Abstract
Young women are more susceptible to orthostatic intolerance than men, though the sex-specific pathophysiology remains unknown. As blood pressure (BP) is regulated through the baroreflex mechanism, we tested the hypothesis that baroreflex control of muscle sympathetic nerve activity (MSNA) during orthostasis is impaired in women and can be affected by the menstrual cycle. MSNA and haemodynamics were measured supine and during a graded upright tilt (30 deg for 6 min, 60 deg for 45 min or till presyncope) in 11 young men and 11 women during the early follicular (EFP) and mid-luteal phase (MLP) of the menstrual cycle. Sympathetic baroreflex sensitivity was quantified using the slope of the linear correlation between total activity and diastolic BP during spontaneous breathing. Baroreflex function was further assessed during a Valsalva manoeuvre (VM). Although MSNA burst frequency responses during tilting were similar between sexes and menstrual phases, increases in total activity were lower in women during EFP than MLP (P= 0.030), while total peripheral resistance and plasma noradrenaline were not similarly lower; upright total activity tended to be lower in women during EFP than men (P= 0.102). Sympathetic baroreflex sensitivity did not differ between sexes (P= 0.676) supine (−281 ± 46 (s.e.m.) units beat−1 mmHg−1 in men vs−252 ± 52 in EFP and −272 ± 40 in MLP in women), at 30 deg tilt (−648 ± 129 vs−611 ± 79 and −487 ± 94), and at 60 deg tilt (−792 ± 135 vs−831 ± 92 and −814 ± 142); this sensitivity was not affected by the menstrual cycle (P= 0.747). Similar sympathetic baroreflex sensitivity between sexes and phases was also observed during the VM. Cardiovagal baroreflex sensitivity assessed during decreasing BP (i.e. early phase II of the VM) was comparable between sexes, but it was greater in men than women during increasing BP (i.e. phase IV); the menstrual cycle had no influences on cardiovagal baroreflex sensitivity. We conclude that the menstrual cycle affects sympathetic neural responses but not sympathetic baroreflex sensitivity during orthostasis, though upright vasomotor sympathetic activity is not clearly different between men and women. Not only sympathetic but also cardiovagal baroreflex sensitivity is similar between sexes and menstrual phases during a hypotensive stimulus. However, cardiovagal baroreflex-mediated bradycardia during a hypertensive stimulus is different between sexes but not affected by the menstrual cycle. Thus, other factors rather than sympathetic baroreflex control mechanisms contribute to sex differences in orthostatic tolerance in young humans.
Young women have a greater incidence of orthostatic intolerance compared with young men (Robertson, 1999; Fu et al. 2004a, 2005a; Meendering et al. 2005), but the underlying mechanisms are not well understood. It is likely that certain sex-specific factors, such as the menstrual cycle or differences in some hormonal levels may affect orthostatic blood pressure regulation.
Sympathetic neural control plays a critical role in arterial pressure maintenance mainly through baroreflex-mediated vasoconstriction during short-term (Wallin & Sundlof, 1982; Fu et al. 2004b) and sustained (Fu et al. 2006) orthostasis in humans. Results regarding sex differences in sympathetic neural control during orthostasis are few and controversial. There are only two studies, with different findings, showing muscle sympathetic nerve activity (MSNA) responses during upright posture in young men and women (Shoemaker et al. 2001; Fu et al. 2005a). Conversely, there is no information available concerning the effects of the menstrual cycle on MSNA responses during orthostasis in humans.
Minson et al. (2000a) demonstrated previously that sympathetic baroreflex sensitivity was influenced by hormonal fluctuations during the normal menstrual cycle in healthy women. One recent study by Tank et al. (2005) showed that baroreflex regulation of MSNA was not different between young men and women. However, the sensitivity of the sympathetic baroreflex was assessed during pharmacological changes in blood pressure in both of these two studies. Hogarth et al. (2007) reported recently that women had a greater sympathetic baroreflex sensitivity than men as assessed during a Valsalva manoeuvre. Whether similar results can be obtained during physiological modulation of blood pressure, such as changes in posture (when the syndrome of orthostatic intolerance is manifest) needs to be determined.
Since young women are more susceptible to orthostatic intolerance, we speculated that their sympathetic baroreflex sensitivity would be lower compared with young men, especially when they are confronted with orthostatic challenges. Based on the findings of Minson et al. (2000a), we further speculated that sympathetic baroreflex sensitivity would be different between different menstrual phases in women, which may also contribute to their susceptibility to orthostatic intolerance. Thus, the purpose of this study was to test the hypothesis that baroreflex control of MSNA during upright posture is impaired in women and can be affected by the menstrual cycle. Sympathetic baroreflex sensitivity was evaluated during spontaneous breathing (Fu et al. 2006). Baroreflex function was further assessed during the Valsalva manoeuvre.
Methods
Subjects
Eleven healthy young men and eleven age-matched pre-menopausal women were studied. No subject smoked, used recreational drugs, or had significant medical problems. None was an endurance-trained athlete (Levine et al. 1991). All women had self-reported regular menstrual cycles of ∼28 days, and had never taken or had not taken oral contraceptives for ≥ 6 months (Minson et al. 2000b). Subjects were screened with a careful medical history, physical examination and electrocardiogram. Individuals with a history of fainting or neurally mediated syncope were excluded. All subjects were informed of the purpose and procedures used in the study and gave their written informed consent to a protocol approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center and Presbyterian Hospital of Dallas. The study followed guidelines set forth in the Declaration of Helsinki. A summary of the descriptive data for the subjects in both groups is presented in Table 1.
Table 1.
Subjects’ characteristics
| Women (n= 11) |
|||
|---|---|---|---|
| Variables | Early follicular phase | Mid-luteal phase | Men (n= 11) |
| Age (years) | 33 ± 3 | 33 ± 3 | 32 ± 3 |
| Height (cm) | 166 ± 2† | 165 ± 2† | 176 ± 2 |
| Weight (kg) | 64 ± 2† | 64 ± 2† | 79 ± 4 |
| Body mass index (kg m−2) | 23 ± 1 | 23 ± 1 | 25 ± 1 |
| Oestradiol (pg ml−1) | 32 ± 2 | 92 ± 14* | — |
| Progesterone (ng ml−1) | 0.9 ± 0.2 | 11.1 ± 1.7* | — |
Values are presented as means ±s.e.m.
P < 0.05 compared with the early follicular phase in women.
P < 0.05 compared with men.
