Abstract
Arterial blood gases are critical in regulation of cerebral blood flow (CBF) and cerebral metabolic rate for O2 (CMRO2). However, the relation of these variables to cortical tissue  (t
(t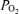 ), and electrocorticographic (ECoG) activity (high voltage low frequency, HVLF, versus low voltage high frequency, LVHF), are not well defined. In the fetus, we tested the hypothesis that ECoG pattern is associated closely with cerebral oxygenation. In fetal sheep (n= 8) with laser Doppler flowmeter, fluorescent O2 probe and ECoG electrodes, we measured laser Doppler CBF (LD-CBF), t
), and electrocorticographic (ECoG) activity (high voltage low frequency, HVLF, versus low voltage high frequency, LVHF), are not well defined. In the fetus, we tested the hypothesis that ECoG pattern is associated closely with cerebral oxygenation. In fetal sheep (n= 8) with laser Doppler flowmeter, fluorescent O2 probe and ECoG electrodes, we measured laser Doppler CBF (LD-CBF), t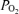 , ECoG and spectral edge frequency-90 (SEF90) in response to 40 min isocapnic hypoxia. In the normoxic fetus, LD-CBF and CMRO2 correlated highly with ECoG state. With a shift from HVLF to LVHF, t
, ECoG and spectral edge frequency-90 (SEF90) in response to 40 min isocapnic hypoxia. In the normoxic fetus, LD-CBF and CMRO2 correlated highly with ECoG state. With a shift from HVLF to LVHF, t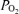 decreased followed by increased LD-CBF (18%) and CMRO2 (13%). With acute hypoxia (
decreased followed by increased LD-CBF (18%) and CMRO2 (13%). With acute hypoxia (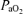 = 12 ± 1 Torr), t
= 12 ± 1 Torr), t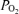 decreased to ∼3 Torr, LD-CBF increased 48 ± 10%, ECoG shifted to chiefly the HVLF state, SEF90 decreased ∼15%, and CMRO2 decreased ∼20% (P < 0.05 for each). For the normoxic fetus, CBF was closely related to ECoG state, but this association was less evident during acute hypoxia. We speculate that, in the otherwise stressed fetus, acute hypoxia may further compromise cerebral oxygenation.
decreased to ∼3 Torr, LD-CBF increased 48 ± 10%, ECoG shifted to chiefly the HVLF state, SEF90 decreased ∼15%, and CMRO2 decreased ∼20% (P < 0.05 for each). For the normoxic fetus, CBF was closely related to ECoG state, but this association was less evident during acute hypoxia. We speculate that, in the otherwise stressed fetus, acute hypoxia may further compromise cerebral oxygenation.
In the developing fetus, cerebral blood flow (CBF) has been shown to be a function of the electrocorticographic activity state (Richardson et al. 1989). In addition, cerebral cortical tissue O2 tension (t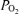 ) and cerebral metabolic rate for O2 (CMRO2) are both functions of, and determinants of, CBF (Pereyra Peña et al. 2007). Nonetheless, many aspects of these interrelations are unknown. Cycles of electrocortical activity appear before birth in species in which significant brain development occurs during late gestation. These include sheep (Mann, 1970; Jost et al. 1972; Ruckebusch, 1972; Boddy et al. 1974; Mann et al. 1974; Ruckebusch et al. 1977; Clapp et al. 1980; Clewlow et al. 1983; Richardson et al. 1985, 1989; Szeto & Hinman, 1985; Szeto et al. 1985), baboons (Stark et al. 1991; Isler et al. 2005), and humans (Osredkar et al. 2005). In sheep, particularly during the last 3 weeks of gestation, e.g. from gestational day 125 to term, the electrocorticogram (ECoG) cycles between a high voltage low frequency (HVLF) state associated with quiet sleep, and low voltage high frequency (LVHF) state associated with rapid eye movement sleep/awake (Richardson et al. 1985, 1989). In the human newborn infant, periodic changes in brain ECoG state are considered an indication of well being (Thornberg & Thiringer, 1990; Scher et al. 1996; Watanabe et al. 1999; Ter Horst et al. 2004; Osredkar et al. 2005).
) and cerebral metabolic rate for O2 (CMRO2) are both functions of, and determinants of, CBF (Pereyra Peña et al. 2007). Nonetheless, many aspects of these interrelations are unknown. Cycles of electrocortical activity appear before birth in species in which significant brain development occurs during late gestation. These include sheep (Mann, 1970; Jost et al. 1972; Ruckebusch, 1972; Boddy et al. 1974; Mann et al. 1974; Ruckebusch et al. 1977; Clapp et al. 1980; Clewlow et al. 1983; Richardson et al. 1985, 1989; Szeto & Hinman, 1985; Szeto et al. 1985), baboons (Stark et al. 1991; Isler et al. 2005), and humans (Osredkar et al. 2005). In sheep, particularly during the last 3 weeks of gestation, e.g. from gestational day 125 to term, the electrocorticogram (ECoG) cycles between a high voltage low frequency (HVLF) state associated with quiet sleep, and low voltage high frequency (LVHF) state associated with rapid eye movement sleep/awake (Richardson et al. 1985, 1989). In the human newborn infant, periodic changes in brain ECoG state are considered an indication of well being (Thornberg & Thiringer, 1990; Scher et al. 1996; Watanabe et al. 1999; Ter Horst et al. 2004; Osredkar et al. 2005).
Several studies have suggested that fetal cerebral blood flow and the cerebral metabolic rate for O2 are related to sleep state, being significantly greater during periods of LVHF as compared to HVLF (Richardson et al. 1985, 1989, 1994; Morrison et al. 2005). In addition, several groups have demonstrated a ‘marginal’ decrease in LVHF activity with acute hypoxia (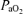 < 16 Torr), and more severe depression in the presence of fetal acidosis (pH < 7.26) (Richardson et al. 1992; Takahashi et al. 2006). In contrast, others have reported a significant increase in time spent in LVHF with a decrease in time spent in HVLF during acute hypoxia (Pulgar et al. 2006). Although one group has reported that fetal cerebral metabolic rate for O2 did not change significantly with variation in ECoG activity, CMRO2per se was not measured (Walker et al. 1984). In an effort to improve the use of ECoG as a diagnostic measure of maturation and predictability, several groups have introduced the concept of spectral edge frequency90 (SEF90), that frequency below which 90% (Szeto, 1990; Thaler et al. 2000; Inder et al. 2003) or 95% (Bell et al. 1991) of the ECoG power resides. This serves as a single quantitative measure of ECoG frequency.
< 16 Torr), and more severe depression in the presence of fetal acidosis (pH < 7.26) (Richardson et al. 1992; Takahashi et al. 2006). In contrast, others have reported a significant increase in time spent in LVHF with a decrease in time spent in HVLF during acute hypoxia (Pulgar et al. 2006). Although one group has reported that fetal cerebral metabolic rate for O2 did not change significantly with variation in ECoG activity, CMRO2per se was not measured (Walker et al. 1984). In an effort to improve the use of ECoG as a diagnostic measure of maturation and predictability, several groups have introduced the concept of spectral edge frequency90 (SEF90), that frequency below which 90% (Szeto, 1990; Thaler et al. 2000; Inder et al. 2003) or 95% (Bell et al. 1991) of the ECoG power resides. This serves as a single quantitative measure of ECoG frequency.
Despite a number of studies on fetal ECoG, including the ontogeny of its development and association with rapid eye movements (Jost et al. 1972; Mirmiran, 1995), breathing-like activity (Dawes et al. 1972; Clewlow et al. 1983; Koos et al. 1986), and body movements (Natale et al. 1981), relatively little is known of its relation to cerebral t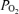 , CMRO2 and CBF. In addition, in the fetus little is known of the extent to which hypoxic-mediated changes with decreased cortical t
, CMRO2 and CBF. In addition, in the fetus little is known of the extent to which hypoxic-mediated changes with decreased cortical t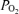 and CMRO2, are associated with altered ECoG state. The question thus arises, as the extent to which evaluation of fetal ECoG state (LVHF versus HVLF) and spectral analysis can provide information in evaluating and correlating neuronal activity changes with measurement of cerebral blood flow, cortical tissue
and CMRO2, are associated with altered ECoG state. The question thus arises, as the extent to which evaluation of fetal ECoG state (LVHF versus HVLF) and spectral analysis can provide information in evaluating and correlating neuronal activity changes with measurement of cerebral blood flow, cortical tissue 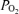 and CMRO2 under normoxic conditions and/or in response to hypoxia. From a basic science standpoint, such information may be of particular value in understanding fundamental physiological mechanisms by the use of pharmacological antagonist/agonist administration designed to investigate CBF regulation. In addition, such information may be of great importance in the evaluation of fetal/neonatal condition particularly in the instance of compromise by hypoxia and/or hypoxia/ischaemia. Thus, in the present study we tested the hypothesis that during normoxia, LVHF ECoG activity is associated with significant increases in CMRO2, decreases in cortical t
and CMRO2 under normoxic conditions and/or in response to hypoxia. From a basic science standpoint, such information may be of particular value in understanding fundamental physiological mechanisms by the use of pharmacological antagonist/agonist administration designed to investigate CBF regulation. In addition, such information may be of great importance in the evaluation of fetal/neonatal condition particularly in the instance of compromise by hypoxia and/or hypoxia/ischaemia. Thus, in the present study we tested the hypothesis that during normoxia, LVHF ECoG activity is associated with significant increases in CMRO2, decreases in cortical t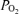 and increases in LD-CBF, and that acute hypoxia is associated with significant alterations in fetal ECoG state as shown by spectral analysis of waveforms and related variables.
and increases in LD-CBF, and that acute hypoxia is associated with significant alterations in fetal ECoG state as shown by spectral analysis of waveforms and related variables.
Methods
Experimental animals, ethical approval, surgery and instrumentation
In eight pregnant sheep, experiments commenced in the fetus at 125 ± 2 days gestation (g.d.; term ∼145 days). We have described details of our experimental surgical technique in numerous reports (Bishai et al. 2003; Tomimatsu et al. 2006; Pereyra Peña et al. 2007). All surgical and experimental procedures were performed within the regulations of the Animal Welfare Act, the National Institutes of Health Guide for the Care and Use of Laboratory Animals, ‘The Guiding Principles in the Care and Use of Animals’ approved by the Council of the American Physiological Society, and the Animal Care and Use Committee of Loma Linda University. In brief, following induction of anaesthesia with thiopental sodium (10 mg kg−1i.v.), the ewe was placed in the supine position, intubated and anaesthesia maintained with inhalation of 1.0% isoflurane in O2. Under aseptic conditions the maternal abdominal wall and uterus were incised, one fetal forelimb delivered, a polyvinyl catheter (2.3 mm o.d.) placed into the brachial artery and advanced to the brachiocephalic artery, and another into the brachial vein and advanced to the superior vena cava. Catheters were anchored subcutaneously and exteriorized. After replacing the forelimb, we repeated this in the other forelimb. We then delivered the fetal head, incised the scalp caudal to the coronal suture exposing the right and left parietal bones, and drilled a 1.3 mm burr hole on the right side 5 mm lateral to the sagittal suture and 15 mm caudal to the coronal suture. We inserted the tip of the composite t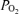 –laser Doppler flow probe with thermocouple (Oxford Optronix, Ltd, Oxford, UK) into the parasagittal parietal lobe cortex to a depth of ∼3 mm below the dura mater, and fixed this to the skull with tissue glue (Bishai et al. 2003; Tomimatsu et al. 2006; Pereyra Peña et al. 2007). We repeated this on the left side. We also inserted a catheter 1.5 cm into the sagittal sinus to sample mixed venous blood from the anterior brain, including the tissue containing the LDF probe, and secured this with tissue glue and latex dental dam. We also placed two pairs of ECoG electrodes (ADInstruments, Colorado Springs, CO, USA), one on either side, through the dura into the parasagittal parietal cortex (5 mm anterior and 5 mm lateral to the bregma) securing these with cyanocrylate glue. A reference electrode was sewn over the occiput, and the fetal scalp incision closed. In addition, we placed a catheter (3.5 mm o.d.) into the amniotic cavity for measurement of that pressure and the administration of antibiotics. We replaced the fetal head into the uterine cavity, sutured the uterine wall in layers, and exteriorized catheters and probes to the ewe's flank. Lastly, we inserted Tygon polyvinyl catheters (2.8 mm o.d.) filled with heparinized saline in the ewe's right femoral artery and vein for blood sampling and infusion of fluids, respectively. Postoperatively, the ewe was given 900 000 U penicillin intramuscularly for 3 days as prophylaxis against wound infection, and the fetus was given cefotaxime (50 mg day−1, i.v.) to prevent meningitis or other infection. We also administered ampicillin (500 mg) and gentamicin (40 mg) into the amniotic fluid daily to prevent chorioamnionitis. We monitored sheep wellbeing and arterial blood gases daily for 4 to 5 days of postoperative recovery before commencing the experiments (Tomimatsu et al. 2006; Pereyra Peña et al. 2007).
