Abstract
The efficacy of infliximab, a chimeric antibody against tumor necrosis factor-α used to treat patients with rheumatoid arthritis (RA), tends to decrease as patients develop human antichimeric antibody against infliximab (HACA). The clinical study reported here was designed to evaluate the efficacy of mizoribine (MZR) pulse therapy in patients who show a reduced or insufficient response to infliximab. Ten RA patients who had active arthritis despite infliximab therapy were treated with MZR pulse therapy at a dose of 100 mg MZR and methotrexate (MTX) and the disease activity assessed at baseline and at weeks 4–8, 12–16, and 20–24. The dose was increased to 150 mg in those patients who showed an insufficient response to MZR. The mean 28-joint disease activity score (DAS28) at weeks 12–16 and 20–24 of therapy was significantly lower than that at baseline. A moderate or good European League against Rheumatism (EULAR) response was achieved in seven patients (70%) at weeks 12–16 and in five patients (50%) at weeks 20–24. The dose of 150 mg MZR was effective in one of the three patients who showed an insufficient response to pulse therapy with 100 mg MZR. Based on these results, we propose that MZR pulse therapy should be attempted before the patient is switched to other biologics.
Keywords: Infliximab, Mizoribine, Rheumatoid arthritis
Introduction
Infliximab is a chimeric anti-tumor necrosis factor-alpha (TNF-α) monoclonal antibody that has proven effective in patients with rheumatoid arthritis (RA) [1–3]. In Japan, mizoribine (MZR; 4-carbamoyl-1-b-d-ribofuranosylimidazolium) is used as an immunosuppressive agent in patients undergoing renal transplantation and receiving treatment for RA and lupus nephritis in Japan. This drug inhibits inosine monophosphate dehydrogenase, a rate-limiting enzyme in the de novo pathway of nucleic acid synthesis, thereby inhibiting lymphocyte proliferation [4, 5]. Mizoribine, which is usually used at a daily dose of 75–150 mg administered in three separate doses, is known for its low rate of side effects [6], but it has been considered comparatively less effective than other disease-modifying antirheumatic drugs (DMARDs) in patients with RA. A correlation between the peak MZR blood concentration and clinical response to the therapy has been observed in patients with lupus nephritis [7]. It has also been recently shown that a drug therapeutic regimen consisting of 100–150 mg MZR once daily is more effective than the three divided doses because the achieved blood concentration was higher with the former [8]. Mizoribine pulse therapy has also been found to be effective in patients with RA who show an inadequate effect of methotrexate (MTX) [9–11]. In the case of MZR pulse therapy, patients receive MZR on one or two days of the week combined with MTX. The basis of this combination therapy is that MZR inhibits the synthesis of purines and MTX inhibits primarily the synthesis of pyrimidines; consequently, both drugs together inhibit the de novo pathway of nuclei acid synthesis. As such, the combined use of these two drugs is considered to inhibit lymphocyte proliferation more effectively than either used solely (monotherapy) [12]. Tokuda et al. [9] reported the efficacy of MZR pulse therapy as additional therapy for nine patients who showed an insufficient effect of MTX. Five of the nine patients responded to the combined MZR pulse + MTX drug therapeutic regimen within 20 weeks. Kohriyama et al. [10] and Murai et al. [11] also reported the efficacy of MZR pulse therapy as additional therapy in patients showing an inadequate effect of MTX.
An important problem associated with the use of infliximab in therapeutic drug regimens is that its efficacy often decreases during prolonged treatment. In Japan, the approved dose of infliximab is up to 3 mg/kg, or 200 mg/body, and that of MTX is up to 8 mg/week. Insufficient doses of these drugs may contribute to a decrease in the clinical efficacy of infliximab. The objective of this clinical study was to evaluate the efficacy of MZR pulse therapy in patients who show reduced or insufficient response to infliximab.
