Abstract
It has been proposed that spinal lamina I neurons with ascending axons that project to the midbrain play a crucial role in hyperalgesia. To test this hypothesis the quantitative properties of lamina I spinoparabrachial neurons in the chronic constriction injury (CCI) model of neuropathic pain were compared to those of unoperated and sham-operated controls. Behavioural testing showed that animals with a CCI exhibited heat hyperalgesia within 4 days of the injury, and this hyperalgesia persisted throughout the 14-day post-operative testing period. In the CCI, nociceptive lamina I spinoparabrachial neurons had heat thresholds that were significantly lower than controls (43.0 ± 2.8°C vs. 46.7 ± 2.6°C; P < 10−4, ANOVA). Nociceptive lamina I spinoparabrachial neurons were also significantly more responsive to graded heat stimuli in the CCI, compared to controls (P < 0.02, 2-factor repeated-measures ANOVA), and increased after-discharges were also observed. Furthermore, the heat-evoked stimulus–response functions of lamina I spinoparabrachial neurons in CCI animals co-varied significantly (P < 0.03, ANCOVA) with the amplitude of heat hyperalgesia determined behaviourally. Taken together these results are consistent with the hypothesis that lamina I spinoparabrachial neurons have an important mechanistic role in the pathophysiology of neuropathic pain.
Damage to, or dysfunction of, the nervous system often leads to sensory disorders that can include neuropathic pain. Clinically, neuropathic pains are heterogeneous in nature, but they share several common features such as spontaneous pain, hyperalgesia (increased sensitivity to noxious stimuli) and allodynia (painful responses to normally innocuous stimuli), suggestive of common mechanisms. Several animal models of neuropathic pain have been developed (Zeltser & Seltzer, 1994), though different models produce different combinations of behavioural signs of neuropathic pain (Kim et al. 1997).
A few studies have shown that peripheral nociceptors can become hyper-responsive after nerve injury (Shea & Perl, 1985; Ahlgren et al. 1992; Tanner et al. 1998; Andrew & Greenspan, 1999; Shim et al. 2005). However, many of the symptoms of neuropathic pain are thought to result from abnormal processing within the central nervous system (Bennett, 1994). It is generally assumed that this abnormal processing occurs within the spinal cord, as it is the site of the first synapse in nociceptive pathways, and considerable integration and modulation can occur there. Previous physiological studies have characterized the receptive properties of nociceptive spinal neurons in animals with neuropathic pain (Paleček et al. 1992; Laird & Bennett, 1993; Takaishi et al. 1996; Chapman et al. 1998). Most of these previous studies did not identify specific neural circuits, but comparisons of quantitative responses to graded noxious stimuli showed that neurons from animals with neuropathic pain were no different to neurons from controls (Laird & Bennett, 1993; Takaishi et al. 1996; Chapman et al. 1998). Surprisingly, in the chronic constriction injury model of neuropathic pain, spinothalamic neurons in the deep dorsal horn were less responsive to noxious heat stimuli compared to controls (Paleček et al. 1992). Clearly these data are inconsistent with the behavioural signs of neuropathic pain, where there is enhanced responsiveness to noxious stimuli.
One possible explanation for these findings is that most previous studies have focused on unidentified neurons in the deep dorsal horn of the spinal cord, which were probably a mixed population of excitatory and inhibitory interneurons, rather than projection neurons (Spike et al. 2003). Also, recent in vitro studies (Ikeda et al. 2003, 2006) have proposed an important role in hyperalgesia for neurons in the superficial layer of the spinal cord (lamina I) that have axons that project to the midbrain. Thus the aim of the current study was to test the hypothesis that sensitization of lamina I neurons with axons that projected to the midbrain parabrachial nucleus could provide a mechanistic explanation for the behavioural signs of neuropathic pain that are observed in vivo after chronic constriction injury (CCI) of the rat sciatic nerve.
Methods
Ethical approval
All experiments were approved by the Ethical Review Panel at Sheffield University, and were licensed under the UK Animals (Scientific Procedures) Act 1986. Experiments were performed on 80 male Sprague–Dawley rats (225–390 g) that were divided into three groups: (1) an unoperated control group (n= 34); (2) animals with neuropathic pain following chronic constriction of a sciatic nerve (n= 33) and (3) sham-operated animals (n= 13).
Chronic constriction injury
Rats were anaesthetized with isoflurane (4% induction, 2% maintenance) and the left sciatic nerve surgically exposed. Four 4/0 chromic gut sutures were tied loosely around the nerve at 1 mm intervals, proximal to the sciatic trifurcation (Bennett & Xie, 1988). The wound was closed in layers and the animals allowed to recover consciousness before being returned to their cages. Sham-operated animals were prepared similarly, except that the nerve was exposed but not constricted.
Behavioural testing
Behavioural tests to determine baseline nociceptive sensitivity were performed 6, 3 and 1 days before surgery, and then 1, 4, 6, 8, 11 and 14 days post-operatively to detect signs of neuropathic pain. On each occasion the animals were placed in a clear plastic cage with a glass floor and allowed to acclimatise until all exploratory and grooming behaviour stopped (15–45 min.). A radiant heat source (IITC, Woodland Hills, CA, USA) was focused on the plantar surface of each hindpaw and the latency to paw withdrawal was measured (Hargreaves et al. 1988). Five latency measurements for each paw were recorded during each testing session and the mean calculated. To prevent thermal injury to the skin the radiant heat stimulus automatically cut off if an animal did not withdraw its paw within 20 s. In order to prevent behavioural sensitization/desensitisation, successive stimuli were applied at 15 min intervals and the order in which the paws were stimulated was varied systematically. Fifty per cent withdrawal thresholds to mechanical stimulation were also determined; the animals were placed in a cage with a wire mesh floor and a series of progressively stiffer von Frey monofilaments were applied using the ‘up–down’ method of Chaplan et al. (1994).
Animal preparation for single-unit recording
Rats were anaesthetized with urethane (1.2 g kg−1) injected intra-peritoneally. Anaesthetic depth was maintained with additional doses of urethane (100 mg) given intravenously. Anaesthetic depth was sufficient that the animals were areflexic to pinching a forepaw. Cannulae were placed into the left carotid artery, right jugular vein and into the trachea. Blood pressure was monitored with a pressure transducer that was connected to the arterial cannula. Body temperature was maintained at 38.0 ± 0.5°C with an electric blanket that was controlled from a rectal thermistor. The long-acting local anaesthetic bupivacaine (0.3 ml of 0.5% solution) was injected subcutaneously at the sites of all incisions, and EMLA (eutectic mixture of local anaesthetics; AstraZeneca, Luton, UK) was applied topically to the ear canals.
The lumbar enlargement of the spinal cord was exposed by dorsal laminectomy and a pool formed from the surrounding skin that was filled with warm Ringer solution. A craniotomy was made to permit the insertion of stimulating electrodes into the right parabrachial nucleus. The animal's head was mounted in a stereotaxic headholder (incisor bar 3 mm below the zero position) and the animal suspended in vertebral clamps. D-Tubocurarine (150 μg) was injected intravenously to induce neuromuscular blockade and the animal ventilated to maintain end-tidal CO2 levels of 3.8–4.2%. During neuromuscular blockade, anaesthetic depth was considered sufficient if blood pressure and heart rate were stable during noxious stimulation.