–laser Doppler flow probe with thermocouple (Oxford Optronix, Ltd, Oxford, UK) into the parasagittal parietal lobe cortex to a depth of ∼3 mm below the dura mater, and fixed this to the skull with tissue glue (Bishai et al. 2003; Tomimatsu et al. 2006; Pereyra Peña et al. 2007). We repeated this on the left side. We also inserted a catheter 1.5 cm into the sagittal sinus to sample mixed venous blood from the anterior brain, including the tissue containing the LDF probe, and secured this with tissue glue and latex dental dam. We also placed two pairs of ECoG electrodes (ADInstruments, Colorado Springs, CO, USA), one on either side, through the dura into the parasagittal parietal cortex (5 mm anterior and 5 mm lateral to the bregma) securing these with cyanocrylate glue. A reference electrode was sewn over the occiput, and the fetal scalp incision closed. In addition, we placed a catheter (3.5 mm o.d.) into the amniotic cavity for measurement of that pressure and the administration of antibiotics. We replaced the fetal head into the uterine cavity, sutured the uterine wall in layers, and exteriorized catheters and probes to the ewe's flank. Lastly, we inserted Tygon polyvinyl catheters (2.8 mm o.d.) filled with heparinized saline in the ewe's right femoral artery and vein for blood sampling and infusion of fluids, respectively. Postoperatively, the ewe was given 900 000 U penicillin intramuscularly for 3 days as prophylaxis against wound infection, and the fetus was given cefotaxime (50 mg day−1, i.v.) to prevent meningitis or other infection. We also administered ampicillin (500 mg) and gentamicin (40 mg) into the amniotic fluid daily to prevent chorioamnionitis. We monitored sheep wellbeing and arterial blood gases daily for 4 to 5 days of postoperative recovery before commencing the experiments (Tomimatsu et al. 2006; Pereyra Peña et al. 2007).
During this recovery period, we recorded LD-CBF, cortical t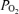 , ECoG, arterial blood gases, blood pressure and heart rate daily to assess the health of the fetus and ewe. All studies were carried out with the ewe standing in a metabolic cart in the laboratory at ∼20°C between 09.00 and 15.00 h. Ewes were given free access to alfalfa pellets and water throughout the study. Following completion of the experiment, the ewe and fetus were killed with an overdose of the proprietary euthanasia solution, Euthasol (pentobarbital sodium; 100 mg kg−1; Virbac, Ft Worth, TX, USA). The fetal body was towel-dried and weighed to the nearest 10 g. We recorded the location of the laser Doppler probes, indicated by dye track, the ECoG electrodes and all catheters.
, ECoG, arterial blood gases, blood pressure and heart rate daily to assess the health of the fetus and ewe. All studies were carried out with the ewe standing in a metabolic cart in the laboratory at ∼20°C between 09.00 and 15.00 h. Ewes were given free access to alfalfa pellets and water throughout the study. Following completion of the experiment, the ewe and fetus were killed with an overdose of the proprietary euthanasia solution, Euthasol (pentobarbital sodium; 100 mg kg−1; Virbac, Ft Worth, TX, USA). The fetal body was towel-dried and weighed to the nearest 10 g. We recorded the location of the laser Doppler probes, indicated by dye track, the ECoG electrodes and all catheters.
Overall experimental design
The protocol was designed to measure several aspects of fetal CBF, cortical t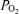 and ECoG during three consecutive periods: normoxic control (60 min), isocapnic hypoxic hypoxia (40 min) and recovery (60 min). Variables included: the relation of CBF to ECoG states, the relation of cortical t
and ECoG during three consecutive periods: normoxic control (60 min), isocapnic hypoxic hypoxia (40 min) and recovery (60 min). Variables included: the relation of CBF to ECoG states, the relation of cortical t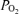 to CMRO2, ECoG state, and O2 dose–response relations. We induced hypoxic hypoxia by having the ewe breathe 10.5% O2 with 3–5% CO2 in nitrogen to maintain maternal and fetal arterial
to CMRO2, ECoG state, and O2 dose–response relations. We induced hypoxic hypoxia by having the ewe breathe 10.5% O2 with 3–5% CO2 in nitrogen to maintain maternal and fetal arterial 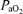
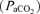 at isocapnic levels. This was administered by passing a 50 : 50 air–nitrogen plus CO2 gas mixture at 30 l min−1 through a large opaque plastic bag placed over the ewe's head. A high rate of air flow to the bag prevented accumulation of CO2 and water vapour. This procedure has been well established in our laboratory, and is tolerated by the ewes with minimal evidence of distress. For the isocapnic hypoxia period, we have determined the time-control reproducibility of LD-CBF for a given hypoxic exposure (Pereyra Peña et al. 2007). To achieve steady state, we measured LD-CBF, t
at isocapnic levels. This was administered by passing a 50 : 50 air–nitrogen plus CO2 gas mixture at 30 l min−1 through a large opaque plastic bag placed over the ewe's head. A high rate of air flow to the bag prevented accumulation of CO2 and water vapour. This procedure has been well established in our laboratory, and is tolerated by the ewes with minimal evidence of distress. For the isocapnic hypoxia period, we have determined the time-control reproducibility of LD-CBF for a given hypoxic exposure (Pereyra Peña et al. 2007). To achieve steady state, we measured LD-CBF, t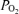 , ECoG and other functions continuously.
, ECoG and other functions continuously.
LD-cerebral blood flow measurements
We measured continuously right and left cerebral cortical blood flow by use of the laser Doppler flowmeter (Oxford Optronix), and t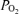 using a fluorescent O2 probe (Oxylite, Oxford Optronix), as we have described previously (Tomimatsu et al. 2006; Pereyra Peña et al. 2007). We also continuously measured ECoG, mean arterial blood pressure and heart rate, the latter by use of a pressure transducer (Argon Medical, Athens, TX, USA), and the data acquisition software. For the LD-CBF, t
using a fluorescent O2 probe (Oxylite, Oxford Optronix), as we have described previously (Tomimatsu et al. 2006; Pereyra Peña et al. 2007). We also continuously measured ECoG, mean arterial blood pressure and heart rate, the latter by use of a pressure transducer (Argon Medical, Athens, TX, USA), and the data acquisition software. For the LD-CBF, t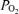 , mean arterial blood pressure and heart rate data we took 1 min averages, and exported the values to a spreadsheet for analysis. As LD-CBF provides a relative measure of cerebral blood flow, these values as well as those for O2 delivery and CMRO2 for each animal were calculated as a percentage of the mean values during the baseline control period (Tomimatsu et al. 2006; Pereyra Peña et al. 2007). Analog outputs (sampling rate 200 Hz) were digitized and stored using an analog-to-digital converter (Powerlab 16/SP; ADInstruments) and data acquisition software (Chart 4, ADInstruments) (see Bishai et al. 2003; Tomimatsu et al. 2006; Pereyra Peña et al. 2007).
, mean arterial blood pressure and heart rate data we took 1 min averages, and exported the values to a spreadsheet for analysis. As LD-CBF provides a relative measure of cerebral blood flow, these values as well as those for O2 delivery and CMRO2 for each animal were calculated as a percentage of the mean values during the baseline control period (Tomimatsu et al. 2006; Pereyra Peña et al. 2007). Analog outputs (sampling rate 200 Hz) were digitized and stored using an analog-to-digital converter (Powerlab 16/SP; ADInstruments) and data acquisition software (Chart 4, ADInstruments) (see Bishai et al. 2003; Tomimatsu et al. 2006; Pereyra Peña et al. 2007).
Blood sampling and calculations
We collected and analysed maternal and fetal blood samples (0.3 ml) for blood gases corrected to body temperature (ABL3, Radiometer, Copenhagen, Denmark) (Lotgering et al. 1983) every 10 min throughout the control period. To standardize the degree of hypoxia, additional blood samples were taken every 5 min during this period, and the inspired gas mixture was adjusted accordingly. We also measured spectrophotometrically haemoglobin concentration [Hgb] and oxyhaemoglobin saturation [HbO2] (OSM2 Hemoximeter, Radiometer), and calculated O2 content (CaO2) as the product of [HbO2]×[Hgb]× 1.34. As previously reported, we calculated relative cerebral O2 delivery (LD-CBF × CaO2), cerebral fractional O2 extraction, i.e. O2 consumed as a fraction of that delivered (CMRO2/cerebral O2 delivery) (Jones et al. 1982; Jones & Traystman, 1984), and the cerebral metabolic rate for O2 (LD-CBF × arterial-to-sagittal sinus O2 content difference) (Tomimatsu et al. 2006; Pereyra Peña et al. 2007).
Electrocorticogram
The fetal ECoG signals were analog-filtered and sampled at 150 Hz before being processed by the 12 bit A/D converter and band-pass filtered between 1–50 Hz, during the 60 min control period, 40 min hypoxia and 60 min recovery. For the ECoG signal analysis, we developed a computer code in-house using MATLAB software (Mathworks, Inc., Natick, MA, USA) to determine the percentage of signal power in the several frequency bands, as well as the spectral edge, median and modal frequencies of ECoG time series data. With this code, we identified HVLF and LVHF epochs, and performed power spectral analysis using fast Fourier transformation. The software provides the total spectral power between 1–30 Hz, as well as power for 1–4 Hz (delta), 4–8 Hz (theta), 8–13 Hz (alpha), 13–20 Hz (beta), 20–30 Hz (gamma) bands, and the spectral edge frequency (SEF90, frequency below which 90% of the spectral power resides). The software uses a threshold derived from the digitized ECoG signal to segregate the electrical signal into periods of HVLF and LVHF epochs, and to provide the number, duration and percentage of total time of HVLF and LVHF epochs present in a particular time period. The signal was full wave-rectified, smoothed at 0.01 Hz, and the threshold applied. Once the software determined the presence of a particular event type (HVLF or LVHF), it calculated the fast Fourier transformation frequency, SEF90 and the duration of the event in minutes using the raw data. We defined the threshold level for each animal on the first day of analysis, and applied this to the subsequent analysis periods. For statistical analysis, all calculations were made on logarithms of intensity (dB) to provide an optimal approximation to the normal distribution (Gasser et al. 1982; Williams et al. 1992).
Data acquisition and statistical analyses
The data were acquired continuously and calculated as 1 min means for each period. We recorded LD-CBF and t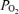 signals from both cerebral hemispheres. Analysis of variance showed no systematic differences between hemispheres. Thus, the two results were averaged to provide a mean estimate of changes in relative blood flow and t
signals from both cerebral hemispheres. Analysis of variance showed no systematic differences between hemispheres. Thus, the two results were averaged to provide a mean estimate of changes in relative blood flow and t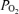 values. We expressed results as means ±s.e.m. Differences were evaluated for statistical significance (P < 0.05) by two-way analysis of variance (ANOVA) followed by Newman–Keuls post hoc test, to compare control and hypoxic periods. All analysis including both linear and non-linear regressions were performed with a standard statistical package (GraphPad Prism, GraphPad Software, San Diego, CA, USA).
values. We expressed results as means ±s.e.m. Differences were evaluated for statistical significance (P < 0.05) by two-way analysis of variance (ANOVA) followed by Newman–Keuls post hoc test, to compare control and hypoxic periods. All analysis including both linear and non-linear regressions were performed with a standard statistical package (GraphPad Prism, GraphPad Software, San Diego, CA, USA).
Results
Maternal blood gas values
In response to 40 min isocapnic hypoxic hypoxia, maternal arterial 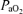 and oxyhaemoglobin saturation decreased significantly to ∼60 ± 5 Torr and 85 ± 6%, respectively, while
and oxyhaemoglobin saturation decreased significantly to ∼60 ± 5 Torr and 85 ± 6%, respectively, while 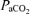 , pH, haemoglobin concentration and base excess remained constant, as we have reported previously (see Pereyra Peña et al. 2007) (data not shown).
