ± 0.1 |
| Triglycerides (mmol l−1) | 0.7 ± 0.1 | 1.2 ± 0.3 |
| MAP (mmHg) | 94 ± 3 | 99 ± 2 |
| Heart rate (beats min−1) | 55 ± 2 | 59 ± 2 |
| Forearm blood flow (ml min−1) | 35 ± 6 | 34 ± 3 |
| Forearm blood flow (ml (100 g)−1 min−1) | 3.4 ± 0.4 | 4.1 ± 0.3 |
| Forearm VC (ml min−1 (100 mmHg)−1) | 36 ± 5 | 34 ± 3 |
Data presented as mean ±s.e.m. FFM = fat free mass; MVC = maximal voluntary contraction; LDL = low density lipoprotein; HDL = high density lipoprotein; MAP = mean arterial pressure; VC = vascular conductance.
P < 0.05 vs. young.
Effect of ascorbic acid on forearm haemodynamic responses to single muscle contractions
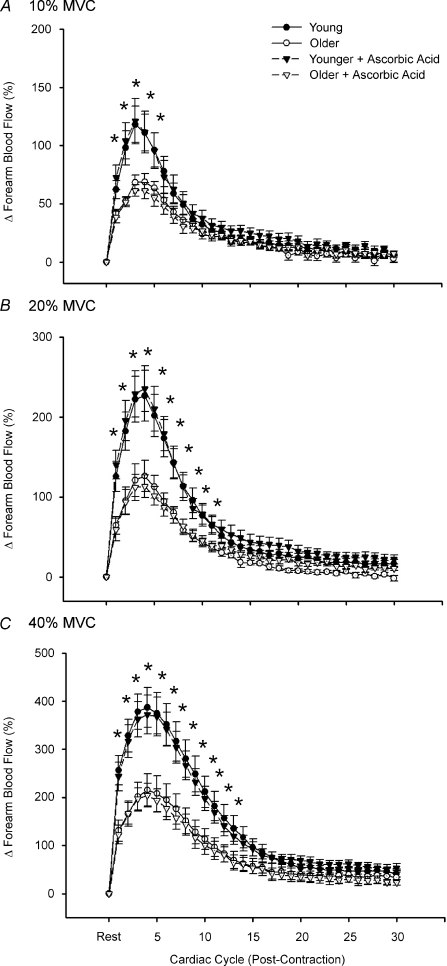
Dynamic blood flow responses following single forearm muscle contractions at 10, 20 and 40% MVC are shown in Fig. 2A–C. At all exercise intensities, both young and older groups demonstrated the typical temporal hyperaemic pattern, with peak hyperaemia occurring ∼3–4 cardiac cycles post-contraction. Consistent with previous findings from our laboratory (Carlson et al. 2008), older adults had a blunted immediate (first beat post-contraction; ∼35–50%) and peak hyperaemic response (∼45%) compared to young adults at all exercise intensities (Fig. 2A–C; P < 0.05). This impairment was observed for a total of 4, 11 and 13 beats post-contraction for contraction intensities of 10, 20 and 40% MVC, respectively. This rapid hyperaemic response was unaffected by infusion of ascorbic acid in young adults. In contrast to our hypothesis, ascorbic acid administration in older adults also did not augment the rapid hyperaemia seen following a brief single muscle contraction at any exercise intensity (Fig. 2A–C). Data were identical when analysed as percentage increases in FVC (not shown).
Figure 2. Dynamic rapid hyperaemic responses to single forearm muscle contractions at 10, 20 and 40% MVC.
The peak hyperaemic response occurred at 3–4 cardiac cycles post-contraction for all exercise intensities regardless of age or ascorbic acid administration. There were significant age group differences in contraction-induced rapid vasodilatation for all intensities, and ascorbic acid did not impact this response in either young or older adults. *P < 0.05 vs. older.
Effect of ascorbic acid on forearm haemodynamics during rhythmic handgrip exercise
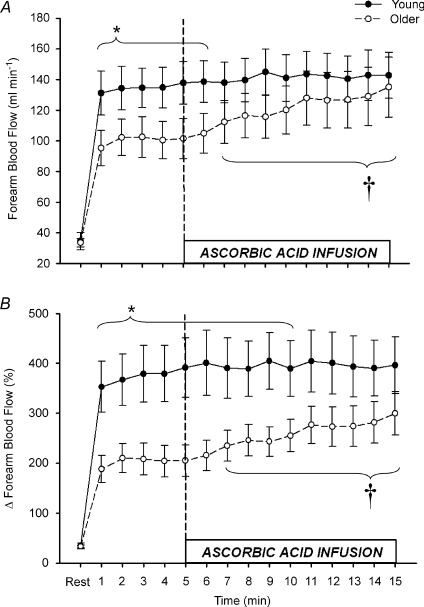
Rhythmic dynamic handgrip exercise performed at 10% MVC significantly increased FBF and FVC from baseline within the first minute and throughout the duration of the exercise trial in both young and older adults (P < 0.05; Table 2, Fig. 3). Absolute FBF and FVC tended to be lower in older compared with young adults when expressed as absolute values (Table 2, Fig. 3A; P= 0.05–0.09 for minutes 1–6), and was significantly lower when expressed as the percentage increase in FBF and FVC from baseline (Table 2, Fig. 3B; P < 0.05 for minutes 1–10). Ascorbic acid infusion had no effect on FBF or FVC in young adults throughout the entire exercise bout (Table 2, Fig. 3A and B; P > 0.05), but significantly and progressively increased FBF and FVC from steady state in older adults beginning at minute 7 (2nd minute of ascorbic acid infusion) until the end of exercise (minute 15) (Table 2, Fig. 3A and B; P < 0.05).