Antidromic activation of spinoparabrachial neurons
An array of stimulating electrodes was placed stereotaxically into the right parabrachial nucleus (0.1 mm rostral – 0.5 mm caudal to lambda, 1.5–2.0 mm from the midline, 6.0–7.0 mm below the cortical surface). The array consisted of three concentric bipolar electrodes (Rhodes SNE-100; David Kopf, Tujunga, CA, USA) that were evenly spaced at 1 mm intervals. The electrodes were staggered vertically so that the tip of the medial electrode was in the vicinity of the internal lateral subnucleus of the parabrachial complex, the middle electrode was near the external lateral subnucleus and the lateral electrode was near the Kölliker–Fuse nucleus. Anatomical tracing studies have shown that these nuclei/subnuclei are the principal targets within the parabrachial complex of ascending spinal inputs (Slugg & Light, 1994; Bernard et al. 1995; Feil & Herbert, 1995). Bipolar stimuli were applied between the inner and outer conductors of individual electrodes, or occasionally between the conductors of adjacent pairs of electrodes. Electrical stimuli were 1 or 2 ms in duration and were up to 1.5 mA in intensity. When the stimulating electrode array was well positioned, antidromic thresholds were of the order of 30–100 μA at 1 ms stimulus duration, but stronger stimuli were needed on the occasions that array position was not optimal.
Identification and classification of lamina I spinoparabrachial neurons
Extracellular recordings of the activity of neurons in the superficial dorsal horn were made with tungsten microelectrodes (impedance 8–10 MΩ; FHC Co., Bowdoin, ME, USA) that were inserted into the left lumbar enlargement of the spinal cord. Neurons were selected for study as long as they had receptive fields on the ventral surface of the hindpaw. Lamina I was identified beneath a layer of fibres having regular ongoing activity (muscle spindles). At these sites brisk responses to squeezing the hindpaw with fine tipped forceps were evoked, although in this region of the rat spinal cord there is an absence of background activity typical of thermoreceptive (cooling-specific) neurons, in contrast to the cat (Craig et al. 2001). Single spinoparabrachial neurons were identified by their antidromic responses to electrical stimulation from the contralateral parabrachial nucleus with the implanted electrode array. The antidromic nature of the activation was confirmed if the neuron showed an all-or-none response at threshold, responded with invariant latency, followed one-for-one a 250 Hz train of six antidromic shocks and collision occurred between antidromic and orthodromic impulses (Fig. 1). In 45 cases electrolytic lesions (+15 μA, 15 s) were made at recording sites and the animal perfused intravascularly with 4% paraformaldehyde. Recording sites and the tracks of the stimulating electrodes were identified in 50 μm transverse sections that were stained with thionin.
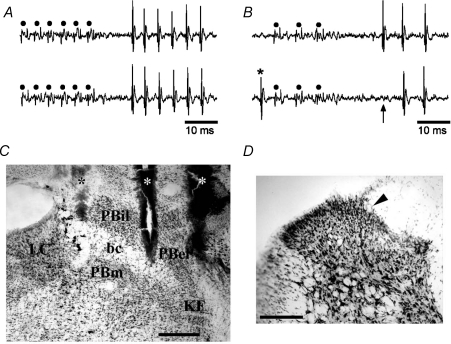
Figure 1. Identification of lamina I spinoparabrachial neurons in vivo.
A, pair of traces showing 1-for-1 following of a train of 6 antidromic electrical stimuli (45 μA, 2 ms, 250 Hz; dots) delivered from the middle stimulating electrode in the contralateral parabrachial nucleus. B, collision of the first antidromic impulse in a train of 3 (150 Hz, upper trace) when an orthodromic impulse (asterisk, lower trace) occurred within the critical interval. The arrow indicates the point at which the first antidromic response should have occurred. C, photomicrograph of a frozen section stained with thionin showing the tracks of the stimulating electrodes (asterisks) in the contralateral parabrachial nucleus. Bar is 0.4 mm. bc, brachium conjunctivum; KF, Kölliker–Fuse nucleus; LC, locus coeruleus; PBel, external lateral subnucleus of the parabrachial area; PBil, internal lateral nucleus of the parabrachial area; PBm, medial subnucleus of the parabrachial area. D, photomicrograph of the contralateral spinal dorsal horn at the level of the 3rd lumbar segment. An arrowhead marks the position of an electrolytic microlesion that was made at the recording site of the cell shown in A and B. Dorsal is up, lateral is left. Bar is 0.2 mm.
Each neuron isolated was classified using the following forms of cutaneous stimulation: cooling with a beaker of wet ice, innocuous brushing, squeezing with blunt forceps, radiant warming and noxious heating. Noxious stimuli were used sparingly, to avoid sensitization, and pinching with serrated forceps and intense heat stimuli were avoided altogether. Neurons were classified based on the scheme developed for lamina I projection neurons in the cat (Craig et al. 2001; Wilson et al. 2002). Thus, neurons maximally responsive to innocuous cooling or warming, and insensitive to other stimulus modalities were classified as COOL or WARM thermoreceptive neurons, respectively; cells that responded to cold stimuli and that were also responsive to noxious mechanical and noxious heat stimuli were classified as polymodal-nociceptive or HPC cells (for heat, pinch and cold); units that responded to noxious mechanical and/or noxious heat stimuli, but not to noxious cold stimulation were classified as nociceptive-specific (NS); finally neurons that responded to innocuous brushing stimuli as well as to noxious mechanical and/or thermal stimuli were classified as wide dynamic range (WDR) neurons.
Quantitative characterization of lamina I spinoparabrachial neurons
Prior to quantitative characterization, the background discharge rate of each cell at room temperature was recorded for 1 min in the absence of stimulation. The extent of each neuron's receptive field was mapped after this period using either a beaker filled with wet ice (for cool- and cold-sensitive cells), or with a series of pinches applied manually with smooth-tipped fine forceps. Receptive fields were drawn onto standardized, calibrated cartoons, and receptive field area was measured digitally (Cell D, Olympus, UK). Quantitative thermal stimuli were applied with a custom-built thermoelectric (Peltier) element (10 × 10 mm) under feedback control. Standard cooling and heating protocols were used that were similar to prior studies on cats (Craig et al. 2001). The cooling stimulus sequence consisted of a descending staircase series of 3°C cooling steps of 20 s duration delivered from a baseline of 34°C to a final skin-thermode interface temperature of 4°C. Warming steps (10 s duration) of 36, 38 and 40°C were added to the end of the cooling sequence to test for inhibition of COOL cells or to aid in the characterization of WARM cells. The heating stimulus sequence consisted of a series of ‘ramp and hold’ steps (rise rate 9°C s−1) from a baseline of 34°C to final temperatures of 42, 44, 46, 48, 50 and 52°C. The stimuli were 10 s in duration and the interstimulus interval was 1 min. Quantitative responses to graded mechanical stimuli (Andrew & Craig, 2002) were not studied in these experiments. In 65 rats only a single neuron was studied per experiment. However in six controls, seven CCI and two sham-operated animals, two neurons were studied. As repeated noxious stimuli can produce sensitization, in the experiments where a second cell was studied, it was selected so that its receptive field never overlapped that of the previously characterized unit.
After unit characterization was completed, the conduction velocities of the afferents fibres supplying a neuron were determined by intracutaneous electrical stimulation. A pair of needle electrodes was inserted into the cutaneous receptive field, and graded electrical stimuli (1 ms stimulus duration, 0.25 Hz) applied. The latencies of different components of the afferent inputs were recorded from oscilloscope traces, and the conduction distance estimated with a suture thread. Animals were killed with an overdose of anaesthetic at the end of the experiment.