Measurements
MSNA
MSNA signals were obtained with the microneurographic technique (Vallbo et al. 1979). Briefly, a recording electrode was placed in the peroneal nerve at the popliteal fossa, and a reference electrode was placed subcutaneously 2–3 cm from the recording electrode. The nerve signals were amplified (gain 70 000–160 000), band-pass filtered (700–2000 Hz), full-wave rectified, and integrated with a resistance-capacitance circuit (time constant 0.1 s). Criteria for adequate MSNA recording included: (1) pulse synchrony, (2) facilitation during the hypotensive phase of the Valsalva manoeuvre, and suppression during the hypertensive overshoot after release, (3) increases in response to breath holding, and (4) insensitivity to emotional stimuli (Vallbo et al. 1979).
Heart rate and blood pressure
Heart rate was determined from the electrocardiogram, and beat-to-beat arterial pressure was measured non-invasively from the middle finger using photoplethysmography (Portapres, TNO Institute of Applied Physics Biomedical Instrumentation, the Netherlands). Cuff blood pressure was measured by electrosphygmomanometry (model 4240, SunTech Medical Instruments Inc., Raleigh, NC, USA) with a microphone placed over the brachial artery to detect Korotkoff sounds. Respiratory excursions were detected by a nasal cannula (model 1265, Respironics California Inc., Carlsbad, CA, USA).
Cardiac output
Cardiac output was measured with the acetylene rebreathing technique (model MGA1100, Marquette, Milwaukee, WI, USA) (Triebwasser et al. 1977). Cardiac index was derived from cardiac output divided by body surface area. Stroke volume was calculated from cardiac output and heart rate, and was normalized to body surface area as stroke index. Total peripheral resistance was calculated as the quotient of mean arterial pressure and cardiac output, multiplied by 80 (expressed as dyne s cm−5). Mean arterial pressure was calculated as (systolic pressure − diastolic pressure)/3 + diastolic pressure, where blood pressure was measured by arm cuff during the rebreathing.
Protocol
Women were studied twice, once during the early follicular phase (1–4 days after the onset of menstruation when both oestrogen and progesterone are low), and once during the mid-luteal phase (19 to 22 days after the onset of menstruation when both hormones are high), with the order counterbalanced. Cycle phase was determined by the onset of menstruation and by the detection of the luteinizing hormone surge by an ovulation prediction kit (OvuQuick, Quidel Corp., San Deigo, CA, USA). It was verified by circulating oestradiol and progesterone concentrations on each study day. Men were studied only once. All subjects were on an isocaloric constant diet consisting of 200 mEq sodium, 100 mEq potassium, and 1000 mg calcium, while fluid intake was ad libitum 2 days prior to testing. Women took a pregnancy test and showed negative results on each study day.
The experiment was performed in the morning or afternoon ≥ 2 h after a light breakfast or lunch, and ≥ 48 h after the last caffeinated or alcoholic beverage in a quiet, environmentally controlled laboratory with an ambient temperature of ∼25°C. The subject was placed in the supine position, and an intravenous catheter was inserted into an antecubital vein of the left arm for blood samples. At least 10 min after an acceptable nerve recording site had been found, baseline data were collected for 6 min. All subjects were then asked to perform two Valsalva manoeuvres at 40 mmHg for 20 s after a normal inspiration, separated by a 2 min recovery period. After sufficient recovery, the subject was tilted passively to 30 deg upright for 6 min, and then 60 deg for 45 min or till presyncope. A belt was placed across the subject's waist to make sure he/she would not fall. A bicycle saddle was used to support approximately two-thirds of the body weight during tilting, while the subject stood on a plate at the end of the tilt bed on one leg, allowing the other leg to be relaxed for microneurography. After that, the subject was returned to supine for recovery.
Heart rate, blood pressure, respiratory waveforms, and MSNA were recorded continuously. Cardiac output was measured while supine, after 5 min of 30 deg tilt, and after 5, 10, 20, 30 and 40 min of 60 deg upright tilt. Blood samples were taken in the supine position, after 5 and 20 min of 60 deg tilt, and immediately after returning to supine because either the subject completed 45 min of tilting or developed presyncope.
Data analysis
Sympathetic bursts were identified by a computer program (Cui et al. 2001), and then were confirmed by an experienced microneurographer. The integrated neurogram was normalized by assigning a value of 100 to the largest amplitude of a sympathetic burst during the 6 min supine baseline. All bursts for that trial were then normalized against that value (Halliwill, 2000). Burst areas of the integrated neurogram, systolic and diastolic pressures, and R-R interval were measured simultaneously on a beat-to-beat basis. Total activity of the burst was defined as the burst area of the rectified and integrated neurogram. The number of bursts per minute (burst frequency), the number of bursts per 100 heart beats (burst incidence), and total activity were used as quantitative indexes.
Assessments of sympathetic baroreflex sensitivity during spontaneous breathing
Baroreflex control of MSNA was assessed by using the slope of the linear correlation between total activity and diastolic pressure during spontaneous breathing in the supine position and during upright tilt as previously described (Fu et al. 2006). To perform a linear regression, values for total activity were averaged over a 3 mmHg diastolic pressure bin. A statistical weighting procedure was adopted; each data point was entered once for each heart beat in the bin, and total activity was expressed as arbitrary units per heart beat (i.e. units beat−1) (Kienbaum et al. 2001; Fu et al. 2006).
Additionally, we used the MSNA and stroke index relationship to further evaluate sympathetic baroreflex control for each subject during changes in posture and during sustained upright tilt, since MSNA has been demonstrated to be related to the change in stroke volume in the supine position (Charkoudian et al. 2005) and during orthostasis (Levine et al. 2002; Convertino et al. 2004; Fu et al. 2005a, 2006).
Assessments of baroreflex function during the Valsalva manoeuvre
Sympathetic baroreflex function was assessed by relating all sympathetic bursts occurring during the 20 s straining period of the Valsalva manoeuvre to the maximum fall of diastolic pressure as measured from the highest level just after the beginning of straining (phase I) to its nadir (Fu et al. 2005b). Cardiovagal baroreflex sensitivity was assessed during early phase II (i.e. a hypotensive stimulus) and phase IV (i.e. a hypertensive stimulus) (Fu et al. 2005b). The slope of the linear relationship between changes in systolic pressure and the corresponding changes in R-R interval was estimated to evaluate cardiovagal baroreflex sensitivity.
Statistical analysis
Data are expressed as means ±s.e.m. Supine MSNA, blood pressure, and heart rate were averaged for 6 min. During 30 deg upright tilt, data were collected and averaged from the 2nd to 5th min (30 deg Tilt). During 60 deg upright tilt, data were averaged from the 2nd to 5th min (Tilt5), 7th to 10th min (Tilt10), 17th to 20th min (Tilt20), 26th to 29th min (Tilt30), 36th to 39th min (Tilt40), and 42nd to 45th min (Tilt45). Since some subjects had presyncope during 45 min of 60 deg upright tilt and the tilt test was terminated at different time points, we used the ‘last stable data carry forward’ method for imputing with missing values (Ali & Talukder, 2005). Data were also averaged in 20 s intervals over the last 3 min prior to presyncope in presyncopal subjects, and from the 42nd to 45th min in non-presyncopal subjects.