Table 2.
Forearm and systemic haemodynamics values during Trial 1 rhythmic exercise
| Saline |
Ascorbic acid |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (min) | Age | Rest | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| HR (beats min−1) | Young | 55 ± 2 | 59 ± 2 | 57 ± 2 | 57 ± 2 | 58 ± 2 | 58 ± 2 | 58 ± 2 | 58 ± 2 | 58 ± 2 | 58 ± 2 | 57 ± 2 | 57 ± 2 | 58 ± 2 | 58 ± 3 | 58 ± 2 | 58 ± 2 |
| Older | 59 ± 2 | 63 ± 2 | 63 ± 2 | 62 ± 2 | 63 ± 2 | 62 ± 2 | 62 ± 2 | 62 ± 2 | 63 ± 2 | 62 ± 2 | 62 ± 2 | 62 ± 2 | 62 ± 2 | 62 ± 2 | 63 ± 2 | 62 ± 2 | |
| MAP (mmHg) | Young | 94 ± 3 | 98 ± 3 | 99 ± 3 | 99 ± 3 | 98 ± 3 | 97 ± 3 | 98 ± 3 | 98 ± 3 | 98 ± 3 | 98 ± 3 | 96 ± 3 | 98 ± 3 | 98 ± 3 | 98 ± 3 | 97 ± 3 | 98 ± 3 |
| Older | 99 ± 2 | 103 ± 2 | 103 ± 2 | 104 ± 2 | 104 ± 2 | 103 ± 2 | 103 ± 2 | 104 ± 2 | 104 ± 2 | 104 ± 2 | 103 ± 2 | 104 ± 2 | 105 ± 2 | 103 ± 3 | 102 ± 2 | 103 ± 2 | |
| FVC (ml min−1 100 mmHg−1) | Young | 36 ± 5 | 132 ± 14 | 135 ± 13 | 135 ± 12 | 136 ± 13 | 141 ± 13 | 141 ± 13 | 140 ± 13 | 141 ± 13 | 146 ± 14 | 145 ± 15 | 145 ± 14 | 144 ± 14 | 142 ± 14 | 145 ± 15 | 144 ± 14 |
| Older | 34 ± 3 | 94 ± 13* | 100 ± 13* | 100 ± 14* | 99 ± 14* | 100 ± 15* | 104 ± 14* | 110 ± 15 | 113 ± 15 | 112 ± 14 | 117 ± 16 | 124 ± 18 | 122 ± 19 | 126 ± 21 | 128 ± 21 | 132 ± 21 | |
| ΔFVC (%) | Young | — | 335 ± 48 | 346 ± 47 | 355 ± 52 | 358 ± 53 | 378 ± 58 | 380 ± 61 | 372 ± 57 | 368 ± 52 | 383 ± 52 | 377 ± 54 | 383 ± 58 | 379 ± 59 | 374 ± 59 | 378 ± 55 | 375 ± 54 |
| Older | — | 177 ± 26* | 198 ± 28* | 195 ± 30* | 191 ± 30* | 194 ± 30* | 204 ± 29* | 220 ± 29*† | 229 ± 29*† | 226 ± 27*† | 241 ± 31*† | 259 ± 35† | 253 ± 37† | 262 ± 40† | 270 ± 42† | 283 ± 41† | |
Data are mean ±s.e.m. HR = heart rate; MAP = mean arterial pressure; FVC = forearm vascular conductance;
P < 0.05 vs. young
P < 0.05 vs. end-control saline (minute 5 of exercise).
Figure 3. Forearm hyperaemic responses to mild rhythmic handgrip exercise before and during ascorbic acid infusion.
Prior to ascorbic acid, absolute forearm blood flow tended to be lower in older vs. young adults during mild intensity steady-state exercise (A; *P = 0.06–0.09 for minutes 1–6). When expressed as percentage increases from baseline, forearm hyperaemic responses were significantly reduced in older adults (B; *P < 0.05 for minutes 1–10). Infusion of ascorbic acid significantly increased forearm blood flow in older, but not young adults during continued exercise. †P < 0.05 vs. steady-state exercise within older group for minutes 7–15.