Data analysis
Electrophysiological data were displayed using conventional oscilloscopic and audio methods. Data were digitized with a computer interface (Power1401; CED, Cambridge, UK) for off-line analysis. Neural records were sampled at 25 kHz and stimulus records at 1 kHz. For quantitative analysis, the average discharge rate was calculated by dividing the total response, beginning at stimulus onset, by the stimulus duration. Background activity was not subtracted from evoked responses as there is evidence that it is of biological significance (Andrew & Craig, 2002). Statistical analyses were performed with parametric tests or non-parametric tests, as appropriate (data not normally distributed or variance not homogeneous). Neuronal stimulus–response functions were compared between different experimental groups using 2-factor, repeated-measures ANOVA, and ANCOVA was used to investigate whether neural responses varied as a function of behavioural measures of neuropathic pain. All tests were performed with Statistica software (Statsoft; Tulsa, OK, USA), and P values < 0.05 were considered significant.
Results
Behaviour
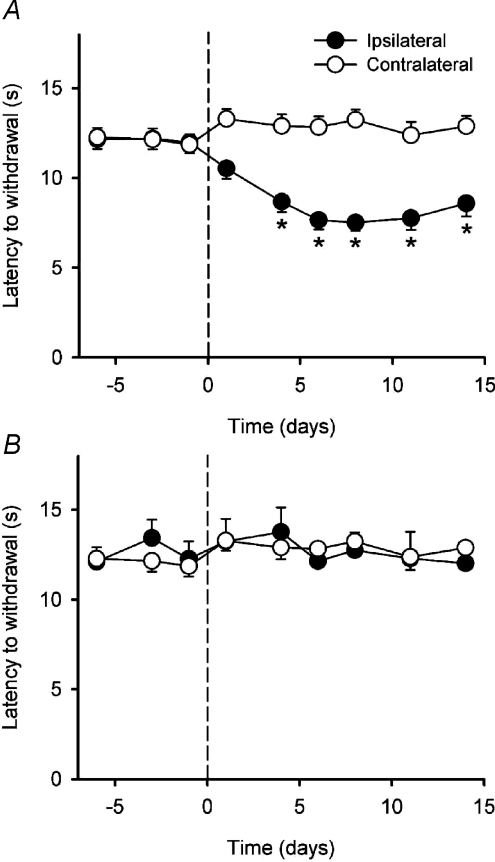
Rats with a CCI showed changes in posture typical of this model of neuropathic pain (Bennett & Xie, 1988; Attal et al. 1990): the hindpaw was everted and the toes ventroflexed. During thermal behavioural testing the operated hindlimb was often withdrawn with an exaggerated flicking movement that was followed by repeated licking. This was never seen in sham-operated rats. The time course of the development of heat hyperalgesia is shown in Fig. 2A. As can be clearly seen, there was a highly significant reduction in paw withdrawal latency after the CCI (P < 10−5; ANOVA). When compared to the contralateral paw, significant differences in latency were first evident 4 days post-surgery (P < 0.02; 2-factor repeated measures ANOVA followed by Tukey's post hoc test) and they persisted to the last testing session (P < 0.03). There was no significant change in withdrawal latency in sham-operated rats over time (P > 0.9, ANOVA) or when compared to the contralateral paw (P > 0.5, 2-factor repeated-measures ANOVA; Fig. 2B).
Figure 2. Time course of the development of the behavioural effects of the CCI.
A, mean (± 1 s.e.m.) paw withdrawal latencies to radiant heat stimulation of the ipsilateral and contralateral hindpaws in the CCI animals (n= 33). The CCI was performed on day 0 (indicated by the dashed line) Asterisks indicate significant differences (P < 0.02; 2-factor repeated-measures ANOVA followed by Tukey's post-hoc test) between the ipsilateral and contralateral paws. B, mean (± 1 s.e.m.) paw withdrawal latencies to radiant heat stimulation of the ipsilateral and contralateral hindpaws in the sham-operated animals (n= 13). The sham operation was performed on day 0 (indicated by the dashed line).
In animals with a CCI, there was no significant difference in 50% paw withdrawal thresholds over time when compared to the contralateral paw (P > 0.7, Kruskall–Wallis ANOVA; data not shown).
General properties of lamina I spinoparabrachial neurons
Terminal experiments were performed on CCI and sham-operated rats after behavioural testing was completed (14 days post-operatively), or when robust behavioural signs of neuropathic pain were evident (left/right paw withdrawal latency difference of ≥ 10 s). This latter approach maximised the chance of identifying potential neuronal mechanisms. On average, terminal experiments were performed on animals with a CCI 8 days after the completion of behavioural testing (s.d. 7.4); terminal experiments on sham-operated rats were performed within a similar period (mean 9 days post-testing completion, s.d. 9.1). Based on prior studies, behavioural signs of neuropathic pain persist for approximately 40 days after the CCI (reviewed in Vierck et al. 2005). Thus despite the variability in the timing of the terminal electrophysiological experiment, it would be expected that all of the CCI animals would still show neuropathic pain behaviours.
Recordings were made from 95 lamina I spinoparabrachial neurons in three groups of animals. Forty neurons were studied in control rats, 40 cells were studied in animals with a CCI and 15 neurons were recorded in sham-operated control rats. All of these neurons followed a 250 Hz train of six antidromic shocks with an invariant latency, and also showed collision of the first impulse in a train with a spontaneous or evoked impulse. The mean antidromic latency of all lamina I spinoparabrachial neurons was 15.8 ms (s.d. 19.7), giving an average central conduction velocity of 8.5 m s−1 (s.d. 5.0). This value is consistent with lightly myelinated (Aδ) axons, as reported previously (Bester et al. 2000; Keller et al. 2007), although five cells (3 control, 2 CCI) had central axons with conduction velocities less than 2.5 m s−1, indicating that they were unmyelinated. Occasionally long-latency (150–200 ms) evoked responses were observed that had substantial jitter (10–20 ms). These cells were typically encountered dorsal to lamina I and they did not follow high frequency stimulus trains; they were assumed to be orthodromically activated (McMahon & Wall, 1988), and were not studied further.
Thirty of the 45 lesions that were made were recovered in histological sections (n= 14 control; n= 12 CCI, n= 4 sham). All of the lesions were located in lamina I, usually just medial to the dorsal root entry zone, such as the example shown in Fig. 1. The location of the lesions is consistent with retrograde tracing studies (Spike et al. 2003) that have shown that spinoparabrachial neurons are concentrated in lamina I.
Most neurons were antidromically activated from the medial (88%) and middle (82%) stimulating electrodes (corresponding to the internal lateral and external lateral subnuclei). Typically (60 of 95 units) neurons were activated from all three stimulating electrodes (Table 1), but all combinations of effective stimulating sites were observed.
Table 1.
Quantitative electrophysiological and receptive field properties of lamina I spinoparabrachial neurons in control, CCI and sham-operated rats
| Central CV (m s−1) | Background firing (impulses s−1) | RF size (mm2) | Proportion of cells with effective antidromic sites |
|||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| Control | 8.5 ± 5.3 | 0.2 ± 0.3 | 96.8 ± 85.3 | 7/40 | 7/40 | 26/40 |
| (n= 40) | (0.6–19.1) | (0–1.4) | (15.7–329.7) | |||
| CCI | 8.1 ± 4.8 | 0.2 ± 0.7 | 86.7 ± 96.7 | 9/40 | 5/40 | 26/40 |
| (n= 40) | (1.4–25.0) | (0–4.1) | (9.7–327.5) | |||
| Sham | 9.6 ± 4.2 | 0.3 ± 0.6 | 73.8 ± 75.4 | 3/15 | 4/15 | 8/15 |
| (n= 15) | (4.7–18.2) | (0–1.6) | (23.9–291.8) | |||
CV: conduction velocity; RF: receptive field. For central CV, background firing rate and RF size values are means ±s.d. with the data range in parentheses.