Patients and methods
Background of the patients
Ten RA patients treated with infliximab between 2005 and 2008 at Tsukuba University Hospital were enrolled in this study. Of these, eight showed a reduced response to infliximab (=high disease activity despite the clinical response to infliximab by week 30 of treatment), and two showed an insufficient response to infliximab (=no clinical response to infliximab during the 30-week treatment period). All ten patients fulfilled the American College of Rheumatology criteria for RA revised in 1987 [13]. They all had active arthritis as defined by the 28-joint disease activity score (DAS28) >3.2 at study entry, with the exception of one patient who wanted to receive MZR therapy despite a DAS28 of 3.0. The mean ± standard deviation (SD) age of the patients was 50.3 ± 12.8 years, the mean ± SD disease duration was 6.5 ± 6.2 years, and the mean ± SD DAS28 using erythrocyte sedimentation rate (ESR; DAS28-ESR) was 5.0 ± 1.5.
All patients were concomitantly receiving MTX, 6–8 mg/week. The doses of concomitant prednisolone and DMARDs, including MTX, were not increased during the last 3 months prior to entry in the study.
Study protocol and clinical response
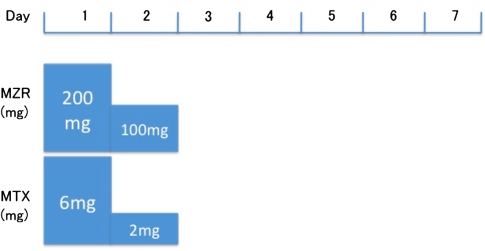
This study was approved by the ethical committee of our hospital. Informed consent was obtained before the study. Patients first received 100 mg of MZR together with MTX. The patients received 300 mg MZR on the first two days of the week: 200 mg MZR on the first day in two divided doses and 100 mg MZR in one dose on the second day (Fig. 1). At the time of each infusion of infliximab, the swollen joint count (SJC), tender joint count (TJC), visual analogue score (VAS), ESR, and DAS28 were recorded. Five of the ten patients were started on MZR pulse therapy between the infusions of infliximab. We therefore we assessed the patients’ DAS28 at baseline and after 12–16 weeks, 20–24 weeks and, thereafter, every 8 weeks.
Fig. 1.
The schedule of mizoribine (MZR) pulse therapy, consisting of combination drug therapy with 100 mg methotrexate (MTX). Total drug therapeutic program was 8 mg/week of MTX
Statistical analysis
We analyzed data using the Student’s t test to assess whether the changes in DAS28 and laboratory data from baseline during the course of the treatment were significant.
Results
The clinical socio-demographic and clinical characteristics of the patients, including previously administered DMARDs (as well as those drugs continued during the study), response to MZR pulse therapy [according to the EULAR (European League Against Rheumatism) response criteria at weeks 12–16, and weeks 20–24], response to infliximab (according to the EULAR response criteria at week 30), and change in the dose of prednisolone (PSL) between baseline and week 24, are shown in Table 1.
Table 1.