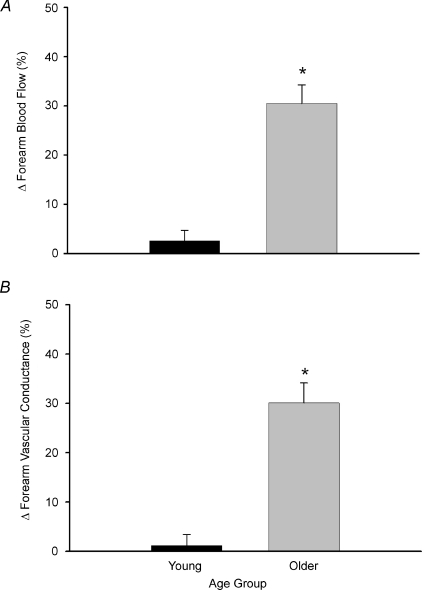
The peak effect of ascorbic acid infusion on FBF and FVC during steady-state exercise was calculated as the percentage increase from minute 5 of exercise (end-saline) to the final minute of exercise (end-ascorbic acid; Fig. 4A and B). Ascorbic acid infusion increased FBF (34 ± 7%) and FVC (32 ± 7%) from steady-state exercise in older adults (both P < 0.05), whereas forearm haemodynamics were unchanged in young adults (ΔFBF = 3 ± 2%; ΔFVC = 1 ± 2%; not significant (n.s.)). MAP and HR were not significantly different between young and older adults at baseline and were not significantly altered throughout the experimental trial (Table 2). Brachial artery diameter at rest was not different in young and older adults (0.43 ± 0.02 vs. 0.42 ± 0.02 cm; P= 0.55) and was similar after 5 min exercise with saline (0.42 ± 0.02 vs. 0.41 ± 0.02 cm) and at the end of ascorbic acid infusion (0.42 ± 0.02 vs. 0.41 ± 0.02 cm).
Figure 4. Peak effect of ascorbic acid on forearm blood flow and vascular conductance during steady-state exercise.
Acute infusion of ascorbic acid increased forearm blood flow by ∼30% in older adults, whereas the increase was minimal and non-significant in young adults (A). Similar responses were observed when quantified as changes in vascular conductance, indicating that the increase in blood flow was due to local vasodilatation (B). *P < 0.05 vs. young.
Effect of prior ascorbic acid on forearm haemodynamics from rest to steady-state exercise
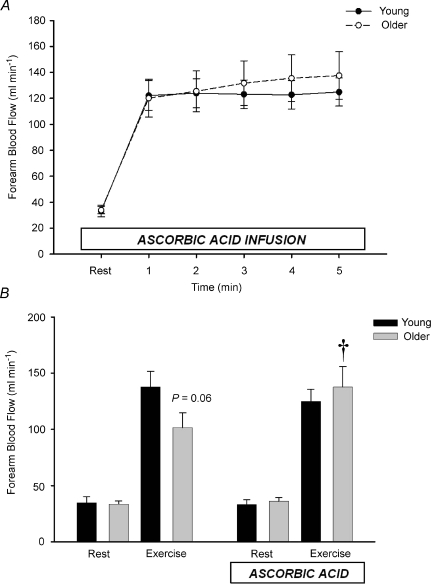
Ascorbic acid had no impact on resting forearm blood flow in either young or older adults (Fig. 5A and B). In contrast to the observed trend for an age-associated impairment in the absolute hyperaemic and vasodilatory responses seen during the transition from rest to steady-state exercise in Trial 1, FBF in older adults during ascorbic acid infusion increased to similar values as young adults within the 1st minute of exercise (Fig. 5A). This pattern was similar throughout all 5 min of exercise (Fig. 5A), and the FBF values at rest and after 5 min of steady-state exercise before and during concurrent ascorbic acid administration are shown in Fig. 5B. Percentage increases in FBF were also not significantly different at minute 1 of exercise in young and older adults (316 ± 40%vs. 255 ± 30%; P= 0.23), and were similar at end-exercise (329 ± 45%vs. 308 ± 40%; P= 0.73). MAP and HR were again not significantly different between young and older adults, thus FVC values were similar to FBF (data not shown).
Figure 5. Effect of ascorbic acid on forearm blood flow from rest to steady-state handgrip exercise.
When ascorbic acid was administered prior to the onset of exercise, forearm blood flow increased to similar levels within 1 min of exercise in young and older adults and this persisted throughout the exercise trial (A). In B, forearm blood flow at rest and after 5 min of steady-state exercise from Trial 1 (no ascorbic acid; control) and Trial 2 (concurrent ascorbic acid infusion) are shown. †P < 0.05 vs. without ascorbic acid within age group.