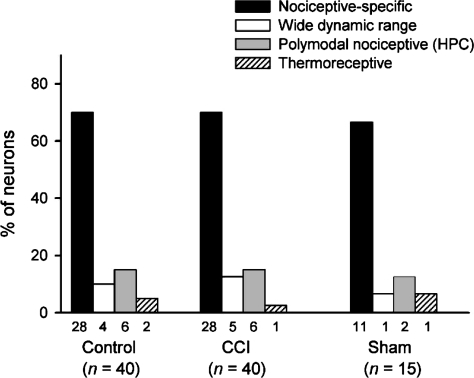
Based on their responses to cutaneous stimulation, all of the neurons studied could be classified into one of the categories listed in Methods; none had an undetectable receptive field (cf. Laird & Bennett, 1993). In each group of rats, the majority of the cells were NS neurons that were only activated by noxious stimuli (Fig. 3), although other cell types were also identified. There was no significant difference between the frequency of wide dynamic range neurons in the CCI group compared to the control group (P > 0.5; χ2 test), nor was there any difference in the proportion of neurons that responded to noxious heat (P > 0.3, χ2 test).
Figure 3. Frequency distribution of different functional classes of lamina I spinoparabrachial neurons in control, CCI and sham-operated animals.
Each neuron isolated was classified using cutaneous stimuli of different modalities (see Methods). The bars show the frequency distribution of the 4 classes of lamina I spinoparabrachial neurons in the three groups of animals studied. There were no significant differences between groups (P > 0.3, χ2 test).
There were no significant differences in the central conduction velocities (P > 0.5, ANOVA), background activity (P > 0.6, ANOVA) and receptive field size (P > 0.6, ANOVA) or in the patterns of projection to the parabrachial nucleus (P > 0.6, general linear model) between neurons from animals with a CCI when compared to unoperated and sham-operated controls (Table 1).
Lamina I spinoparabrachial neurons received inputs from peripheral nerve fibres with both A- and C-fibre conduction velocities, consistent with earlier studies (Bester et al. 2000). A-fibre inputs were in the range 7.4–16.7 m s−1, classifying them as Aδ fibres (5–25 m s−1). C-fibre inputs, i.e. those less than 2.5 m s−1, to lamina I spinoparabrachial neurons usually showed a bimodal distribution of conduction velocities: one peak of activity corresponded with fibres having a conduction velocity of 0.9 ± 0.4 m s−1 (mean ±s.d.) and the other having a conduction velocity of 0.5 ± 0.1 m s−1. There were no obvious qualitative or quantitative differences between neurons in controls and neurons in animals that had received a CCI.
Responses to graded cool and cold stimuli
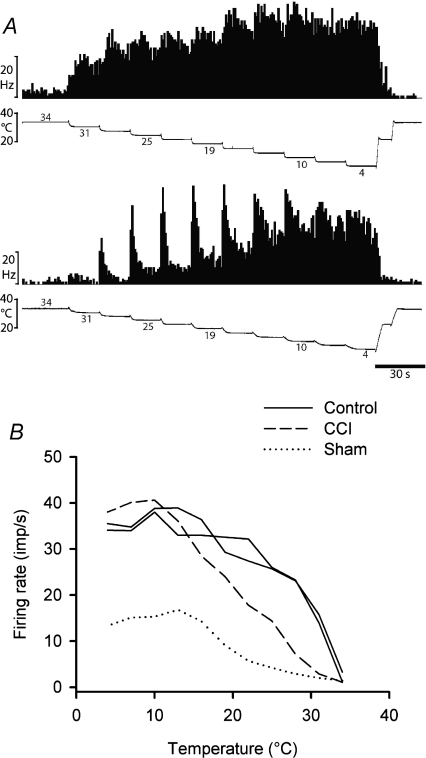
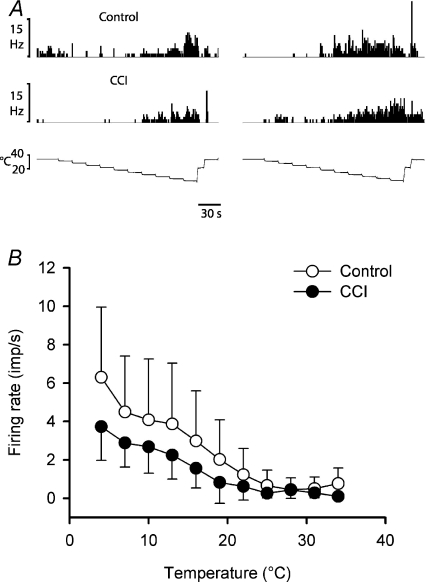
Four neurons were activated by innocuous cooling stimuli (n= 2 control; n= 1 CCI, n= 1 sham-operated) and not by other stimulus modalities; these were classified as COOL neurons. Three of these COOL cells were also inhibited by warming in the range 36–40°C. Cooling-specific lamina I spinoparabrachial neurons have not been reported previously in the rat (Bester et al. 2000), though they are common in lamina I spinoparabrachial and spinothalamic projections in the cat (Light et al. 1993; Craig et al. 2001), and COOL-like responses have been described in lamina I spinothalamic projections from the rat cervical spinal cord (Zhang et al. 2006). Examples of quantitative responses from two neurons are shown in Fig. 4, along with the stimulus–response curves of all four units. All of the neurons had thresholds of 31°C, and their stimulus–response curves reached saturation at 10–16°C, typical of cooling-specific lamina I spinothalamic neurons in the cat (Craig et al. 2001). The sample size was not large enough to distinguish statistical differences between neurons from different groups of animals.
Figure 4. Stimulus encoding by lamina I spinoparabrachial COOL neurons.
A, peristimulus time histograms from two cooling-specific neurons showing their responses to graded intensity cooling stimuli. The upper pair of records was from an unoperated control animal and the lower pair was from an animal that had received a CCI. B, stimulus–response curves of all 4 cooling-specific neurons isolated in the current study.
Fourteen neurons were activated by cold stimuli (n= 6 control, n= 6 CCI, n= 2 sham-operated), and in addition were also responsive to noxious heat stimuli and noxious mechanical stimuli. These cells were classified as HPC (polymodal nociceptive) neurons. Representative examples of the cold-evoked activity of four HPC lamina I spinoparabrachial neurons are shown in Fig. 5, along with the population stimulus–response curves. As can been seen by comparison with Fig. 4, the thresholds for activation of HPC neurons are colder than those of COOL neurons, HPC neurons do not show dynamic responses to individual cooling steps and HPC responses do not saturate like COOL cell activity does at the lowest temperatures. Also, HPC neurons were not inhibited by warming stimuli. The existence of HPC neurons in the rat spinoparabrachial pathway has been alluded to in prior experiments (Bester et al. 2000), but their presence could not be explicitly confirmed due to differences in stimulation methods. The use of graded cold stimuli in the present experiments has provided unambiguous confirmation of HPC cells in the rat lamina I spinoparabrachial pathway.
Figure 5. Encoding of cool and cold temperatures by HPC lamina I spinoparabrachial neurons.
A, individual histogram responses from 4 HPC neurons (2 control, 2 CCI) to the standard cold stimulus sequence. B, mean (± 1 s.d.) stimulus–response curves to cold stimuli for HPC neurons in controls and CCI animals (n= 6 in each group). There was no significant difference between groups when the stimulus–response curves were compared (P > 0.2, 2-factor ANOVA).