, pH, haemoglobin concentration and base excess remained constant, as we have reported previously (see Pereyra Peña et al. 2007) (data not shown).
Fetal systemic responses
Table 1 presents data on fetal arterial blood gas values during the normoxic control period, at 20 and 40 min following the onset of acute isocapnic hypoxia, and during the recovery period. During the control period, the change in fetal ECoG state from HVLF to LVHF had no significant effect on arterial blood gas values, mean arterial blood pressure or fetal heart rate. In response to acute hypoxia, 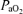 decreased from 24 ± 1 to ∼12 ± 1 Torr, with an accompanying decrease in [HbO2] (P < 0.05 for each). As also evident in Table 1, acute hypoxia was associated with a significant increase in MABP (∼12%) and decrease in FHR (∼13%).
decreased from 24 ± 1 to ∼12 ± 1 Torr, with an accompanying decrease in [HbO2] (P < 0.05 for each). As also evident in Table 1, acute hypoxia was associated with a significant increase in MABP (∼12%) and decrease in FHR (∼13%).
Table 1.
Fetal arterial blood gas values and other variables
| Normoxia |
20 min Hypoxia |
40 min Hypoxia |
Recovery |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HVLF | LVHF | Δ | HVLF | LVHF | Δ | HVLF | LVHF | Δ | HVLF | LVHF | Δ | |
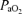 (Torr) (Torr) |
24 | 24 | – | 13 | 14 | 1 | 11 | 13 | 2 | 26 | 24 | −2 |
| (± 1) | (± 1) | (± 1)* | (± 1)* | (± 1)* | (± 1)* | (± 2) | (± 2) | |||||
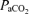 (Torr) (Torr) |
46 | 46 | – | 47 | 44 | −3 | 49 | 44 | −5 | 45 | 45 | – |
| (± 1) | (± 1) | (± 2) | (± 1) | (± 2) | (± 2) | (± 1) | (± 1) | |||||
| pH | 7.38 | 7.38 | – | 7.33 | 7.34 | 0.01 | 7.23 | 7.35 | 0.12 | 7.24 | 7.26 | 0.02 |
| (± 0.01) | (± 0.01) | (± 0.02) | (± 0.01) | (± 0.03) | (± 0.02) | (± 0.03) | (± 0.02) | |||||
| BE (mm) | 1.8 | 1.8 | – | −1.4 | 0.0 | 1.4 | −9.4 | −1.8 | 7.6 | −8.1 | −9.5 | −1.4 |
| (± 0.4) | (± 0.6) | (± 1.0) | (± 1.0) | (± 1.0) | (± 1.0) | (± 2.0) | (± 1.0) | |||||
| O2 content (mm) | 7.6 | 7.5 | −0.1 | 3.8 | 3.8 | – | 2.7 | 3.7 | 1.0 | 6.9 | 7.2 | 0.3 |
| (± 0.4) | (± 0.2) | (± 0.7)* | (± 0.3)* | (± 0.3)* | (± 0.3)* | (± 0.3) | (± 0.4) | |||||
| Hgb (g dl−1) | 8.1 | 7.9 | −0.2 | 9.2 | 8.1 | −1.1 | 9.2 | 8.1 | −1.1 | 8.5 | 8.8 | 0.3 |
| (± 0.2) | (± 0.3) | (± 0.3) | (± 0.3) | (± 0.3) | (± 0.5) | (± 0.2) | (± 0.2) | |||||
| [HbO2] (%) | 65.9 | 67.1 | 2 | 31.7 | 35.3 | 3.6 | 29.7 | 33.7 | 14 | 60.5 | 62.2 | 1.7 |
| (± 5.4) | (± 6.8) | (± 6.1)* | (± 3.3)* | (± 2.3)* | (± 4.0)* | (± 2.6) | (± 3.9) | |||||
| Mean arterial BP (mmHg) | 48 | 48 | – | 52 | 56 | 5 | 56 | 52 | −4 | 50 | 55 | 5 |
| (± 2) | (± 2) | (± 4) | (± 3)* | (± 1)* | (± 4) | (± 2) | (± 1) | |||||
| Fetal heart rate (beats min−1) | 173 | 177 | 4 | 154 | 153 | −1 | 153 | 155 | −8 | 198 | 148 | −50 |
| (± 5) | (± 3) | (± 11)* | (± 6)* | (± 9)* | (± 11)* | (± 7) | (± 20) | |||||
N= 8; values at time of blood sampling are means ±s.e.m.;
P < 0.05, as compared to control values. BE, base excess;
[Hb], haemoglobin concentration; [HbO2], oxyhaemoglobin saturation; MABP, mean arterial blood pressure.
Fetal cerebral responses
Table 2 presents data on sagittal sinus blood gas values, cortical tissue 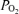 , relative LD-CBF and CMRO2 during the control period, at 20 and 40 min hypoxia, and the recovery period. During the normoxic control period, although both LD-CBF and CMRO2 increased significantly with change in ECoG state (from HVLF to LVHF), the changes in sagittal sinus blood gas values and cortical t
, relative LD-CBF and CMRO2 during the control period, at 20 and 40 min hypoxia, and the recovery period. During the normoxic control period, although both LD-CBF and CMRO2 increased significantly with change in ECoG state (from HVLF to LVHF), the changes in sagittal sinus blood gas values and cortical t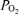 were not significant. With acute hypoxia, sagittal sinus
were not significant. With acute hypoxia, sagittal sinus 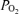 and oxyhaemoglobin saturation decreased significantly, as did cortical t
and oxyhaemoglobin saturation decreased significantly, as did cortical t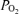 and CMRO2, despite significant increases in relative LD-CBF. Also with hypoxia, fractional O2 extraction remained relatively constant (Table 2).
and CMRO2, despite significant increases in relative LD-CBF. Also with hypoxia, fractional O2 extraction remained relatively constant (Table 2).
Table 2.
Fetal sagittal sinus blood gas values and other variables
| Normoxia |
20 min Hypoxia |
40 min Hypoxia |
Recovery |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HVLF | LVHF | Δ | HVLF | LVHF | Δ | HVLF | LVHF | Δ | HVLF | LVHF | Δ | |
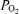 (Torr) (Torr) |
17.3 | 17.7 | 0.4 | 10.0 | 10.6 | 0.6 | 8.6 | 7.0 | −1.6 | 16.4 | 17.2 | 0.8 |
| (± 0.4) | (± 0.4) | (± 0.3)* | (± 1.0)* | (± 0.5)* | (± 0.9)* | (± 0.8) | (± 0.5) | |||||
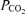 (Torr) (Torr) |
51.1 | 51.4 | 0.3 | 47.8 | 51.4 | 3.6 | 44.7 | 50.0 | 5.3 | 49.5 | 49.9 | 0.4 |
| (± 0.4) | (± 0.7) | (± 1.2) | (± 1.9) | (± 1.5) | (± 1.7) | (± 1.2) | (± 1.1) | |||||
| pH | 7.35 | 7.35 | – | 7.35 | 7.29 | −0.06 | 7.34 | 7.22 | −0.12 | 7.21 | 7.22 | 0.01 |
| (± 0.1) | (± 0.1) | (± 0.1) | (± 0.2) | (± 0.1) | (± 0.4) | (± 0.2) | (± 0.3) | |||||
| [HbO2] (%) | 47.3 | 48.1 | 0.8 | 23.4 | 24.7 | 1.3 | 19.5 | 14.2 | −4.7 | 38.2 | 40.1 | 5.9 |
| (± 2.4) | (± 3.2) | (± 1.8)* | (± 3.0)* | (± 1.2)* | (± 2.2)* | (± 2.7)* | (± 2.2)* | |||||
| BE (mM) | 1.4 | 0.9 | −0.5 | 0.8 | −2.3 | −1.5 | −1.5 | −7.7 | −6.2 | −9.9 | −7.8 | −2.1 |
| (± 0.3) | (± 0.4) | (± 0.4) | (± 1.0) | (± 0.6) | (± 2.3) | (± 0.8) | (± 2.2) | |||||
| Cortical tPO2 (Torr) | 9.7 | 9.8 | 0.1 | 2.7 | 2.6 | −0.1 | 3.0 | 3.2 | 0.2 | 9.8 | 9.8 | – |
| (± 0.6) | (± 0.8) | (± 0.2)* | (± 0.3)* | (± 0.1)* | (± 0.7)* | (± 0.2) | (± 0.2) | |||||
| O2 content (mm) | 4.9 | 5.0 | 0.1 | 2.5 | 2.8 | 0.3 | 2.2 | 1.5 | −0.7 | 3.8 | 4.2 | 0.4 |
| (± 0.3) | (± 0.1) | (± 0.1)* | (± 0.5)* | (± 0.2)* | (± 0.2)* | (± 0.3) | (± 0.2) | |||||
| Arterial to sagittal sinus ΔO2 (mM) | 2.7 | 2.5 | −0.2 | 1.5 | 0.8 | −0.7 | 1.1 | 0.9 | −0.2 | 2.5 | 2.5 | |
| (± 0.1) | (± 0.2) | (± 0.1)* | (± 0.3)* | (± 0.2)* | (± 0.1)* | (± 0.1) | (± 0.3) | |||||
| Relative LD-CBF (% control) | 100 | 118 | 18 | 148 | 135 | −13 | 140 | 137 | −3 | 107 | 101 | −6 |
| (± 4)* | (± 10)* | (± 10)* | (± 10)* | (± 12)* | (± 6) | (± 9) | ||||||
| Fractional O2 extraction | 0.34 | 0.36 | 0.02 | 0.29 | 0.38 | 0.09 | 0.38 | 0.33 | −0.05 | 0.38 | 0.42 | 0.04 |
| (± 0.03) | (± 0.03) | (± 0.03) | (± 0.02) | (± 0.03) | (± 0.03) | (± 0.04) | (± 0.05) | |||||
| Relative CMRO2 (% control) | 100 | 113 | 13 | 80 | 77 | −3 | 78 | 70 | −8 | 139 | 137 | −2 |
| (± 8)* | (± 18)* | (± 13)* | (± 22)* | (± 11)* | (± 17) | (± 22) | ||||||
N= 8; values at time of blood sampling are means ±s.e.m.;
P < 0.05 between values during given period, or from value during normoxic control period. BE, base excess; LD-CBF, laser Doppler cerebral blood flow;
cortical t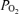 , cortical tissue
, cortical tissue 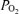 ; arterial-sagittal sinus ΔO2, arterio-venous O2 content difference; relative CMRO2, relative cerebral metabolic rate for O2.
; arterial-sagittal sinus ΔO2, arterio-venous O2 content difference; relative CMRO2, relative cerebral metabolic rate for O2.
Table 3 presents data on the fetal HVLF versus LVHF ECoG states, in terms of mean epoch length (min), root mean square ECoG intensity (μV), the 90% spectral edge frequency (SEF90; Hz), and the relative per cent power of the several ECoG waveforms. During the normoxic control period, the mean epoch lengths of HVLF and LVHF did not differ significantly. By definition, ECoG intensity e.g. voltage power, was significantly greater during HVLF than LVHF. Over a given time period of 40 to 60 min, the duration occupied by HVLF and LVHF ECoG were 58 ± 5% and 34 ± 4%, respectively, of the total time, with 8 ± 3% for transitional waveforms. Again, by definition, the SEF90 values increased significantly during LVHF as compared to HVLF. Also during the two ECoG states in the control period, the relative power of the several waveforms did not differ significantly, thus mean values are given. In contrast, in response to isocapnic hypoxia the HVLF state occupied proportionally a much greater time period, and this was associated with a marked decrease in LVHF mean epoch length and SEF90 (P < 0.05 for each). Acute hypoxia also was associated with modest, but significant, alterations of the several waveforms, with increased power of the delta and beta waveforms. During the recovery period, ECoG activity returned to near control values within 5 to 10 min.
Table 3.