Clinical and socio-demographic characteristics of the patient cohort
| Case no. | Sex | Age (years) | Duration of RA (years) | Stagea | Previous DMARDsb | Response to IFXc | Baseline DAS28d | Response to MZRe/DAS28 (weeks 12–16) | Response to MZR/DAS28 (weeks 20–24) | Dose of predonisolone |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 64 | 8 | II | SASP BC | Moderate | 7.9 | Good 3.0 | Moderate 4.5 | No change |
| 2 | F | 48 | 4 | III | SASP BC | Good | 4.0 | Good 2.5 | Good 2.5 | No change |
| 3 | F | 32 | 2.5 | II | SASP BC | Good | 4.8 | Moderate 4.2 | No 4.7 | No change |
| 4 | F | 41 | 1.5 | I | SASP BC | Moderate | 6.9 | No 7.3 | No 7.3 | 8 → 3 mg |
| 5 | F | 55 | 2.5 | II | SASP BC | No | 5.2 | Moderate 4.2 | Moderate 4.3 | 8 → 7 mg |
| 6 | M | 29 | 20 | IV | SASP BC D-PC | No | 4.1 | Moderate 3.4 | No 4.2 | No change |
| 7 | F | 65 | 2 | I | SASP BC | Moderate | 3.0 | No 3.1 | No 2.8 | No change |
| 8 | M | 59 | 1 | I | SASP | Good | 3.8 | Moderate 2.9 | No 3.3 | No change |
| 9 | F | 50 | 11 | IV | SASP BC GST | Good | 4.2 | No 3.9 | Good 2.7 | 10 → 8 mg |
| 10 | M | 60 | 12 | II | BC | Moderate | 5.6 | Moderate 4.3 | Moderate 5.0 | No change |
RA rheumatoid arthritis, IFX infliximab, MZR mizoribine
aSteinbrocker stage of radiographs
bDisease-modifying antirheumatic drugs, including drugs continued during the study. SASP salazosulfapyridine, BC bucillamine, d-PCd-penicillamine, GST gold sodium thiomalate
cEULAR (European League Against Rheumatism) response criteria, at week 30
dDAS28-ESR, 28-joint disease activity score based on erythrocyte sedimentation rate
eEULAR response criteria
All of the patients were followed for more than 24 weeks. The MZR pulse therapy was well tolerated, and none of the patients discontinued the therapy. Seven patients (70%) had achieved a moderate or good EULAR response at weeks 12–16, and five patients (50%) had achieved a moderate or good EULAR response at weeks 20–24,.
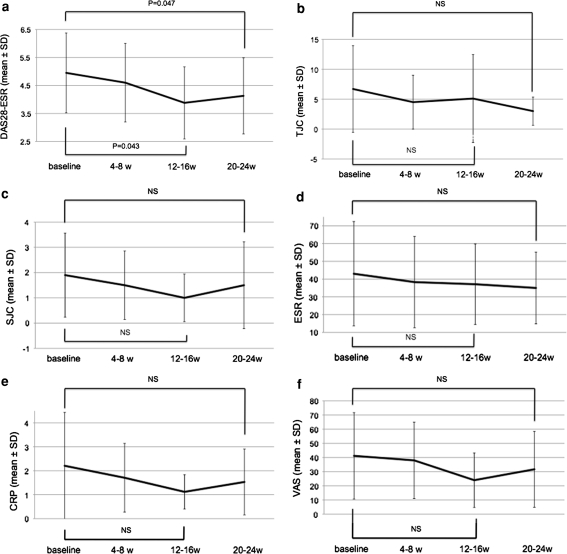
The mean DAS28 decreased from 5.0 at baseline to 3.9 (P = 0.047) at week 16, and 4.1 (P = 0.043) at week 24 (Fig. 2). The mean C-reactive protein (CRP) and ESR levels decreased, although not significantly during MZR pulse therapy.
Fig. 2.
a Changes in the 28-joint disease activity score (DAS28) from baseline and during the MZR pulse therapy regimen, at weeks 4–8, 12–16, and 20–24. The DAS28 significantly decreased at 12–16 weeks (P = 0.047) and at 20–24 weeks (P = 0.043). b Change in the tender joint count (TJC) at baseline and during therapy, at 4–8, 12–16, and 20–24 weeks. c Change in the swollen joint count (SJC) at baseline and during therapy, at 4–8, 12–16, and 20–24 weeks. d Change in the erythrocyte sedimentation rate (ESR) at baseline and during therapy, at 4–8, 12–16, and 20–24 weeks. e Change in the C-reactive protein (CRP) at baseline and during therapy, at 4–8, 12–16, and 20–24 weeks. f Change in the visual analog scale (VAS) at baseline and during therapy, at 4–8, 12–16, and 20–24 weeks. NS Not significant
Three patients showed insufficient or reduced response to MZR pulse therapy after 24 weeks; we therefore increased the dose of MZR up to 150 mg in these patients. One of these patients showed a favorable response to the higher dose (case 2). None of the patients had an adverse reaction to the higher dose, not even a minor infection, nor were there any abnormalities in the laboratory data. A complete blood count, including white blood cells, neutrophils, lymphocytes, hemoglobin, and platelet counts, demonstrated the absence of any significant changes that could be related to MZR pulse therapy (Table 2).