The responsiveness of HPC neurons did not differ between controls and rats with a CCI. The mean cold threshold of HPC neurons in animals with a CCI (18.0°C, range 7–25°C, s.d. 6.8) was not significantly different from that of controls (17.0°C, range 13–22°C, s.d. 3.1; P > 0.5, Mann–Whitney U-test). Also the cold-evoked stimulus–response curves for HPC neurons in controls and CCI rats were not significantly different either (P > 0.2, 2-factor repeated measures ANOVA).
Responses to graded heat stimuli
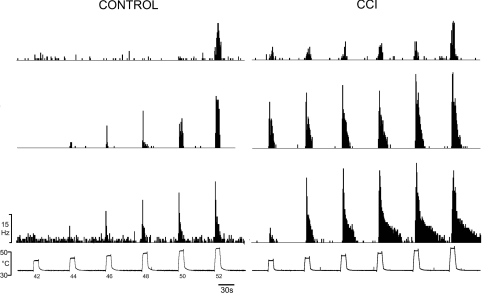
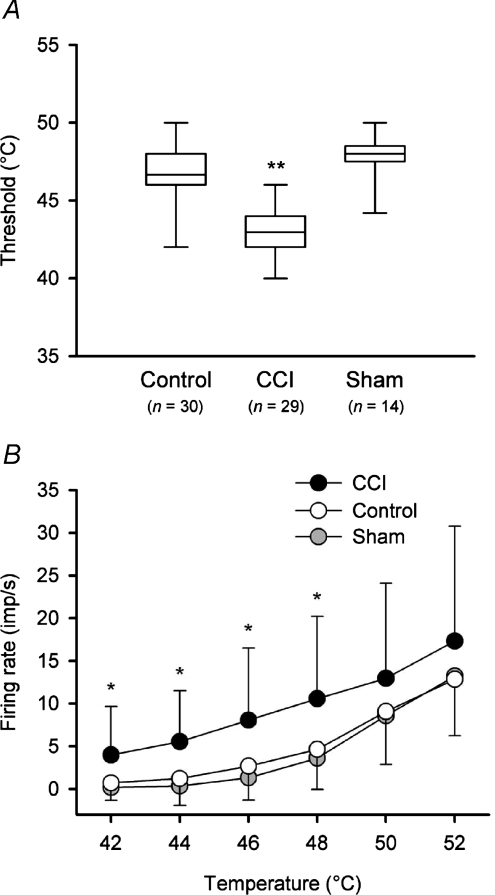
CCI produced significant changes in the heat-encoding properties of nociceptive lamina I spinoparabrachial neurons, when compared to both unoperated and sham-operated controls. Examples of the discharge of three neurons from controls and three neurons from animals with a CCI are shown in Fig. 6; these peristimulus time histograms show the activity of the neuron that had a maximal firing rate in the 25th percentile of the group, the activity of the neuron that had a maximal firing rate that was the median of the group and the discharge of the neuron that had a maximal firing rate that was in the 75th percentile. The mean heat threshold of neurons in animals with a CCI (43.0°C, range 40–52°C, s.d. 2.8, n= 29) was significantly lower (P < 10−4, ANOVA; Fig. 7A) than the mean threshold of neurons in both unoperated (mean 46.7°C, range 42–52°C, s.d. 2.6, n= 30) and sham-operated controls (mean 47.8°C, range 44–50°C, 1.8°C, n= 14). Neuronal heat thresholds lower than 42°C were never seen in controls (cf. Bester et al. 2000). There was no significant difference between the thresholds of unoperated controls and sham-operated controls (P > 0.2, ANOVA).
Figure 6. Heat responsiveness of nociceptive lamina I spinoparabrachial neurons in control and neuropathic rats.
Peristimulus time histograms of the discharge of 6 different lamina I spinoparabrachial neurons (3 control, 3 CCI) in response to graded heat stimulation. For each group of neurons (control and CCI), the middle histogram is from the cell whose maximum firing rate was the median of all of the neurons in that group; the top histogram is from the neuron whose maximum response was the 25th percentile of the population, and the bottom histogram is from the 75th percentile neuron. As can be seen, thresholds were lower in neurons recorded in CCI rats and suprathreshold responsiveness and after-discharge were also greater in those cells.
Figure 7. Quantitative differences in neuronal heat encoding in animals with a CCI.
A, box plots of heat thresholds of nociceptive neurons in each of the control, CCI and sham-operated groups. The horizontal line within the box is the median value, the box boundaries are the 25th and 75th percentiles and the bars indicate the data range. Thresholds were significantly lower in animals with a CCI (P < 10−4, ANOVA; asterisks) compared to both control and sham-operated rats. B, stimulus–response curves (mean ± 1 s.d.) of heat encoding by neurons in controls, CCI rats and sham-operated animals. Neurons in animals with a CCI were significantly more responsive to temperatures in the range 42–48°C (P < 0.003; 2-factor ANOVA followed by Tukey's post hoc test; asterisks).
As well as having lower heat thresholds, neurons in animals with a CCI were significantly more responsive to suprathreshold heat stimuli than neurons in controls (Fig. 7B; P < 0.02, 2-factor repeated-measures ANOVA). In the CCI, lamina I spinoparabrachial neurons were significantly more responsive than controls at temperatures of 42°C (P < 0.002, Tukey's post hoc test), 44°C (P < 0.0004, Tukey's post hoc test), 46°C (P < 0.002, Tukey's post hoc test) and 48°C (P < 0.003, Tukey's post hoc test), but not at temperatures of 50 or 52°C (P > 0.09, Tukey's post hoc test). Stimulus–response curves of neurons from unoperated controls were not significantly different from those from sham-operated animals (P > 0.9, 2-factor repeated-measures ANOVA).
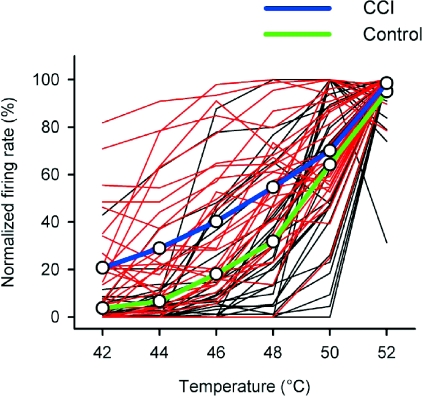
Plotting the response of each neuron on a normalized scale (with the maximal discharge of each cell set at 100%) further illustrates the enhanced responsiveness of neurons from animals with a CCI (Fig. 8). As can be seen, compared to unoperated controls, the normalized firing rates of neurons in animals with a CCI were greater over the range 42–48°C. Independent statistical verification of the difference between the control and CCI groups was obtained (P < 0.0002, general linear model) and post hoc tests confirmed that this effect was restricted to the temperature range 42–48°C (P < 0.002).
Figure 8. Normalized heat-evoked responses from nociceptive lamina I spinoparabrachial neurons.
The individual stimulus–response functions of nociceptive lamina I spinoparabrachial neurons have been plotted, normalized to the maximal discharge of each cell. Red lines are from neurons in animals with a CCI and black lines are from neurons in unoperated controls. Population means are shown with thick blue (CCI) and green (control) lines. The discharge of neurons in animals with a CCI was significantly greater than that of controls at temperatures of 42–48°C (P < 0.002, general linear model).