Supine baseline values and baroreflex sensitivity assessed during the Valsalva manoeuvre between menstrual phases were compared using Wilcoxon's signed rank tests, and between sexes were compared with Mann–Whitney rank sum tests. MSNA and haemodynamic responses during upright tilt between sexes and phases were analysed using two-way repeated measures analysis of variance (ANOVA) with factors for sex/phase, posture, and the interaction between sex/phase and posture. The Bonferroni–Holm method was used post hoc for multiple comparisons. The relationship between total activity and diastolic pressure during spontaneous breathing in the supine position and during sustained upright tilt was determined for each subject by least-squares linear regression analysis, and the slopes were also compared using two-way repeated measures ANOVA. The correlation between MSNA and stroke index during changes in posture was determined by least-squares linear regression for each subject, while the slopes between phases were compared using Wilcoxon's signed rank tests, and between sexes were compared using Mann–Whitney rank sum tests. All statistical analyses were performed with a personal computer-based analysis program (SigmaStat (Systat Software Inc., San Jose, CA, USA), SPSS (SPSS Inc., Chicago, IL, USA). A P value of < 0.05 was considered statistically significant.
Results
Supine MSNA and haemodynamics
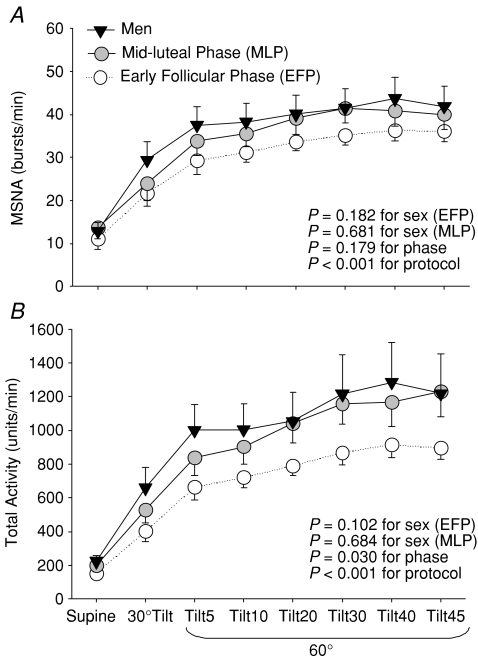
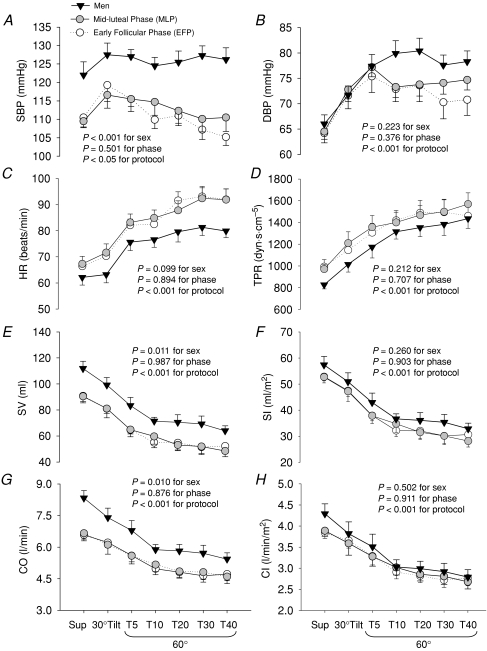
Supine MSNA and plasma catecholamine concentrations were not different between sexes (Fig. 1 and Table 2). Systolic pressure was greater in men than women, which was attributable to greater cardiac output and stroke volume; however, diastolic pressure, heart rate, stroke index, cardiac index, and total peripheral resistance did not differ between the groups (Fig. 2).
Figure 1. Muscle sympathetic nerve activity (MSNA) burst frequency (A) and total activity (B) responses during a graded upright tilt in men and women during the early follicular phase (when both oestrogen and progesterone are low) and the mid-luteal phase (when both sex hormones are high).
Values are means ±s.e.m. Tilt5, Tilt10, Tilt20, Tilt30, Tilt40 and Tilt45, 5, 10, 20, 30, 40 and 45 min after 60 deg upright tilt.
Table 2.
Plasma catecholamine responses to upright tilt in men and women during the two different menstrual phases
| Women (n= 11) |
||||
|---|---|---|---|---|
| Variables | Early follicular phase | Mid-luteal phase | Men (n= 11) | |
| Noradrenaline (pg ml−1) | Supine | 176 ± 16 | 201 ± 13 | 207 ± 20 |
| 60 deg Tilt5 | 372 ± 32* | 371 ± 28* | 338 ± 49* | |
| 60 deg Tilt20 | 447 ± 39* | 482 ± 46* | 409 ± 52* | |
| Adrenaline (pg ml−1) | Supine | 29 ± 5 | 26 ± 7 | 29 ± 5 |
| 60 deg Tilt5 | 51 ± 12 | 35 ± 6 | 52 ± 18 | |
| 60 deg Tilt20 | 57 ± 12* | 54 ± 12* | 99 ± 22* | |
Values are presented as means ±s.e.m.
P < 0.05 compared with supine within the group and the same menstrual phase in women.
Figure 2. Systolic blood pressure (SBP, A), diastolic blood pressure (DBP, B), heart rate (HR, C), total peripheral resistance (TPR, D), stroke volume (SV, E), stroke index (SI, F), cardiac output (CO, G), and cardiac index (CI, H) responses during a graded upright tilt in men and women during the two different menstrual phases.
Values are means ±s.e.m. Sup, supine; T5, T10, T20, T30 and T40, 5, 10, 20, 30 and 40 min after 60 deg upright tilt.
Even though oestradiol and progesterone were markedly greater in the mid-luteal phase than the early follicular phase in women, supine MSNA, plasma catecholamine concentrations, and haemodynamic variables were not different between the two different menstrual phases (Tables 1 and 2, Figs 1 and 2).
Responses during upright tilt
MSNA burst frequency increased at 30 deg, further increased at 60 deg tilt, and remained elevated throughout 45 min of tilting; these responses were not different between sexes and menstrual phases (Fig. 1A). However, women during the early follicular phase had less increases in total activity during upright tilt compared with the mid-luteal phase (Fig. 1B, P= 0.030). Upright total activity tended to be lower in women during the early follicular phase than men (Fig. 1B, P= 0.102).