Fetal electrocorticographic activity
| Normoxia |
20 min Hypoxia |
40 min Hypoxia |
Recovery |
|||||
|---|---|---|---|---|---|---|---|---|
| HVLF | LVHF | HVLF | LVHF | HVLF | LVHF | HVLF | LVHF | |
| Mean epoch length (min) | 8.1 ± 1.2 | 6.8 ± 1.0 | 7.4 ± 1.6 | 2.5 ± 0.5* | 6.7 ± 1.5 | 4.0 ± 0.5* | 10.2 ± 2.0 | 6.4 ± 1.3 |
| RMS ECoG intensity (μV) | 13.7 ± 0.4 | 11.7 ± 0.5 | 12.6 ± 0.6 | 10.1 ± 0.3 | 10.3 ± 0.5 | 10.5 ± 0.4 | 11.7 ± 0.2 | 14.7 ± 0.7 |
| Total duration of epoch (min) | 34.7 ± 4.8 | 20.5 ± 5.0 | 13.6 ± 0.8 | 6.3 ± 0.7 | 13.3 ± 1.4 | 6.0 ± 1.7 | 30.7 ± 9.7 | 19.2 ± 6.0 |
| Mean spectral edge frequency (Hz) (90%) | 7.6 ± 0.2 | 10.9 ± 0.3 | 6.1 ± 0.2* | 7.6 ± 0.5* | 7.5 ± 8.4 | 6.7 ± 0.3* | 7.6 ± 0.3 | 11.0 ± 0.5 |
| δ waves (% power) | 77.8 ± 0.2 | 81.6 ± 0.5* | 82.2 ± 0.6* | 79.7 ± 0.5 | ||||
| θ waves (% power) | 11.6 ± 0.2 | 11.0 ± 0.3 | 10.6 ± 0.3 | 11.1 ± 0.2 | ||||
| α waves (% power) | 4.6 ± 0.1 | 3.9 ± 0.2* | 3.3 ± 0.1* | 3.9 ± 0.1 | ||||
| β waves (% power) | 4.0 ± 0.1 | 3.2 ± 0.2* | 2.7 ± 0.2* | 4.0 ± 0.2 | ||||
| γ waves (% power) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | ||||
n= 8. Values are mean ±s.e.m. ECoG, electrocorticographic activity; Hz, Hertz frequency; 1–4 Hz (delta, δ), 4–8 Hz (theta, θ), 8–13 Hz (alpha, α), 13–20 Hz (beta, β); 20–30 (gamma, γ). The total duration of HVLF and LVHF epochs is calculated for 60 min control and recovery periods, and for the 20 min hypoxia periods;
P < 0.05 from value during normoxic control period.
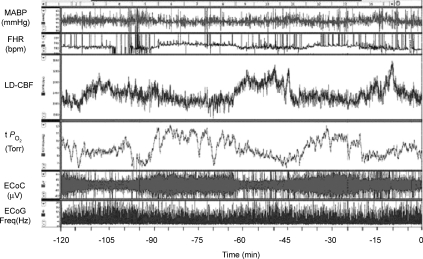
Figure 1 illustrates a 2 h recording during a normoxic control period in a sheep fetus at 135 ± 1 days gestation. Shown are the continuous record of mean arterial blood pressure (MABP), heart rate (FHR), laser Doppler cerebral blood flow (LD-CBF), cortical tissue 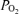 (t
(t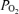 ), electrocortical activity (ECoG, μV) and ECoG frequency (Hz). As is evident, the fetal ECoG is characterized by two distinct states of activity: high voltage low frequency (HVLF) and low voltage high frequency (LVHF). Of note, during these studies we often observed that the early portion of LVHF ECoG state was accompanied by a significant decrease in cortical t
), electrocortical activity (ECoG, μV) and ECoG frequency (Hz). As is evident, the fetal ECoG is characterized by two distinct states of activity: high voltage low frequency (HVLF) and low voltage high frequency (LVHF). Of note, during these studies we often observed that the early portion of LVHF ECoG state was accompanied by a significant decrease in cortical t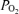 , which then returned to baseline value during the latter part of that state, and with a significant ∼18 ± 4% increase in LD-CBF (Table 2). Although in most instances, LVHF was associated with increased LD-CBF, as shown in this example cortical t
, which then returned to baseline value during the latter part of that state, and with a significant ∼18 ± 4% increase in LD-CBF (Table 2). Although in most instances, LVHF was associated with increased LD-CBF, as shown in this example cortical t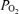 did not fall initially in every case.
did not fall initially in every case.
Figure 1. Two hour normoxic control period in the sheep fetus.
Recording of 2 h normoxic control period in the sheep fetus at 135 ± 1 days gestation, showing temporal relations of mean arterial blood pressure (MABP, mmHg), fetal heart rate (FHR, beats min–1), laser Doppler cerebral blood flow (LD-CBF), cerebral cortical tissue 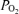 (t
(t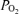 , Torr) electrocortical activity (ECoG, μV) and ECoG frequency (Hz). Shown are the associations of low voltage high frequency ECoG to increased LD-CBF, and in some instances decreased t
, Torr) electrocortical activity (ECoG, μV) and ECoG frequency (Hz). Shown are the associations of low voltage high frequency ECoG to increased LD-CBF, and in some instances decreased t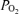 .
.
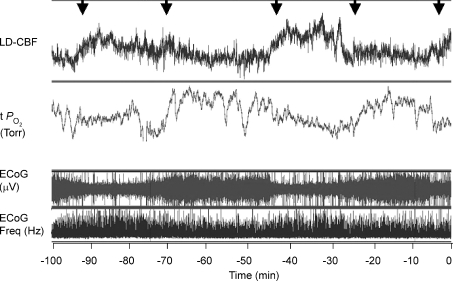
The question arises as to the temporal relationships of the changes noted in Fig. 1 during the normoxic control period. Figure 2 shows a 100 min recording (same animal as in Fig. 1), with an expanded time scale to display these changes in greater detail. Noteworthy, are the temporal relations among the onset of LVHF activity, decline in cortical t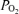 and increase in LD-CBF. For all studies during the normoxic control period, with change in ECoG state from HVLF to LVHF, t
and increase in LD-CBF. For all studies during the normoxic control period, with change in ECoG state from HVLF to LVHF, t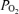 decreased 2.8 ± 0.3 Torr and this lasted slightly less than 1 min as CBF increased significantly. As noted above and is evident in both Figs 1 and 2, while in many instances cortical t
decreased 2.8 ± 0.3 Torr and this lasted slightly less than 1 min as CBF increased significantly. As noted above and is evident in both Figs 1 and 2, while in many instances cortical t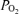 decreased significantly with LVHF ECoG activity, this was not always the case. In one of eight of the experimental animals, there was no association of ECoG state with cortical tissue t
decreased significantly with LVHF ECoG activity, this was not always the case. In one of eight of the experimental animals, there was no association of ECoG state with cortical tissue t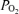 . This may reflect placement of the cortical tissue
. This may reflect placement of the cortical tissue 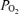 probe in relation to arterioles or other vessels.
probe in relation to arterioles or other vessels.
Figure 2. Relationships of cerebral blood flow, cortical tissue PO2, and ECoG state.
Recording of 100 min in the 135 ± 1 days gestation sheep fetus, illustrating relationships of LD-CBF and cortical t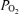 (Torr) to the ECoG state (μV) and ECOG frequency (Hz) (same fetus as Fig. 1). Noteworthy are the temporal relationships among the onset of LVHF ECoG activity with a decline in cortical t
(Torr) to the ECoG state (μV) and ECOG frequency (Hz) (same fetus as Fig. 1). Noteworthy are the temporal relationships among the onset of LVHF ECoG activity with a decline in cortical t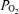 , and increase in LD-CBF. Arrows indicate change in ECoG state.
, and increase in LD-CBF. Arrows indicate change in ECoG state.
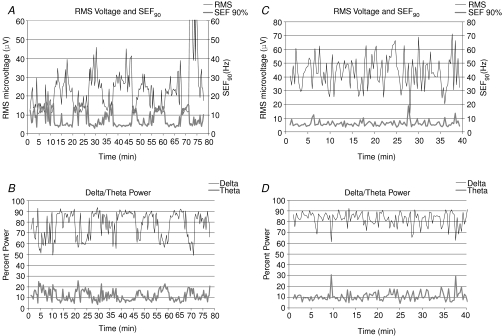
To explore the ECoG patterns in greater detail, we examined the root mean square microvoltage, 90% spectral edge frequency, and performed a power analysis of the several waveforms. As shown in Fig. 3A, during the normoxic control period the root mean square ECoG microvoltage signal showed a distinct cyclic periodicity (upper trace), as did the SEF90 (lower trace). These variables were calculated for 60 s epochs. Figure 3B illustrates the delta (1–4 Hz; upper trace) and theta (4–8 Hz; lower trace) per cent power for these 60 s epochs under control conditions (also see Table 3). When calculated per 90, 120, 150, 180, 240 and 300 s epochs, some fidelity of these variables was lost, as compared to calculations per 60 s epoch, although the cyclic patterns remained clear (data not shown).
Figure 3. Root mean square microvoltage, 90% spectral edge frequency, and waveforms during normoxia and hypoxia.
A, root mean square (RMS) microvoltage (μV) and 90% spectral edge frequencey (SEF90) in a 135 ± 1 days gestation day fetus during the normoxic control period. These values are calculated for 60 s epochs. B, delta (1–4 Hz; upper trace; 78%) and theta (4–8 Hz; lower trace; 12%) per cent power calculated for 60 s epochs in the same fetus. C, RMS microvoltage (μV) and 90% SEF90 in 135 ± 1 days gestation fetus during 40 min acute, isocapnic hypoxia calculated for 60 s epochs. D, delta (1–4 Hz) and theta (4–8 Hz) per cent power calculated for 60 s epochs in the same fetus during acute hypoxia. The upper dotted line is the value for mean delta power (82%), while the lower dotted line is mean theta power (11%). As is evident, the alpha, beta and gamma signals contain only a small fraction of the power (see Table 3).
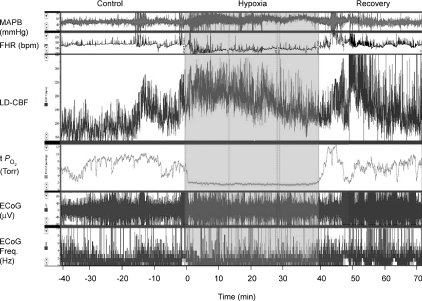
Figure 4 shows a continuous recording in a 135 ± 1 gestational day fetus of MABP, FHR, LD-CBF, t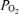 , ECoG and root mean square ECoG frequency during the last 40 min of the control period, 40 min of isocapnic hypoxia, and 30 min of the recovery period. As noted, the onset of acute hypoxia was associated with a rapid increase in mean arterial blood pressure and decrease in heart rate, accompanied by a rapid fall in cortical t
, ECoG and root mean square ECoG frequency during the last 40 min of the control period, 40 min of isocapnic hypoxia, and 30 min of the recovery period. As noted, the onset of acute hypoxia was associated with a rapid increase in mean arterial blood pressure and decrease in heart rate, accompanied by a rapid fall in cortical t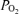 to 2 to 3 Torr, and an increase in LD-CBF. Despite the increased LD-CBF, cortical t
to 2 to 3 Torr, and an increase in LD-CBF. Despite the increased LD-CBF, cortical t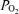 remained low. In several cases, following its initial fall, over a period of ∼10 min, t
remained low. In several cases, following its initial fall, over a period of ∼10 min, t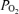 rose to a level midway between its nadir and the control value. Also of note, acute hypoxia was accompanied by a significant decrease of LVHF state with replacement by HVLF ECoG signal. During the recovery period the cycling of LVHF and HVLF resumed within 10 min. The figure also illustrates the rapid rise in cortical t
rose to a level midway between its nadir and the control value. Also of note, acute hypoxia was accompanied by a significant decrease of LVHF state with replacement by HVLF ECoG signal. During the recovery period the cycling of LVHF and HVLF resumed within 10 min. The figure also illustrates the rapid rise in cortical t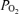 and brief overshoot with return to normoxia. Cyclicity in LD-CBF pattern also returned within 5 to 10 min.
and brief overshoot with return to normoxia. Cyclicity in LD-CBF pattern also returned within 5 to 10 min.
Figure 4. Responses to acute isocapnic hypoxia in a sheep fetus.