Heat hyperalgesia and the activity of lamina I spinoparabrachial neurons
The results from the present study provide the first quantitative evidence that changes in the heat sensitivity of lamina I spinoparabrachial neurons in the CCI could potentially underlie the heat hyperalgesia that is seen in this model of neuropathic pain. If the activity of lamina I spinoparabrachial neurons is sufficient to account for the behavioural signs of neuropathic pain in the CCI model, then it would be predicted that the activity of these neurons would be greatly enhanced in animals that displayed pronounced heat hyperalgesia. Conversely, in animals that displayed little heat hyperalgesia, there would be comparably little sensitization of lamina I spinoparabrachial neurons; or, in other words, the heat-evoked activity of lamina I spinoparabrachial neurons should co-vary as a function of the magnitude of heat hyperalgesia. The magnitude of the heat hyperalgesia can be determined by calculating the difference between the withdrawal latency of the hindpaw on the operated side and the withdrawal latency of the contralateral hindpaw. The CCI model offers an advantage for this type of analysis, compared to other models of neuropathic pain, because there can be considerable inter-animal variability in the magnitude of the heat hyperalgesia (Bennett & Xie, 1988). However, using ANCOVA in this type of study will probably only identify large effects, because nearly all of the data points are from separate experiments. Therefore there is an assumption that the cell (or cells) recorded in a single animal are representative of all of the lamina I spinoparabrachial neurons in that region of the spinal cord in that animal. Clearly this is unlikely to be the case, but nonetheless, if the effect is large enough then the method is potentially sensitive enough to detect it.
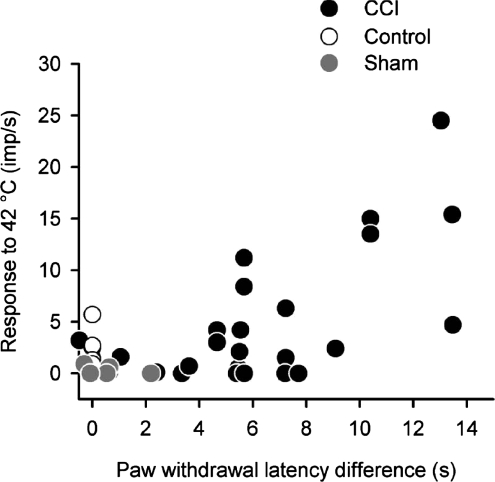
The relationship between neuronal firing and behaviour is shown in Fig. 9. Here the response of nociceptive lamina I spinoparabrachial neurons to a 42°C heat stimulus is plotted as a function of the difference in withdrawal latency of the right and left hind paws. As can be seen, there is a general trend that within the CCI group, as latency difference increases, so does the response to the 42°C heat stimulus. When comparing neuronal stimulus–response curves from animals with a CCI to sham-operated controls (behavioural data was not collected for unoperated controls), ANCOVA confirmed that neuronal responsiveness covaried significantly as a function of left–right paw withdrawal latency difference (P < 0.03). Thus sensitization of heat evoked responses from nociceptive lamina I spinoparabrachial neurons is sufficient to account for the heat hyperalgesia in the CCI model of neuropathic pain.
Figure 9. Relationship between lamina I spinoparabrachial activity and behaviour.
The discharge evoked by a 10 s duration 42°C heat stimulus has been plotted against the difference in heat-evoked withdrawal latency between the ipsilateral and contralateral hindpaws. Paw withdrawal latency difference was a significant co-variant in the heat-evoked stimulus–response curves of animals with a CCI when compared to sham-operated controls (P < 0.03, ANCOVA). As withdrawal latency differences for unoperated controls were not measured they have been assigned to zero for convenience.
Discussion
The principal finding in the current study is that nociceptive lamina I spinoparabrachial neurons become sensitized to heat in the CCI model of neuropathic pain; this sensitization is sufficient to account for the heat hyperalgesia that is observed in those animals. This is the first study to identify quantitative changes in a specific neural circuit that could account for behavioural alterations in neuropathic pain.
Technical considerations
It was necessary to perform the present experiments on anaesthetized animals, as a sufficiently large number of neurons could only be obtained in this type of preparation. It would not have been possible to control for the effects of general anaesthesia by using decerebrate (unanaesthetized) animals, due to the requirement to antidromically identify spinoparabrachial neurons. Urethane produces long-lasting, stable anaesthesia for non-recovery experiments (Flecknell, 1996), and it has minimal effects on spinal reflexes (Maggi & Meli, 1986). Although urethane does potentiate GABAergic and glycinergic currents in Xenopus oocytes, its effects on these inhibitory systems seems to be less marked than other commonly used general anaesthetics (Hara & Harris, 2002). Consistent with this, the discharge rates of lamina I spinoparabrachial neurons were comparable to those of lamina I spinothalamic and spinoparabrachial neurons in barbiturate-anaesthetized cats (Light et al. 1993; Craig et al. 2001) and lamina I neurons in unanaesthetized and decerebrate cats (Christensen & Perl, 1969). It is notable that rat lamina I spinoparabrachial neurons are about twice as responsive under halothane/nitrous oxide anaesthesia (Bester et al. 2000) compared to urethane anaesthesia, but it is known that halothane sensitizes nociceptors (Campbell et al. 1984). Halothane-induced sensitization of nociceptors might also account for some of the other differences noted between the present study and that of Bester et al. (2000), for example, neuronal heat thresholds < 42°C and an increased proportion of wide dynamic range neurons in their sample of cells.
In my hands there was no evidence that the CCI model produced mechanical allodynia/hyperalgesia, as there was no significant change in 50% paw withdrawal thresholds after the CCI. The original study of Bennett & Xie (1988) did not find any changes in withdrawal thresholds to noxious pressure (Randall-Sellito), and others have noted that mechanical allodynia is difficult to demonstrate in the CCI model (Chaplan et al. 1994). However, some investigators have reported behavioural changes in the CCI model that were interpreted as mechanical allodynia (e.g. Attal et al. 1990; Kim et al. 1997). Different methods of measuring paw withdrawal thresholds to mechanical stimuli might account for differences in results, and even the material that the floor of the behavioural testing cage is made from seems to be important (Pitcher et al. 1999). As the results from the present study confirm an important role for lamina I spinoparabrachial neurons in heat hyperalgesia, it seems likely that these neurons could also be involved in mechanical hyperalgesia and allodynia. One potential mechanism of mechanical allodynia could involve the recruitment of novel low-threshold inputs to nociceptive lamina I spinoparabrachial neurons, either by disinhibition or by potentiation of glutamatergic transmission (Woolf & Salter, 2000). Thus, the observation that there was no significant change in the proportion of lamina I spinoparabrachial neurons that were activated by inputs from low-threshold mechanoreceptors strongly suggests that the CCI model is not a robust model of mechanical allodynia. On the other hand, the spinal nerve ligation model (Kim & Chung, 1992) produces a rapidly developing and marked mechanical allodynia, and it remains to be determined whether the proportion of wide dynamic range lamina I spinoparabrachial neurons is increased in this model of neuropathic pain.
Comparison with previous studies of spinal neurons in neuropathic pain models
Previous in vivo studies have characterized the quantitative response properties of spinal nociceptive neurons in the CCI model (Paleček et al. 1992; Laird & Bennett, 1993), the spinal nerve ligation model (Chapman et al. 1998) and the partial sciatic tight ligation model (Takaishi et al. 1996). Although these studies mainly recorded the activity of unidentified, deep dorsal horn neurons, only Paleček et al. (1992) studied identified (spinothalamic) neurons. All of these studies reported qualitative differences between neurons in controls and neurons in animals with neuropathic pain, but none of them were able to demonstrate quantitative changes in neuronal responsiveness to graded noxious stimuli. Furthermore, spinothalamic neurons in the CCI were significantly less responsive to noxious heat compared to controls (Paleček et al. 1992).