Systolic pressure was stable in men, but decreased progressively in women during sustained upright tilt; it was greater in men compared with women (Fig. 2A). Diastolic pressure increased during upright tilt, and was not different between sexes (Fig. 2B). Heart rate increased progressively during graded upright tilt, and tended to be greater in women than men (Fig. 2C). Total peripheral resistance increased progressively during graded upright tilt, and the responses were similar between sexes (Fig. 2D). Both stroke volume and cardiac output decreased gradually during sustained upright tilt, and were greater in men than women (Fig. 2E and G). However, both stroke index and cardiac index were not different between sexes during tilting (Fig. 2F and H). The menstrual cycle had no significant impacts on haemodynamic responses during upright tilt in women, with similar total peripheral resistance and plasma noradrenaline concentration despite the differences in total activity (Figs 1 and 2, Table 2).
Sympathetic baroreflex sensitivity during tilting
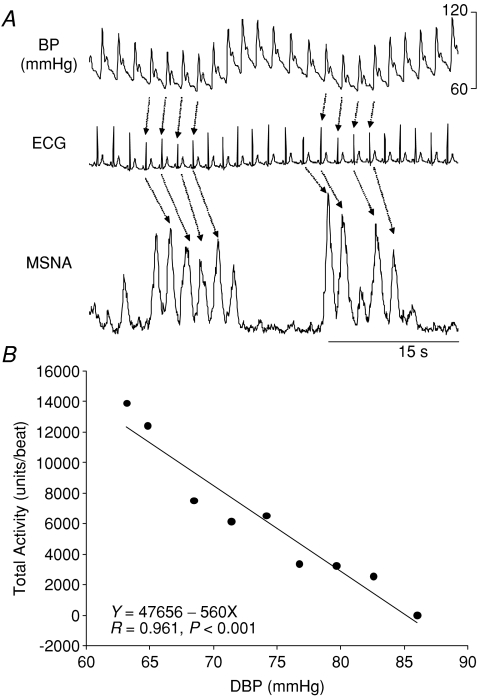
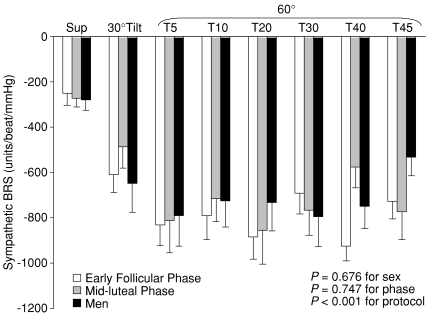
Figure 3 depicts the assessment of sympathetic baroreflex sensitivity during spontaneous breathing from one representative subject. The sensitivity increased at 30 deg, further increased at 60 deg tilt, and remained elevated during sustained upright tilt; the responses were not different between sexes and the menstrual phases (Fig. 4).
Figure 3.
A, sympathetic baroreflex sensitivity assessment during spontaneous breathing from one representative subject. B, the slope of the linear correlation between total activity and diastolic blood pressure (DBP) indicates sympathetic baroreflex sensitivity.
Figure 4. Effects of sex and the menstrual cycle on sympathetic baroreflex sensitivity (BRS) during a graded upright tilt in healthy humans.
Values are means ±s.e.m. Sup, supine; T5, T10, T20, T30, T40 and T45, 5, 10, 20, 30, 40 and 45 min after 60 deg upright tilt.
Changes in MSNA were associated with changes in stroke index during changes in posture, while the slopes of the line relating these two variables were not different between sexes (−1.05 ± 0.16 bursts min−1 ml−1 m–2 in men vs−0.72 ± 0.11 in the early-follicular phase and −0.92 ± 0.10 in the mid-luteal phase in women; P= 0.264 and 0.768), and the menstrual phases (P= 0.765).
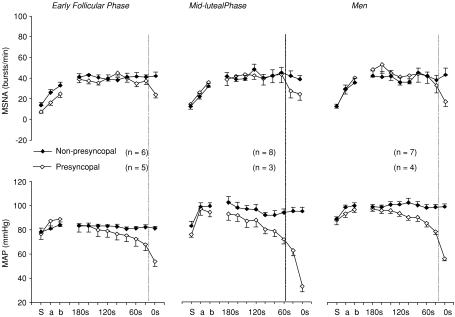
The time to presyncope during the 45 min 60 deg upright tilt did not differ between sexes and phases in the present study (Fig. 5). Figure 6 depicts MSNA and blood pressure responses prior to presyncope in men and women during the two different menstrual phases. Mean arterial pressure started to fall approximately 120 s before the onset of presyncope, while MSNA burst frequency decreased rapidly about 20 s prior to presyncope; these responses seemed to be similar between sexes and phases. This earliest drop in blood pressure may be due to a reduction in cardiac output (Jardine et al. 2002) and/or active vasodilatation unrelated to sympathetic withdrawal.
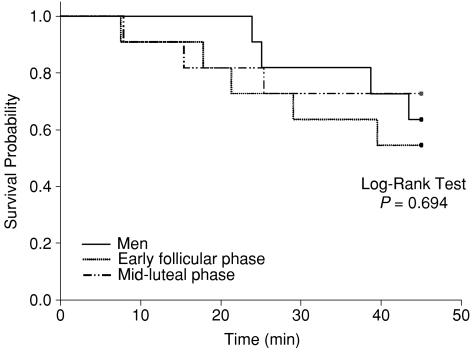
Figure 5.
Survival probability during sustained (i.e. 45 min) 60 deg upright tilt in men and women during the two different menstrual phases
Figure 6. MSNA and mean arterial pressure (MAP) responses prior to presyncope in men and women during the two different menstrual phases.
Values are means ±s.e.m. S, supine; a, 30 deg Tilt; b, between the 2nd and 5th min of 60 deg upright tilt. Data were also averaged in 20 s intervals over the last 3 min prior to presyncope in presyncopal subjects, and from the 42nd to 45th min in non-presyncopal subjects. The vertical lines indicate the critical time point for both falls in blood pressure and sympathetic nerve activity.
