Abstract
Classic neurotransmitter phenotypes are generally predetermined and develop as a consequence of target-independent lineage decisions. A unique mode of target-dependent phenotype instruction is the acquisition of the cholinergic phenotype in the peripheral sympathetic nervous system. A body of work suggests that the sweat gland plays an important role to determine the cholinergic phenotype at this target site. A key issue is whether neurons destined to innervate the sweat glands express cholinergic markers before or only after their terminals make target contact. We employed cholinergic-specific over-expression of the vesicular acetylcholine transporter (VAChT) in transgenic mice to overcome sensitivity limits in the detection of initial cholinergic sweat gland innervation. We found that VAChT immunoreactive nerve terminals were present around the sweat gland anlage already from the earliest postnatal stages on, coincident selectively at this sympathetic target with tyrosine hydroxylase–positive fibers. Our results provide a new mechanistic model for sympathetic neuron–target interaction during development, with initial selection by the target of pioneering nerve terminals expressing a cholinergic phenotype, and subsequent stabilization of this phenotype during development.
Keywords: acetylcholine, development, transgene, vesicular transporter
Noradrenaline is the principal neurotransmitter in the mammalian peripheral sympathetic nervous system (Elfvin et al., 1993). However, some 4% (Masliukov and Timmermans, 2004) of postganglionic neurons in most of the paravertebral sympathetic chain ganglia utilize acetylcholine (ACh) instead of noradrenaline. One target organ of these cholinergic neurons is the eccrine sweat glands in the skin. A functional cholinergic innervation of sweat glands is a prerequisite to produce and secrete sweat in most mammalian species, and sweating can be potently blocked by anti-cholinergic agents, e.g. atropine (Stevens and Landis, 1987).
In addition to the cholinergic phenotype, which is characterized by the expression of choline acetyltransferase (ChAT), the ACh synthesizing enzyme, and the vesicular acetylcholine transporter (VAChT), the protein needed to package ACh into small synaptic vesicles (Eiden et al., 2004), the so-called sudomotor fibers variably express a full (e.g. human), or incomplete (i.e. vesicular monoamine transporter 2–deficient; e.g. rodents) noradrenergic co-phenotype (Weihe et al., 2005).
How and when cholinergic sudomotor neurons are generated during development in rodents has been studied extensively for almost two decades. Despite considerable insight into this critical developmental process, key features of cholinergic sudomotor neuronal development are still open for debate. It has been hypothesized that cholinergic sympathetic innervation of sweat glands in rat and mouse is the consequence of a postnatal switch from a fully functional noradrenergic phenotype to a cholinergic phenotype under the influence of target-derived factors (Francis and Landis, 1999). On the other hand some cholinergic sympathetic neurons are generated prenatally and hence long before target innervation, i.e. are target-independent (Schafer et al., 1997). The central question therefore is whether sympathetic neurons destined to innervate the sweat gland anlage express cholinergic markers before or only after they have connected with their target (Burau et al., 2004; Francis and Landis, 1999; Schafer et al., 1997; Stanke et al., 2006; Weihe and Eiden, 2000).
To prove our concept of the target-independent acquisition of cholinergic sweat gland innervation during development required a method to overcome detection sensitivity issues inherent to the presumed low-level expression of cholinergic proteins in sudomotor nerve terminals during the initial stages of target formation and innervation. We addressed this issue by generating transgenic mouse lines that over-expressed VAChT in cholinergic neurons. However, all transgenic attempts that used up to 11.2 kb of sequence from the human cholinergic gene locus (CGL), although resulting in an enhanced VAChT expression in central cholinergic nuclei, failed to do so in neurons of the peripheral nervous system (Schutz et al., 2000, 2003). Faithful transgene expression, not only for the CGL (Eiden, 1998) but also for a number of other mammalian genes, requires regulatory information often found tens of kilobases upstream and downstream of the coding sequences. Thus, mice harboring modified bacterial artificial chromosomes (BAC) have recently become the transgenic system of choice for the analysis of mammalian gene expression (Heintz, 2001). To facilitate the detection of protein products of the CGL by their over-expression in a tissue-appropriate fashion, BAC transgenic mice that expressed enhanced green fluorescent protein (EGFP) under the control of the ChAT promoter were generated (von Engelhardt et al., 2007). EGFP expression was observed in all central areas known to contain cholinergic neurons, e.g. cortex, basal forebrain, brain stem, and spinal cord, with a cellular distribution faithfully copying the cholinergic phenotype. However, the EGFP expression pattern in the peripheral nervous system was not further analyzed.
Here, we asked if EGFP expression also mimics peripheral cholinergic expression in parasympathetic, sympathetic and intestinal intrinsic neurons, establishing that this transgene is an appropriate model for the study of the structural requirements for cholinergic expression in the peripheral nervous system of adult mice as well. Moreover, we analyzed if also the expression of the VAChT gene is enhanced in these mice, resulting from its expression from both the two endogenous alleles and the multiple BAC copies. Aided by the increased VAChT expression at the mRNA and protein levels, we discovered a cholinergic/noradrenergic co-phenotype in pioneering nascent sweat gland innervation, already present before and during target formation.
EXPERIMENTAL PROCEDURES
Mouse breeding, genotyping, tissue harvesting and processing
ChAT-EGFP transgenic mice (von Engelhardt et al., 2007) and non-transgenic siblings were housed in groups of three to five in single ventilated cages (Ehret, Emmendingen, Germany) under specific pathogen-free conditions. They were kept on a 12-h light/dark cycle and had access to food and water ad libitum. Care of the animals and conduct of all experiments followed the guidelines of the German Animal Protection Law (issued in 2006) and were approved by the Regierungspräsidium Gieβen. Hemizygous transgenic mice were mated with wild type C57BL/6 mice to obtain litters for experimental analysis. The identification of transgenic offspring was performed by PCR for the transgene-encoded EGFP (von Engelhardt et al., 2007).
For the immediate detection of native EGFP fluorescence, mice were killed by carbon dioxide intoxication and pieces from skeletal muscle, trachea, sympathetic ganglia, and small intestine squeezed between objective slides and coverslips. For the detection of mouse VAChT transcripts by in situ hybridization histochemistry (ISH), pups and 2 to 4-month-old mice were killed by carbon dioxide intoxication, then whole embryos and pups, or selected tissue from 2 to 4-month-old mice (males and females) was quickly dissected, snap-frozen in −40 °C cold isopentane, and processed as described before (Schafer et al., 1997). For immunohistochemical procedures, embryos (E15–E19), pups (P1–P8), and selected tissues from 2 to 4-month-old mice (males and females) were quickly dissected, embedded in paraffin, and sectioned (Schafer et al., 1997).