Effect of ascorbic acid on endothelium-dependent and -independent vasodilatation
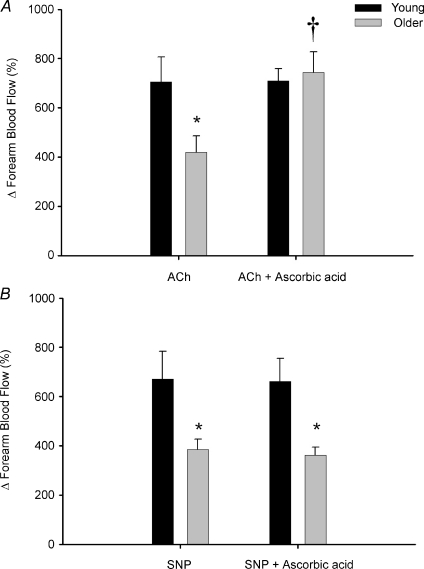
Under control conditions, the increase in FBF in response to acetylcholine was blunted in older (ΔFBF = 420 ± 67%) compared with young adults (ΔFBF = 706 ± 100%; P < 0.05), and this impairment was no longer observed during ascorbic acid administration (older ΔFBF = 744 ± 83%; young = 709 ± 44%; n.s.; Fig. 6A). The increase in FBF to sodium nitroprusside was also attenuated in older compared with young adults under control conditions (ΔFBF = 385 ± 43%vs. 671 ± 113%, respectively; P < 0.05); however, these responses were unaffected by ascorbic acid infusion (older ΔFBF = 365 ± 32%; young = 662 ± 93%; Fig. 6B). No significant differences in HR or MAP were observed between or within groups (P > 0.05), thus changes in FVC were similar to FBF (not shown).
Figure 6. Forearm vasodilatation to intra-arterial infusion of acetylcholine and sodium nitroprusside in young and older adults.
At baseline, percentage increases in forearm blood flow to acetylcholine (ACh) (endothelium dependent) were significantly impaired in older compared with young adults, and this age-associated impairment was abolished during ascorbic acid infusion (A). Older adults also had an impaired forearm blood flow response to sodium nitroprusside (SNP) (endothelium independent) at baseline, and this was unaffected during ascorbic acid infusion (B). *P < 0.05 vs. young; †P < 0.05 vs. without ascorbic acid within age group.
Plasma markers of oxidative stress
At baseline, plasma oxidized LDL was greater in the older compared with young subjects (36.5 ± 1.7 vs. 28.8 ± 2.4 U l−1; P < 0.05). Infusion of ascorbic acid did not affect these plasma levels in either young (29.8 ± 2.2 U l−1) or older adults (37.5 ± 2.7 U l−1), which most likely reflects the fact that the ascorbic acid was administered locally via brachial artery catheter and was dose-adjusted to forearm volume. Importantly, the improvements in acetylcholine-mediated vasodilatation (see above) in older subjects is consistent with previous studies and provides evidence that ascorbic acid was effective at the level of the forearm vasculature (Taddei et al. 2000, 2001).
Discussion
In the present study, we directly determined whether acutely improving endothelial vasodilator function would augment blood flow responses to contracting muscle in ageing humans. The primary new findings of the present study are as follows. First, acute infusion of ascorbic acid does not impact the rapid hyperaemic responses to single, brief mild-to-moderate intensity muscle contractions in young or older adults. Second, local ascorbic acid infusion during rhythmic handgrip exercise increased forearm blood flow by ∼30% in older adults during continuous exercise, and this was due to significant increases in forearm vascular conductance (vasodilatation). In contrast, ascorbic acid did not influence forearm haemodynamics in the young subjects. Third, when older adults transitioned from rest to steady-state exercise with concurrent ascorbic acid infusion, steady-state blood flow was significantly improved and the impaired responses under control conditions were no longer observed compared with young adults. Finally, age-related impairments in the vasodilator responses to the endothelium-dependent agonist acetylcholine were no longer evident during ascorbic acid infusion. To the best of our knowledge, these data are the first to demonstrate that acute improvements in endothelium-dependent vasodilatation are associated with augmented blood flow responses to dynamically contracting skeletal muscles of ageing humans.