Baroreflex sensitivity during the Valsalva manoeuvre
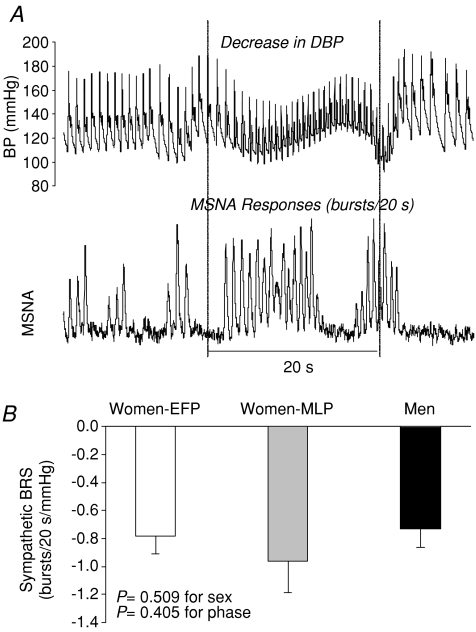
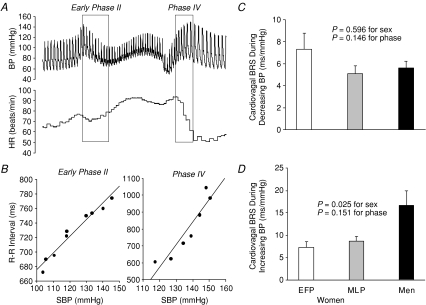
Sympathetic baroreflex sensitivity, as assessed by relating all sympathetic bursts occurring during the 20 s straining period of the Valsalva manoeuvre to the maximum fall in diastolic pressure (Fig. 7A), did not differ between sexes and was not affected by the menstrual cycle in women (Fig. 7B). Figure 8A and B depicts assessments of cardiovagal baroreflex sensitivity during the Valsalva manoeuvre. The sensitivity during decreasing blood pressure (i.e. early phase II) was comparable between sexes and phases (Fig. 8C). However, the sensitivity during increasing blood pressure (i.e. phase IV) was significantly greater in men than women, but was not affected by the menstrual cycle (Fig. 8D).
Figure 7.
A, sympathetic baroreflex sensitivity (BRS) assessed during the Valsalva manoeuvre. B, the sensitivity was not different between sexes and menstrual phases. Values are means ±s.e.m. EFP, the early follicular phase; MLP, the mid-luteal phase.
Figure 8.
A, cardiovagal baroreflex sensitivity (BRS) assessed during early phase II and phase IV of the Valsalva manoeuvre. B, the slope of the linear relationship between R-R interval and systolic BP indicates cardiovagal BRS. C, the sensitivity during decreasing BP was not different between sexes and phases. D, however, it was greater in men than women of both phases during increasing BP. Values are means ±s.e.m. EFP, the early follicular phase; MLP, the mid-luteal phase.
Discussion
The major findings from this study are that (1) MSNA burst frequency responses during sustained upright tilt were similar between sexes and were not affected by the menstrual cycle; (2) upright total activity was lower in women during the early follicular phase than the mid-luteal phase, with similar increases in total activity during tilting compared with men; (3) despite this difference, plasma noradrenaline concentration was not affected by the sex or the menstrual cycle, and downstream vasoconstriction was also similar; (4) sympathetic baroreflex sensitivity was comparable between sexes and menstrual phases during orthostasis; and (5) cardiovagal baroreflex sensitivity during a hypotensive stimulus was similar between sexes and phases; however, the sensitivity was significantly greater in men than women during a hypertensive stimulus.
These results suggest that even though vasomotor sympathetic activity during orthostasis can be affected by the menstrual cycle, it is not clearly different between sexes. Sympathetic baroreflex sensitivity remains stable in women during the two different menstrual phases and is not different from men. Thus, other factors (i.e. stroke volume and cardiac output) rather than neural control mechanisms may contribute to sex differences in orthostatic tolerance in young healthy humans.
Sex and sympathetic neural control during orthostasis
We found that MSNA responses during sustained (i.e. 45 min) upright tilt were similar between men and women in the mid-luteal phase, which is consistent with our previous report in an entirely different group of subjects during acute (i.e. 6 min) upright tilt (Fu et al. 2005a). In the previous study, we did not control menstrual phases in female subjects, but none of them were tested during menstruation (Fu et al. 2005a). The present study showed that women during menstruation (i.e. the early follicular phase) tended to have lower increases in total activity during orthostasis compared with men, but these differences were small and not statistically robust. Both supine and upright sympathetic baroreflex sensitivity did not differ between men and women during the two different menstrual phases. In addition, sympathetic baroreflex sensitivity assessed during the Valsalva manoeuvre was similar between sexes. These observations are in agreement with the study of Tank et al. (2005) but different from that of Hogarth et al. (2007). Our results provide strong evidence that healthy young men and women have comparable sympathetic baroreflex control during orthostasis.
Tank et al. (2005) evaluated sympathetic baroreflex function during pharmacological changes in blood pressure. Their finding was confirmed during physiological modulation of blood pressure during orthostasis in our subjects. However, they showed that the sympathetic baroreflex curve was shifted toward lower systolic pressure values in women, even though the sensitivity was not different between sexes. Different from their method, we used diastolic pressure (Wallin et al. 1974; Sundlof & Wallin, 1978; Kienbaum et al. 2001) rather than systolic pressure as an input stimulus for MSNA, since it has been demonstrated that when, for each cardiac cycle, the occurrence of a sympathetic burst is correlated with different blood pressure parameters there is regularly a close negative correlation to diastolic pressure, a low correlation to systolic, and an intermediary negative correlation to mean blood pressure (Sundlof & Wallin, 1978). We assessed sympathetic baroreflex sensitivity during spontaneous breathing. Although the entire sympathetic baroreflex stimulus−response curve cannot be evaluated, our data can be used to reveal the physiological modulation of sympathetic neural control around the prevailing and regulated operating point in humans (Fu et al. 2006). Our finding was further confirmed by the negative linear correlation between MSNA and stroke index during changes in posture. Since diastolic pressure was similar between sexes in the study of Tank et al. (2005), the sympathetic baroreflex curve would have been superimposed in men and women if diastolic pressure had been used in the assessment of sympathetic baroreflex function.
Hogarth et al. (2007) showed that women had a greater sympathetic baroreflex sensitivity compared with men. It is of note that Hogarth et al. (2007) studied both pre- and post-menopausal women, while we enrolled only young pre-menopausal women who had regular menstrual cycles and did not take oral contraceptives. It has been demonstrated that MSNA increases with advancing age, and the increment is greater in women than men (Matsukawa et al. 1998; Narkiewicz et al. 2005). It seems possible that middle-aged and/or elderly women may have a greater sympathetic baroreflex control compared with their male counterparts, even in the healthy population. This speculation needs to be tested in future studies, especially in patients with hypertension (Fu et al. 2008).
Menstrual cycle and sympathetic baroreflex sensitivity during orthostasis
We found for the first time that total activity responses during sustained upright tilt were affected by the menstrual cycle. Since MSNA burst frequency during tilting was not different between the two different menstrual phases, it could be argued that the blunted upright total activity could be explained by displacement of the recording electrodes during tilting. However, this possibility seems unlikely given the low variability in total activity response during both phases. Moreover, we cannot think of any reasons that the displacement only occurred during the early follicular phase but not the mid-luteal phase. It has been suggested that total activity is more closely related to noradrenaline spillover in humans compared with burst frequency (Hjemdahl et al. 1989), suggesting that the smaller increases in total activity during tilting are consistent with a blunted sympathetic activation in the early follicular phase.