ISH
A complementary RNA probe for the detection of mouse VAChT transcripts in tissue sections was generated from mouse C57BL/6 brainstem cDNA. A 755 nucleotide long DNA fragment (GenBank acc. no. NM_021712, nucleotides 1021−1775) was amplified by PCR, subcloned into pGEM-T (Promega), and the sequence confirmed by double-stranded sequencing. Gene-specific transcripts were generated using SP6 (for antisense probe) and T7 (for sense probe) RNA polymerases. The ISH procedure was essentially performed as described in detail earlier (Schafer et al., 1997; Schutz et al., 2000).
Semiquantitative real-time reverse transcriptase–polymerase chain reaction (RT-PCR)
Single pieces from lumbar spinal cord, and stellate ganglia (pooled from one animal) from wild type and hemizygous ChAT-EGFP transgenic mice were quickly dissected, frozen in liquid nitrogen and stored at −70 °C. Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) and dissolved in water. The integrity of the RNA was analyzed by gel electrophoresis. Contaminating genomic DNA was removed by digesting 2 μg of total RNA for 15 min at room temperature with DNase I (Invitrogen). The RNA was reverse transcribed into cDNA using Superscript II RT (Invitrogen). To check for the complete absence of contaminating genomic DNA, a non-RT reaction was included for each sample. In all subsequent PCR reactions, 1 μl of cDNA sample was used.
Gene-specific primer pairs were selected with Primer Express™ 1.0 software (PE Applied Biosystems, Foster City, CA, USA). For the detection of mouse VAChT transcripts, a forward primer, 5′-CGT GAC CCC TCC CAG TCT AA-3′, was used in combination with a reverse primer, 5′-ATC ACC GGT ACA CCT CTG CC-3′, resulting in the amplification of a 101 bp fragment from the 3-prime non-coding region of the VAChT gene (GenBank acc. No. NM_021712, nt. 2176−2276). A β-actin primer set was used for normalization and consisted of a forward primer, 5′-AGC TTC TTT GCA GCT CCT TCG-3′, and a reverse primer, 5′-AGG GTC AGG ATA CCT CTC TTG CT-3′, generating a 251 bp amplicon from exons 1−3 (GenBank acc. No. NM_007393, nt. 30−280). An interleukin-2 (IL2) primer set was used for normalization of VAChT signals in genomic DNA from tail biopsies and consisted of a forward primer, 5′-CTA GGC CAC AGA ATT GAA AGA TCT-3′, and a reverse primer, 5′-GTA GGT GGA AAT TCT AGC ATC ATC C-3′, resulting in a 324 bp amplicon (GenBank acc. No. NT_162143.2).
Semiquantitative PCR analysis was performed with a MX3000P device (Stratagene, Amsterdam, The Netherlands), using SYBR Green as fluorescent dye for signal detection, as previously described (Schutz et al., 2004). Before analysis of experimental samples, the optimal primer concentrations were determined using a range of different concentration combinations. The forward/reverse primer concentrations resulting from these tests were 300/300 nM for VAChT, 900/300 nM for β-actin, and 300/300 nM for IL2. In addition, several fourfold dilutions of reverse-transcribed spinal cord cDNA from wild type mice were analyzed to check for linear amplification efficiency of VAChT and β-actin under experimental conditions. All PCR reactions were performed using SYBR Green Jumpstart Taq Ready Mix (Sigma-Aldrich). Amplification conditions were: 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s, and 55 °C for 30 s. Following PCR, a dissociation curve analysis confirmed the amplification of a single product.
Fluorescent PCR signals were normalized to the passive internal reference dye, ROX, and a threshold cycle (CT) was determined within the exponential phase of the reaction. Each PCR reaction was performed in triplicate, and the mean CT value calculated. Relative abundances of VAChT transcripts in a given tissue were calculated as differences in CT between β-actin and VAChT (ΔCT=CTVAChT–CTβ-actin) in individual samples and expressed as percentage of β-actin expression by using the equation “Expression [% β-actin]=2−ΔCT×100.” Data from all samples of an analysis group were averaged and expressed as mean±standard error.
Antibodies
Mouse VAChT was detected using a previously characterized polyclonal antiserum from rabbit (VAChT 80259; 1:1000 diluted) (Weihe et al., 1996). TH was detected using a polyclonal antiserum from sheep (1:800 diluted; Chemicon, Temecula, CA, USA). Details about controls for the specificity of immunoreactions for the antisera employed in this study have been summarized recently in detail by our group (Weihe et al., 2005).
Immunohistochemistry
Paraffin-embedded sections mounted on microscopic slides were deparaffinized with xylene, rehydrated through a graded series of 2-propanol, incubated in methanol/hydrogen peroxide to block endogenous peroxidase activity and then incubated in 10 mM sodium citrate buffer (pH 6.0) at 95 °C for 10 min for antigen retrieval. Unspecific antibody binding was blocked by incubation in 5% bovine serum albumin (BSA)/50 mM PBS (pH 7.4) for 1 h. For single-labeling brightfield immunohistochemistry, primary antibodies were applied in 1% BSA/PBS and incubated at 16 °C overnight followed by 2 h at 37 °C. Antibody concentration were 1:1000 for VAChT, and 1:800 for TH. After 3×10 min wash in PBS, sections were incubated with species-specific biotinylated secondary antibodies (Dianova, Hamburg, Germany; diluted 1:500 in 1% BSA/PBS) for 45 min at 37 °C, and the antigen–antibody complexes visualized with the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA), using diaminobenzidine/nickel as substrate. For confocal double-immunofluorescence microscopy, a combination of the two primary antibodies was co-applied in 1% BSA/PBS, as described (Weihe et al., 2005, 2006). Incubation was carried out overnight at 16 °C, followed by 2 h at 37 °C. After extensive washing with 1% BSA/PBS over 30 min, immunoreactions were visualized for the first primary antibody by species-specific secondary antibodies labeled with Alexa Fluor 647 (MoBiTec, Göttingen, Germany; dilution 1:200 in 1% BSA/PBS) and for the second primary antibody by species-specific biotinylated secondary antisera (Dianova; diluted 1:200 in 1% BSA/PBS) followed by streptavidin-conjugated Alexa Fluor 488 (1:200 in 1% BSA/PBS). All secondary antibodies and streptavidin conjugates were applied for 2 h at 37 °C. Sections were extensively washed before they were coverslipped with FluorSave reagent (Calbiochem, Merck Biosciences, Schwalbach, Germany). Immunofluorescence staining was documented as digitized false-color images (eight-bit Tiff format) obtained with an Olympus BX50WI laser scanning microscope (Olympus Optical, Hamburg, Germany) and Olympus Fluoview 2.1 software. Adobe Photoshop 9.0 CS2 software was used to compose and label the plates from single Tiff images without manipulations of contrast or brightness.