Table 2.
Results of a complete blood count among the patient cohort at baseline and at 20–24 weeks after the initiation of MZR pulse therapy
| Laboratory blood tests | Baseline | At weeks 20–24 | P |
|---|---|---|---|
| WBC (/µl) | 7410 ± 2100 | 7000 ± 1510 | NS |
| Lymphocyte (/µl) | 2050 ± 1690 | 1750 ± 1110 | NS |
| Neutrocyte (/µl) | 5030 ± 1870 | 4470 ± 940 | NS |
| Hemoglobin (g/dl) | 12.7 ± 1.1 | 12.6 ± 1.5 | NS |
| Platelet count (×104/µl) | 32.0 ± 8.0 | 33.3 ± 9.9 | NS |
Values are given as the mean ± standard deviation of white blood cell (WBC), lymphocyte, neutrocyte, hemoglobin, and platelet counts before (baseline) and 20–24 weeks after the initiation of MZR pulse therapy
NS not significant
Two successful cases of MZR pulse therapy are described below in detail.
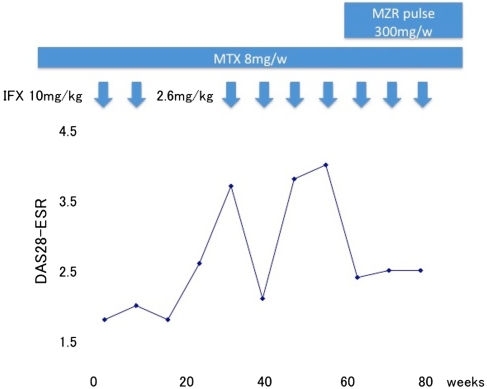
Case 1 was a 48-year-old woman who had been successfully treated with 10 mg/kg of infliximab during a clinical trial for 54 weeks. Her DAS28 had been less than 2.6 during the trial, but infliximab therapy was stopped after the eighth infusion because the trial was finished. Thereafter, her disease activity increased, (DAS28 3.7), and infliximab therapy was restarted at a dose of 2.6 mg/kg (the maximum approved dose is 200 mg and her body weight was 77 kg; therefore, she was administered 2.6 mg/kg infliximab). However, her disease activity did not decrease despite three additional infusions of infliximab. We therefore considered that 2.6 mg/kg infliximab had limited efficacy in this patient and added MZR pulse therapy at a dose of 100 mg together with MTX. By 4 weeks after the iniation of the MZR pulse thereapy, her DAS28 had decreased to 2.4. At week 20 on MZR pulse therapy, she achieved a good EULAR response (Fig. 3).
Fig. 3.
Response to therapy by patient 1 (case 1). IFX Infliximab
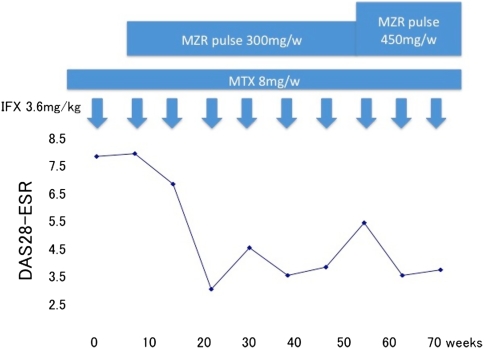
Case 2 was a 64-year-old man whose disease had been successfully controlled with infliximab, but who showed an increase of the disease activity while still on this drug. We therefore added MZR pulse therapy at a dose of 100 mg together with MTX 4 weeks before the 19th infusion of infliximab. Twenty weeks later, his DAS28 had decreased to 3.0 ,and he had achieved a good EULAR response. Thereafter, his disease activity was under control for over 24 weeks. At the time of the 25th infusion of infliximab, his DAS28 was 5.4, and his disease activity had increased again. We then increased the dose of MZR to 150 mg. Eight weeks later, his DAS28 had decreased to 3.5 (Fig. 4).