We did not measure noradrenaline spillover in this study; however, plasma noradrenaline concentration during upright tilt was similar between the menstrual phases. It is possible that body fluid retention (volume expansion) under different hormonal conditions (Stachenfeld & Taylor, 2004, 2005) may influence plasma concentrations of noradrenaline in these women. Conversely, downstream vasoconstriction during orthostaisis did not differ between phases. These results suggest that for the same degree of peripheral vasoconstriction, less efferent sympathetic nerve discharges seem to be needed in the early follicular phase than the mid-luteal phase. This may be attributable to the fluctuations of female sex hormones (i.e. oestrogen and progesterone) (Minson et al. 2000a). Meyer et al. (1997) showed that oestrogen replacement attenuated resistance artery adrenergic sensitivity via endothelial vasodilators such as nitric oxide and prostaglandins in female rats. It was also found that oestrogen supplementation selectively attenuated vasoconstrictor responses in perimenopausal women (Sudhir et al. 1997). It is likely that the low level of oestrogen during the early follicular phase could have the opposite effects on adrenergic sensitivity and vasoconstrictor responsiveness in these women.
It is evident that both oestrogen and progesterone have significant impacts on autonomic circulatory control. Whether these two sex hormones have counteractive or additive effects is unknown. Oestrogen was reported to enhance renal and splanchnic sympathetic baroreflex sensitivity through a central mechanism in rats (He et al. 1998). It was also found that oestrogen may influence nitric oxide production by increasing the enzyme nitric oxide synthase expression (Geary et al. 2000), and thereby, increase vasomotor sympathetic outflow in humans (Skarphedinsson et al. 1997). However, previous studies in post-menopausal women showed that oestrogen replacement therapy decreased (Vongpatanasin et al. 2001) or did not change (Hunt et al. 2001) MSNA at rest, suggesting that the effect of oestrogen on sympathetic neural control is complex and can probably be affected by other factors. Conversely, progesterone was found to exert sympathoinhibitory effect and to attenuate sympathetic baroreflex responses via a central mechanism in rats (Heesch & Rogers, 1995). Thus, it is likely that the opposite impacts of oestrogen and progesterone could lead to an unaltered sympathetic neural control, and, therefore, a stable haemodynamic homeostasis during orthostasis in women.
Blunted cardiovagal baroreflex-mediated bradycardia in women
Our finding that cardiovagal baroreflex sensitivity during the hypotensive stimulus (early phase II of the Valsalva manoeuvre) was similar between sexes and was not affected by the menstrual cycle in women is in agreement with some (Minson et al. 2000a; Tank et al. 2005), but not all (Tanaka et al. 2003) previous reports. Interestingly, we found that cardiovagal baroreflex sensitivity during the hypertensive stimulus was significantly lower in women than men. The overshoot (phase IV) of the Valsalva manoeuvre, when the arterial pressure rises quickly, represents a physiological challenge for the baroreflex.
Baroreflex-mediated bradycardia involves reciprocal changes in vagal activation and sympathetic activity inhibition (Coleman, 1980). It has been demonstrated that an increase in vagal tone occurs rapidly, whereas sympathetic withdrawal happens more slowly (Katona et al. 1970). Sex dimorphism in cardiovagal baroreflex sensitivity has been proposed, at least partly, from a smaller increase in vagal outflow to the heart in response to baroreceptor activation (Abdel-Rahman, 1999). This notion is supported by the findings from one previous study in anaesthetized humans (under the condition of parasympathetic nervous system activation) showing that baroreflex sensitivity assessed by the pharmacological method in men was significantly greater than women (Tanaka et al. 2004). Abdel-Rahman et al. (1994) showed that the relatively depressed baroreflex control of heart rate in women was almost entirely dependent on the pattern of blood pressure elevation, brief versus sustained. It is possible that an adaptive response can occur within the central nervous system (Kunze, 1986). On the other hand, it was reported that women had lower carotid artery distensibility compared with men (Hayward & Kelly, 1997). Carotid distensibility was found to be associated with cardiovagal baroreflex sensitivity (Bonyhay et al. 1996). The lower levels of carotid artery distensibility in women would result in a smaller mechanical transduction of arterial pressure into barosensory stretch. This, in turn, would result in an attenuated cardiovagal baroreflex response. Even though cardiovagal baroreflex sensitivity was reduced in women, the baroreflex threshold and saturation, operating range, or operating point were found to be similar between sexes (Beske et al. 2001).
Orthostatic tolerance
In the present study, we found that five out of the 11 women had presyncope in the early-follicular phase, while three of them had presyncope in the mid-luteal phase of their menstrual cycles. Surprisingly, the survival probability during sustained (i.e. 45 min) upright tilt was not different between men and women, as well as between the menstrual phases. However, these observations do not allow us to conclude that there are no sex and/or menstrual phase differences in orthostatic tolerance in humans. Since not all subjects had presyncope during upright tilt, we cannot determine each person's maximal orthostatic tolerance in the present study. A progressive lower body negative pressure to presyncope as we previously used (Fu et al. 2004a) or a tilt-table test in combination with lower body negative pressure to presyncope needs be utilized in future studies to determine the effects of sex and the menstrual cycle on maximal orthostatic tolerance in humans.
Study limitations
There are at least three limitations in this study. First, sympathetic baroreflex sensitivity was quantified during spontaneous breathing. We recognize that the blood pressure fluctuations during spontaneous breathing are not as large as those obtained using other methods such as the neck-chamber technique or invasive pharmacological manipulation. Therefore the entire baroreflex stimulus–response curve cannot be evaluated. Consequently, we cannot determine whether the operating point on this stimulus–response curve has simply shifted to a steeper part of the curve during upright posture or whether an entirely new relationship is achieved. Additionally, spontaneous variations in blood pressure and sympathetic activity may be in part due to the baroreflex, but in part due to other influences. However, our results were confirmed by other non-invasive approaches (i.e. the Valsalva manoeuvre, and the correlation between MSNA and stroke volume/index). Second, the initial premise that women would have greater orthostatic intolerance than men was not duplicated in this study, simply because we applied upright tilt for a fixed time period (i.e. 60 deg for 45 min) and did not bring every subject to presyncope. More prolonged and/or intensive orthostatic challenges would be needed to confirm this observation. Third, all our subjects were healthy individuals with no previous history of syncope. Still, about 40% of them had presyncope during tilting, which is actually common in both healthy individuals and syncopal patients (Mosqueda-Garcia et al. 1997; Jardine et al. 2002; Fu et al. 2006; Ichinose et al. 2006).