RESULTS
VAChT mRNA is up-regulated in cholinergic neurons of adult ChAT-EGFP transgenic mice
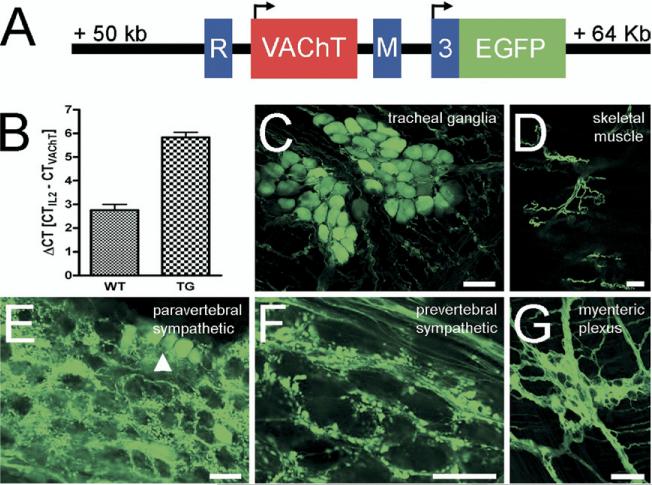
Forepaw sweat glands are innervated by postganglionic sympathetic neurons originating in the stellate ganglion (SG) (Anderson et al., 2006). Tyrosine hydroxylase (TH)–positive nerve fibers have been found to be the first sympathetic fibers appearing around the forming forepaw sweat glands at postnatal day (P) 2 in rat (Guidry and Landis, 1998). The first cholinergic nerve fibers displaying immunoreactivity for VAChT have been detected at P4 (Schafer et al., 1997; Guidry and Landis, 1998). The failure to detect cholinergic nerve fibers around forming sweat gland coils as early as seen with TH could be due to absence of cholinergic expression at the level of CGL expression, or merely to a low-level expression of cholinergic proteins at this developmental time point. To overcome potential sensitivity issues that would obscure the second of these two possibilities, we took advantage of BAC transgenic mice that express EGFP under the control of the ChAT promoter (Fig. 1A) specifically in cholinergic neurons in both the central (von Engelhardt et al., 2007) and peripheral nervous system. Hemizygous ChAT-EGFP mice that contain eight BAC copies (Fig. 1B) displayed intense EGFP fluorescence in parasympathetic, sympathetic and gut intrinsic neurons, nerve fibers and terminals (Fig. 1C–G).
Fig. 1.
EGFP is expressed in a cholinergic-specific manner from the CGL in ChAT-EGFP transgenic mice. (A) Schematic representation of the core region from the CGL, as modified in the ChAT-EGFP BAC clone. The EGFP coding sequence (green color) is integrated into the first ChAT coding exon (no. 3, blue color). Two additional, 3-prime non-coding ChAT exons (R and M) flank a single VAChT-encoding exon (red color). Translation start sites for the two genes are marked by arrows. (B) Estimation of the VAChT, and hence BAC copy number in hemizygous transgenic mice by qPCR. In wild type (WT) mice, a ΔCT=2.75±0.25 (n=5) equals two VAChT copies per diploid genome. In hemizygous ChAT-EGFP transgenic mice, a ΔCT=5.83±0.23 (n=7) equals additional eight transgene copies. (C–G) Examples for EGFP fluorescence in native preparations from selected authentic cholinergic peripheral nerves. Parasympathetic tracheal ganglia (C), motor nerves in skeletal muscle (D), post-ganglionic cholinergic neurons (arrowhead) and pre-ganglionic nerve terminals in a paravertebral sympathetic ganglion (E), pre-ganglionic nerve terminals in a prevertebral sympathetic ganglion (F), and the myenteric plexus (G) display EGFP fluorescence. Scale bars=50 μm.
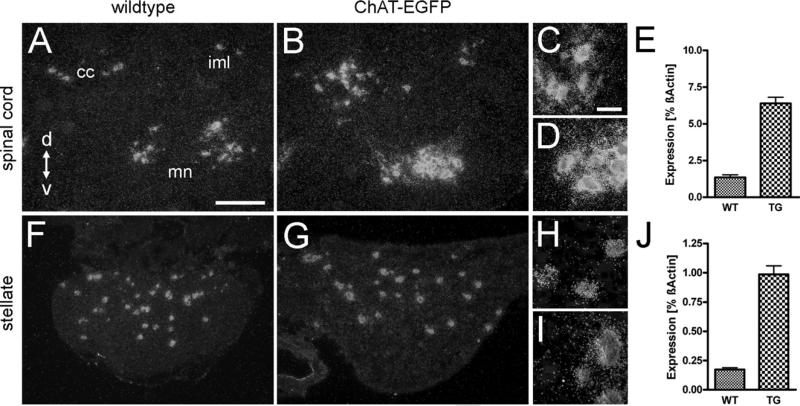
From this expression pattern and the close genomic interdigitation of the ChAT and VAChT genes (Eiden, 1998) it could be inferred that the transgene also contained all necessary cis-regulatory elements for additional expression of the embedded VAChT gene, thereby enhancing VAChT detectability in cell bodies and nerve endings. Thus, we analyzed adult hemizygous ChAT-EGFP transgenic mice by ISH and semi-quantitative RT-PCR for VAChT mRNA expression levels in the spinal cord and in the SG (Fig. 2). In the lumbar spinal cord, pre-ganglionic sympathetic neurons located in the intermediolateral cell column (lamina VII), ventral horn motor neurons (lamina IX), and neurons around the central canal (lamina X), all showed robust VAChT ISH signals in wild type mice (Fig. 2A, C). In ChAT-EGFP transgenic mice (Fig. 2B, D), VAChT ISH signals appeared more intense than in wild type. Estimation of VAChT mRNA levels by RT-PCR revealed an approximately fivefold higher level in transgenic (6.39±0.42; n=13) compared with wild type (1.351±0.185; n=8) mice (Fig. 2E).
Fig. 2.
VAChT mRNA is up-regulated in adult ChAT-EGFP transgenic mice. Darkfield microphotographs of VAChT ISH signals (left three columns), and changes in VAChT mRNA expression levels determined by RT-PCR (right column) from wild type (WT) and ChAT-EGFP transgenic (TG) mice. (A) Transverse section through upper lumbar spinal cord. VAChT mRNA expressing neurons are around the central canal (cc), in the intermediolateral cell column (iml), and the ventral horn motor neuron area (mn). Scale bar=100 μm (also applying to B, F, and G). d=Dorsal, v=ventral. (B) Comparable section to (A) from a TG mouse, showing intensification of VAChT ISH signals as compared with WT. (C) Higher magnification of motor neuron area from (A). Scale bar=25 μm (also applying to D, H, and I). (D) Higher magnification of motor neuron area from (B). (E) VAChT mRNA is up-regulated fivefold in spinal cord of TG compared with WT mice. (F) SG from a WT mouse. Several neurons exhibit VAChT ISH signals. (G) SG from a ChAT-EGFP mouse. ISH signal intensities for VAChT are increased as compared with WT. (H, I) Higher magnification from (F) and (G), respectively. (J) VAChT mRNA is up-regulated fivefold in TG SG as compared with WT.