Recording of responses to acute isocapnic hypoxia in a sheep fetus at 134 ± 1 days gestation. Following a 40 min control period, acute isocapnic hypoxia was introduced for 40 min. Shown are mean arterial blood pressure (MABP; mmHg), fetal heart rate (FHR; beats min–1), laser Doppler cerebral blood flow (LD-CBF), cortical tissue 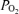 (t
(t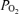 ; Torr), ECoG microvoltage (μV) and electrocortical frequency (Hz). As is evident, during the control period ECoG state cycled between high and low frequency. In response to acute hypoxia (gray, shaded area), the ECoG state shifted to a HVLF state with less LVHF activity. Also, with the onset of acute hypoxia, mean arterial blood pressure increased, fetal heart rate decreased and LD-CBF increased about 20%. Also notable, in response to acute hypoxia, t
; Torr), ECoG microvoltage (μV) and electrocortical frequency (Hz). As is evident, during the control period ECoG state cycled between high and low frequency. In response to acute hypoxia (gray, shaded area), the ECoG state shifted to a HVLF state with less LVHF activity. Also, with the onset of acute hypoxia, mean arterial blood pressure increased, fetal heart rate decreased and LD-CBF increased about 20%. Also notable, in response to acute hypoxia, t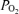 decreased significantly with less evidence of cycling. During the recovery period the cycling pattern of LVHF and HVLF resumed within 10 min. Notable during the recovery period was the rapid increase and overshoot of t
decreased significantly with less evidence of cycling. During the recovery period the cycling pattern of LVHF and HVLF resumed within 10 min. Notable during the recovery period was the rapid increase and overshoot of t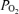 and LD-CBF before returning to pre-hypoxic levels with resumption of cycling pattern.
and LD-CBF before returning to pre-hypoxic levels with resumption of cycling pattern.
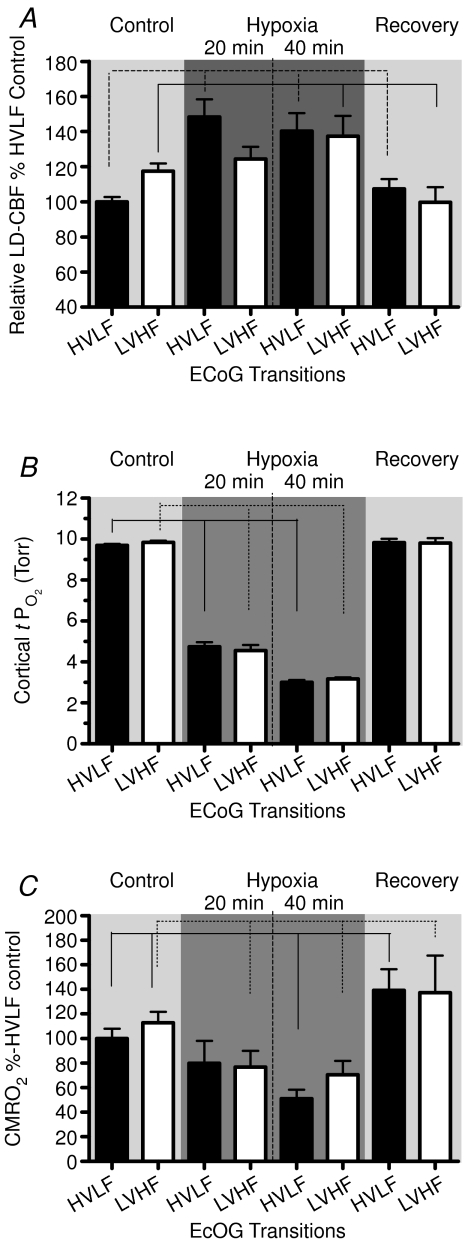
Figure 5 illustrates the mean values of the several variables in relation to ECoG state during the 60 min control period, 20 and 40 min hypoxia, and 60 min recovery period. Figure 5A shows the relative LD-CBF, with that during control HVLF assigned as 100%, rising to 118 ± 4 with transition to LVHF state. In response to isocapnic hypoxia, LD-CBF increased significantly during both HVLF and LVHF ECoG states. With return to the normoxic recovery period, the LD-CBF values fell to near normal values during both ECoG states (see Table 2). Figure 5B shows the mean values of cortical t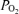 during the two ECoG states during the control period, with significant decreases in response to 20 and 40 min acute isocapnic hypoxia (see Table 2). Figure 5C shows the cerebral metabolic rate for O2, with the HVLF state during the control period being assigned the basal rate of 100%. During normoxia, the LVHF state was associated with significant 13% increase in CMRO2, while in response to isocapnic hypoxia CMRO2 fell ∼20% during both HVLF and LVHF states (see Table 2). Upon return to normoxia in the recovery period, the CMRO2 was increased significantly during both ECoG states. Figure 3C illustrates the root mean square (RMS) microvoltage (μV) and 90% spectral edge frequency in 135 ± 1 days gestation fetus during the 40 min of acute isocapnic hypoxia, calculated for 60 s epochs. Figure 3D shows the delta (1–4 Hz) and theta (4–8 Hz) per cent power calculated for 60 s epochs in response to hypoxia. As is evident, with hypoxia the well-defined cyclic patterns observed during the control period (Fig. 3A and B) disappeared, and the ECoG state became rather chaotic with little defined LVHF state, and SEF90 decreased significantly (see Table 3).
during the two ECoG states during the control period, with significant decreases in response to 20 and 40 min acute isocapnic hypoxia (see Table 2). Figure 5C shows the cerebral metabolic rate for O2, with the HVLF state during the control period being assigned the basal rate of 100%. During normoxia, the LVHF state was associated with significant 13% increase in CMRO2, while in response to isocapnic hypoxia CMRO2 fell ∼20% during both HVLF and LVHF states (see Table 2). Upon return to normoxia in the recovery period, the CMRO2 was increased significantly during both ECoG states. Figure 3C illustrates the root mean square (RMS) microvoltage (μV) and 90% spectral edge frequency in 135 ± 1 days gestation fetus during the 40 min of acute isocapnic hypoxia, calculated for 60 s epochs. Figure 3D shows the delta (1–4 Hz) and theta (4–8 Hz) per cent power calculated for 60 s epochs in response to hypoxia. As is evident, with hypoxia the well-defined cyclic patterns observed during the control period (Fig. 3A and B) disappeared, and the ECoG state became rather chaotic with little defined LVHF state, and SEF90 decreased significantly (see Table 3).
Figure 5. Values of LD-CBF, cortical tPO2 and CMRO2.
A, mean values of relation of LD-CBF normalized to the per cent high voltage low frequency ECoG state (HVLF and LVHF), during 60 min control period, first and second 20 min periods of hypoxia, and 60 min recovery period. The continuous and dotted lines represent significant differences (P < 0.01) in LD-CBF for HVLF and LVHF states, respectively, between the control and hypoxic periods. As seen, LD-CBF increased significantly in response to hypoxia during both HVLF and LFHF states. B, cortical tissue 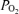 values (Torr) as function of ECoG state during control, hypoxia and recovery periods. C, cerebral metabolic rate for O2 as per cent of HVLF control, during periods of normoxia, 20 and 40 min isocapnic hypoxia, and recovery. As is evident, CMRO2 decreased significantly for the LVHF state during both periods of hypoxia (P < 0.05). Values are mean ±s.e.m., n for each value 20–200; HVLF, high voltage low frequency; LVHF, low voltage high frequency.
values (Torr) as function of ECoG state during control, hypoxia and recovery periods. C, cerebral metabolic rate for O2 as per cent of HVLF control, during periods of normoxia, 20 and 40 min isocapnic hypoxia, and recovery. As is evident, CMRO2 decreased significantly for the LVHF state during both periods of hypoxia (P < 0.05). Values are mean ±s.e.m., n for each value 20–200; HVLF, high voltage low frequency; LVHF, low voltage high frequency.
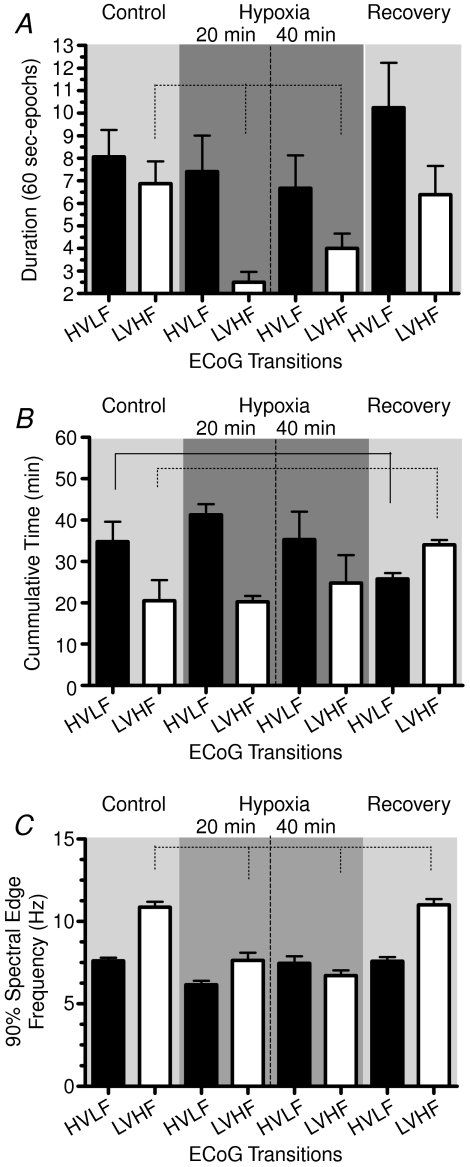
Figure 6 demonstrates several aspects of the ECoG states during the periods of baseline control, 20 and 40 min acute hypoxia, and recovery. Panel A shows the duration (min) of individual ECoG states calculated per 60 s epochs. Particularly striking during the hypoxic period, were the significant decreases in LVHF duration from the control value. With the return to normoxia the epoch durations returned to control values (see Table 3). Figure 6B illustrates the cumulative time of HVLF and LVHF epochs per 60 min control and recovery periods. For each 20 min period of hypoxia, the total epoch duration for each ECoG state was calculated per 60 min, to compare properly with the 60 min control and recovery periods. As is evident, during hypoxia the total duration of LVHF epochs did not differ significantly from that of the control period; however, LVHF state increased significantly during recovery. Figure 6C shows the 90% spectral edge frequency during HVLF and LVHF states. In response to hypoxia, SEF90 fell significantly during both HVLF and LVHF, returning to control values during the recovery period (see Table 3).
Figure 6. Values of ECoG state duration, cumulative duration, and 90% spectral edge frequency.
A, mean duration of individual HVLF and LVHF epochs (min) during control, hypoxia, and recovery periods. As seen, the duration of LVHF epochs decreased significantly with hypoxia. B, cumulative time (min) spent in HVLF and LVHF states per 60 min control and recovery periods, and calculated per 60 min of each of the 20 min epochs of hypoxia. C, 90% spectral edge frequency during the normoxic control period, 20 and 40 min acute hypoxia, and recovery. During both periods of hypoxia the decrease in SEF90 during the LVHF states was significant (P < 0.01). Values are mean ±s.e.m., n for each value 20–200; HVLF, high voltage low frequency; LVHF, low voltage high frequency.
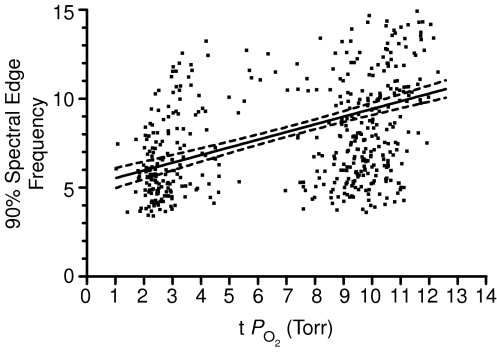
On the basis of the above findings, the question arises as to the relation of 90% spectral edge frequency to cortical tissue 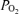 . As seen in Fig. 7, which includes over 500 data points, SEF90 decreased significantly from ∼10 ± 2 during the normoxic control state with t
. As seen in Fig. 7, which includes over 500 data points, SEF90 decreased significantly from ∼10 ± 2 during the normoxic control state with t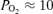 Torr, to ∼6 ± 2 during hypoxia with t
Torr, to ∼6 ± 2 during hypoxia with t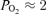 to 3 Torr (r2= 0.43 ± 0.04, P < 0.001).
to 3 Torr (r2= 0.43 ± 0.04, P < 0.001).