In summary, we found in this study that the menstrual cycle affected vasomotor sympathetic responses but not sympathetic baroreflex sensitivity during orthostasis. Not only sympathetic but also cardiovagal baroreflex sensitivity was similar between sexes and menstrual phases during the hypotensive stimulus. However, cardiovagal baroreflex-mediated bradycardia during the hypertensive stimulus was different between sexes but not affected by the menstrual cycle. Thus, other factors (i.e. stroke volume and cardiac output) rather than sympathetic baroreflex control mechanisms may contribute to sex differences in orthostatic tolerance in humans.
Acknowledgments
The time and effort put forth by the subjects is greatly appreciated. The authors thank M. Dean Palmer, Daniel L. Creson, Diane Bedenkop, Peggy Fowler, Murugappan Ramanathan, and Cyrus Oufi for their valuable laboratory assistance. This study was supported by National Institutes of Health K23 grant (HL075283) and the Clinical and Translational Research Center (formerly, the General Clinical Research Center) grant (RR00633).
Glossary
Abbreviations
- BP
blood pressure
- EFP
early follicular phase
- MLP
mid-luteal phase
- MSNA
muscle sympathetic nerve activity
- VM
Valsalva manoeuvre
Disclosure statement
The authors have no conflicts to disclose.
References
- Abdel-Rahman AA. Gender difference in baroreflexmediated bradycardia in young rats: role of cardiac sympathetic and parasympathetic components. Can J Physiol Pharmacol. 1999;77:358–366. [PubMed] [Google Scholar]
- Abdel-Rahman AR, Merrill RH, Wooles WR. Gender-related differences in the baroreceptor reflex control of heart rate in normotensive humans. J Appl Physiol. 1994;77:606–613. doi: 10.1152/jappl.1994.77.2.606. [DOI] [PubMed] [Google Scholar]
- Ali MW, Talukder E. Analysis of longitudinal binary data with missing data due to dropouts. J Biopharm Stat. 2005;15:993–1007. doi: 10.1080/10543400500266692. [DOI] [PubMed] [Google Scholar]
- Beske SD, Alvarez GE, Ballard TP, Davy KP. Gender difference in cardiovagal baroreflex gain in humans. J Appl Physiol. 2001;91:2088–2092. doi: 10.1152/jappl.2001.91.5.2088. [DOI] [PubMed] [Google Scholar]
- Bonyhay I, Jokkel G, Kollai M. Relation between baroreflex sensitivity and carotid artery elasticity in healthy humans. Am J Physiol Heart Circ Physiol. 1996;271:H1139–1144. doi: 10.1152/ajpheart.1996.271.3.H1139. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol. 2005;568:315–321. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman TG. Arterial baroreflex control of heart rate in the conscious rat. Am J Physiol Heart Circ Physiol. 1980;238:H515–520. doi: 10.1152/ajpheart.1980.238.4.H515. [DOI] [PubMed] [Google Scholar]
- Convertino VA, Ludwig DA, Cooke WH. Stroke volume and sympathetic responses to lower-body negative pressure reveal new insight into circulatory shock in humans. Auton Neurosci. 2004;111:127–134. doi: 10.1016/j.autneu.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Shibasaki M, Hodges NA, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during posthandgrip muscle ischemia in humans. J Appl Physiol. 2001;91:1679–1686. doi: 10.1152/jappl.2001.91.4.1679. [DOI] [PubMed] [Google Scholar]
- Fu Q, Arbab-Zadeh A, Perhonen MA, Zhang R, Zuckerman JH, Levine BD. Hemodynamics of orthostatic intolerance: implications for gender differences. Am J Physiol Heart Circ Physiol. 2004;286:H449–457. doi: 10.1152/ajpheart.00735.2002. [DOI] [PubMed] [Google Scholar]
- Fu Q, Shook RP, Okazaki K, Hastings JL, Shibata S, Conner CL, Palmer MD, Levine BD. Vasomotor sympathetic neural control is maintained during sustained upright posture in humans. J Physiol. 2006;577:679–687. doi: 10.1113/jphysiol.2006.118158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Vongpatanasin W, Levine BD. Neural and nonneural mechanisms for sex differences in elderly hypertension: can exercise training help? Hypertension. 2008;52:787–794. doi: 10.1161/HYPERTENSIONAHA.108.118927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Witkowski S, Levine BD. Vasoconstrictor reserve and sympathetic neural control of orthostasis. Circulation. 2004;110:2931–2937. doi: 10.1161/01.CIR.0000146384.91715.B5. [DOI] [PubMed] [Google Scholar]
- Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol. 2005;289:R109–116. doi: 10.1152/ajpregu.00013.2005. [DOI] [PubMed] [Google Scholar]
- Fu Q, Zhang R, Witkowski S, Arbab-Zadeh A, Prasad A, Okazaki K, Levine BD. Persistent sympathetic activation during chronic antihypertensive therapy: a potential mechanism for long term morbidity? Hypertension. 2005;45:513–521. doi: 10.1161/01.HYP.0000158312.63381.c1. [DOI] [PubMed] [Google Scholar]
- Geary GG, Krause DN, Duckles SP. Estrogen reduces mouse cerebral artery tone through endothelial NOS- and cyclooxygenase-dependent mechanisms. Am J Physiol Heart Circ Physiol. 2000;279:H511–519. doi: 10.1152/ajpheart.2000.279.2.H511. [DOI] [PubMed] [Google Scholar]
- Halliwill JR. Segregated signal averaging of sympathetic baroreflex responses in humans. J Appl Physiol. 2000;88:767–773. doi: 10.1152/jappl.2000.88.2.767. [DOI] [PubMed] [Google Scholar]
- Hayward CS, Kelly RP. Gender-related differences in the central arterial pressure waveform. J Am Coll Cardiol. 1997;30:1863–1871. doi: 10.1016/s0735-1097(97)00378-1. [DOI] [PubMed] [Google Scholar]
- He XR, Wang W, Crofton JT, Share L. Effects of 17β-estradiol on sympathetic activity and pressor response to phenylephrine in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1202–1208. doi: 10.1152/ajpregu.1998.275.4.R1202. [DOI] [PubMed] [Google Scholar]
- Heesch CM, Rogers RC. Effects of pregnancy and progesterone metabolites on regulation of sympathetic outflow. Clin Exp Pharmacol Physiol. 1995;22:136–142. doi: 10.1111/j.1440-1681.1995.tb01970.x. [DOI] [PubMed] [Google Scholar]
- Hjemdahl P, Fagius J, Freyschuss U, Wallin BG, Daleskog M, Bohlin G, Perski A. Muscle sympathetic activity and norepinephrine release during mental challenge in humans. Am J Physiol Endocrinol Metab. 1989;257:E654–664. doi: 10.1152/ajpendo.1989.257.5.E654. [DOI] [PubMed] [Google Scholar]