About 4% of all SG neurons are cholinergic (Masliukov and Timmermans, 2004) in mice and have major projections to sweat glands (Schafer et al., 1998; Ernsberger and Rohrer, 1999), and periost (Asmus et al., 2000). Both in wild type (Fig. 2F+H) and ChAT-EGFP transgenic mice (Fig. 2G+I), a comparable number of these neurons were detectable by VAChT ISH. However, VAChT mRNA signals were found to be five- to sixfold greater in transgenic (0.987±0.073; n=4) than in wild type mice (0.173±0.015; n=4) mice (Fig. 2J).
Enhanced VAChT mRNA expression levels were also observed in other classic cholinergic neurons in the peripheral nervous system in ChAT-EGFP mice as compared with wild type, e.g. parasympathetic and intestine, but were never seen ectopically at non-cholinergic neuronal and neuroendocrine sites, e.g. adrenal medulla, and pre-vertebral sympathetic ganglia (data not shown).
VAChT immunoreactivity is intensified in the SG and sweat gland terminal fields of adult ChAT-EGFP transgenic mice
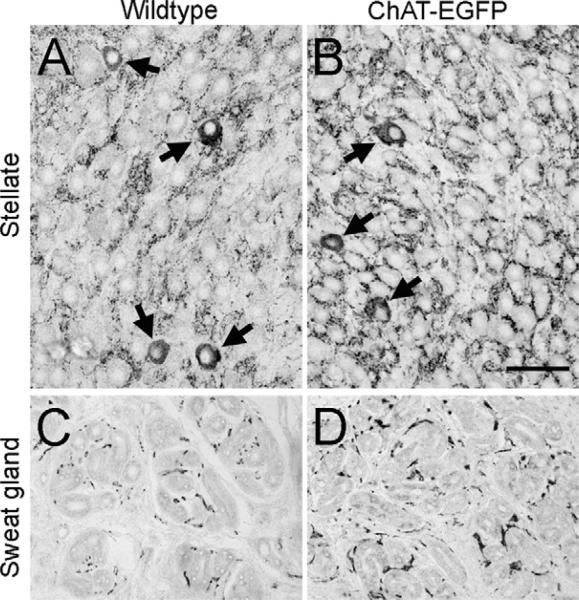
We next investigated whether the up-regulated transcription of the VAChT gene also translated into an enhanced visibility of the VAChT protein in vivo. In the SG, the number of VAChT immunoreactive cell bodies appeared not to be altered in transgenic (Fig. 3B) compared with wild type (Fig. 3A) mice, whereas the preganglionic input was intensified in this sympathetic ganglion. Concomitantly, a stronger VAChT immunoreactivity pattern was found in sweat gland terminal fields of transgenic (Fig. 3D) compared with wild type (Fig. 3C) mice.
Fig. 3.
VAChT immunoreactivity is intensified in the SG and sweat gland terminal fields of adult ChAT-EGFP transgenic mice. (A) SG from a WT mouse. VAChT immunoreactivity labels pre-ganglionic input and few post-ganglionic neuronal cell bodies (arrows). (B) SG from a ChAT-EGFP mouse. Intensified VAChT immunoreactivity in pre-ganglionic fibers and no apparent change in VAChT-positive cell bodies of post-ganglionic neurons as compared with WT (arrows) Scale bar=50 μm (applying to all panels). (C) Forepaw sweat gland field from a WT mouse. (D) Forepaw sweat gland field from a ChAT-EGFP mouse. Note intensified VAChT innervation pattern of sweat glands in TG as compared with WT.
VAChT mRNA and immunoreactivity are detected in many SG neurons of ChAT-EGFP transgenic mice at P1
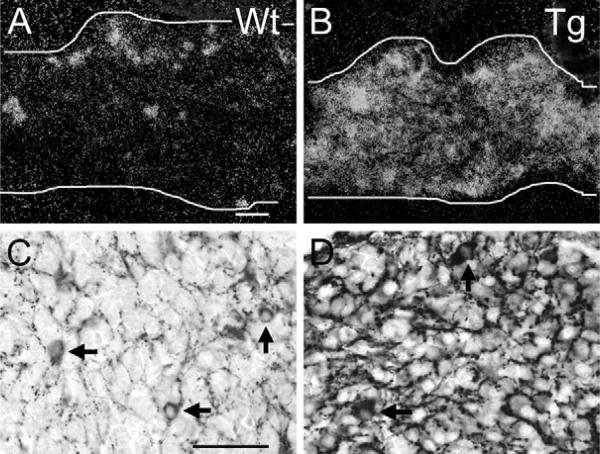
The enhanced detection of VAChT mRNA and immunoreactivity in the SG, and in sweat gland terminal fields of adult ChAT-EGFP transgenic mice prompted us to investigate whether VAChT mRNA and protein levels are proportionately enhanced in the SG at the earliest stages of the formation of sweat glands and their innervation, i.e. shortly after birth.
In P1 wild type mice, strong VAChT mRNA (Fig. 4A) and immunoreactivity (Fig. 4C) were found restricted to the minor subpopulation of SG neurons in which endogenous VAChT expression is seen by ISH in wild-type mice, with weak VAChT mRNA signals, however, even visible over a wider population of cells in the SG (Fig. 4A). In ChAT-EGFP transgenic mice, VAChT mRNA signals covered almost the entire SG, suggesting proportional intensification in both stellate neuron populations (strongly and weakly VAChT-expressing) present in wild-type mice (Fig. 4B). Concomitantly, VAChT immunoreactivity was present at low to intermediate levels in the vast majority of neurons (Fig. 4D). A minor subpopulation still displayed much higher VAChT mRNA and immunoreactivity levels, suggesting that this is the population also seen in WT mice (Fig. 4C). Similar differences between wild type and ChAT-EGFP transgenic mice in VAChT mRNA and immunoreactivity patterns were observed at stages E17, E19 and P3 (data not shown).
Fig. 4.
VAChT over-expression in SG post-ganglionic neurons and pre-ganglionic input in ChAT-EGFP transgenic mice at P1 as compared with wild type. (A) P1 SG (demarcated by white lines in A, also in B) from a WT mouse. Clusters and single neurons are strongly VAChT ISH-positive. Many additional neurons have weak VAChT mRNA levels. Scale bar=50 μm (A), (also applying to B). (B) P1 SG from ChAT-EGFP transgenic mouse. Many neurons are VAChT ISH-positive. (C) P1 SG from a WT mouse. In addition to the pre-ganglionic input, a few randomly distributed neurons show VAChT immunoreactivity. Scale bar=50 μm (C) (also applying to D). (D) P1 SG from a ChAT-EGFP transgenic mouse. Note stronger immunoreactions in pre-ganglionic fiber input and in a few neuronal cell bodies (arrows) as compared with WT, and weak to intermediate immunoreactions in many other post-ganglionic neurons.