Ageing and contraction-induced rapid vasodilatation: effect of ascorbic acid
In young healthy adults, single (brief) muscle contractions evoke a rapid hyperaemic response that is graded with contraction intensity (Corcondilas et al. 1964; Tschakovsky et al. 2004; Kirby et al. 2007). Although the exact mechanisms underlying this phenomenon are unclear, the impact of a ‘muscle pump’ effect appears negligible and thus vascular smooth muscle cell hyperpolarization and subsequent vasodilatation is obligatory to observe this response (Hamann et al. 2004). Recent studies implicate a role for several potential contributors to this rapid vasodilatation including mechanical effects on the vasculature (which is in part dependent on an intact endothelium) (Clifford et al. 2006; Kirby et al. 2007), K+ released during muscle activation (Armstrong et al. 2007), and acetylcholine spillover from motor nerves (VanTeeffelen & Segal, 2006). Further, data from Duza & Sarelius (2004) obtained from skeletal muscle arterioles of mice indicate that an intact endothelium is obligatory for the initiation of contraction-induced vasodilatation. In agreement with recent findings from our laboratory (Carlson et al. 2008), the data from the present study indicate that the rapid hyperaemic responses to single, brief muscle contractions of mild-to-moderate intensities are significantly impaired in older compared with young healthy adults. Further, our data indicate that improving acetylcholine-mediated (endothelium-dependent) vasodilatation via ascorbic acid infusion does not impact on the rapid hyperaemic responses in young, and more importantly, older humans. Together, these observations might provide further support for the hypothesis that the ‘normal’ rapid vasodilator responses are not mediated via acetylcholine in humans (Brock et al. 1998; Dyke et al. 1998; Naik et al. 1999). Although we cannot completely rule out a possible role of impaired endothelial function in this blunted rapid vasodilatory response with age, our data indicate that this is insensitive to the improvements in vascular function mediated via ascorbic acid. We speculate that this age-related impairment may be due to impaired K+ signalling (either release of, or responsiveness to) or impaired mechanically induced vasodilatation, but future investigations will be required to elucidate the specific underlying mechanisms.
Ageing and exercise hyperaemia during rhythmic handgrip exercise: effect of ascorbic acid
In the present study, we infused ascorbic acid into the brachial artery during rhythmic handgrip exercise to determine whether muscle blood flow would increase during sustained (continuous) exercise in young and older healthy adults. Our findings clearly indicate that blood flow increased ∼30% in older adults, whereas ascorbic acid was without effect in young adults (Fig. 2). Further, when the subjects transitioned from rest to exercise with concurrent ascorbic acid infusion (Fig. 5), similar results were obtained, indicating that this effect of ascorbic acid on muscle blood flow in older subjects cannot be attributed to a drift in haemodynamics over the course of the original 15 min exercise bout. Given that there were no changes in heart rate or mean arterial pressure throughout the exercise trials, the increases in muscle blood flow were due to a corresponding increase in vascular conductance (i.e. vasodilatation). Further, we demonstrated that the age-related decrease in endothelium-dependent vasodilatation (via acetylcholine) is abolished after infusion of ascorbic acid. Taken together, these data are the first to demonstrate that acute improvements in endothelial function increases muscle blood flow during continuous exercise via local vasodilatation in older healthy humans.
Endothelial dysfunction is one hallmark of vascular ageing, and represents a significant risk for cardiovascular disease risk and progression (Luscher et al. 1993b; Shimokawa, 1999). In addition to its role in maintaining vascular health, the endothelium produces vasodilator (e.g. nitric oxide, prostacyclin, endothelium-derived hyperpolarizing factor) and vasoconstrictor (e.g. endothelin-1, thromboxane) substances that significantly influence vascular tone (Luscher et al. 1993a). Indeed, recent evidence implicates a significant role for endothelium-derived substances in controlling muscle blood flow and vascular tone in contracting muscle of young humans (Hillig et al. 2003; Clifford & Hellsten, 2004; Saltin, 2007). As such, there has been much speculation that age-related reductions in endothelium-dependent vasodilatation play a role in the impaired skeletal muscle perfusion often observed during exercise in older healthy and diseased adults (Drexler & Hornig, 1996; Proctor & Parker, 2006). Consistent with this, work by Schrage and colleagues indicate that the normal contributions of nitric oxide and vasodilating prostaglandins to exercise hyperaemia are impaired with age (Schrage et al. 2007). In the present study, we extend these observations by demonstrating that acute improvements of endothelial vasodilator function (achieved via ascorbic acid administration) is associated with significant improvements in muscle blood flow during exercise in older but not young adults. These collective observations provide support for the hypothesis that endothelial dysfunction is mechanistically linked with altered vascular control during dynamic forearm exercise in ageing humans.
Potential mechanisms
Although we clearly demonstrate that ascorbic acid significantly improves muscle blood flow to contracting muscle during continuous exercise via vasodilatation in older adults, the signalling mechanism(s) underlying this improvement remains unclear. Consistent with previous data (Taddei et al. 2000, 2001), ascorbic acid did not influence resting forearm haemodynamics, indicating that the improvements in blood flow during exercise (and acetylcholine infusion) in older adults is only observed during stimulation of the endothelium. It is possible that ascorbic acid is acting to increase nitric oxide bioavailability, an event that could be due to direct scavenging of free radicals (e.g. O2−) (Nishikimi, 1975), or via the stabilization of tetrahydrobiopterin (BH4), an important cofactor for nitric oxide synthesis (Heller et al. 2001). In this context, recent work in older experimental animals and humans supports the hypothesis that increasing BH4 concentrations significantly improve endothelium-dependent vasodilatation (Eskurza et al. 2005; Delp et al. 2008). Whether this translates to improved vascular control in contracting muscle of ageing humans is unknown. Another possibility is that age-associated oxidative stress increases endothelin-mediated vasoconstriction, and that ascorbic acid acutely reverses this detrimental effect of endothelin on local vasodilator function, thereby allowing for greater hyperaemic responses during exercise in older adults (Bohm et al. 2007; Van Guilder et al. 2007). Clearly, future studies will be needed to determine the specific mechanisms involved in the ascorbic acid-mediated improvement in muscle blood flow and vascular control during exercise of ageing humans.