Figure 7. 90% spectral edge frequency as a function of cortical tissue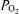 .
.
n= 502 values, r2= 0.43 ± 0.04, P < 0.0001.
Discussion
The present study confirms that in the near-term fetus during the normoxic control period, electrocorticographic activity cycles between HVLF and LVHF states, and that this latter state is associated with significant increases in both LD-CBF and CMRO2. In many, but not all, instances of the LVHF state, cortical t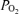 decreased briefly, but this was rapidly restored to baseline values in association with the increase in CBF (Fig. 2). These control period changes in ECoG waveform also were associated with changes in intensity (root mean square voltage), and 90% spectral edge frequency (Table 3, Fig. 3). The ECoG state changes occurred without significant alteration of fetal arterial blood gases, mean arterial pressure or heart rate. Acute isocapnic hypoxia was associated with significant decreases in cortical t
decreased briefly, but this was rapidly restored to baseline values in association with the increase in CBF (Fig. 2). These control period changes in ECoG waveform also were associated with changes in intensity (root mean square voltage), and 90% spectral edge frequency (Table 3, Fig. 3). The ECoG state changes occurred without significant alteration of fetal arterial blood gases, mean arterial pressure or heart rate. Acute isocapnic hypoxia was associated with significant decreases in cortical t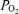 and CMRO2, despite significantly increased CBF. With hypoxia, fetal electrocortical activity also changed significantly, as evidenced by decreased LVHF epoch length and decreased SEF90 with increased duration of HVLF state. Acute hypoxia also was associated with significant changes of ECoG waveforms as per cent power. We believe that these findings in the developing brain are of great importance in laying the groundwork for understanding the physiological basis for the interrelations of these variables, as well as in establishing ‘norms’ for establishing the extent of abnormality in the compromised fetus/newborn.
and CMRO2, despite significantly increased CBF. With hypoxia, fetal electrocortical activity also changed significantly, as evidenced by decreased LVHF epoch length and decreased SEF90 with increased duration of HVLF state. Acute hypoxia also was associated with significant changes of ECoG waveforms as per cent power. We believe that these findings in the developing brain are of great importance in laying the groundwork for understanding the physiological basis for the interrelations of these variables, as well as in establishing ‘norms’ for establishing the extent of abnormality in the compromised fetus/newborn.
Fetal electrocortical activity
Fetal cerebral tissue oxygenation is determined by a number of factors, including fetal arterial O2 content and partial pressure, cerebral blood flow, cerebral metabolic rate for O2, and the position of the oxyhaemoglobin dissociation curve (Jones & Traystman, 1984; Pereyra Peña et al. 2007). In the present study, we demonstrate that fetal electrocortical activity constitutes an additional important determinant in this regard. Electrocortical activity of the developing brain has been of interest for several decades, and initial studies in the newborn infant raised a number of issues regarding phasic ECoG activity and its role in neural development. Perhaps unexpectedly, the newborn infant ECoG has significantly more LVHF state activity (∼50%) than that recorded in older children and adults (∼20%) (Roffwarg et al. 1966). Beginning in the 1970s, developmental physiologists discovered that cycles of electrocortical activity appear in the fetus prior to birth in the sheep (Jost et al. 1972; Boddy et al. 1974) and other species in which significant brain development occurs during late gestation, such as humans (Roffwarg et al. 1966; Osredkar et al. 2005) and baboons (Stark et al. 1991). Particularly during the last 3 weeks of gestation in sheep, the ECoG cycles between a low voltage high frequency state associated with rapid eye movement sleep/awake state, and a high voltage low frequency state associated with quiet sleep. The relative durations of these change with developmental maturation (Szeto & Hinman, 1985). The LVHF state also is associated with fetal breathing activity (Dawes et al. 1972). In addition, the relative duration of LVHF, as compared with HVLF, activity shows circadian periodicity, LVHF duration being somewhat greater between 20.00 and 24.00 h, as compared with 00.00 to 06.00 h (Shinozuka & Nathanielsz, 1998). In the human newborn, these periodic changes in ECoG state are considered an indication of infant well being (Thornberg & Thiringer, 1990; Scher et al. 1996; Watanabe et al. 1999; Ter Horst et al. 2004; Osredkar et al. 2005).
Examination of the basic mechanisms of fetal ECoG activity was beyond the scope of the present study. Low voltage high frequency activity appears to originate from ascending impulses transmitted from pons to thalamus, and on to higher centres, e.g. ponto-geniculo-cortical waves. Importantly, in the developing brain LVHF ECoG appears to play a critical role in activity-dependent neural growth and development (Mirmiran, 1986, 1995; Marks et al. 1995; Spitzer, 2006), including maturation and myelinization of higher centres (Roffwarg et al. 1966), and the inhibition of programmed cell death (Morrissey et al. 2004). Thus, ECoG activity state may serve both as an indicator of the degree of brain maturation, and as a stimulus or mediator of further development (Mirmiran, 1995). Nonetheless, a major challenge to our understanding of the biological function of ECoG states is to elucidate their role in neural development and function of the various regions of the central nervous system (Marks et al. 1995), and to understand their relation to cerebral metabolism and response to various pharmacological interventions (Czikk et al. 2003; MacLachlan et al. 2008). As considered by several groups, spectral edge frequency, e.g. that frequency below 90% of the ECoG power (Szeto, 1990; Thaler et al. 2000; Inder et al. 2003), may serve as a useful measure of cerebral maturation, and in identifying the newborn infant with hypoxic-ischemic encephalopathy (Scher et al. 2002; Osredkar et al. 2005), or other brain damage (Inder et al. 2003).
In the present study in the 130 to 135 day fetus, with continuous recording, we confirm a significant 18 ± 4% increase in LD-CBF in association with LVHF state. This compares with the reports of CBF increase with a shift from HVLF to LVHF ECoG activity of 17 ± 2% (Richardson et al. 1985, 1994), 23 ± 5% (Grant et al. 2005), to 35 ± 5% (Richardson et al. 1989). We also demonstrate that the onset of LVHF activity may be associated with a brief, but significant, decrease in cortical t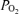 . This t
. This t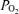 decrease appears to signal a compensatory increase in CBF, with increased cerebral metabolic rate for O2. In addition, we demonstrate that evaluation of both fetal LVHF and HVLF ECoG state per se and spectral analysis, can provide information of value in evaluating and correlating neuronal activity changes with altered CMRO2 and t
decrease appears to signal a compensatory increase in CBF, with increased cerebral metabolic rate for O2. In addition, we demonstrate that evaluation of both fetal LVHF and HVLF ECoG state per se and spectral analysis, can provide information of value in evaluating and correlating neuronal activity changes with altered CMRO2 and t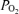 . Although one group has reported that fetal O2 consumption changed only a small amount or not at all with variation in ECoG activity; CMRO2per se was not measured (Walker et al. 1984).
. Although one group has reported that fetal O2 consumption changed only a small amount or not at all with variation in ECoG activity; CMRO2per se was not measured (Walker et al. 1984).
Acute hypoxia and fetal ECoG states
In the present study, we demonstrate the significant role of hypoxic-mediated decreases in cortical t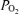 , increases in CBF and brain neuronal activity as reflected in ECoG state. Particularly striking during acute hypoxia was the profound and sustained decrease in cortical t
, increases in CBF and brain neuronal activity as reflected in ECoG state. Particularly striking during acute hypoxia was the profound and sustained decrease in cortical t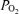 , with rather chaotic RMS microvoltage, and little evidence of LVHF ECoG state, with significant decrease in SEF90. Thus, we believe this study lays the groundwork for future investigation to study the mechanisms of cerebrovascular regulation, with correlation of these parameters in response to hypoxia without or with concomittant pharmacological antagonist/agonist administration. A number of investigators have examined the relation of fetal ECoG to the state of oxygenation, and several ‘models’ of fetal hypoxia have been used for such studies. As may be expected, fetal ECoG activity depends upon
, with rather chaotic RMS microvoltage, and little evidence of LVHF ECoG state, with significant decrease in SEF90. Thus, we believe this study lays the groundwork for future investigation to study the mechanisms of cerebrovascular regulation, with correlation of these parameters in response to hypoxia without or with concomittant pharmacological antagonist/agonist administration. A number of investigators have examined the relation of fetal ECoG to the state of oxygenation, and several ‘models’ of fetal hypoxia have been used for such studies. As may be expected, fetal ECoG activity depends upon 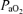 , oxyhaemoglobin saturation and related variables. Nonetheless, considerable difference exists among the various reports. In one of the first studies along this line, in near-term sheep aged 119 to 133 gestational days subjected to acute hypoxaemia (
, oxyhaemoglobin saturation and related variables. Nonetheless, considerable difference exists among the various reports. In one of the first studies along this line, in near-term sheep aged 119 to 133 gestational days subjected to acute hypoxaemia (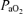 = 16 ± 1 Torr) for 1 h, LVHF activity decreased from 33 ± 1 min h−1 (55% of time) to 18 ± 1 min h−1 (30% of time), with a return to control value following re-establishment of normal oxygenation (Boddy et al. 1974). These authors also reported that during the normoxic control period, changes from LVHF to HVLF activity occurred 2.4 ± 1 h−1, while during hypoxaemia this increased to 5.6 ± 1 h−1 (Boddy et al. 1974). With fetal hypoxia others have demonstrated a similar shift from LVHF to HVLF activity (Clewlow et al. 1983). Results of the present study agree with these findings (Table 3; Fig. 4). In contrast, in another study of hypoxic hypoxia in which
= 16 ± 1 Torr) for 1 h, LVHF activity decreased from 33 ± 1 min h−1 (55% of time) to 18 ± 1 min h−1 (30% of time), with a return to control value following re-establishment of normal oxygenation (Boddy et al. 1974). These authors also reported that during the normoxic control period, changes from LVHF to HVLF activity occurred 2.4 ± 1 h−1, while during hypoxaemia this increased to 5.6 ± 1 h−1 (Boddy et al. 1974). With fetal hypoxia others have demonstrated a similar shift from LVHF to HVLF activity (Clewlow et al. 1983). Results of the present study agree with these findings (Table 3; Fig. 4). In contrast, in another study of hypoxic hypoxia in which 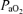 was decreased from 28 ± 1 to 17 ± 1 Torr for 60 min, the incidence of LVHF activity (32 ± 1 min h−1 or 53%) did not change significantly, although rapid eye movements decreased ∼50%, and breathing activity essentially ceased (Koos & Sameshima, 1988). Similarly, with several degrees of carbon monoxide hypoxia (from 16 to 28%[HbCO]), the incidence of LVHF and HVLF were ∼30 and 26%, respectively, similar to the control values (Koos et al. 1988). Perhaps surprisingly, one group has reported that in response to hypoxaemia (
was decreased from 28 ± 1 to 17 ± 1 Torr for 60 min, the incidence of LVHF activity (32 ± 1 min h−1 or 53%) did not change significantly, although rapid eye movements decreased ∼50%, and breathing activity essentially ceased (Koos & Sameshima, 1988). Similarly, with several degrees of carbon monoxide hypoxia (from 16 to 28%[HbCO]), the incidence of LVHF and HVLF were ∼30 and 26%, respectively, similar to the control values (Koos et al. 1988). Perhaps surprisingly, one group has reported that in response to hypoxaemia (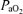 = 14 ± 1 Torr) maintained for 5 days, the time spent in LVHF increased, as compared with HVLF which decreased (Pulgar et al. 2006). In another study, near-term fetal sheep were subjected to prolonged, graded hypoxaemia for 4 days (
= 14 ± 1 Torr) maintained for 5 days, the time spent in LVHF increased, as compared with HVLF which decreased (Pulgar et al. 2006). In another study, near-term fetal sheep were subjected to prolonged, graded hypoxaemia for 4 days (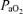 = 18 or 16 Torr, with [HbO2] 30–40% or < 30% saturation, respectively) (Richardson et al. 1992). Under control conditions, LVHF activity averaged 53 ± 1% of the total time, and showed only marginal decrease between 30 to 60%[HbO2]. Not until arterial [HbO2] was < 30% and pH was ∼7.26, did LVHF activity decrease to 35 ± 4% of the total time with cycling between the two activity states still evident (Richardson et al. 1992). In yet another study in near-term fetal sheep, hypoxaemia/partial asphyxia produced by 5 min occlusion of either unilateral or bilateral middle uterine artery (so that
= 18 or 16 Torr, with [HbO2] 30–40% or < 30% saturation, respectively) (Richardson et al. 1992). Under control conditions, LVHF activity averaged 53 ± 1% of the total time, and showed only marginal decrease between 30 to 60%[HbO2]. Not until arterial [HbO2] was < 30% and pH was ∼7.26, did LVHF activity decrease to 35 ± 4% of the total time with cycling between the two activity states still evident (Richardson et al. 1992). In yet another study in near-term fetal sheep, hypoxaemia/partial asphyxia produced by 5 min occlusion of either unilateral or bilateral middle uterine artery (so that 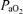 decreased to 17 ± 2 or 15 ± 4 Torr, respectively), resulted in a switch to HVLF from LVHF state (Harding et al. 1981). In response to maternal common iliac (uterine) artery occlusion for 2 h to reduce
decreased to 17 ± 2 or 15 ± 4 Torr, respectively), resulted in a switch to HVLF from LVHF state (Harding et al. 1981). In response to maternal common iliac (uterine) artery occlusion for 2 h to reduce 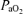 to 16 ± 1 Torr, the time spent in LVHF state decreased from 58 ± 3 to 38 ± 4% (Bocking & Harding, 1986). In this latter study, the effects of hypoxaemia became less apparent in the older fetus (Bocking & Harding, 1986). In contrast, in a further study along this line, by use of an occluder on the maternal blood supply to the uterus (common uterine artery) for 48 h, with a similar reduction in fetal
to 16 ± 1 Torr, the time spent in LVHF state decreased from 58 ± 3 to 38 ± 4% (Bocking & Harding, 1986). In this latter study, the effects of hypoxaemia became less apparent in the older fetus (Bocking & Harding, 1986). In contrast, in a further study along this line, by use of an occluder on the maternal blood supply to the uterus (common uterine artery) for 48 h, with a similar reduction in fetal 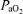 , the per cent of time spent in LVHF remained at the control value of 51 ± 2% (Bocking et al. 1988).
, the per cent of time spent in LVHF remained at the control value of 51 ± 2% (Bocking et al. 1988).
In a model of fetal cerebral ischaemia produced by bilateral carotid artery occlusion for 10, 20, 30 or 40 min, the ECoG pattern rapidly became isoelectric, with the duration of depression being a function of the duration of cerebral ischaemia (Williams et al. 1992). Although with occlusion durations of 10 or 20 min the ECoG activity pattern slowly recovered, that after 30 or 40 min occlusion did not, and was associated with both epileptiform activity and the development of cortical neuronal loss and laminar necrosis (Williams et al. 1992). With 30 min bilateral carotid artery occlusion in the 95 day gestation sheep fetus (0.65 gestation), ECoG intensity decreased briefly for ∼10 min in the ‘mild’ hypoxaemia group, and with a more prolonged 30 to 60 min decrease in the more ‘severe’ group (Fraser et al. 2005). Similarly, spectral edge frequency decreased ∼2 Hz in the ‘mild” occlusion group, as opposed to ∼4 Hz in the more ‘severe’ group, and persistent changes in ECoG intensity and SEF were associated with neuropathological injury, particularly of the parasagittal cortex and subcortical white matter (Fraser et al. 2005).
With umbilical cord occlusion for 20 min, in fetal sheep at 91 ± 1 gestation day (0.6 gestation), ECoG activity fell rapidly from 17 ± 1 dB, and remained depressed (4 ± 1 dB) during the period of occlusion, returning to control value with release of the occlusion (George et al. 2004). In contrast, in those fetuses subjected to 30 min occlusion, ECoG amplitude not only failed to return to control value, but displayed epiliptiform activity and abnormal muscular movements. Another group examined two patterns of repeated umbilical cord occlusion: ‘mild’ (40 s occlusion with release for 80 s, repeated 15 times) or ‘severe’ (60 s occlusion with release for 60 s, repeated 15 times). With ‘mild’ occlusion, an ECoG pattern of persistent HVLF activity became apparent, while with ‘severe’ compression and acidaemia (pH < 7.20) epileptiform ECoG spikes appeared, and spectral edge frequency decreased significantly (Takahashi et al. 2006).
The present study affirms the significant decrease in fetal cortical t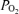 and 20% decrease in CMRO2, in association with acute hypoxia (Pereyra Peña et al. 2007). Of importance here is an attempt to understand better the mechanisms of these interrelations. The fact that LD-CBF increases with a change from HVLF to LVHF ECoG state, and CMRO2 increases, suggests that CMRO2 is driving CBF under normoxic conditions. In response to acute hypoxia, however, CBF increases despite a change to predominantly HVLF state and decrease in CMRO2. This suggests strongly that during hypoxia, other factors such as an increase in eNOS activity work to increase CBF. In addition, the HVLF state during hypoxia may differ in some respects from that during normoxia as it contains a greater percentage of delta waves (Table 3). We must confess, however, that we do not know the biological importance of this observation. Currently, we are exploring these aspects of ECoG interrelations CMRO2 and CBF.
and 20% decrease in CMRO2, in association with acute hypoxia (Pereyra Peña et al. 2007). Of importance here is an attempt to understand better the mechanisms of these interrelations. The fact that LD-CBF increases with a change from HVLF to LVHF ECoG state, and CMRO2 increases, suggests that CMRO2 is driving CBF under normoxic conditions. In response to acute hypoxia, however, CBF increases despite a change to predominantly HVLF state and decrease in CMRO2. This suggests strongly that during hypoxia, other factors such as an increase in eNOS activity work to increase CBF. In addition, the HVLF state during hypoxia may differ in some respects from that during normoxia as it contains a greater percentage of delta waves (Table 3). We must confess, however, that we do not know the biological importance of this observation. Currently, we are exploring these aspects of ECoG interrelations CMRO2 and CBF.
Perspective
For the developing fetus, as in the infant, optimal cerebral blood flow and cerebral oxygen consumption are essential for proper neural growth and development. In turn, optimal ECoG activity both reflects and is vital for neurogenesis and brain development. Thus, a complex positive feed-forward system of ECoG→ CMRO2→CBF→neuronal function→ exists. Understanding in a deep sense these interrelations and their mechanisms is a major challenge for our time. The present study presents the first measurements of fetal ECoG state with continuous measurements of CBF and cortical tissue 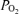 , in concert with cerebral metabolic rate for O2. During normoxia, the present study demonstrates the significant relationship among fetal ECoG states and these variables, with LVHF activity being associated with a decrease in cortical t
, in concert with cerebral metabolic rate for O2. During normoxia, the present study demonstrates the significant relationship among fetal ECoG states and these variables, with LVHF activity being associated with a decrease in cortical t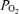 and increase in CBF to maintain optimal CMRO2. The study also uses spectral edge frequency as a quantitative measure of ECoG state.
and increase in CBF to maintain optimal CMRO2. The study also uses spectral edge frequency as a quantitative measure of ECoG state.
These interrelations also are of great importance in considering the response to hypoxia. As we and others have shown, acute hypoxia is associated with a moderate decrease in CMRO2, with an increase in CBF, depending upon the degree of hypoxaemia. It also is associated with significant decreases in cortical t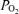 . Although some disagreement exists regarding the extent to which fetal ECoG activity is altered by isocapnic hypoxia, the present study demonstrates significant decreases in LVHF state and SEF90. Thus, we believe that this study lays the ground work for in-depth exploration of the complex mechanisms which regulate CBF, CMRO2 and related responses in terms of vascular and/or neural function. Critical questions remain as to the fundamental mechanisms involved in the neural regulation of LVHF versus HVLF ECoG states, their association with cerebral tissue
. Although some disagreement exists regarding the extent to which fetal ECoG activity is altered by isocapnic hypoxia, the present study demonstrates significant decreases in LVHF state and SEF90. Thus, we believe that this study lays the ground work for in-depth exploration of the complex mechanisms which regulate CBF, CMRO2 and related responses in terms of vascular and/or neural function. Critical questions remain as to the fundamental mechanisms involved in the neural regulation of LVHF versus HVLF ECoG states, their association with cerebral tissue 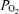 , CMRO2 and cerebral blood flow, and the role of these functions in the compromised fetus/newborn subjected to hypoxia and/or hypoxia-ischaemia.
, CMRO2 and cerebral blood flow, and the role of these functions in the compromised fetus/newborn subjected to hypoxia and/or hypoxia-ischaemia.
Acknowledgments
We thank Jimin Suh and Brenda Kreutzer for assistance with preparation of the manuscript. Jorge Pereyra Peña was a recipient of a Postdoctoral Fellowship Award from the American Heart Association Western Affiliate. This work was supported by the United States Public Health Service National Institutes of Health Grants HD/HL-03807-39.