VAChT and TH immunoreactive nerve fibers appear simultaneously in nerve fibers around developing forepaw sweat glands in ChAT-EGFP transgenic mice
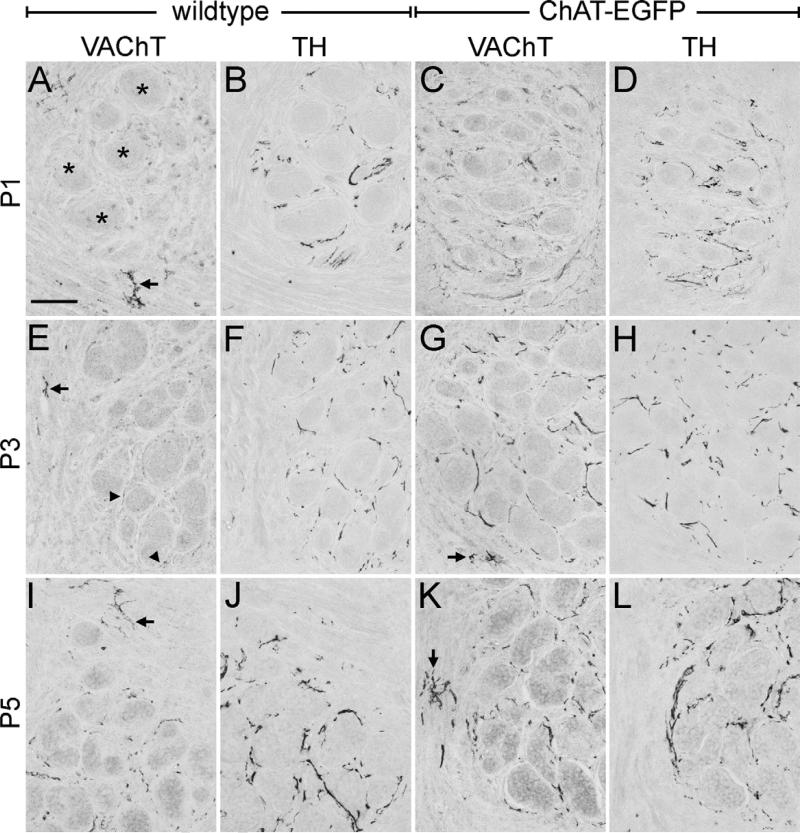
We next analyzed if the increased mRNA expression and immunohistochemical detection of VAChT in the SG at birth resulted in a proportionately enhanced detectability in the developing sweat gland terminal field and other sympathetic targets. We examined forepaw sweat gland territories from the day of birth onward in 1 day intervals and compared the appearances of noradrenergic, TH-positive, and cholinergic, VAChT-positive fibers (Fig. 5). In wild-type mice, a cholinergic innervation of adjacent sweat gland fields was undetectable in P1, although VAChT immunoreactivity was readily detectable in neuromuscular junctions (Fig. 5A). The presence of a sympathetic innervation, however, was confirmed by TH staining around sweat gland coils at this age (Fig. 5B). Similar to the previous analyses in rats and mice (Schafer et al., 1997; Guidry and Landis, 1998), a faint VAChT-positive innervation of sweat glands was reliably visible from P3 on in wild type mice (Fig. 5E), and had intensified by P5 (Fig. 5I). TH immunoreactivity also intensified from P3 (Fig. 5F) to P5 (Fig. 5J).
Fig. 5.
Coincident appearance of VAChT and TH immunoreactive nerve fibers around developing forepaw sweat glands in ChAT-EGFP transgenic mice. (A) VAChT immunoreactivity is present in neuromuscular endplates (arrow), but undetectable in an adjacent sweat gland territory (asterisks) of a P1 WT mouse. Scale bar=50 μm (applying to all panels). (B) TH immunoreactivity labels sympathetic sweat gland innervation on an adjacent section to (A), but is absent from the cholinergic neuromuscular junctions. (C, D) In a P1 ChAT-EGFP mouse, both VAChT and TH immunoreactive nerve fibers are present around sweat gland tubules. (E) In a P3 WT mouse, VAChT immunoreactivity is detectable in both neuromuscular (arrow) and, for the first time, sweat gland innervation (arrowheads). (F) TH immunoreactivity labels exclusively sympathetic sweat gland innervation on an adjacent section to (E). (G, H) Sweat gland territory of a P3 ChAT-EGFP mouse. Both VAChT and TH immunoreactivities are present in sympathetic fibers around sweat gland coils. In addition, VAChT immunoreactive fibers label a neuromuscular junction (arrow in (G)). (I, J) In a P5 WT mouse sweat gland territory, both VAChT and TH immunoreactivities have intensified. Arrow in (I) points to VAChT immunoreactive neuromuscular endplate. (K, L) Sweat gland territory of a P5 ChAT-EGFP mouse. Note stronger VAChT-immunoreactive sudomotor fibers compared with P5-WT. The arrow points to a neuromuscular endplate. TH staining is comparable to P3 WT.
In ChAT-EGFP transgenic mice, VAChT immunoreactivity (Fig. 5C) also increased during the early neonatal periods, but is clearly seen to appear simultaneously with TH (Fig. 5D) in the sweat gland territory already at P1. The staining intensities of TH and VAChT were similarly strong by P3 (Fig. 5G, H), and did not significantly increase thereafter until P5 (Fig. 5K, L).
A parallel analysis of EGFP and ChAT immunoreactivities in sweat gland nerve fibers revealed similar staining patterns in wild type and ChAT-EGFP mice. Both markers were present around forepaw sweat gland coils from P6 on (data not shown), indicating that the production and detectability of ChAT, as expected, was not altered in ChAT-EGFP mice. EGFP appears to be a less valuable marker compared with VAChT for labeling distal nerve fibers and terminals during development, perhaps because of relative VAChT protein concentrations in small synaptic vesicles in distal forming nerve terminals, compared with EGFP expression in their relatively scanty cytoplasm.