If the ascorbic acid in the present study is working specifically to reduce the accumulation of reactive oxygen species during exercise and this in turn improves vascular control in older adults, we do not know whether these species are being generated within the blood vessels or from the contracting skeletal muscle. Our finding that acetylcholine-mediated vasodilatation was restored in older adults during ascorbic acid does provide indirect evidence that at least part of this stress is present at rest and most likely within the endothelial cells (Donato et al. 2007). However, it is well known that muscle contractions can increase reactive oxygen species production (Bailey et al. 2007), and this coupled with evidence of reduced antioxidant defence systems in skeletal muscle of ageing humans (Pansarasa et al. 1999), clearly suggests a possible interaction between ascorbic acid and free radicals generated from the muscle tissue. Again, future investigations will be required to determine the potential sources of free radical generation during muscle contractions in ageing humans.
Experimental considerations
Several experimental considerations exist for the present study. First, it is possible that the absolute forearm blood flow and vascular conductance values during steady-state exercise in Trial 1 (prior to ascorbic acid) tended to be lower in older adults due to the trend for an age-related decline in MVC (and thus exercise workload) compared with young subjects. Although this was not statistically significant (∼18%; P= 0.1), this could partially explain why steady-state haemodynamics were lower in our older subjects, especially when viewed in the light of a recent study showing no difference in forearm blood flow during handgrip exercise with age (Donato et al. 2006). However, we do not believe this influences our single contraction data, as we have previously demonstrated that the impairment in the immediate hyperaemic response in older adults is independent of any age-related difference in workload (Carlson et al. 2008). Regardless, we would like to emphasize that this should not influence the interpretation of our data as they relate to the ascorbic acid-mediated improvement in forearm blood flow and vascular conductance of ascorbic acid during rhythmic handgrip exercise.
Second, with regard to our pharmacological tests, we found that the vasodilator responses to both endothelium-dependent (acetylcholine) and -independent (sodium nitroprusside) agonists were impaired with age. This latter observation is in contrast to some (DeSouza et al. 2000, 2002; Taddei et al. 2000, 2001), but not all (Taddei et al. 2000; Newcomer et al. 2005; Parker et al. 2006) previous studies showing that vascular smooth muscle cell responsiveness is preserved with age in humans. The reasons for this discrepancy are unclear, but could suggest that preserved smooth muscle cell function is simply not a universal finding with healthy ageing. However, it is important to note that in the present study, the age-associated differences in vasodilatation to acetylcholine were abolished during ascorbic acid infusion, whereas the responses to sodium nitroprusside were unaffected. Thus, the ascorbic acid-mediated increases in forearm blood flow we observed during rhythmic exercise were associated specifically with improved endothelium-dependent vasodilator function, and were not related to changes in smooth muscle cell responsiveness.
Finally, we employed mild-to-moderate intensity exercise of a small muscle mass (forearm) to isolate the local effects of muscle contraction on vascular tone, and limit the potential modulatory influences of cardiac output (systemic arterial flow) and the sympathetic nervous system on muscle blood flow responses (Dinenno et al. 2005; Koch et al. 2005). Further, the forearm vasculature is not under greater tonic sympathetic vasoconstriction at rest (Dinenno et al. 2002), whereas the leg circulation is characterized by augmented basal α-adrenergic vasoconstrictor tone (Dinenno et al. 2001). Future studies will be required to determine whether ascorbic acid is capable of improving vascular control in ageing humans during higher intensity exercise or larger muscle mass exercise (e.g. cycling) where cardiac output and sympathetic neural influences are involved in the integrative control of skeletal muscle blood flow.
Conclusions
The collective findings from the present investigation indicate that acute improvements in endothelial vasodilator function are associated with augmented blood flow responses to contracting muscles of ageing humans. However, this improved hyperaemia was only observed during continuous dynamic exercise, as the responses to single, brief contractions were not influenced by ascorbic acid and thus still significantly impaired with age. The exact signalling mechanisms underlying this improved exercise hyperaemia during continuous exercise need to be elucidated, and whether other longer-term interventions that improve endothelial vasodilator function in humans (e.g. aerobic exercise training) translate to improved muscle blood flow during exercise in older adults remains to be determined. Finally, the divergent effects of ascorbic acid on contraction-induced rapid vasodilatation versus sustained exercise hyperaemia highlight the complex alterations in vascular physiology with human ageing.
Acknowledgments
We would like to thank Whitney Lewis for her assistance in these experiments, as well as the subjects who volunteered to participate. This research was supported by National Institutes of Health awards AG022337, AG027150, and HL087952 (F. A. Dinenno).