One possible explanation for the mismatch between the behavioural results and the electrophysiological results of the above studies is that the composition of neuronal types differed between the control and neuropathic groups. The paucity of projection neurons in the deep dorsal horn of the rat (Spike et al. 2003) suggests that most of the neurons characterized in studies of ‘unidentified’ deep dorsal horn neurons will have been a mixture of excitatory and inhibitory interneurons. As nerve injury is likely to produce different effects on different types of neurons, variability in the composition of the control and neuropathic groups of neurons could mask any true physiological changes that are present. Clearly this explanation cannot account for the results of Paleček et al. (1992), who studied identified neurons, but selectively destroying superficial dorsal horn neurons that express the neurokinin 1 receptor (which includes 80% of lamina I spinoparabrachial neurons; Todd et al. 2000) abolishes behavioural signs of mechanical allodynia in the spinal nerve ligation model (Nicholls et al. 1999), suggesting a crucial role for lamina I neurons in neuropathic pain.
Not all previous physiological studies have reported a discrepancy between the electrophysiological and behavioural data: Chen & Pan (2002) described mechanical sensitization of deep dorsal horn spinothalamic neurons in the streptozotocin model of diabetic neuropathy, and Keller et al. (2007) reported mechanical sensitization of lamina I spinoparabrachial neurons in the sciatic nerve constriction model of neuropathic pain. However, neither of these studies used quantitative stimuli (Andrew & Greenspan, 1999; Andrew & Craig, 2002) in the mechanical characterization of neurons.
Central vs. peripheral mechanisms in the sensitization of lamina I spinoparabrachial neurons
There has been considerable debate over the relative importance of changes in the peripheral nervous system versus changes in the central nervous system in the pathophysiology of chronic pain (see Devor, 2006). Models of central sensitization (Woolf & Salter, 2000) make several predictions regarding spontaneous activity, receptive field size and neuronal responsiveness that can be compared to the results of the present study. However, with in vivo studies it is difficult to draw definitive conclusions regarding whether changes seen in spinal neurons are simply the central reflection of peripheral changes, or whether they truly are intrinsic changes within the spinal cord (Balasubramanyan et al. 2006). Comparisons of central neurons with peripheral nerve fibres can be useful, especially if changes seen centrally are not found peripherally, but there is little quantitative data on peripheral nerve fibres in models of neuropathic pain. The CCI causes spontaneous activity in up to 30% of the constricted fibres (Kajander & Bennett, 1992), but only about 5% of them retain their connections to sensory receptors in the skin (Xie et al. 1995). Most of the spontaneously active fibres are myelinated, though a few are unmyelinated (Kajander & Bennett, 1992). However, by the time that the terminal electrophysiological experiments were performed in the current study, virtually all of the spontaneously active myelinated fibres should have degenerated (Kajander & Bennett, 1992). The observation that there was no significant difference in spontaneous activity of lamina I spinoparabrachial neurons in CCI animals compared to controls is consistent with this. Thus the peripheral fibres that survive the CCI and that innervate lamina I spinoparabrachial neurons do not seem to be the fibres that are spontaneously active (cf. Laird & Bennett, 1993). In the current study receptive field sizes of lamina I spinoparabrachial neurons were also similar to controls. Lamina I projection neurons show expanded receptive fields in inflammatory pain (Hylden et al. 1989), but receptive field sizes of spinal neurons, including spinothalamic neurons, are similar to controls in the chronic constriction injury (Paleček et al. 1992; Laird & Bennett, 1993) and the partial sciatic nerve ligation (Takaishi et al. 1996) models.
CCI of the rat saphenous nerve caused increased heat sensitivity of unmyelinated nociceptors: in response to a standard heat stimulus (32 to 47°C in 15 s) approximately 30% more impulses were evoked in nociceptors from animals with a CCI compared to controls (Koltzenburg et al. 1994). In the CCI, lamina I spinoparabrachial neurons were 130% more responsive to a 10 s 48°C stimulus compared to controls. Thus the heat sensitization of lamina I spinoparabrachial neurons may be the central representation of peripheral sensitization, with the increased magnitude of sensitization simply being due to summation of inputs from many fibres. However, the data of Koltzenburg et al. (1994) do not exclude the possibility of central sensitization in the CCI, because no data were reported on nociceptor heat thresholds. Nonetheless, based on the current experiments it seems that the CCI is predominantly a model of peripheral nociceptor sensitization.
Acknowledgments
This work was supported by the Wellcome Trust.
Author contributions
All of the experiments described in this manuscript were performed at the University of Sheffield. D.A. has sole responsibility for this paper, having designed the study, collected and analysed the data and drafted the article. He has approved the final version to be published.
References
- Ahlgren SC, White DM, Levine JD. Increased responsiveness of sensory neurons in the saphenous nerve of the streptozotocin-diabetic rat. J Neurophysiol. 1992;68:2077–2085. doi: 10.1152/jn.1992.68.6.2077. [DOI] [PubMed] [Google Scholar]
- Andrew D, Greenspan JD. Modality-specific hyper-responsivity of regenerated cat cutaneous nociceptors. J Physiol. 1999;516:897–906. doi: 10.1111/j.1469-7793.1999.0897u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D, Craig AD. Quantitative responses of spinothalamic lamina I neurones to graded mechanical stimulation in the cat. J Physiol. 2002;545:913–931. doi: 10.1113/jphysiol.2002.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attal N, Jazat F, Kayser V, Guilbaud G. Further evidence for ‘pain-related’ behaviours in a model of unilateral peripheral neuropathy. Pain. 1990;41:235–251. doi: 10.1016/0304-3959(90)90022-6. [DOI] [PubMed] [Google Scholar]
- Balasubramanyan S, Stemkowski PL, Stebbing MJ, Smith PA. Sciatic chronic constriction produces cell-type-specific changes in the electrophysiological properties of rat substantia gelatinosa neurons. J Neurophysiol. 2006;96:579–590. doi: 10.1152/jn.00087.2006. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie Y-K. A peripheral neuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bennett GJ. Neuropathic pain. In: Wall PD, Melzack R, editors. Textbook of Pain. New York: Livingstone; 1994. pp. 201–221. [Google Scholar]
- Bernard JF, Dallel R, Raboisson P, Villneuva L, LeBars D. Organisation of the efferent projections from the spinal cervical enlargement to the parabrachial area and periaqueductal grey: a PHA-L study in the rat. J Comp Neurol. 1995;353:480–505. doi: 10.1002/cne.903530403. [DOI] [PubMed] [Google Scholar]
- Bester H, Champman V, Besson J-M, Bernard J-F. Physiological properties of the lamina I spinoparabrachial neurons in the rat. J Neurophysiol. 2000;83:2239–2259. doi: 10.1152/jn.2000.83.4.2239. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Raja SN, Meyer RA. Halothane sensitises cutaneous nociceptors in monkeys. J Neurophysiol. 1984;52:762–770. doi: 10.1152/jn.1984.52.4.762. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chapman V, Suzuki R, Dickenson AH. Electrophysiological characterization of spinal neuronal response properties on anaesthetized rats after ligation of spinal nerves L5–L6. J Physiol. 1998;507:881–894. doi: 10.1111/j.1469-7793.1998.881bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S-R, Pan H-L. Hypersensitivity of spinothalamic tract neurons associated with diabetic neuropathic pain in rats. J Neurophysiol. 2002;87:2726–2733. doi: 10.1152/jn.2002.87.6.2726. [DOI] [PubMed] [Google Scholar]