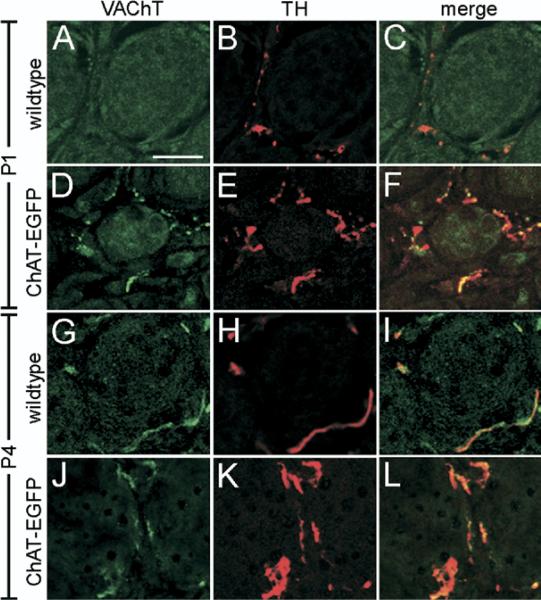
Finally, we confirmed that VAChT and TH were co-expressed in sympathetic fibers by performing a double immunofluorescence analysis. While VAChT was undetectable and TH present around P1 sweat gland coils from wild type mice (Fig. 6A–C), a clear detection of VAChT and co-incidence with TH was observed in P1 transgenic mice (Fig. 6D–F). At P4, both in wild type (Fig. 6G–I) and transgenic mice (Fig. 6J–L), VAChT and TH co-localized in the same fibers. Other noradrenergically innervated sympathetic targets, e.g. major blood vessels and white fat, did not display cholinergic innervation in the ChAT-EGFP transgenic mice. This indicated that the VAChT/TH IR fibers seen around P1 sweat glands originated from the strongly immunoreactive neurons in the SG, the population of cholinergic neurons that is also detected in wild type mice, but not from the many other neurons showing a weak IR pattern.
Fig. 6.
Co-expression of VAChT and TH in sudomotor innervation. (A–C) Absence of VAChT (green), and presence of TH (red) immunoreactive nerve fibers in forepaw sweat gland innervation of a P1 WT mouse. Scale bar=20 μm (applying to all panels). (D–F) Co-existence of VAChT and TH immunoreactive fibers around a sweat gland coil of a P1 ChAT-EGFP mouse. (G–I) Co-existence of VAChT and TH immunoreactive fibers around a sweat gland coil of a P4 WT mouse. (J–L) Co-existence of VAChT and TH immunoreactive fibers around a sweat gland coil of a P4 ChAT-EGFP mouse.
DISCUSSION
Two possible mechanisms could account for the developmental acquisition of the cholinergic phenotype in peripheral sympathetic neurons. In one, the peripheral target structure, e.g. the sweat gland, instructs sudomotor neurons uncommitted to the cholinergic phenotype, to activate the genes, and produce de novo the proteins, required for the synthesis and vesicular storage of ACh (Francis and Landis, 1999). A second mechanism is based on the assumption that most, if not all, genes encoding proteins required for classic neurotransmission in a given class of neurons, are expressed prior to target innervation, and that with new target selection a pre-existing phenotype is enhanced and shifted to a preferential and target-specific neurotransmitter choice (Schafer et al., 1997; Weihe and Eiden, 2000).
Introduction of several copies of the CGL into mice increased the sensitivity of the detection of cholinergic nerve cell bodies and fibers through EGFP over-expression from the ChAT promoter. While EGFP-based fluorescence turned out to be a valuable marker for central cholinergic neurons (von Engelhardt et al., 2007), and for peripheral ganglia and nerve fibers in the adult mouse (this report), it did not increase the sensitivity of cholinergic nerve terminal detection in the developing sympathetic nervous system. This may be due to differentially lower expression levels from the ChAT relative to the VAChT promoter in the PNS in general, and especially during development (Schutz et al., 2001).
The proportional fivefold greater expression of VAChT in adult ChAT-EGFP transgenic mice, suggested to us that re-investigation of the developmental expression pattern of VAChT during the course of sweat gland innervation might prove fruitful in these animals. We found that VAChT mRNA and protein expression was indeed proportionately higher, and present in many SG neurons around birth, indicating that in addition to a small number of purely cholinergic neurons (Guidry and Landis, 1998; Masliukov and Timmermans, 2004), many additional postganglionic sympathetic neurons in the SG transiently have a cholinergic phenotype, albeit weak, during development (Masliukov and Timmermans, 2004; Weihe and Eiden, 2000). Thus, from embryonic stages on and into adulthood, the sympathetic cholinergic phenotype passes through a successive stabilization in expression, rather than through a target-dependent induction. This mode of a cholinergic/noradrenergic duality during ontogeny likely is also the basis for the phenotypic plasticity observed as a rapid functional switch between noradrenergic and cholinergic neurotransmission in neonatal sympathetic neurons innervating cardiac myocytes in culture (Yang et al., 2002), and helps to explain the transplantation-induced switch from noradrenergic to cholinergic neurotransmission, when sweat gland primordia are placed into hairy skin early postnatally (Schotzinger and Landis, 1988). Noteworthy, cholinergic/monoaminergic duality is not unique to vertebrates but an early evolutionary trait, as VAChT and the vesicular monoamine transporter 2 co-exist in a subset of motor neurons in adult Caenorhabditis elegans (Duerr et al., 2001).
Due to the enhanced VAChT detectability in the ChAT-EGFP mice we were able to observe a coincident appearance of VAChT and TH immunoreactive nerve fiber terminals around forepaw sweat gland primordia in the initial course of target formation, i.e. at P1. This indicates that pioneering sudomotor axons that express a cholinergic phenotype successfully connect cholinergically committed neuronal cell bodies with their appropriate target without being instructed to switch from a noradrenergic phenotype. According to the target-dependent instruction mode one would expect to see a significant increase in the number of cholinergic neurons between the initial noradrenergic target innervation and the subsequent appearance of cholinergic markers, leaving a time window for a target-dependent switch of just a few days. Thus, a detailed analysis of cholinergic neuron numbers in the sympathetic ganglion of interest across the critical developmental time points is highly informative. In one study some 30% of all cholinergic neurons in the SG were found to be generated by P2, while the remaining 70% were generated thereafter until P60, using VAChT and vasoactive intestinal peptide (VIP) immunoreactivity as markers (Stanke et al., 2006). This postnatal increase was interpreted in favor of the target-dependent mode of cholinergic phenotype aquisition. However, we (Weihe and Eiden, 2000) and others (Masliukov and Timmermans, 2004) found that the fraction of cholinergic neurons compared with all neurons in the SG remained stable at around 4% until P10, and declined again to 3% in 2-month-old mice. According to Stanke and colleagues this proportion would be expected to increase significantly until P60.
