Summary
We used functional MRI to determine the cerebral structures required during the recollection of episodic autobiographical memories according to five time-periods covering the whole lifespan to test the two concurring models of memory consolidation which propose either a temporary (standard model) or a permanent (multiple-trace model) role of the hippocampus in episodic memory retrieval. The experimental paradigm was specially designed to engage subjects (67.17 ± 5.22 years old) in the retrieval of episodic autobiographical memories, whatever the time-period, from personally relevant cues selected by questioning a family member. Moreover, the nature of the memories was checked at debriefing by means of behavioral measures to control the degree of episodicity. Behavioral data showed that recollected memories were characterized by specificity and details whatever their remoteness. Main neuroimaging data (SPM99) revealed the activation of a network including the left superior frontal gyri, bilateral precuneus/posterior cingulate and lingual gyri, left angular gyrus and left hippocampus, although the subtraction analyses detected subtle differences between certain time-periods. Small volume correction (SVC) centered on the hippocampus detected left hippocampal activation for all time-periods and additional right hippocampal activation for the intermediate periods. Further confirmation was provided by using a three-way ANOVA on BOLD values which revealed hippocampal activation, whatever the time-interval. The present data challenge the standard model of memory consolidation and support the multiple-trace model, instead. The comparison with previous literature stresses the idea that a bilateral involvement of the hippocampus characterizes rich episodic autobiographical memory recollection.
Keywords: Aged, physiology, psychology, Brain Mapping, Cues, Female, Health Status, Hippocampus, growth & development, physiology, Humans, Interviews as Topic, Magnetic Resonance Imaging, Memory, physiology, Reference Values
Keywords: autobiographical memory, episodic memory, fMRI, hippocampus, memory consolidation, autonoetic consciousness
Introduction
Theories of memory consolidation have postulated that, according to the retention interval (i.e. the number of years since the event), memory traces rely in part on different cerebral regions. According to the “standard model”, which follows from the work of David Marr (1971), the role of the medial temporal lobe (MTL) is critical in encoding, as well as in memory consolidation, but the MTL would contribute only temporarily to the retrieval of events stored in the neocortex (Squire and Alvarez, 1995; McClelland et al., 1995; Murre et al., 1996; Bayley et al., 2005). The duration of the consolidation period is still uncertain, ranging from 2 years (Graham and Hodges, 1997; Schmidke and Vollmer, 1997) to more than 10 years (Reed and Squire, 1998; Rempel-Clower et al., 1996). Moreover, the standard model does not distinguish between the two components of declarative memory, episodic and semantic, a distinction which has been clearly reaffirmed in the literature (Vargha-Khadem et al., 1997; Gadian et al., 2000; Guillery et al., 2001). Nadel, Moscovitch and collaborators (Nadel and Moscovitch, 1997; Hayes et al., 2004; Nadel et al., 2001, 2004; Moscovitch et al., 2005) have proposed an “alternative model”, termed the Multiple-Trace Theory (or MTT), which takes into account the episodic and semantic dichotomy in declarative memory (Tulving, 1972, 1985) based on observations of lifelong episodic retrograde amnesia in patients suffering from MTL damage (Fujii et al., 2000; Rosenbaum et al., 2001; Cipolotti et al., 2001). This latter model suggests that storage and retrieval of personal semantic memories ultimately depends on neocortical regions, particularly in the lateral temporal lobe, while storage and retrieval of episodic memories remain dependent on the MTL. Thus, this episodic and semantic distinction proves to be crucial for understanding the organization of remote memories and for testing the models of consolidation.
Episodic memory can be apprehended through laboratory studies which commonly use encoding and retrieval of lists of items that are far from removed concerning detailed event-specific autobiographical memories. Episodic autobiographical memory is more closely related to the current definition of episodic memory (Wheeler, 1999; Wheeler et al., 1997, 2004) as it makes a distinction between its different components (content and context), presupposes very lengthy retention intervals, autobiographical reference and phenomenological aspects of memory. Autobiographical memory refers to information and personally-relevant events acquired in a specific spatio-temporal context that a person has accumulated starting from his or her very early childhood, and which enables him or her to construct a sense of identity and continuity (Conway and Pleydell-Pearce, 2000). It is formed of different types of representations, from general knowledge about oneself (semantic component) to very specific personal events (episodic component; Tulving et al., 1988; Conway, 2001). The episodic component is characterized by an autonoetic state of consciousness which enables a conscious recollection of the personal event, in its original encoding context, and implies a mental time travel (Tulving, 1985, 2001, 2002; Wheeler et al., 1997). The semantic component is characterized by a noetic state of consciousness in which one is capable of retrieving the general facts about an event, without re-experiencing it. Retrieving an episodic autobiographical memory entails a complex generative retrieval process based on executive functions and autobiographical semantic knowledge (Conway and Pleydell-Pearce, 2000).
Several studies have used functional imaging to investigate the neural correlates of autobiographical memory in healthy subjects. The overall pattern associated with the retrieval of autobiographical memories involves an extensive network, predominantly left-lateralized, including the hippocampus, parahippocampal gyrus, medial frontal cortex, retrosplenial/posterior cingulate cortex, precuneus, temporo-parietal junction, temporal pole and cerebellum (Maguire, 2001; Moscovitch et al., 2005). It is widely agreed that the hippocampus and adjacent MTL structures are initially involved in the storage and retrieval of episodic memories. Yet, the question remains whether it is involved during the retrieval of episodic memories over long retention intervals.
While some neuroimaging studies have found evidence of greater activation of the MTL during the retrieval of recent relative to remote episodic memories (Haist et al., 2001; Niki and Luo, 2002; Piefke et al., 2003) favouring the standard model, most results show a permanent involvement of the MTL in memory retrieval, whatever the time-interval, concordant with MTT (Conway et al., 1999; Mayes et al., 2000; Ryan et al., 2001; Maguire et al., 2001, 2003a; Piolino et al., 2004; Gilboa et al., 2004; Addis et al., 2004a; Rekkas et al., 2005; Steinvorth et al., 2006). A “mixed” result (i.e. concordant with both models) showed that activity in the right hippocampus declined the more remote the autobiographical memories, while the left hippocampus remained permanently involved, whatever memory remoteness, suggesting that both models are valid, depending on hippocampal laterality (Maguire et al., 2003a).
However, in most of these studies, the true remoteness of autobiographical memories may be questionable due to recent retrievals (e.g. pre-scan interviews, re-activations) and the age of subjects. