Abstract
There is minimal in vivo data in humans evaluating myocardial substrate utilization during increased heart work. This study was performed to determine the balance of myocardial glucose and lactate metabolism during rest and increased heart work induced by atrial pacing in seven healthy men and women (age, 49.7 ± 3.9 years; body mass index, 23.4 ± 1.1 kg m−2, maximum oxygen consumption, 35.5 ± 3.0 ml kg−1 min−1, ejection fraction, 68 ± 3%). After 3 days of dietary control, catheters were placed in coronary sinus, femoral arterial and venous, and peripheral venous blood vessels. Subjects received a primed continuous infusion of [3,3,3-2H]lactate and [6,6-2H]glucose throughout the study. Arterial and coronary sinus blood sampling and measurements of coronary sinus blood flow were made during rest and atrial pacing at approximately 111 beats min–1. Myocardial oxygen consumption increased (P= 0.04) from rest to atrial pacing. Net glucose uptake increased (P= 0.04) from rest to atrial pacing with unchanged fractional extraction (rest: 9.1 ± 2.7%, atrial pacing 9.8 ± 2.9%). The percentage of whole body glucose disposal from myocardial uptake also increased from rest to atrial pacing. Isotopically measured lactate uptake also increased significantly from rest to atrial pacing with no significant differences in fractional extraction. The myocardium released lactate throughout the experiment, which increased significantly from rest and atrial pacing (P < 0.05). The heart accounted for a significantly greater percentage of whole body lactate disposal during atrial pacing (15.0 ± 4.4%) compared to rest (4.9 ± 0.9%, P= 0.03). These data suggest: (1) in the absence of ischaemia the myocardium is constantly taking up and releasing lactate at rest which increases during atrial pacing, and (2) when arterial substrate delivery is unchanged, increased myocardial work is accomplished with similar proportions of glucose and lactate utilization in healthy humans in vivo.
Myocardial substrate utilization is an important factor influencing energy efficiency (Burkhoff et al. 1991), tissue survival during ischaemia (Kantor et al. 1999), and cardiac efficiency (Peterson et al. 2004). Alterations in myocardial substrate utilization have been implicated in changes in ventricular performance in heart failure (Stanley et al. 2005; Ashrafian et al. 2007), obesity (Peterson et al. 2004), type 1 (Avogaro et al. 1990b) and type 2 diabetes (Stanley et al. 1997). Therefore, a thorough understanding of factors controlling myocardial substrate utilization in individuals without known heart disease is important to understand how myocardial substrate use may influence disease states.
Data from animal models and isolated perfused heart preparations indicate the majority of myocardial energy demand is met by the oxidation of free fatty acids at rest after an overnight fast (Opie, 1968; Stanley et al. 2005). Plasma free fatty acid (FFA) concentration dictates, in large part, the relative contribution of glucose, lactate and FFA to myocardial oxidation (Stanley et al. 2005). There are conflicting data regarding myocardial substrate selection during states of increased work in isolated perfused hearts. Some data suggest increased myocardial work is selective for increased carbohydrate oxidation (Collins-Nakai et al. 1994; Goodwin et al. 1998a), other studies suggest fat and carbohydrate utilization increase similarly (Crass et al. 1969; Neely et al. 1969; Neely et al. 1976), while others report a greater increase in carbohydrate than fat oxidation (Goodwin et al. 1998b). Studies on resting myocardial substrate utilization in vivo have largely corroborated findings in animal models, starting with the early work of Bing et al. (1953, 1954).
There is less in vivo data available in humans describing the balance of myocardial glucose and lactate utilization during increased heart work. Increased myocardial glucose and lactate utilization have been found during whole body exercise in humans (Kaijser et al. 1972; Gertz et al. 1988; Camici et al. 1989; Kemppainen et al. 2002). Alterations in substrate selection during exercise were related to changes in plasma concentration, underscoring the importance of arterial substrate delivery to myocardial substrate utilization.
This study was done to investigate the hypothesis that with similar arterial metabolite concentrations, glucose and lactate uptake would increase during atrial pacing on an absolute basis, with no significant changes in uptake relative to total myocardial work. Atrial pacing was used to increase heart work instead of exercise because, unlike exercise, atrial pacing does not alter arterial substrate concentration.
Methods
Ethical approval
The Colorado Multiple Institutional Review Board at the University of Colorado Denver approved this study prior to its commencement. Informed consent for participation was required in writing for subjects to enter the study. All research-related activity conformed to the principles outlined in the Declaration of Helsinki (updated 2004).
Subjects
Seven healthy men and women were recruited for this study. Subjects gave written informed consent, and were excluded if they: smoked, had diabetes, thyroid disease, hyperlipidaemia, liver or kidney disease, were taking medications which affect glucose or lipid metabolism, and/or regularly engage in vigorous exercise (> 2 h per week). Subjects were excluded if they had a body mass index < 20 kg m−2 or > 28 kg m−2. Women were taking oral contraceptives and were studied during the mid-follicular phase of their menstrual cycle to minimize effects of menstrual cycle phase on substrate metabolism. All subjects had a negative history for angina-like chest pain.
General experimental design
After preliminary screening, subjects participated in one metabolic trial. The subjects were provided with a diet from the metabolic kitchen at the General Clinical Research Center (GCRC) for 3 days prior to the study. During the metabolic trial, a right heart catheterization was combined with measurement of glucose and lactate kinetics at rest and during atrial pacing to increase heart work.
Preliminary testing
Subjects reported to the GCRC for the screening procedures following a 12 h overnight fast. They were given a health and physical examination, followed by fasting blood measurements. Body composition was determined using dual energy X-ray absorptiometry (DEXA) analysis (Lunar DPX-IQ, Lunar Corporation, Madison, WI, USA). Resting metabolic rate (RMR) was measured using indirect calorimetry via a metabolic cart system (Sensormedics 2900, Sensormedics, Yorba Linda, CA, USA). Subjects rested supine for 30 min, then a ventilated canopy was placed over their head and measurements continued for 15–20 min. Metabolic rate was calculated from the flow rate of expired air, in conjunction with measuring differences in the oxygen (O2) and carbon dioxide (CO2) concentrations using standard equations (Wier, 1949). Subjects were included in the study if they had normal fasting glucose, defined by the American Diabetes Association as fasting serum glucose concentration < 100 mg dl−1. On a separate screening day, subjects arrived at the GCRC and completed a maximum oxygen consumption 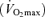 test using the Balke treadmill test with a 12-lead ECG. No subjects had electrocardiographic indications of ischaemia at rest and all subjects were negative for ischaemia during the stress test. A resting echocardiogram was also performed to exclude subjects with myocardial or valvular disease and left ventricular hypertrophy (Sonos 5500, Royal Philips Electronics, the Netherlands).
test using the Balke treadmill test with a 12-lead ECG. No subjects had electrocardiographic indications of ischaemia at rest and all subjects were negative for ischaemia during the stress test. A resting echocardiogram was also performed to exclude subjects with myocardial or valvular disease and left ventricular hypertrophy (Sonos 5500, Royal Philips Electronics, the Netherlands).
Diet control
Three day diet records were analysed for each subject to determine the macronutrient and energy content of their diet. Dieticians used the RMR with an activity factor of 1.4, combined with each subject's typical dietary intake, to make a 3 day diet which was provided to each subject prior to the metabolic study. The diet followed the American Heart Association recommendations for macronutrient composition (30% fat, 15% protein and 55% carbohydrate). Subjects were asked to maintain normal daily physical activity during the period of dietary control, and refrain from planned exercise for 3 days prior to the metabolic study. Pre-study nutritional control ensured subjects were close to energy balance, and therefore, differences in energy status and glycogen stores prior to testing were controlled for between subjects. Subjects spent the evening before the metabolic study at the GCRC at University of Colorado Hospital to ensure compliance with the overnight fast.
Metabolic study
Subjects were taken to the cardiac catheterization laboratory on the morning of the metabolic study after an overnight fast where catheters were placed into the right internal jugular vein for coronary sinus access (6 Fr, Cordis Multipurpose A-2, Johnson and Johnson Corporation, Piscataway, NJ, USA) and Doppler flow (0.014 Doppler Flow Wire, size 300, Cardiometrics Inc., Mountain View, CA, USA), femoral artery for arterial blood (Cordis 4 Fr arterial sheath, Johnson and Johnson Corp, Piscataway, NJ, USA), right femoral vein for pulmonary artery access (8 Fr venous sheath, Boston Scientific, Natick, MA, USA, and 7.5 Fr VIP thermodilution pulmonary artery catheter, Baxter-Edwards, Dearfield, IL, USA), right femoral vein for atrial pacer wire (6 Fr venous sheath, Boston Scientific and Cordis 6 Fr atrial pacing wire, Johnson and Johnson), and a peripheral venous catheter for isotope infusion using standard catheterization techniques. Heparin was administered at 15 units h−1 to maintain patency of the arterial line during the 3 h study, and did not change arterial FFA concentration (Bergman et al. 2009). After insertion of the coronary sinus catheter, an angiogram was performed using 30 ml of a non-ionic contrast agent to measure the diameter of the coronary sinus and to determine the position of the multipurpose catheter for both blood sampling and positioning of the Doppler flow wire. This angiogram was performed prior to the initiation of the isotope infusion and there was an interval of at least 60 min between the angiogram and the measurement of coronary sinus flow velocity with the Doppler wire. Initial cardiac haemodynamic measurements were made with a pulmonary artery catheter with the determination of cardiac output by thermodilution and Fick methods and the recording of right atrial, pulmonary artery and pulmonary capillary wedge pressures. Systemic arterial pressure was determined from the femoral arterial sheath. Determination of rate–pressure product, mean arterial pressure, and systemic and pulmonary vascular resistances were calculated by standard methods. Following these measurements, blood was withdrawn from the arterial and coronary sinus catheters for background glucose and lactate enrichment. Then through a peripheral venous catheter a priming bolus of [3,3,3-2H]lactate and [6,6-2H]glucose was initiated containing 150 × resting infusion rate of glucose and lactate. The 2H2 glucose was then infused at a rate of 0.03 mg kg−1 min−1, and 2H3 lactate at 0.036 mg kg−1 min−1 until the end of the study. Additionally, we infused [1-13C]palmitate at 0.012 mg kg−1 min−1 and [1-14C]acetate at 0.2 nCi kg−1 min−1 to measure myocardial FFA uptake and oxidation which are reported separately (Bergman et al. 2009). Measurements of resting coronary sinus blood flow were determined from Doppler flow velocities in the coronary sinus starting at minutes 30 and 45 of the infusion. Indirect calorimetry was performed for 15 min starting at 50 min of the infusion. Simultaneous arterial and coronary sinus blood sampling was performed at 60, 70, 80 and 90 min of rest. Blood was obtained for glucose, lactate, FFA, glycerol, plasma catecholamines, insulin, glucagon, isotopic enrichment of glucose and lactate, arterial blood gasses, haemoglobin, haematocrit and O2 content. Blood for the determination of lactate concentration was collected in 8% perchloric acid. Atrial pacing was then started at a heart rate of 120 beats min–1 in an attempt to standardize myocardial oxygen consumption (Table 2, Fig. 1B). Measurements of paced coronary sinus blood flow were made starting at minutes 15 and 55 of pacing. Simultaneous arterial and coronary sinus blood samples were obtained following 20, 30, 40 and 50 min of atrial pacing. Cardiac output during atrial pacing was determined as during rest using thermodilution and Fick methods. Blood samples were obtained during pacing for the same measurements as during rest. Another angiogram of the coronary sinus was performed with intravenous contrast during atrial pacing after completion of all coronary sinus Doppler flow velocity measurements and blood sampling. At the completion of the study, all catheters were removed and the subjects returned to the GCRC for 7 h of observation before discharge.
Table 2.
Haemodynamics and indirect calorimetry during rest and atrial pacing
| Parameter | Rest | Atrial pacing |
|---|---|---|
| Heart rate (beats min–1) | 70 ± 4 | 111 ± 5§ |
| Systolic BP (mmHg) | 137 ± 3 | 142 ± 8 |
| Diastolic BP (mmHg) | 72 ± 3 | 79 ± 5 |
| Mean BP – MAP (mmHg) | 97 ± 3 | 100 ± 5 |
| Cardiac output (l min−1) (thermodilution) | 5.28 ± 0.25 | 5.55 ± 0.44 |
| Stroke volume (ml) | 77 ± 7 | 53 ± 7§ |
| Coronary sinus CSA (cm2) | 0.53 ± 0.10 | 0.43 ± 0.09§ |
| Flow velocity (cm s−1) | 6.2 ± 0.7 | 9.6 ± 1.7§ |
| Rate–pressure product (beats min–1× mmHg) | 9587 ± 640 | 15 419 ± 1130§ |
| Systemic vascular resistance (dyn s cm−5) | 1443 ± 80 | 1445 ± 71 |
| Right atrial pressure (mmHg) | 3 ± 1 | 1 ± 1 |
| Systolic pulmonary artery pressure (mmHg) | 20 ± 2 | 14 ± 3§ |
| Diastolic PA pressure (mmHg) | 7 ± 2 | 7 ± 3 |
| Mean PA pressure (mmHg) | 13 ± 2 | 10 ± 3§ |
| Pulmonary capillary wedge pressure (mmHg) | 6 ± 2 | 4 ± 2 |
| Mixed venous O2 saturation (%) | 74 ± 2 | 73 ± 1 |
| a–v O2 content difference (vol%) | 4.1 ± 0.4 | 4.3 ± 0.3 |
| Coronary sinus O2 saturation (%) | 34 ± 3 | 30 ± 1 |
| a–cs O2 content difference (vol%) | 12.2 ± 0.7 | 12.9 ± 0.9 |
 (ml min−1) (ml min−1) |
248.9 ± 21.7 | 272.3 ± 21.3 |
| Whole body RER | 0.83 ± 0.03 | 0.86 ± 0.05 |
| Myocardial RQ | 0.83 ± 0.03 | 0.78 ± 0.04 |
Values are means ±s.e.m. for n= 7. Data were averaged from two measurements during rest and two measurements during atrial pacing. BP, blood pressure (S, systolic; D, diastolic); PA, pulmonary artery; cs, coronary sinus; a, arterial; v, mixed venous (PA); CSA, cross sectional area; RER, respiratory exchange ratio; RQ, respiratory quotient.
Significantly different from rest at P < 0.05. Mean arterial pressure, MAP = 0.33 (SBP – DBP) + DBP; rate–pressure product = SBP × heart rate; systemic vascular resistance = (MAP – right atrial pressure) × 80/cardiac output.
Figure 1.
Coronary sinus blood flow (A) and myocardial 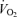 (B) during rest and atrial pacing in men and women. Values are means ±s.e.m. § Significantly different from rest, P < 0.05.
(B) during rest and atrial pacing in men and women. Values are means ±s.e.m. § Significantly different from rest, P < 0.05.
Metabolite and hormone analyses
Insulin (Wide & Porath, 1966) (Clinical Assays Gamma Coat RIA) and glucagon (Aguilar-Parada et al. 1969) were determined by radioimmunoassay. Catecholamines were determined by high performance liquid chromatography with electrochemical detection (Causon et al. 1981). Standard enzymatic assays were used to measure lactate (Sigma Kit no. 826), glucose and triglycerides (Olympus America Diagnostics AU400e Chemistry Immuno Analyzer), glycerol (Boehringer Mannheim Diagnostics) and FFA (NEFA Kit, Wako).
Gas chromatography/mass spectroscopy methods
Metabolite isotopic enrichment was measured using gas chromatography–mass spectrometry (GCMS; GC model 5890 series II and MS model 5989A, Hewlett-Packard). The [6,6-2H]glucose, and [3,3,3-2H]lactate were derivatized by the penta-acetate and t-butyldimethylsilyl (TBDMS) derivative, respectively.
Calculations
The rate of appearance (Ra) and rate of disappearance (Rd) of glucose and lactate were calculated using equations defined by Steele, modified for stable isotopes (Wolfe, 1992).
 |
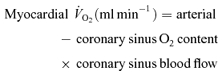 |
Coronary sinus blood flow (ml min−1) = (coronary sinus diameter)2× 0.785 × (mean flow velocity) × heart rate.
The mean flow velocity was determined by the area under the curve of the carotid sinus (CS) Doppler flow velocity.
 |
 |
where F represents isotope infusion rate, IE1 and IE2 are isotopic tracer to tracee ratios at sampling time points 1 (t1) and 2 (t2), respectively. C1 and C2 are metabolite concentrations at t1 and t2; V is the estimated volume distribution of glucose and lactate (180 ml kg−1) as previously published (Friedlander et al. 1997). All isotopic enrichments were corrected for background enrichments from blood samples taken before isotope infusion.
Blood 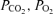 , pH and haemoglobin were measured on both arterial and venous samples and used in the calculations by Douglas et al. (1988) for determination of blood CO2 content. CO2 solubility and apparent dissociation constant were estimated from the equations of Kelman (1967). Haemoglobin concentration, saturation and content of O2 were directly measured using the OSM-3 haemocytometer (Radiometer, Copenhagen). Blood O2 content was also calculated with haemoglobin concentration, and saturation was determined from an equation from Nunn (1993), as described previously (Bergman et al. 1999a). Art Ep is the arterial plasma enrichment, while [Art] is the arterial concentration of the metabolite of interest. CS Ep is the coronary sinus plasma enrichment, while [CS] is the coronary sinus concentration of the metabolite of interest. Art Hct and CS Hct are the arterial and coronary sinus haematocrit concentrations, respectively.
, pH and haemoglobin were measured on both arterial and venous samples and used in the calculations by Douglas et al. (1988) for determination of blood CO2 content. CO2 solubility and apparent dissociation constant were estimated from the equations of Kelman (1967). Haemoglobin concentration, saturation and content of O2 were directly measured using the OSM-3 haemocytometer (Radiometer, Copenhagen). Blood O2 content was also calculated with haemoglobin concentration, and saturation was determined from an equation from Nunn (1993), as described previously (Bergman et al. 1999a). Art Ep is the arterial plasma enrichment, while [Art] is the arterial concentration of the metabolite of interest. CS Ep is the coronary sinus plasma enrichment, while [CS] is the coronary sinus concentration of the metabolite of interest. Art Hct and CS Hct are the arterial and coronary sinus haematocrit concentrations, respectively.
Myocardial respiratory quotient (RQ) was calculated from the ratio of coronary sinus–arterial CO2 difference to arterial–coronary sinus O2 difference. When the RQ was below 0.70 or above 1.0, a theoretical limit was assumed. Myocardial oxygen extraction ratio (OER, %), which is the relative contribution of a substrate to myocardial oxygen consumption, was calculated as described by Lassers et al. (1972).
Statistics
Data are presented as mean ±s.e.m. Differences between measures during rest and atrial pacing were analysed using Student's paired t test. Differences in arterial and coronary sinus metabolite concentrations were analysed using Student's paired t test (SPSS, Chicago, IL, USA). Linear regression analyses were performed to correlate arterial substrate concentration and myocardial uptake or release. An α level of 0.05 was used throughout.
Results
Subject characteristics
Anthropometric data for subjects are reported in Table 1. Subjects were weight stable in the 6 months prior to participation in this research study. The men and women in this study had a normal BMI.  was average for this age range, and left ventricular size and ejection fraction were also normal.
was average for this age range, and left ventricular size and ejection fraction were also normal.
Table 1.
Subject demographics
| Values | |
|---|---|
| n (Men/Women) | 7 (5/2) |
| Age (years) | 49.7 ± 3.9 |
| Weight (kg) | 72.57 ± 4.5 |
| Height (cm) | 175.9 ± 2.2 |
| BMI (kg m−2) | 23.4 ± 1.1 |
| % Body fat | 28.7 ± 1.5 |
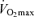 (ml kg−1 min−1) (ml kg−1 min−1) |
35.5 ± 3.0 |
| LV ejection fraction (%) | 68 ± 3 |
Values are means ±s.e.m. BMI, body mass index; LV, left ventricular.
Indirect calorimetry
As shown in Table 2, rates of whole body oxygen consumption were not significantly different during rest and atrial pacing. Whole body respiratory exchange ratio and myocardial RQ were not significantly different from rest to atrial pacing.
Haemodynamics
At baseline, cardiac output, systemic arterial pressure, intracardiac filling pressures and vascular resistances were within normal limits (Table 2). The arterial–coronary sinus O2 content difference indicated an O2 extraction rate of 66%, compared to 22% in the systemic circulation. Thus, even at rest, the myocardium had a high level of O2 extraction. With atrial pacing there was an expected significant increase in heart rate (Table 2). Although the target heart rate was 120 beats min–1, three subjects had intermittent AV Wenckebach with pacing at that rate. These subjects were paced and studied at a lower heart rate that maintained normal rhythm, which resulted in a mean pacing heart rate of less than 120 beats min–1. There were significant increases in rate–pressure product, an estimate of myocardial O2 consumption, with atrial pacing. Atrial pacing did not result in an increase in cardiac output and there was no increase in either systemic or coronary sinus O2 extraction. Although right atrial and pulmonary capillary wedge pressures did not change with pacing, there was a reduction in pulmonary artery systolic and mean pressures.
Metabolite concentrations
Atrial pacing did not significantly change FFA, glycerol, adrenaline (epinephrine), noradrenaline (norepinephrine), or insulin concentrations compared to rest (Table 3). However, triglyceride and glucagon concentrations increased significantly during atrial pacing compared to rest (P < 0.02).
Table 3.
Concentrations of hormones and substrates during rest and atrial pacing in men and women
| Rest | Atrial pacing | |
|---|---|---|
| FFAs (μmol l−1) | 1083.4 ± 141.1 | 1224.5 ± 104.2 |
| Glycerol (μmol l−1) | 108.7 ± 30.3 | 114.6 ± 28.6 |
| Triglycerides (mg dl−1) | 95.8 ± 12.3 | 100.8 ± 12.3§ |
| Adrenaline (pg ml−1) | 61.4 ± 12.4 | 62.6 ± 16.1 |
| Noradrenaline (pg ml−1) | 398.6 ± 86.6 | 391.7 ± 96.5 |
| Insulin (pg ml−1) | 4.8 ± 0.5 | 4.6 ± 0.7 |
| Glucagon (pg ml−1) | 68.2 ± 4.7 | 72.2 ± 4.7§ |
Values are means ±s.e.m. FFAs, free fatty acids;
significantly different from rest at P < 0.05.
Myocardial blood flow
As expected, atrial pacing and greater myocardial work increased coronary sinus blood flow compared to rest (Fig. 1A, P= 0.02). Similarly, myocardial oxygen consumption increased significantly from rest to atrial pacing as expected (Fig. 1B, P= 0.04).
Tracer kinetics
Glucose and lactate enrichment increased over time during rest and atrial pacing (Fig. 2A and B). There were no differences between arterial and coronary sinus glucose enrichments during rest or atrial pacing. Coronary sinus lactate enrichment was significantly lower than arterial enrichment during both rest and atrial pacing (P < 0.05).
Figure 2.
Glucose and lactate arterial and coronary sinus enrichment in lean men and women during 90 min of rest and 50 min of atrial pacing. Values are means ±s.e.m. ¥ Significantly different from arterial, P < 0.05.
Glucose metabolism
Arterial and coronary sinus glucose concentrations were stable and did not change significantly during rest and atrial pacing as shown in Fig. 3A. Coronary sinus glucose concentration was significantly lower than arterial glucose concentration during rest (P= 0.04) and atrial pacing (P= 0.02). The arterial–coronary sinus glucose concentration difference was positive and was not significantly different from rest to atrial pacing (Fig. 3B). When multiplied by coronary sinus blood flow, myocardial net glucose uptake (Fig. 3C) increased significantly (P= 0.04) from rest to atrial pacing. We did not observe a relationship between arterial FFA and myocardial glucose Rd (P= 0.55). Myocardial glucose fractional extraction (Fig. 3D) was also not different from rest to atrial pacing. Therefore, the increase in net glucose uptake during atrial pacing was due to greater glucose delivery via increased blood flow. As a result of increased net glucose uptake, the heart accounted for a significantly greater percentage of whole body glucose disposal during atrial pacing compared to rest (Fig. 3E, P= 0.04). The relative proportion of glucose uptake to myocardial oxygen consumption (OER) was 42.3 ± 14.4% at rest and 40.2 ± 11.6% during atrial pacing.
Figure 3.
Arterial and coronary sinus glucose concentration (A), arterial–coronary sinus glucose difference (B), and myocardial net glucose uptake (C), myocardial glucose fractional extraction (D), and percentage of glucose disappearance (Rd) from myocardial glucose uptake (E) during rest and atrial pacing in men and women. Values are means ±s.e.m. § Significantly different from rest, P < 0.05. ¥ Significantly different from arterial, P < 0.05.
Lactate metabolism
Arterial and coronary sinus lactate concentration was not significantly different between rest and atrial pacing (Fig. 4A). Coronary sinus lactate concentration was significantly lower than arterial lactate concentration during rest (P= 0.007) and atrial pacing (P= 0.02). Arterial–coronary sinus lactate difference (Fig. 4B) was not significantly different from rest to atrial pacing. However, isotope tracer-measured net lactate uptake increased significantly from rest to atrial pacing (Fig. 4C, P= 0.006). The myocardium released lactate throughout the experiment, which increased significantly from rest to atrial pacing (Fig. 4D, P < 0.05). There was a significant relationship between myocardial lactate uptake and lactate release (r2= 0.34, P= 0.03). There were no significant differences in lactate fractional extraction during rest and atrial pacing with the myocardium extracting the bulk of lactate delivery (Fig. 4E). As a result of increased isotopically measured lactate uptake, the heart accounted for a significantly greater percentage of whole body lactate disposal during atrial pacing compared to rest (Fig. 4F, P= 0.03). The relative proportion of tracer-measured lactate uptake to myocardial oxygen consumption (OER) was 16.5 ± 3.3% at rest and 16.1 ± 4.9% during atrial pacing.
Figure 4.
Arterial and coronary sinus lactate concentration (A), arterial–coronary sinus lactate difference (B), and myocardial net lactate uptake (C), myocardial lactate release (D), myocardial lactate fractional extraction (E), and percentage of lactate Rd from myocardial lactate uptake (F) during rest and atrial pacing in men and women. Values are means ±s.e.m. § Significantly different from rest, P < 0.05. ¥ Significantly different from arterial, P < 0.05.
Discussion
We performed this study to test the hypothesis that without a change in arterial substrate concentration, myocardial glucose and lactate uptake increase from rest to atrial pacing, but account for a similar proportion of total substrate uptake by the heart. These data support our hypothesis and suggest myocardial glucose and lactate uptake increase proportionally with a moderate increase in work. Thus, our data suggest that in the working human heart in vivo there is no intrinsic alteration in myocardial substrate selection for glucose or lactate. Thus, alterations in myocardial substrate preferences during moderate whole body exercise are probably due to alterations in substrate delivery and sympathetic drive.
The current myocardial RQ data are similar to others (Camici et al. 1989) indicating preferential myocardial fat oxidation after an overnight fast that does not change significantly during atrial pacing (Table 2). The oxygen extraction ratios for glucose and lactate also indicate a similar contribution of each substrate to myocardial energy provision during rest and atrial pacing. A similar contribution of FFAs during rest and atrial pacing was also obtained using oxygen extraction ratios and [1-13C]palmitate uptake in this study (Bergman et al. 2009). These data suggest the heart receives the majority of energy provision from fat during rest after an overnight fast, which is not altered during moderate atrial pacing without a change in plasma substrate concentrations. In contrast, when whole body exercise is performed, myocardial substrate use in humans shifts to greater carbohydrate use (Gertz et al. 1988), probably due to increased lactate delivery and oxidation (Stanley, 1991). As exercise duration progresses and lactate concentration falls, there is a concomitant decrease in myocardial lactate uptake and increase in FFA uptake despite unchanged arterial FFA concentration (Gertz et al. 1988). The current data suggest there is no intrinsic alteration in myocardial substrate utilization during a moderate increase in heart work. Taken together, these data suggest arterial lactate concentration plays a central role in regulating myocardial substrate utilization during whole body exercise.
Myocardial glucose uptake increased significantly from rest to atrial pacing (Fig. 3C) due to increased coronary sinus blood flow (Fig. 1A), since there was no difference in fractional extraction (Fig. 3D). Our data are consistent with others who reported significant myocardial glucose uptake at rest which increases during atrial pacing (Camici et al. 1989; Neglia et al. 2007), or cycle ergometry (Gertz et al. 1988; Kemppainen et al. 2002). Glucose uptake roughly doubled from rest to atrial pacing in this study (Fig. 3C) which is quantitatively similar to previous reports (Gertz et al. 1988; Kemppainen et al. 2002). Only 20–25% of myocardial glucose uptake is oxidized in humans at rest (Wisneski et al. 1985b; Gertz et al. 1988), with approximately 13% released as lactate and the remainder probably stored as glycogen (Wisneski et al. 1985b). Gertz et al. (1988) reported the percentage of glucose extracted undergoing rapid oxidation increased during whole body cycle ergometry exercise to approximately 53%. Those data suggest that even during exercise, a proportion of myocardial glucose uptake is diverted to glycogen storage. Glucose and lactate uptake exceeded myocardial carbohydrate oxidation at rest and during atrial pacing in the current study. Although not directly measured, glycogen storage is likely to be the major fate of myocardial glucose uptake in excess of glucose oxidation. Since previous studies have shown that 85–100% of myocardial lactate uptake was oxidized in humans at rest (Gertz et al. 1981, 1988), it is likely that excess myocardial glucose uptake accounted for the majority of positive myocardial carbohydrate balance.
Other investigators reported tracer-measured myocardial lactate release in excess of glucose uptake (Gertz et al. 1988; Camici et al. 1989), consistent with intramyocardial glycogen degradation during increased heart work. We found myocardial lactate release at rest and during atrial pacing was less than net glucose uptake. These data are less suggestive of net glycogen degradation during a modest increase in heart work. Other investigators have speculated that glycogen contributes to myocardial energy provision during high, but not low intensity atrial pacing (Camici et al. 1989) and cycle ergometry (Gertz et al. 1988). However, only lactate release in excess of glucose uptake would be measured as ‘glycogen degradation’ with the methods used in this study. Therefore, we cannot rule out that glycogen degradation contributed to tracer-measured lactate release during atrial pacing.
Many other investigators have reported in both skeletal muscle (Stanley et al. 1986; Bergman et al. 1999b) and heart (Gertz et al. 1981; Wisneski et al. 1985a; Gertz et al. 1988; Neglia et al. 2007) that net tissue lactate uptake underestimates actual lactate uptake since lactate is released under fully aerobic conditions. Therefore, isotope methodology is required to quantify tissue lactate exchange (Gertz et al. 1981; Stanley et al. 1986). Using isotopes, significant myocardial lactate fractional extraction has been measured in humans in vivo (Gertz et al. 1981; Wisneski et al. 1985b; Gertz et al. 1988; Neglia et al. 2007). Our data are similar as we found 63% of lactate fractional extraction determined isotopically that was not significantly different during atrial pacing (Fig. 4E). Gertz et al. also found unchanged lactate fractional extraction from rest during supine cycle ergometry at 40% , despite an increase in arterial lactate concentration (Gertz et al. 1988). These data suggest lactate uptake increases with greater myocardial work due to increased delivery and not a change in fractional extraction.
, despite an increase in arterial lactate concentration (Gertz et al. 1988). These data suggest lactate uptake increases with greater myocardial work due to increased delivery and not a change in fractional extraction.
Similar to others (Stanley et al. 1988) we found that the heart accounted for a small percentage (4.9%) of whole body lactate uptake at rest (Fig. 4F). During greater heart work, increased lactate uptake by the heart could account for 15% of whole body lactate disposal (Fig. 4F). These data are similar to others (Stanley et al. 1988) who reported whole body lactate disposal from heart lactate uptake increased similarly during cycle ergometry at 40% . At rest, it is likely that gluconeogenic tissues are responsible for the majority of lactate disposal, which is displaced slightly during atrial pacing with greater myocardial lactate uptake.
. At rest, it is likely that gluconeogenic tissues are responsible for the majority of lactate disposal, which is displaced slightly during atrial pacing with greater myocardial lactate uptake.
Using isotopes we measured myocardial lactate release at rest, which increased during atrial pacing (Fig. 4D). Our results are similar to others who reported myocardial lactate release at rest and during exercise (Wisneski et al. 1985b; Gertz et al. 1988; Avogaro et al. 1990a). Our data are in contrast to a previous study (Kaijser & Berglund, 1992) reporting no lactate release in normal healthy human hearts at rest or during atrial pacing using [14C]lactate infusion and arterial–coronary sinus balance methods. It is not clear what caused these differences between our studies. Subjects in the Kaijser & Berglund study were 24 years old, while subjects in the present experiment were twice as old. Thus, we cannot rule out that myocardial lactate release is an ageing effect of the heart. However, other investigators reported myocardial lactate release using a younger population so this is less likely (Wisneski et al. 1985b; Gertz et al. 1988). Another possibility is that subjects in the current study had undiagnosed isolated regional cardiac ischaemia which promoted lactate release. However, our subjects had no history of heart disease, normal echocardiogram, left ventricular mass and  for their age, and normal ECG readings during the
for their age, and normal ECG readings during the 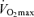 test. Therefore, we feel it is unlikely that myocardial ischaemia can explain these findings. The current study used a much longer isotopic equilibration time (60 min) before starting 30 min of resting measurements, compared to a 17 min equilibration in the Kaijser & Berglund study. Therefore, it is possible that lack of isotopic steady state influenced previous measures of myocardial lactate release (Kaijser & Berglund, 1992). Unchanged lactate release during atrial pacing was also reported by Neglia et al., but this result is hard to interpret since their control group had a history of angina and/or a stress test suggestive of myocardial ischaemia (Neglia et al. 2007). Our data are similar to those of Wisneski et al. who studied patients who underwent coronary angiography for chest pain, but who did not develop clinical symptoms of ischaemia during atrial pacing (Wisneski et al. 1985a). They found that isotopically measured lactate release more than doubled from rest to atrial pacing. Our data corroborate these findings and suggest that in the absence of ischaemia the myocardium is constantly releasing lactate at rest, which increases during atrial pacing.
test. Therefore, we feel it is unlikely that myocardial ischaemia can explain these findings. The current study used a much longer isotopic equilibration time (60 min) before starting 30 min of resting measurements, compared to a 17 min equilibration in the Kaijser & Berglund study. Therefore, it is possible that lack of isotopic steady state influenced previous measures of myocardial lactate release (Kaijser & Berglund, 1992). Unchanged lactate release during atrial pacing was also reported by Neglia et al., but this result is hard to interpret since their control group had a history of angina and/or a stress test suggestive of myocardial ischaemia (Neglia et al. 2007). Our data are similar to those of Wisneski et al. who studied patients who underwent coronary angiography for chest pain, but who did not develop clinical symptoms of ischaemia during atrial pacing (Wisneski et al. 1985a). They found that isotopically measured lactate release more than doubled from rest to atrial pacing. Our data corroborate these findings and suggest that in the absence of ischaemia the myocardium is constantly releasing lactate at rest, which increases during atrial pacing.
There are several limitations in this study. We did not observe an increase in cardiac output during atrial pacing compared to the resting state. Therefore, atrial pacing increases myocardial oxygen consumption and does not influence arterial concentration, but it is not a completely physiological model since increased heart work is performed without an increase in cardiac output. Additionally, it is not known if our data would be similar with a more dramatic increase in myocardial work induced by a greater atrial pacing heart rate. During this study, we infused [1-13C]palmitate to simultaneously measure myocardial palmitate uptake and oxidation, which is reported separately (Bergman et al. 2009). Therefore, we needed to use a non-carbon label to simultaneously measure lactate metabolism. We chose [3,3,3-2H]lactate because it does not recycle, which was required to be able to simultaneously measure glucose uptake and turnover. However, there is concern that deuterated lactate labels over-estimate whole body lactate disappearance compared with carbon lactate labels (Shiota et al. 1984). To the extent that [3,3,3-2H]lactate overestimates whole body lactate turnover, this would decrease the calculated proportion of myocardial lactate uptake to whole body lactate disappearance. Finally, we infused a small amount of heparin to maintain patency of the arterial line in each study. To mobilize lipoprotein lipase, which would increase FFA concentration, a heparin bolus of approximately 60 units kg−1, or 4400 units would be required for the average subject in this study (Imamura et al. 2008). We only infused 15 units per hour, and as a result did not observe changes in FFA concentration outside the normal increase with increased duration of fasting.
The determination of glucose and lactate substrate utilization by the human myocardium under a variety of physiological conditions has important clinical relevance to disease states such as heart failure, diabetes and coronary artery disease. In patients with heart failure, increased plasma FFA and decreased carbohydrate oxidation increase the oxygen cost of metabolism over preferential use of glucose and lactate (Lommi et al. 1998). Pharmacological approaches to increase glucose utilization in heart failure have successfully enhanced pyruvate oxidation, increased stroke volume and stroke work, and increased left ventricle (LV) mechanical efficiency (Bersin et al. 1994; Bersin & Stacpoole, 1997; Nikolaidis et al. 2005). Patients in chronic heart failure can develop insulin resistance, thus limiting the uptake of glucose and thereby influencing myocardial metabolism (Ashrafian et al. 2007). The importance of glucose and lactate utilization in patients with cardiac dysfunction remains unclear; however, the pathophysiology of myocardial dysfunction is associated with abnormalities in myocardial glucose metabolism. Substrate utilization along with oxidative phosphorylation and ATP transfer and utilization are major factors determining myocardial metabolism and the effect on cardiac contractile function, especially under conditions of enhanced adrenergic stimulation such as exercise, heart failure, myocardial ischaemia and poorly controlled diabetes. Thus, determining the fate of glucose and lactate in the normal human myocardium under a variety of physiological conditions is an important step in pursuing the metabolic derangements and potential therapeutic approaches in myocardial disease.
To conclude, our data suggest that increased heart work in humans in vivo without a change in arterial substrate concentration results in a similar proportion of glucose and lactate uptake relative to myocardial energy demands. Based on myocardial RQ and the relative contributions of glucose and lactate to total myocardial energy expenditure, the relative proportion of myocardial fat and carbohydrate oxidation did not change significantly during atrial pacing compared to rest. These data also show myocardial lactate release during rest and atrial pacing despite isotopically measured lactate uptake. Therefore, in the absence of ischaemia the myocardium is constantly releasing lactate at rest which increases during atrial pacing.
Acknowledgments
This work was partially supported by the National Institutes of Health General Clinical Research Center grant RR-00036, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants to B. C. Bergman (DK-059739), and a National Institutes of Health Clinical Nutrition Research Unit pilot project grant from UCHSC. We would also like to thank Ronald Zolty, MD, for his assistance during some of the invasive metabolic studies.
References
- Aguilar-Parada E, Eisentraut AM, Unger RH. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci. 1969;257:415–419. doi: 10.1097/00000441-196906000-00008. [DOI] [PubMed] [Google Scholar]
- Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation. 2007;116:434–448. doi: 10.1161/CIRCULATIONAHA.107.702795. [DOI] [PubMed] [Google Scholar]
- Avogaro A, Nosadini R, Doria A, Fioretto P, Velussi M, Vigorito C, Sacca L, Toffolo G, Cobelli C, Trevisan R, Duner E, Razzolini R, Crepaldi G. Myocardial metabolism in insulin-deficient diabetic humans without coronary artery disease. Am J Physiol Endocrinol Metab. 1990;258:E606–E618. doi: 10.1152/ajpendo.1990.258.4.E606. [DOI] [PubMed] [Google Scholar]
- Avogaro A, Nosadini R, Doria A, Fioretto P, Velussi M, Vigorito C, Sacca L, Toffolo G, Cobelli C, Trevisan R. Myocardial metabolism in insulin-deficient diabetic humans without coronary artery disease. Am J Physiol Endocrinol Metab. 1990;258:E606–E618. doi: 10.1152/ajpendo.1990.258.4.E606. [DOI] [PubMed] [Google Scholar]
- Bergman BC, Butterfield GE, Wolfel EE, Lopaschuk GD, Casazza GA, Horning MA, Brooks GA. Muscle net glucose uptake and glucose kinetics after endurance training in men. Am J Physiol Endocrinol Metab. 1999;277:E81–E92. doi: 10.1152/ajpendo.1999.277.1.E81. [DOI] [PubMed] [Google Scholar]
- Bergman BC, Tsvetkova T, Lowes B, Wolfel EE. Myocardial FFA metabolism during rest and atrial pacing in humans. Am J Physiol Endocrinol Metab. 2009;296:E358–E366. doi: 10.1152/ajpendo.90747.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman BC, Wolfel EE, Butterfield GE, Lopaschuk GD, Casazza GA, Horning MA, Brooks GA. Active muscle and whole body lactate kinetics after endurance training in men. J Appl Physiol. 1999;87:1684–1696. doi: 10.1152/jappl.1999.87.5.1684. [DOI] [PubMed] [Google Scholar]
- Bersin RM, Stacpoole PW. Dichloroacetate as metabolic therapy for myocardial ischemia and failure. Am Heart J. 1997;134:841–855. doi: 10.1016/s0002-8703(97)80007-5. [DOI] [PubMed] [Google Scholar]
- Bersin RM, Wolfe C, Kwasman M, Lau D, Klinski C, Tanaka K, Khorrami P, Henderson GN, de Marco T, Chatterjee K. Improved hemodynamic function and mechanical efficiency in congestive heart failure with sodium dichloroacetate. J Am Coll Cardiol. 1994;23:1617–1624. doi: 10.1016/0735-1097(94)90665-3. [DOI] [PubMed] [Google Scholar]
- Bing RJ, Siegel A, Ungar I, Gilbert M. Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism. Am J Med. 1954;16:504–515. doi: 10.1016/0002-9343(54)90365-4. [DOI] [PubMed] [Google Scholar]
- Bing RJ, Siegel A, Vitale A, Balboni F, Sparks E, Taeschler M, Klapper M, Edwards S. Metabolic studies on the human heart in vivo. I. Studies on carbohydrate metabolism of the human heart. Am J Med. 1953;15:284–296. doi: 10.1016/0002-9343(53)90082-5. [DOI] [PubMed] [Google Scholar]
- Burkhoff D, Weiss RG, Schulman SP, Kalil-Filho R, Wannenburg T, Gerstenblith G. Influence of metabolic substrate on rat heart function and metabolism at different coronary flows. Am J Physiol Heart Circ Physiol. 1991;261:H741–H750. doi: 10.1152/ajpheart.1991.261.3.H741. [DOI] [PubMed] [Google Scholar]
- Camici P, Marraccini P, Marzilli M, Lorenzoni R, Buzzigoli G, Puntoni R, Boni C, Bellina CR, Klasen GA, L’Abbate A, Ferrannini E. Coronary hemodynamics and myocardial metabolism during and after pacing stress in normal humans. Am J Physiol Endocrinol Metab. 1989;257:E309–E317. doi: 10.1152/ajpendo.1989.257.3.E309. [DOI] [PubMed] [Google Scholar]
- Causon RC, Carruthers ME, Rodnight R. Assay of plasma catecholamines by liquid chromatography with electrochemical detection. Anal Biochem. 1981;116:223–226. doi: 10.1016/0003-2697(81)90347-x. [DOI] [PubMed] [Google Scholar]
- Collins-Nakai RL, Noseworthy D, Lopaschuk GD. Epinephrine increases ATP production in hearts by preferentially increasing glucose metabolism. Am J Physiol Heart Circ Physiol. 1994;267:H1862–H1871. doi: 10.1152/ajpheart.1994.267.5.H1862. [DOI] [PubMed] [Google Scholar]
- Crass MF, 3rd, McCaskill ES, Shipp JC. Effect of pressure development on glucose and palmitate metabolism in perfused heart. Am J Physiol. 1969;216:1569–1576. doi: 10.1152/ajplegacy.1969.216.6.1569. [DOI] [PubMed] [Google Scholar]
- Douglas A, Jones N, Reed J. Calculation of whole blood CO2 content. J Appl Physiol. 1988;65:473–477. doi: 10.1152/jappl.1988.65.1.473. [DOI] [PubMed] [Google Scholar]
- Friedlander AL, Casazza GA, Horning MA, Huie MJ, Brooks GA. Training-induced alterations of glucose flux in men. J Appl Physiol. 1997;82:1360–1369. doi: 10.1152/jappl.1997.82.4.1360. [DOI] [PubMed] [Google Scholar]
- Gertz EW, Wisneski JA, Neese RA, Bristow JD, Searle GL, Hanlon JT. Myocardial lactate metabolism: evidence of lactate release during net chemical extraction in man. Circulation. 1981;63:1273–1279. doi: 10.1161/01.cir.63.6.1273. [DOI] [PubMed] [Google Scholar]
- Gertz EW, Wisneski JA, Stanley WC, Neese RA. Myocardial substrate utilization during exercise in humans: dual carbon-labeled carbohydrate isotope experiments. J Clin Invest. 1988;82:2017–2025. doi: 10.1172/JCI113822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GW, Ahmad F, Doenst T, Taegtmeyer H. Energy provision from glycogen, glucose, and fatty acids on adrenergic stimulation of isolated working rat hearts. Am J Physiol Heart Circ Physiol. 1998;274:H1239–H1247. doi: 10.1152/ajpheart.1998.274.4.H1239. [DOI] [PubMed] [Google Scholar]
- Goodwin GW, Taylor CS, Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem. 1998;273:29530–29539. doi: 10.1074/jbc.273.45.29530. [DOI] [PubMed] [Google Scholar]
- Imamura S, Kobayashi J, Nakajima K, Sakasegawa S, Nohara A, Noguchi T, Kawashiri MA, Inazu A, Deeb SS, Mabuchi H, Brunzell JD. A novel method for measuring human lipoprotein lipase and hepatic lipase activities in postheparin plasma. J Lipid Res. 2008;49:1431–1437. doi: 10.1194/jlr.M700528-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaijser L, Berglund B. Myocardial lactate extraction and release at rest and during heavy exercise in healthy men. Acta Physiol Scand. 1992;144:39–45. doi: 10.1111/j.1748-1716.1992.tb09265.x. [DOI] [PubMed] [Google Scholar]
- Kaijser L, Lassers BW, Wahlqvist ML, Carlson LA. Myocardial lipid and carbohydrate metabolism in fasting men during prolonged exercise. J Appl Physiol. 1972;32:847–858. doi: 10.1152/jappl.1972.32.6.847. [DOI] [PubMed] [Google Scholar]
- Kantor PF, Dyck JR, Lopaschuk GD. Fatty acid oxidation in the reperfused ischemic heart. Am J Med Sci. 1999;318:3–14. doi: 10.1097/00000441-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Kelman GR. Digital computer procedure for the conversion of PCO2 into blood CO2 content. Respir Physiol. 1967;3:111–115. doi: 10.1016/0034-5687(67)90028-x. [DOI] [PubMed] [Google Scholar]
- Kemppainen J, Fujimoto T, Kalliokoski KK, Viljanen T, Nuutila P, Knuuti J. Myocardial and skeletal muscle glucose uptake during exercise in humans. J Physiol. 2002;542:403–412. doi: 10.1113/jphysiol.2002.018135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassers BW, Kaijser L, Carlson LA. Myocardial lipid and carbohydrate metabolism in healthy, fasting men at rest: studies during continuous infusion of 3H-palmitate. Eur J Clin Invest. 1972;2:348–358. doi: 10.1111/j.1365-2362.1972.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Lommi J, Kupari M, Yki-Jarvinen H. Free fatty acid kinetics and oxidation in congestive heart failure. Am J Cardiol. 1998;81:45–50. doi: 10.1016/s0002-9149(97)00804-7. [DOI] [PubMed] [Google Scholar]
- Neely JR, Bowman RH, Morgan HE. Effects of ventricular pressure development and palmitate on glucose transport. Am J Physiol. 1969;216:804–811. doi: 10.1152/ajplegacy.1969.216.4.804. [DOI] [PubMed] [Google Scholar]
- Neely JR, Whitmer M, Mochizuki S. Effects of mechanical activity and hormones on myocardial glucose and fatty acid utilization. Circ Res. 1976;38:I22–I30. [PubMed] [Google Scholar]
- Neglia D, De Caterina A, Marraccini P, Natali A, Ciardetti M, Vecoli C, Gastaldelli A, Ciociaro D, Pellegrini P, Testa R, Menichetti L, L’Abbate A, Stanley WC, Recchia FA. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2007;293:H3270–H3278. doi: 10.1152/ajpheart.00887.2007. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Doverspike A, Hentosz T, Zourelias L, Shen YT, Elahi D, Shannon RP. Glucagon-like peptide-1 limits myocardial stunning following brief coronary occlusion and reperfusion in conscious canines. J Pharmacol Exp Ther. 2005;312:303–308. doi: 10.1124/jpet.104.073890. [DOI] [PubMed] [Google Scholar]
- Nunn JF. Nunn's Applied Respiratory Physiology. Boston: Butterworth-Heineman; 1993. [Google Scholar]
- Opie LH. Metabolism of the heart in health and disease. I. Am Heart J. 1968;76:685–698. doi: 10.1016/0002-8703(68)90168-3. [DOI] [PubMed] [Google Scholar]
- Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–2196. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- Shiota M, Golden S, Katz J. Lactate metabolism in the perfused rat hindlimb. Biochem J. 1984;222:281–292. doi: 10.1042/bj2220281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley WC. Myocardial lactate metabolism during exercise. Med Sci Sport Ex. 1991;23:920–924. [PubMed] [Google Scholar]
- Stanley WC, Gertz EW, Wisneski JA, Neese RA, Morris DL, Brooks GA. Lactate extraction during net lactate release in legs of humans during exercise. J Appl Physiol. 1986;60:1116–1120. doi: 10.1152/jappl.1986.60.4.1116. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res. 1997;34:25–33. doi: 10.1016/s0008-6363(97)00047-3. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Neese RA, Wisneski JA, Gertz EW. Lactate kinetics during exercise in humans: studies with isotopic tracers. J Cardiopulmonary Rehabil. 1988;8:331–340. [Google Scholar]
- Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- Wide L, Porath J. Radioimmunoassay of proteins with the use of Sephadex-coupled antibodies. Biochim Biophys Acta. 1966;130:257–260. [Google Scholar]
- Wier JBdeV. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisneski JA, Gertz EW, Neese RA, Gruenke LD, Craig JC. Dual carbon-labeled isotope experiments using d−[6-14C] glucose and l−[1,2,3-13C3] lactate: a new approach for investigating human myocardial metabolism during ischemia. J Am Coll Cardiol. 1985;5:1138–1146. doi: 10.1016/s0735-1097(85)80016-4. [DOI] [PubMed] [Google Scholar]
- Wisneski JA, Gertz EW, Neese RA, Gruenke LD, Morris DL, Craig JC. Metabolic fate of extracted glucose in normal human myocardium. J Clin Invest. 1985;76:1819–1827. doi: 10.1172/JCI112174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe R. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss; 1992. [Google Scholar]