Fig. 4.
Response to therapy by patient 2 (case 2)
This second case suggests that increasing the dose of MZR may be effective. The clinical response to MZR pulse therapy was most clearly observed in cases 1 and 2, probably because infliximab showed some degree of efficacy in these patients. In case 4, although the patient’s DAS28 did not decrease until week 24 of treatment, MZR pulse therapy was considered to be clinically effective because we were able to decrease the dose of PSL from 8 to 3 mg.
Discussion
In Japan, infliximab has been used to treat RA patients since 2003. Although it efficacy in Japanese RA patients was demonstrated in the RECONFIRM study [14], the results of this study also indicated that the clinical response to infliximab may decline after 30 weeks of drug therapy. This reduced effect of infliximab therapy in relation to the development of human antichimeric antibody against infliximab (HACA) has been reported in several studies [15, 16]. An increase of the dose of infliximab beyond 3 mg/kg (e.g., 5, 10 mg/kg) or the shortening of the interval between infliximab infusions (e.g., every 6 weeks) has proven to be effective in such cases [2, 17, 18]. However, these methods are not approved by the Japanese Ministry of Health, Labor and Welfare. Etanercept is another biological product available in Japan. Alternating anti-TNF therapies, such as switching between etanercept and infliximab, has been reported to be effective in patients who do not respond to their first anti-TNF drug [19, 20]. However, such switching of anti-TNF therapy was strictly limited in Japan because only two biologics were available when we started this study. Tocilizumab and adalimumab were approved in Japan in April 2008, thereby doubling the number of biologics that can be used to treat patients with RA (four); however, the choice of biologics is still limited because some patients refuse self-injection. In our opinion, it is better to use one biological agent as long as possible—and not a combination—because it is still unclear whether the other biologics decrease the effect.
The objective of this study was to evaluate the efficacy and safety of MZR pulse therapy in patients who showed a reduced or insufficient response to infliximab. We observed significant efficacy at weeks 12–16 and at weeks 20–24. The decrease in the number of patients who responded to the therapy at weeks 20–24 (relative to weeks 12–16) may suggest a decline in the response of our patients to MZR pulse therapy. In this situation, a higher dose of MZR combined with MTX may be effective, as shown in case 1. Further studies are needed to confirm the efficacy of the higher dose of MZR.
The response rate using MZR pulse therapy that we obtained in this study in patients who showed a reduced response to infliximab (70%; 7/10 patients with a moderate or good EULAR score) was higher than that reported in previous studies using MZR pulse therapy (16–50%) in patients who showed inadequate response to MTX (without infliximab) [9–11]. This difference in response rate suggests that MZR pulse therapy may have some additional effect other than that as a DMARD. Although we could not measure anti-infliximab antibody levels, it would appear that MZR pulse therapy administered concomitantly with infliximab, in addition to its effect as a DMARD, also inhibits HACA.
In conclusion, in our small study cohort of patients with RA, MZR pulse therapy proved to be effective in patients who showed a reduced response to infliximab. We suggest that, in cases where infliximab is ineffective, MZR pulse therapy should be attempted before the patient is switched to another biologic.
Conflict of interest statement
None.