- Christensen BN, Perl ER. Spinal neurons specifically excited by noxious or thermal stimuli: marginal zone of the dorsal horn. J Neurophysiol. 1969;33:293–307. doi: 10.1152/jn.1970.33.2.293. [DOI] [PubMed] [Google Scholar]
- Craig AD, Krout K, Andrew D. Quantitative response characteristics of thermoreceptive and nociceptive lamina I spinothalamic neurons in the cat. J Neurophysiol. 2001;86:1459–1480. doi: 10.1152/jn.2001.86.3.1459. [DOI] [PubMed] [Google Scholar]
- Devor M. Centralization, central sensitization and neuropathic pain. Focus on “Sciatic chronic constriction injury produces cell-type-specific changes in the electrophysiological properties of rat substantia gelatinosa neurons”. J Neurophysiol. 2006;96:522–523. doi: 10.1152/jn.00365.2006. [DOI] [PubMed] [Google Scholar]
- Feil K, Herbert H. Topographic organization of spinal and trigeminal sensory pathways to the rat parabrachial and Kölliker-Fuse nuclei. J Comp Neurol. 1995;353:506–528. doi: 10.1002/cne.903530404. [DOI] [PubMed] [Google Scholar]
- Flecknell P. Laboratory Animal Anaesthesia. London: Elsevier; 1996. [Google Scholar]
- Hara K, Harris RA. The anesthetic mechanisms of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg. 2002;94:313–318. doi: 10.1097/00000539-200202000-00015. [DOI] [PubMed] [Google Scholar]
- Hargreaves KM, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hylden JLK, Nahin RL, Traub RJ, Dubner R. Expansion of receptive fields of spinal lamina I projection neurons in rats with unilateral adjuvant-induced inflammation: the contribution of dorsal horn mechanisms. Pain. 1989;37:229–243. doi: 10.1016/0304-3959(89)90135-8. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Heinke B, Ruscheweyh R, Sandkühler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science. 2003;299:1237–1240. doi: 10.1126/science.1080659. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jäger T, Sandkühler J. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science. 2006;312:1659–1662. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- Kajander KC, Bennett GJ. Onset of painful peripheral neuropathy in rat: a partial and differential deafferentation and spontaneous discharge in Aβ and Aδ primary afferent neurons. J Neurophysiol. 1992;68:734–744. doi: 10.1152/jn.1992.68.3.734. [DOI] [PubMed] [Google Scholar]
- Keller AF, Beggs S, Salter MW, De Koninck Y. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol Pain. 2007;3:27. doi: 10.1186/1744-8069-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Yoon YW, Chung JM. Comparison of three rodent neuropathic pain models. Exp Brain Res. 1997;113:200–206. doi: 10.1007/BF02450318. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Kees S, Budweiser S, Ochs G, Toyka KV. The properties of unmyelinated nociceptive afferents change in a painful chronic constriction neuropathy. In: Gebhart GF, Hammond DL, Jensen TS, editors. Progress in Pain Research and Management. Vol. 2. Seattle: IASP Press; 1994. pp. 511–522. [Google Scholar]
- Laird JMA, Bennett GJ. An electrophysiological study of dorsal horn neurons in the spinal cord of rats with an experimental peripheral neuropathy. J Neurophysiol. 1993;69:2072–2085. doi: 10.1152/jn.1993.69.6.2072. [DOI] [PubMed] [Google Scholar]
- Light AR, Sedivec MJ, Casale EJ, Jones SJ. Physiological and morphological characteristics of spinal neurons projecting to the parabrachial region of the cat. Somatosens Mot Res. 1993;10:309–325. doi: 10.3109/08990229309028840. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Wall PD. Descending excitation and inhibition of spinal cord lamina I projection neurons. J Neurophysiol. 1988;59:1204–1219. doi: 10.1152/jn.1988.59.4.1204. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: general considerations. Experientia. 1986;42:109–114. doi: 10.1007/BF01952426. [DOI] [PubMed] [Google Scholar]
- Nicholls ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- Paleček J, Palečeková V, Dougherty PM, Carlton SM, Willis WD. Responses of spinothalamic tract cells to mechanical and thermal stimulation of skin in rats with experimental peripheral neuropathy. J Neurophysiol. 1992;67:1562–1573. doi: 10.1152/jn.1992.67.6.1562. [DOI] [PubMed] [Google Scholar]
- Pitcher GM, Ritchie J, Henry JL. Paw withdrawal threshold in the von Frey hair test is influenced by the surface on which the rat stands. J Neurosci Methods. 1999;87:185–193. doi: 10.1016/s0165-0270(99)00004-7. [DOI] [PubMed] [Google Scholar]
- Shea VK, Perl ER. Regeneration of cutaneous unmyelinated (C) fibres after transection. J Neurophysiol. 1985;54:502–512. doi: 10.1152/jn.1985.54.3.502. [DOI] [PubMed] [Google Scholar]
- Shim B, Kim D-W, Kim BH, Nam TS, Leem JW, Chung JM. Mechanical and heat sensitization of cutaneous nociceptors in rats with experimental peripheral neuropathy. Neuroscience. 2005;132:193–201. doi: 10.1016/j.neuroscience.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Slugg RM, Light AR. Spinal cord and trigeminal projections to the pontine parabrachial region in the rat as demonstrated with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1994;339:49–61. doi: 10.1002/cne.903390106. [DOI] [PubMed] [Google Scholar]
- Spike RC, Puskár Z, Andrew D, Todd AJ. A quantitative and morphological study of projection neurons in lamina I of the rat lumbar spinal cord. Eur J Neurosci. 2003;18:2433–2448. doi: 10.1046/j.1460-9568.2003.02981.x. [DOI] [PubMed] [Google Scholar]
- Takaishi K, Eisele JH, Carstens E. Behavioral and electrophysiological assessment of hyperalgesia and changes in dorsal horn responses following partial sciatic nerve ligation in rats. Pain. 1996;66:297–306. doi: 10.1016/0304-3959(96)03023-0. [DOI] [PubMed] [Google Scholar]
- Tanner KD, Reichling DB, Levine JD. Nociceptor hyper-responsiveness during vincristine-induced painful peripheral neuropathy in the rat. J Neurosci. 1998;18:6480–6491. doi: 10.1523/JNEUROSCI.18-16-06480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ, McGill MM, Shehab SA. Neurolinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. Eur J Neurosci. 2000;12:689–700. doi: 10.1046/j.1460-9568.2000.00950.x. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Acosta-Rua AJ, Johnson RD. Bilateral chronic constriction of the sciatic nerve: a model of long-term cold hyperalgesia. J Pain. 2005;6:507–517. doi: 10.1016/j.jpain.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Wilson LB, Andrew D, Craig AD. Activation of spinobulbar lamina I neurons by static muscle contraction. J Neurophysiol. 2002;87:1641–1645. doi: 10.1152/jn.00609.2001. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: Increasing the gain in pain. Science. 2000;288:1765–1768. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Xie Y, Zhang J, Petersen M, LaMotte RH. Functional changes in dorsal root ganglion cells after chronic nerve constriction in the rat. J Neurophysiol. 1995;73:1811–1820. doi: 10.1152/jn.1995.73.5.1811. [DOI] [PubMed] [Google Scholar]
- Zeltser R, Seltzer Z. A practical guide for the use of animals models in the study of neuropathic pain. In: Boivie J, Hansson P, Lindblom U, editors. Touch, Temperature and Pain in Health and Disease: Mechanisms and Assessment, Progress in Pain Research and Management. Seattle: IASP Press; 1994. pp. 295–338. [Google Scholar]
- Zhang X, Davidson S, Giesler GJ. Thermally identified subgroups of marginal zone neurons project to distinct regions of the ventral posterior lateral nucleus in rats. J Neurosci. 2006;26:5215–5223. doi: 10.1523/JNEUROSCI.0701-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]