References
- Armstrong ML, Dua AK, Murrant CL. Potassium initiates vasodilatation induced by a single skeletal muscle contraction in hamster cremaster muscle. J Physiol. 2007;581:841–852. doi: 10.1113/jphysiol.2007.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DM, Lawrenson L, McEnemy J, Young IS, James PE, Jackson SK, Henry RR, Mathieu-Costello O, McCord JM, Richardson RS. Electron paramagnetic spectroscopic evidence of exercise-induced free radical accumulation in human skeletal muscle. Free Rad Res. 2007;41:182–190. doi: 10.1080/10715760601028867. [DOI] [PubMed] [Google Scholar]
- Bohm F, Settergren M, Pernow J. Vitamin C blocks vascular dysfunction and release of interleukin-6 induced by endothelin-1 in humans in vivo. Atherosclerosis. 2007;190:408–415. doi: 10.1016/j.atherosclerosis.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M, Kjaer M. Combined inhibition of nitric oxide and prostaglandins reduces skeletal muscle blood flow during exercise. J Physiol. 2002;543:691–698. doi: 10.1113/jphysiol.2002.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock RW, Tschakovsky ME, Shoemaker JK, Halliwill JR, Joyner MJ, Hughson RL. Effects of acetylcholine and nitric oxide on forearm blood flow at rest and after a single contraction. J Appl Physiol. 1998;85:2249–2254. doi: 10.1152/jappl.1998.85.6.2249. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Clifford PS. Autonomic control of skeletal muscle blood flow at the onset of exercise. Am J Physiol Heart Circ Physiol. 1999;277:H1872–H1877. doi: 10.1152/ajpheart.1999.277.5.H1872. [DOI] [PubMed] [Google Scholar]
- Carlson RE, Kirby BS, Voyles WF, Dinenno FA. Evidence for impaired skeletal muscle contraction-induced rapid vasodilation in aging humans. Am J Physiol Heart Circ Physiol. 2008;294:H1963–H1970. doi: 10.1152/ajpheart.01084.2007. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol. 2006;572:561–567. doi: 10.1113/jphysiol.2005.099507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcondilas A, Koroxenidis GT, Shepherd JT. Effect of a brief contraction of forearm muscles on forearm blood flow. J Appl Physiol. 1964;19:142–146. doi: 10.1152/jappl.1964.19.1.142. [DOI] [PubMed] [Google Scholar]
- Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol. 2008;586:1161–1168. doi: 10.1113/jphysiol.2007.147686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza CA, Clevenger CM, Greiner JJ, Smith DT, Hoetzer GL, Shapiro LF, Stauffer BL. Evidence for agonist-specific endothelial vasodilator dysfunction with ageing in healthy humans. J Physiol. 2002;542:255–262. doi: 10.1113/jphysiol.2002.019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional α-adrenergic vasoconstriction in healthy men. Circulation. 2002;106:1349–1354. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol. 2003;553:281–292. doi: 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments α-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol. 2004;287:H2576–H2584. doi: 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ, Halliwill JR. Failure of systemic hypoxia to blunt sympathetic neural vasoconstriction in the human forearm. J Physiol. 2003;549:985–994. doi: 10.1113/jphysiol.2003.042507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic α-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol. 2005;567:311–321. doi: 10.1113/jphysiol.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Tanaka H, Stauffer BL, Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented α-adrenergic vasoconstriction. J Physiol. 2001;536:977–983. doi: 10.1111/j.1469-7793.2001.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-κB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol. 2006;290:H272–H278. doi: 10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- Drexler H, Hornig B. Importance of endothelial function in chronic heart failure. J Cardiovasc Pharmacol. 1996;27:S9–12. doi: 10.1097/00005344-199600002-00003. [DOI] [PubMed] [Google Scholar]
- Duza T, Sarelius IH. Increase in endothelial cell Ca2+ in response to mouse cremaster muscle contraction. J Physiol. 2004;555:459–469. doi: 10.1113/jphysiol.2003.051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke CK, Dietz NM, Lennon RL, Warner DO, Joyner MJ. Forearm blood flow responses to handgripping after local neuromuscular blockade. J Appl Physiol. 1998;84:754–758. doi: 10.1152/jappl.1998.84.2.754. [DOI] [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann JJ, Buckwalter JB, Clifford PS. Vasodilatation is obligatory for contraction-induced hyperaemia in canine skeletal muscle. J Physiol. 2004;557:1013–1020. doi: 10.1113/jphysiol.2004.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, Werner ER. l-Ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem. 2001;276:40–47. doi: 10.1074/jbc.M004392200. [DOI] [PubMed] [Google Scholar]