References
- 1.Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomized phase III trial. Lancet. 1999;354:1932–9. [DOI] [PubMed]
- 2.Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Eng J Med. 2000;343:1594–602. [DOI] [PubMed]
- 3.St Clair EW, van der Heijde DM, Smolen JS, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis. A randomized, controlled trial. Arthritis Rheum. 2004;50:3432–43. [DOI] [PubMed]
- 4.Ishikawa H. Mizoribine and mycophenolate mofetil. Curr Med Chem. 1999;6:575–97. [PubMed]
- 5.Koyama H, Tsuji M. Genetic and biochemical studies on the activation and cytotoxic mechanism of bredinin, a potent inhibitor of purine biosynthesis in mammalian cells. Biochem Pharmacol. 1983;32:3547–53. [DOI] [PubMed]
- 6.Tanaka E, Inoue E, Kawaguchi Y, et al. Acceptability and usefulness of mizoribine in the management of rheumatoid arthritis in methotrexate-refractory patients and elderly patients, based on analysis of data from a large-scale observational cohort study. Mod Rheumatol. 2006;16:214–9. [DOI] [PubMed]
- 7.Kuroda T, Hirose S, Tanabe N, et al. Mizoribine therapy for patients with lupus nephritis: the association between peak mizoribine concentration and clinical efficacy. Mod Rheumatol. 2007;17:206–12. [DOI] [PubMed]
- 8.Shida J, Shuto T, Tokito T, et al. Clinical investigation of efficacy by administration of mizoribine once a day for rheumatoid arthritis (in Japanese). Kyushu Riumachi. 2006;26:9–14.
- 9.Tokuda M, Dobashi H, Hiraishi M, et al. Effect of mizoribine pulse therapy on the disease activity of rheumatoid arithritis refractory to the treatment with methotrexate (in Japanese). Rheumatology. 1998;20:519–26.
- 10.Kohriyama K, Hiramatsu Y, Aoyama T, et al. Efficacy of combination pulse therapy with methotrexate and mizoribine for patients with rheumatoid arthritis showing escape phenomenon to low-dose methotrexate therapy (in Japanese). Rinsyo Riumachi. 2003;15:227–34.
- 11.Murai T, Arai K, Fujisawa J, et al. Evaluation of combination pulse therapy with methotrexate and mizoribine for patients with methotrexate-resistant rheumatoid arthritis (in Japanese). Jpn J Joint Dis (in press).
- 12.Nishimura K, Itoh K, Kuga Y, et al. Prevention of joint destruction in rheumatoid arthritis patients receiving combination methotrexate and mizoribine therapy: a two-year, multicenter open-comparison study to methotrexate monotherapy. Prog Med. 2006;26:2163–72.
- 13.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:3315–24. [DOI] [PubMed]
- 14.Yamanaka H, Tanaka Y, Sekiguchi N, et al. Retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a rheumatoid arthritis management group in Japan (RECONFIRM). Mod Rheumatol. 2007;17(2):178. [DOI] [PubMed]
- 15.Gerrit JW, Marijin V, Willem L, et al. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arithritis. Arthritis Rheum. 2006;54:711–5. [DOI] [PubMed]
- 16.van der Bijl AE, Breedveld FC, Antoni CE, et al. An open-label pilot study of the effectiveness of adalimumab in patients with rheumatoid arthritis and previous infliximab treatment: relationship to reasons for failure and anti-infliximab antibody status. Clin Rheumatol. 2008;27:1021–8. [DOI] [PMC free article] [PubMed]
- 17.St. Clair EW, Carrie LW, Adedigbo A, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1451–9. [DOI] [PubMed]
- 18.Elisabeth H, Mikkel O, Jan P, et al. Do rheumatoid arthritis patients in clinical practice benefit from switching from infliximab to a second tumor necrosis factor alpha inhibitor? Ann Rheum Dis. 2007;66:1184–9. [DOI] [PMC free article] [PubMed]
- 19.Van Vollenhoven R, Harju A, Brannemark S, Klareskog L. Treatment with infliximab (Remicade) when etanercept (Enbrel) has failed or vice versa: data from the STURE registry showing that switching tumour necrosis factor α blockers can make sense. Ann Rheum Dis. 2003;62:1195–8. [DOI] [PMC free article] [PubMed]
- 20.Cohen G, Courvoisier N, Cohen JD, et al. The efficiency of switching from infliximab to etanercept and vice versa in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2005;23:795–800. [PubMed]