If it does not control acquisition of the neurotransmitter phenotype per se, what then is the specific function of the target in this process? One function of the target organ could be the generation of an innervation factor (Glebova and Ginty, 2005). Developing sweat glands have been found to express neurturin mRNA shortly after birth in mice (Hiltunen and Airaksinen, 2004). Neurturin signals through the GDNF family receptor GFRα2 and the Ret receptor tyrosine kinase (Airaksinen and Saarma, 2002), and GFRα2 immunoreactivity is present in mouse SG neurons that also express VIP (Hiltunen and Airaksinen, 2004). In Gfra2 knockout mice only the density of cholinergic but not noradrenergic sympathetic innervation of sweat glands was found reduced by 50−70% at P21, while the number of cholinergic neurons in the SG was unaffected (Hiltunen and Airaksinen, 2004). This study suggested that single cholinergic nerve fiber plexus arborization and final target innervation may require neurturin signaling.
The target may also release classic neurotransmitter-selective neurotrophins, that not only function as survival factors (Glebova and Ginty, 2005), but also preferentially regulate the sorting of vesicle-type-specific proteins, e.g. VMAT2, VAChT and their subsequent selective delivery to the synapse, thus influencing the type of neurotransmitter released at the varicose ending (Felder and Dechant, 2007). All the previously proposed sweat gland factors, e.g. leukemia inhibitory factor, ciliary neurotrophic factor, and cardiotrophin 1 (Habecker et al., 1995, 1997; Francis et al., 1997), are target-derived candidate molecules/activities that presumably act in combination in this selection process.
Enhancement of the expression of cholinergic genes and thus generation of larger amounts of cholinergic-specific proteins may also be driven by the synaptic interaction between neuron and target. Experiments with dissociated avian embryonic sympathetic neuron cultures revealed that up-regulation of ChAT expression and activity could be completely blocked by tetrodotoxin, a Na+-channel blocker, indicating that depolarization could be a factor selectively enhancing the expression of the cholinergic phenotype during ontogeny (Smith et al., 1993). Moreover, differential sensitivities of cholinergic vs. noradrenergic genes in response to neurotrophins trigger phenotype-specific transcriptional and proteomic changes upon neuron–target interaction (Apostolova et al., 2007). Discrepancies in the ability to detect the cholinergic phenotype on the ganglionic vs. target level during the first Ps likely are based on these mechanisms.
Finally, the target might also be responsible for influencing the acquisition of additional neuropeptides in the cholinergic neurons, since interfering with the expression of leukemia inhibitory factor receptor beta, a protein that forms heterodimers with gp130, strongly reduced the developmental expression of the VIP gene in chick sympathetic neurons (Duong et al., 2002).
CONCLUSION
In conclusion, our findings indicate that postganglionic sympathetic neurons that express a cholinergic/noradrenergic co-phenotype and are present before sweat gland formation are the pioneering neurons for target innervation. While maintenance, trophic support, anterograde transport of neurotransmitter-specific proteins and survival of these neurons may all be regulated in a target-dependent manner (Hippenmeyer et al., 2004), there does not appear to be target-dependent de novo specification of the cholinergic phenotype at the level of the sweat gland. This finding suggests neurotransmitter specification via selection rather than instruction for cholinergic postganglionic sympathetic neurons, allowing functional “switching” of chemical phenotypes during development via amplification of pre-existing cholinergic expression in some neurons, and down-regulation in others.
Acknowledgments
We thank H. Hlawaty, P. Lattermann, R. Weber, and M. Schneider for technical assistance.
Abbreviations
- ACh
acetylcholine
- BAC
bacterial artificial chromosome
- BSA
bovine serum albumin
- CGL
cholinergic gene locus
- ChAT
choline acetyltransferase
- CT
threshold cycle
- EGFP
enhanced green fluorescent protein
- IL2
interleukin-2
- ISH
in situ hybridization histochemistry
- P
postnatal day
- RT-PCR
reverse transcriptase–polymerase chain reaction
- SG
stellate ganglion
- TH
tyrosine hydroxylase
- VAChT
vesicular acetylcholine transporter
- VIP
vasoactive intestinal peptide
REFERENCES
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Anderson CR, Bergner A, Murphy SM. How many types of cholinergic sympathetic neuron are there in the rat stellate ganglion? Neuroscience. 2006;140:567–576. doi: 10.1016/j.neuroscience.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Apostolova G, Dorn R, Ka S, Hallbook F, Lundeberg J, Liser K, Hakim V, Brodski C, Michaelidis TM, Dechant G. Neurotransmitter phenotype-specific expression changes in developing sympathetic neurons. Mol Cell Neurosci. 2007;35:397–408. doi: 10.1016/j.mcn.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Asmus SE, Parsons S, Landis SC. Developmental changes in the transmitter properties of sympathetic neurons that innervate the periosteum. J Neurosci. 2000;20:1495–1504. doi: 10.1523/JNEUROSCI.20-04-01495.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burau K, Stenull I, Huber K, Misawa H, Berse B, Unsicker K, Ernsberger U. c-ret Regulates cholinergic properties in mouse sympathetic neurons: evidence from mutant mice. Eur J Neurosci. 2004;20:353–362. doi: 10.1111/j.1460-9568.2004.03500.x. [DOI] [PubMed] [Google Scholar]
- Duerr JS, Gaskin J, Rand JB. Identified neurons in C. elegans coexpress vesicular transporters for acetylcholine and monoamines. Am J Physiol Cell Physiol. 2001;280:C1616–C1622. doi: 10.1152/ajpcell.2001.280.6.C1616. [DOI] [PubMed] [Google Scholar]
- Duong CV, Geissen M, Rohrer H. The developmental expression of vasoactive intestinal peptide (VIP) in cholinergic sympathetic neurons depends on cytokines signaling through LIFRbeta-containing receptors. Development. 2002;129:1387–1396. doi: 10.1242/dev.129.6.1387. [DOI] [PubMed] [Google Scholar]
- Eiden LE. The cholinergic gene locus. J Neurochem. 1998;70:2227–2240. doi: 10.1046/j.1471-4159.1998.70062227.x. [DOI] [PubMed] [Google Scholar]
- Eiden LE, Schafer MK, Weihe E, Schutz B. The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pflugers Arch. 2004;447:636–640. doi: 10.1007/s00424-003-1100-5. [DOI] [PubMed] [Google Scholar]
- Elfvin LG, Lindh B, Hokfelt T. The chemical neuroanatomy of sympathetic ganglia. Annu Rev Neurosci. 1993;16:471–507. doi: 10.1146/annurev.ne.16.030193.002351. [DOI] [PubMed] [Google Scholar]
- Ernsberger U, Rohrer H. Development of the cholinergic neurotransmitter phenotype in postganglionic sympathetic neurons. Cell Tissue Res. 1999;297:339–361. doi: 10.1007/s004410051363. [DOI] [PubMed] [Google Scholar]
- Felder E, Dechant G. Neurotrophic factors acutely alter the sorting of the vesicular acetyl choline transporter and the vesicular monoamine transporter 2 in bimodal sympathetic neurons. Mol Cell Neurosci. 2007;34:1–9. doi: 10.1016/j.mcn.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Francis NJ, Asmus SE, Landis SC. CNTF and LIF are not required for the target-directed acquisition of cholinergic and peptidergic properties by sympathetic neurons in vivo. Dev Biol. 1997;182:76–87. doi: 10.1006/dbio.1996.8464. [DOI] [PubMed] [Google Scholar]
- Francis NJ, Landis SC. Cellular and molecular determinants of sympathetic neuron development. Annu Rev Neurosci. 1999;22:541–566. doi: 10.1146/annurev.neuro.22.1.541. [DOI] [PubMed] [Google Scholar]
- Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Annu Rev Neurosci. 2005;28:191–222. doi: 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- Guidry G, Landis SC. Target-dependent development of the vesicular acetylcholine transporter in rodent sweat gland innervation. Dev Biol. 1998;199:175–184. doi: 10.1006/dbio.1998.8929. [DOI] [PubMed] [Google Scholar]
- Habecker BA, Pennica D, Landis SC. Cardiotrophin-1 is not the sweat gland-derived differentiation factor. Neuroreport. 1995;7:41–44. [PubMed] [Google Scholar]
- Habecker BA, Symes AJ, Stahl N, Francis NJ, Economides A, Fink JS, Yancopoulos GD, Landis SC. A sweat gland-derived differentiation activity acts through known cytokine signaling pathways. J Biol Chem. 1997;272:30421–30428. doi: 10.1074/jbc.272.48.30421. [DOI] [PubMed] [Google Scholar]
- Heintz N. BAC to the future: the use of bac transgenic mice for neuroscience research. Nat Rev Neurosci. 2001;2:861–870. doi: 10.1038/35104049. [DOI] [PubMed] [Google Scholar]
- Hiltunen PH, Airaksinen MS. Sympathetic cholinergic target innervation requires GDNF family receptor GFR alpha 2. Mol Cell Neurosci. 2004;26:450–457. doi: 10.1016/j.mcn.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Kramer I, Arber S. Control of neuronal phenotype: what targets tell the cell bodies. Trends Neurosci. 2004;27:482–488. doi: 10.1016/j.tins.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Masliukov PM, Timmermans JP. Immunocytochemical properties of stellate ganglion neurons during early postnatal development. Histochem Cell Biol. 2004;122:201–209. doi: 10.1007/s00418-004-0692-y. [DOI] [PubMed] [Google Scholar]
- Schafer MK, Eiden LE, Weihe E. Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. II. The peripheral nervous system. Neuroscience. 1998;84:361–376. doi: 10.1016/s0306-4522(97)80196-0. [DOI] [PubMed] [Google Scholar]
- Schafer MK, Schutz B, Weihe E, Eiden LE. Target-independent cholinergic differentiation in the rat sympathetic nervous system. Proc Natl Acad Sci U S A. 1997;94:4149–4154. doi: 10.1073/pnas.94.8.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotzinger RJ, Landis SC. Cholinergic phenotype developed by noradrenergic sympathetic neurons after innervation of a novel cholinergic target in vivo. Nature. 1988;335:637–639. doi: 10.1038/335637a0. [DOI] [PubMed] [Google Scholar]
- Schutz B, Chen L, Schafer MK, Weihe E, Eiden LE. Somato-motor neuron-specific expression of the human cholinergic gene locus in transgenic mice. Neuroscience. 2000;96:707–722. doi: 10.1016/s0306-4522(99)00587-4. [DOI] [PubMed] [Google Scholar]
- Schutz B, Damadzic R, Weihe E, Eiden LE. Identification of a region from the human cholinergic gene locus that targets expression of the vesicular acetylcholine transporter to a subset of neurons in the medial habenular nucleus in transgenic mice. J Neurochem. 2003;87:1174–1183. doi: 10.1046/j.1471-4159.2003.02095.x. [DOI] [PubMed] [Google Scholar]
- Schutz B, Mauer D, Salmon AM, Changeux JP, Zimmer A. Analysis of the cellular expression pattern of beta-CGRP in alpha-CGRP-deficient mice. J Comp Neurol. 2004;476:32–43. doi: 10.1002/cne.20211. [DOI] [PubMed] [Google Scholar]
- Schutz B, Weihe E, Eiden LE. Independent patterns of transcription for the products of the rat cholinergic gene locus. Neuroscience. 2001;104:633–642. doi: 10.1016/s0306-4522(01)00100-2. [DOI] [PubMed] [Google Scholar]
- Smith J, Vyas S, Garcia-Arraras JE. Selective modulation of cholinergic properties in cultures of avian embryonic sympathetic ganglia. J Neurosci Res. 1993;34:346–356. doi: 10.1002/jnr.490340312. [DOI] [PubMed] [Google Scholar]
- Stanke M, Duong CV, Pape M, Geissen M, Burbach G, Deller T, Gascan H, Otto C, Parlato R, Schutz G, Rohrer H. Target-dependent specification of the neurotransmitter phenotype: cholinergic differentiation of sympathetic neurons is mediated in vivo by gp 130 signaling. Development. 2006;133:141–150. doi: 10.1242/dev.02189. [DOI] [PubMed] [Google Scholar]
- Stevens LM, Landis SC. Development and properties of the secretory response in rat sweat glands: relationship to the induction of cholinergic function in sweat gland innervation. Dev Biol. 1987;123:179–190. doi: 10.1016/0012-1606(87)90440-4. [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Eliava M, Meyer AH, Rozov A, Monyer H. Functional characterization of intrinsic cholinergic interneurons in the cortex. J Neurosci. 2007;27:5633–5642. doi: 10.1523/JNEUROSCI.4647-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihe E, Depboylu C, Schutz B, Schafer MK, Eiden LE. Three types of tyrosine hydroxylase-positive CNS neurons distinguished by dopa decarboxylase and VMAT2 co-expression. Cell Mol Neurobiol. 2006;26:659–678. doi: 10.1007/s10571-006-9053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihe E, Eiden LE. Chemical neuroanatomy of the vesicular amine transporters. FASEB J. 2000;14:2435–2449. doi: 10.1096/fj.00-0202rev. [DOI] [PubMed] [Google Scholar]
- Weihe E, Schutz B, Hartschuh W, Anlauf M, Schafer MK, Eiden LE. Coexpression of cholinergic and noradrenergic phenotypes in human and nonhuman autonomic nervous system. J Comp Neurol. 2005;492:370–379. doi: 10.1002/cne.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihe E, Tao-Cheng JH, Schafer MK, Erickson JD, Eiden LE. Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles. Proc Natl Acad Sci U S A. 1996;93:3547–3552. doi: 10.1073/pnas.93.8.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Slonimsky JD, Birren SJ. A rapid switch in sympathetic neurotransmitter release properties mediated by the p75 receptor. Nat Neurosci. 2002;5:539–545. doi: 10.1038/nn0602-853. [DOI] [PubMed] [Google Scholar]