- Hillig T, Krustrup P, Fleming I, Osada T, Saltin B, Hellsten Y. Cytochrome P450 2C9 plays an important role in the regulation of exercise-induced skeletal muscle blood flow and oxygen uptake in humans. J Physiol. 2003;546:307–314. doi: 10.1113/jphysiol.2002.030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO, Kohrt WM. Handbook of Physiology. Aging. Bethesda, MD, USA: American Physiological Society; 1995. Exercise; pp. 633–666. [Google Scholar]
- Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol. 2007;583:861–874. doi: 10.1113/jphysiol.2007.131250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Carslon RE, Dinenno FA. Graded sympatholytic effect of exogenous ATP on postjunctional α-adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol. 2008;586:4305–4316. doi: 10.1113/jphysiol.2008.154252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch DW, Newcomer SC, Proctor DN. Blood flow to exercising limbs varies with age, gender, and training status. Can J Appl Physiol. 2005;30:554–575. doi: 10.1139/h05-141. [DOI] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol. 2003;285:H1023–H1031. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- Luscher TF, Boulanger CM, Zhihong Y, Noll G, Dohi Y. Interactions between endothelium-derived relaxing and contracting factors in health and cardiovascular disease. Circulation. 1993;87:V-36–V-44. [Google Scholar]
- Luscher TF, Tanner FC, Tschudi MR, Noll G. Endothelial dysfunction in coronary artery disease. Annu Rev Med. 1993;44:395–418. doi: 10.1146/annurev.me.44.020193.002143. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Gonzalez-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol. 2007;581:853–861. doi: 10.1113/jphysiol.2006.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik JS, Valic Z, Buckwalter JB, Clifford PS. Rapid vasodilation in response to a brief tetanic muscle contraction. J Appl Physiol. 1999;87:1741–1746. doi: 10.1152/jappl.1999.87.5.1741. [DOI] [PubMed] [Google Scholar]
- Newcomer SC, Leuenberger UA, Hogeman CS, Proctor DN. Heterogeneous vasodilator responses of human limbs: influence of age and habitual endurance training. Am J Physiol Heart Circ Physiol. 2005;289:H308–H315. doi: 10.1152/ajpheart.01151.2004. [DOI] [PubMed] [Google Scholar]
- Nishikimi M. Oxidation of ascorbic acid with superoxide anion generated by the xanthine-xanthine oxidase system. Biochem Biophys Res Commun. 1975;17:463–468. doi: 10.1016/0006-291x(75)90710-x. [DOI] [PubMed] [Google Scholar]
- Pansarasa O, Bertorelli L, Vecchiet J, Felzani G, Marzatico F. Age-dependent changes of antioxidant activities and markers of free radical damage in human skeletal muscle. Free Radic Biol Med. 1999;27:617–622. doi: 10.1016/s0891-5849(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Parker BA, Ridout SJ, Proctor DN. Age and flow-mediated dilation: a comparison of dilatory responsiveness in the brachial and popliteal arteries. Am J Physiol Heart Circ Physiol. 2006;291:H3043–H3049. doi: 10.1152/ajpheart.00190.2006. [DOI] [PubMed] [Google Scholar]
- Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol. 2003;284:H1251–H1259. doi: 10.1152/ajpheart.00790.2002. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol. 2003;95:1963–1970. doi: 10.1152/japplphysiol.00472.2003. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Parker BA. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation. 2006;13:315–327. doi: 10.1080/10739680600618967. [DOI] [PubMed] [Google Scholar]
- Saltin B. Exercise hyperaemia: magnitude and aspects on regulation in humans. J Physiol. 2007;583:819–823. doi: 10.1113/jphysiol.2007.136309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998;162:421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol. 2007;579:227–236. doi: 10.1113/jphysiol.2006.124313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduce forearm exercise hyperaemia in human. J Physiol. 2004;557:599–611. doi: 10.1113/jphysiol.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Victor RG. Regulation of muscle sympathetic nerve activity during exercise in humans. Exerc Sport Sci Rev. 1991;19:313–349. [PubMed] [Google Scholar]
- Shimokawa H. Primary endothelial dysfunction: atherosclerosis. J Mol Cell Cardiol. 1999;31:23–37. doi: 10.1006/jmcc.1998.0841. [DOI] [PubMed] [Google Scholar]
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM. Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol. 2004;96:639–644. doi: 10.1152/japplphysiol.00769.2003. [DOI] [PubMed] [Google Scholar]
- Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension. 2007;50:403–409. doi: 10.1161/HYPERTENSIONAHA.107.088294. [DOI] [PubMed] [Google Scholar]
- VanTeeffelen JW, Segal SS. Rapid dilation of arterioles with single contraction of hamster skeletal muscle. Am J Physiol Heart Circ Physiol. 2006;290:H119–H127. doi: 10.1152/ajpheart.00197.2005. [DOI] [PubMed] [Google Scholar]
- Wu KK, Thiagarajan P. Role of endothelium in thrombosis and hemostasis. Annu Rev Med. 1996;47:315–331. doi: 10.1146/annurev.med.47.1.315. [DOI] [PubMed] [Google Scholar]