- Hogarth AJ, Mackintosh AF, Mary DA. Gender-related differences in the sympathetic vasoconstrictor drive of normal subjects. Clin Sci (Lond) 2007;112:353–361. doi: 10.1042/CS20060288. [DOI] [PubMed] [Google Scholar]
- Hunt BE, Taylor JA, Hamner JW, Gagnon M, Lipsitz LA. Estrogen replacement therapy improves baroreflex regulation of vascular sympathetic outflow in postmenopausal women. Circulation. 2001;103:2909–2914. doi: 10.1161/01.cir.103.24.2909. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Fujii N, Kondo N, Nishiyasu T. Modulation of the control of muscle sympathetic nerve activity during severe orthostatic stress. J Physiol. 2006;576:947–958. doi: 10.1113/jphysiol.2006.117507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine DL, Melton IC, Crozier IG, English S, Bennett SI, Frampton CM, Ikram H. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. Am J Physiol Heart Circ Physiol. 2002;282:H1804–1809. doi: 10.1152/ajpheart.00640.2001. [DOI] [PubMed] [Google Scholar]
- Katona PG, Poitras JW, Barnett GO, Terry BS. Cardiac vagal efferent activity and heart period in the carotid sinus reflex. Am J Physiol. 1970;218:1030–1037. doi: 10.1152/ajplegacy.1970.218.4.1030. [DOI] [PubMed] [Google Scholar]
- Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol. 2001;531:861–869. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze DL. Acute resetting of baroreceptor reflex in rabbits: a central component. Am J Physiol Heart Circ Physiol. 1986;250:H866–870. doi: 10.1152/ajpheart.1986.250.5.H866. [DOI] [PubMed] [Google Scholar]
- Levine BD, Buckey JC, Fritsch JM, Yancy CW, Jr, Watenpaugh DE, Snell PG, Lane LD, Eckberg DL, Blomqvist CG. Physical fitness and cardiovascular regulation: mechanisms of orthostatic intolerance. J Appl Physiol. 1991;70:112–122. doi: 10.1152/jappl.1991.70.1.112. [DOI] [PubMed] [Google Scholar]
- Levine BD, Pawelczyk JA, Ertl AC, Cox JF, Zuckerman JH, Diedrich A, Biaggioni I, Ray CA, Smith ML, Iwase S, Saito M, Sugiyama Y, Mano T, Zhang R, Iwasaki K, Lane LD, Buckey JC, Jr, Cooke WH, Baisch FJ, Eckberg DL, Blomqvist CG. Human muscle sympathetic neural and haemodynamic responses to tilt following spaceflight. J Physiol. 2002;538:331–340. doi: 10.1113/jphysiol.2001.012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1600–1604. doi: 10.1152/ajpregu.1998.275.5.R1600. [DOI] [PubMed] [Google Scholar]
- Meendering JR, Torgrimson BN, Houghton BL, Halliwill JR, Minson CT. Menstrual cycle and sex affect hemodynamic responses to combined orthostatic and heat stress. Am J Physiol Heart Circ Physiol. 2005;289:H631–642. doi: 10.1152/ajpheart.00029.2005. [DOI] [PubMed] [Google Scholar]
- Meyer MC, Cummings K, Osol G. Estrogen replacement attenuates resistance artery adrenergic sensitivity via endothelial vasodilators. Am J Physiol Heart Circ Physiol. 1997;272:H2264–2270. doi: 10.1152/ajpheart.1997.272.5.H2264. [DOI] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM, Joyner MJ. Sympathetic activity and baroreflex sensitivity in young women taking oral contraceptives. Circulation. 2000;102:1473–1476. doi: 10.1161/01.cir.102.13.1473. [DOI] [PubMed] [Google Scholar]
- Mosqueda-Garcia R, Furlan R, Fernandez-Violante R, Desai T, Snell M, Jarai Z, Ananthram V, Robertson RM, Robertson D. Sympathetic and baroreceptor reflex function in neurally mediated syncope evoked by tilt. J Clin Invest. 1997;99:2736–2744. doi: 10.1172/JCI119463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45:522–525. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. 1999;317:75–77. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol. 2001;281:H2028–2035. doi: 10.1152/ajpheart.2001.281.5.H2028. [DOI] [PubMed] [Google Scholar]
- Skarphedinsson JO, Elam M, Jungersten L, Wallin BG. Sympathetic nerve traffic correlates with the release of nitric oxide in humans: implications for blood pressure control. J Physiol. 1997;501:671–675. doi: 10.1111/j.1469-7793.1997.671bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachenfeld NS, Taylor HS. Effects of estrogen and progesterone administration on extracellular fluid. J Appl Physiol. 2004;96:1011–1018. doi: 10.1152/japplphysiol.01032.2003. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS, Taylor HS. Progesterone increases plasma volume independent of estradiol. J Appl Physiol. 2005;98:1991–1997. doi: 10.1152/japplphysiol.00031.2005. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Elser MD, Jennings GL, Komesaroff PA. Estrogen supplementation decreases norepinephrine-induced vasoconstriction and total body norepinephrine spillover in perimenopausal women. Hypertension. 1997;30:1538–1543. doi: 10.1161/01.hyp.30.6.1538. [DOI] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Kimura T, Goyagi T, Nishikawa T. Gender differences in baroreflex response and heart rate variability in anaesthetized humans. Br J Anaesth. 2004;92:831–835. doi: 10.1093/bja/aeh143. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Sato M, Umehara S, Nishikawa T. Influence of menstrual cycle on baroreflex control of heart rate: comparison with male volunteers. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1091–1097. doi: 10.1152/ajpregu.00162.2003. [DOI] [PubMed] [Google Scholar]
- Tank J, Diedrich A, Szczech E, Luft FC, Jordan J. Baroreflex regulation of heart rate and sympathetic vasomotor tone in women and men. Hypertension. 2005;45:1159–1164. doi: 10.1161/01.HYP.0000165695.98915.9a. [DOI] [PubMed] [Google Scholar]
- Triebwasser JH, Johnson RL, Burpo RP, Campbell JC, Reardon WC, Blomqvist CG. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Environ Med. 1977;48:203–209. [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Vongpatanasin W, Tuncel M, Mansour Y, Arbique D, Victor RG. Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation. 2001;103:2903–2908. doi: 10.1161/01.cir.103.24.2903. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Delius W, Sundlof G. Human muscle nerve sympathetic activity in cardiac arrhythmias. Scand J Clin Lab Invest. 1974;34:293–300. doi: 10.3109/00365517409049883. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Sundlof G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982;6:287–291. doi: 10.1016/0165-1838(82)90001-7. [DOI] [PubMed] [Google Scholar]