References
- Bell AH, McClure BG, McCullah PJ, McClelland RJ. Spectral edge frequency of the EEG in healthy neonates and variation with behavioural state. Biol Neonate. 1991;60:69–74. doi: 10.1159/000243390. [DOI] [PubMed] [Google Scholar]
- Bishai JM, Blood AB, Hunter CJ, Longo LD, Power GG. Fetal lamb cerebral blood flow (CBF) and oxygen tensions during hypoxia: a comparison of laser Doppler and microsphere measurements of CBF. J Physiol. 2003;546:869–878. doi: 10.1113/jphysiol.2002.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocking AD, Gagnon R, Milne KM, White SE. Behavioral activity during prolonged hypoxemia in fetal sheep. J Appl Physiol. 1988;65:2420–2426. doi: 10.1152/jappl.1988.65.6.2420. [DOI] [PubMed] [Google Scholar]
- Bocking AD, Harding R. Effects of reduced uterine blood flow on electrocortical activity, breathing, and skeletal muscle activity in fetal sheep. Am J Obstet Gynecol. 1986;154:655–662. doi: 10.1016/0002-9378(86)90625-3. [DOI] [PubMed] [Google Scholar]
- Boddy K, Dawes GS, Fisher R, Pinter S, Robinson JS. Foetal respiratory movements, electrocortical and cardiovascular responses to hypoxaemia and hypercapnia in sheep. J Physiol. 1974;243:599–618. doi: 10.1113/jphysiol.1974.sp010768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp JF, III, Szeto HH, Abrams R, Larrow R, Mann LI. Physiologic variability and fetal electrocortical activity. Am J Obstet Gynecol. 1980;136:1045–1050. doi: 10.1016/0002-9378(80)90635-3. [DOI] [PubMed] [Google Scholar]
- Clewlow F, Dawes GS, Johnston BM, Walker DW. Changes in breathing, electrocortical and muscle activity in unanaesthetized fetal lambs with age. J Physiol. 1983;341:463–476. doi: 10.1113/jphysiol.1983.sp014817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czikk MJ, Sweeley JC, Homan JH, Milley JR, Richardson BS. Cerebral leucine uptake and protein synthesis in the near-term ovine fetus: relation to fetal behavioral state. Am J Physiol Regul Integr Comp Physiol. 2003;284:R200–R207. doi: 10.1152/ajpregu.00190.2002. [DOI] [PubMed] [Google Scholar]
- Dawes GS, Fox HE, Leduc BM, Liggins GC, Richards RT. Respiratory movements and rapid eye movement sleep in the foetal lamb. J Physiol. 1972;220:119–143. doi: 10.1113/jphysiol.1972.sp009698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser M, Bennet L, Gunning M, Williams C, Gluckman PD, George S, Gunn AJ. Cortical electroencephalogram suppression is associated with post-ischemic cortical injury in 0.65 gestation fetal sheep. Brain Res Dev Brain Res. 2005;154:45–55. doi: 10.1016/j.devbrainres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Gasser T, Bächer P, Möcks J. Transformations towards the normal distribution of broad band spectral parameters of the EEG. Electroencephalogr Clin Neurophysiol. 1982;53:119–124. doi: 10.1016/0013-4694(82)90112-2. [DOI] [PubMed] [Google Scholar]
- George S, Gunn AJ, Westgate JA, Brabyn C, Guan J, Bennet L. Fetal heart rate variability and brain stem injury after asphyxia in preterm fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2004;287:R925–R933. doi: 10.1152/ajpregu.00263.2004. [DOI] [PubMed] [Google Scholar]
- Grant DA, Franzini C, Wild J, Eede KJ, Walker AM. Autoregulation of the cerebral circulation during sleep in newborn lambs. J Physiol. 2005;564:923–930. doi: 10.1113/jphysiol.2005.083352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding R, Poore ER, Cohen GL. The effect of brief episodes of diminished uterine blood flow on breathing movements, sleep states and heart rate in fetal sheep. J Dev Physiol. 1981;3:231–243. [PubMed] [Google Scholar]
- Inder TE, Buckland L, Williams CE, Spencer C, Gunning MI, Darlow BA, Volpe JJ, Gluckman PD. Lowered electroencephalographic spectral edge frequency predicts the presence of cerebral white matter injury in premature infants. Pediatrics. 2003;111:27–33. doi: 10.1542/peds.111.1.27. [DOI] [PubMed] [Google Scholar]
- Isler JR, Garland M, Stark RI, Grieve PG. Local coherence oscillations in the EEG during development in the fetal baboon. Clin Neurophysiol. 2005;116:2121–2128. doi: 10.1016/j.clinph.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Jones MD, Jr, Rosenberg AA, Simmons MA, Molteni RA, Koehler RC, Traystman RJ. Oxygen delivery to the brain before and after birth. Science. 1982;216:324–325. doi: 10.1126/science.6801768. [DOI] [PubMed] [Google Scholar]
- Jones MD, Jr, Traystman RJ. Cerebral oxygenation of the fetus, newborn, and adult. Semin Perinatol. 1984;8:205–216. [PubMed] [Google Scholar]
- Jost RG, Quilligan EJ, Yeh S-Y, Anderson G. Intrauterine electroencephalogram of the sheep fetus. Am J Obstet Gynecol. 1972;114:535–539. doi: 10.1016/0002-9378(72)90216-5. [DOI] [PubMed] [Google Scholar]
- Koos BJ, Matsuda K, Power GG. Fetal breathing and sleep state responses to graded carboxyhemoglobinemia in sheep. J Appl Physiol. 1988;65:2118–2123. doi: 10.1152/jappl.1988.65.5.2118. [DOI] [PubMed] [Google Scholar]
- Koos BJ, Sameshima H. Effects of hypoxaemia and hypercapnia on breathing movements and sleep state in sinoaortic-denervated fetal sheep. J Dev Physiol. 1988;10:131–144. [PubMed] [Google Scholar]
- Koos BJ, Sameshima H, Power GG. Fetal breathing movement, sleep state and cardiovascular responses to an inhibitor of mitochondrial ATPase in sheep. J Dev Physiol. 1986;8:67–75. [PubMed] [Google Scholar]
- Lotgering FK, Gilbert RD, Longo LD. Exercise responses in pregnant sheep: blood gases, temperatures, and fetal cardiovascular system. J Appl Physiol. 1983;55:842–850. doi: 10.1152/jappl.1983.55.3.842. [DOI] [PubMed] [Google Scholar]
- MacLachlan JN, Hemstreet S, Matushewski B, McCallum JD, Richardson BS. Induced hyper and hypo amino acidemia in the ovine fetus near term: Effects on electrocortical activity. Reprod Sci. 2008;15:710–719. doi: 10.1177/1933719108317152. [DOI] [PubMed] [Google Scholar]
- Mann LI. Fetal brain function and metabolism. Clin Obstet Gynecol. 1970;13:638–651. doi: 10.1097/00003081-197009000-00011. [DOI] [PubMed] [Google Scholar]
- Mann LI, Duchin S, Weiss RR. Fetal EEG sleep stages and physiologic variability. Am J Obstet Gynecol. 1974;119:533–538. doi: 10.1016/0002-9378(74)90215-4. [DOI] [PubMed] [Google Scholar]
- Marks GA, Shaffery JP, Oksenberg A, Speciale SG, Roffwarg HP. A functional role for REM sleep in brain maturation. Behav Brain Res. 1995;69:1–11. doi: 10.1016/0166-4328(95)00018-o. [DOI] [PubMed] [Google Scholar]
- Mirmiran M. The importance of fetal/neonatal REM sleep. Eur J Obstet Gynecol Reprod Biol. 1986;21:283–291. doi: 10.1016/0028-2243(86)90006-7. [DOI] [PubMed] [Google Scholar]
- Mirmiran M. The function of fetal/neonatel rapid eye movement sleep. Behav Brain Res. 1995;69:13–22. doi: 10.1016/0166-4328(95)00019-p. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Carmichael L, Homan J, White S, Richardson BS. Cerebral blood flow during spontaneous and cholinergically induced behavioral states in the sheep fetus. Pediatr Res. 2005;57:667–673. doi: 10.1203/01.PDR.0000156210.27381.12. [DOI] [PubMed] [Google Scholar]
- Morrissey MJ, Duntley SP, Anch AM, Nonneman R. Active sleep and its role in the prevention of apoptosis in the developing brain. Med Hypotheses. 2004;62:876–879. doi: 10.1016/j.mehy.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Natale R, Clewlow F, Dawes GS. Measurement of fetal forelimb movements in the lamb in utero. Am J Obstet Gynecol. 1981;140:545–551. doi: 10.1016/0002-9378(81)90231-3. [DOI] [PubMed] [Google Scholar]
- Osredkar D, Toet MC, van Rooij LGM, van Huffelen AC, Groenendaal F, de Vries LS. Sleep-wake cycling on amplitude-integrated electroencephalography in term newborns with hypoxic-ischemic encephalopathy. Pediatrics. 2005;115:327–332. doi: 10.1542/peds.2004-0863. [DOI] [PubMed] [Google Scholar]
- Pereyra Peña J, Tomimatsu T, Hatran DP, McGill LL, Longo LD. Cerebral blood flow and oxygenation in ovine fetus: responses to superimposed hypoxia at both low an high altitude. J Physiol. 2007;578:359–370. doi: 10.1113/jphysiol.2006.119925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulgar VM, Zhang J, Massmann A, Figueroa JP. Prolonged mild hypoxia alters fetal sheep electrocorticogram activity. J Soc Gynecol Investig. 2006;13:404–411. doi: 10.1016/j.jsgi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Richardson BS, Caetano H, Homan J, Carmichael L. Regional brain blood flow in the ovine fetus during transition to the low-voltage electrocortical state. Brain Res Dev Brain Res. 1994;81:10–16. doi: 10.1016/0165-3806(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Richardson BS, Carmichael L, Homan J, Gagnon R. Cerebral oxidative metabolism in lambs during perinatal period: relationship to electrocortical state. Am J Physiol Regul Integr Comp Physiol. 1989;257:R1251–R1257. doi: 10.1152/ajpregu.1989.257.5.R1251. [DOI] [PubMed] [Google Scholar]
- Richardson BS, Carmichael L, Homan J, Patrick JE. Electrocortical activity, electroocular activity, and breathing movements in fetal sheep with prolonged and graded hypoxemia. Am J Obstet Gynecol. 1992;167:553–558. doi: 10.1016/s0002-9378(11)91452-5. [DOI] [PubMed] [Google Scholar]
- Richardson BS, Patrick JE, Abduljabbar H. Cerebral oxidative metabolism in the fetal lamb: relationship to electrocortical state. Am J Obstet Gynecol. 1985;153:426–431. doi: 10.1016/0002-9378(85)90081-x. [DOI] [PubMed] [Google Scholar]
- Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. The prime role of “dreaming sleep” in early life may be in the development of the central nervous system. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- Ruckebusch Y. Development of sleep and wakefulness in the foetal lamb. Electroencephalogr Clin Neurophysiol. 1972;32:119–128. doi: 10.1016/0013-4694(72)90134-4. [DOI] [PubMed] [Google Scholar]
- Ruckebusch Y, Gaujoux M, Eghbali B. Sleep cycles and kinesis in the foetal lamb. Electroencephalogr Clin Neurophysiol. 1977;42:226–237. doi: 10.1016/0013-4694(77)90029-3. [DOI] [PubMed] [Google Scholar]
- Scher MS, Steppe DA, Banks DL. Prediction of lower developmental performances of healthy neonates by neonatal EEG-sleep measures. Pediatr Neurol. 1996;14:137–144. doi: 10.1016/0887-8994(96)00013-6. [DOI] [PubMed] [Google Scholar]
- Scher MS, Steppe DA, Beggarly ME, Salerno DG, Banks DL. Neonatal EEG-sleep disruption mimicking hypoxic-ischemic encephalopathy after intrapartum asphyxia. Sleep Med. 2002;3:411–415. doi: 10.1016/s1389-9457(02)00071-0. [DOI] [PubMed] [Google Scholar]
- Shinozuka N, Nathanielsz PW. Electorcortical activity in fetal sheep in the last seven days of gestation. J Physiol. 1998;513:273–281. doi: 10.1111/j.1469-7793.1998.273by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- Stark RI, Haiken J, Nordli D, Myers MM. Characterization of electroencephalographic state in fetal baboons. Am J Physiol Regul Integr Comp Physiol. 1991;261:R496–R500. doi: 10.1152/ajpregu.1991.261.2.R496. [DOI] [PubMed] [Google Scholar]
- Szeto HH. Spectral edge frequency as a simple quantitative measure of the maturation of electrocortical activity. Pediatr Res. 1990;27:289–292. doi: 10.1203/00006450-199003000-00018. [DOI] [PubMed] [Google Scholar]
- Szeto HH, Hinman DJ. Prenatal development of sleep-wake patterns in sheep. Sleep. 1985;8:347–355. doi: 10.1093/sleep/8.4.347. [DOI] [PubMed] [Google Scholar]
- Szeto HH, Vo TDH, Dwyer G, Dogramajian ME, Cox MJ, Senger G. The ontogeny of fetal lamb electrocortical activity: A power spectral analysis. Am J Obstet Gynecol. 1985;153:462–466. doi: 10.1016/0002-9378(85)90088-2. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Sugawara J, Chisaka H, Imai N, Saito M, Murakami T, Kimura Y, Okamura K. Appearance of abnormal electrocorticogram patterns during umbilical cord compression in sheep fetus. Tohoku J Exp Med. 2006;208:9–17. doi: 10.1620/tjem.208.9. [DOI] [PubMed] [Google Scholar]
- Ter Horst HJ, Sommer C, Bergman KA, Fock JM, Van Weerden TW, Bos AF. Prognostic significance of amplitude-integrated EEG during the first 72 hours after birth in severely asphyxiated neonates. Pediatr Res. 2004;55:1026–1033. doi: 10.1203/01.pdr.0000127019.52562.8c. [DOI] [PubMed] [Google Scholar]
- Thaler I, Boldes R, Timor-Tritsch I. Real-time spectral analysis of the fetal EEG: A new approach to monitoring sleep states and fetal condition during labor. Pediatr Res. 2000;48:340–345. doi: 10.1203/00006450-200009000-00013. [DOI] [PubMed] [Google Scholar]
- Thornberg E, Thiringer K. Normal pattern of the cerebral function monitor trace in term and preterm neonates. Acta Paediatr Scand. 1990;79:20–25. doi: 10.1111/j.1651-2227.1990.tb11324.x. [DOI] [PubMed] [Google Scholar]
- Tomimatsu T, Pereyra Peña J, Longo LD. Fetal hypercapnia and cerebral tissue oxygenation: Studies in near-term sheep. Pediatr Res. 2006;60:711–716. doi: 10.1203/01.pdr.0000246308.37154.ce. [DOI] [PubMed] [Google Scholar]
- Walker AM, Fleming J, Smolich J, Stunden R, Horne R, Maloney J. Fetal oxygen consumption, umbilical circulation and electrocortical activity transitions in fetal lambs. J Dev Physiol. 1984;6:267–274. [PubMed] [Google Scholar]
- Watanabe K, Hayakawa F, Okumura A. Neonatal EEG: a powerful tool in the assessment of brain damage in preterm infants. Brain Dev. 1999;21:361–372. doi: 10.1016/s0387-7604(99)00034-0. [DOI] [PubMed] [Google Scholar]
- Williams CE, Gunn AJ, Mallard C, Gluckman PD. Outcome after ishemia in the developing sheep brain: an electroencephalographic and histological study. Ann Neurol. 1992;31:14–21. doi: 10.1002/ana.410310104. [DOI] [PubMed] [Google Scholar]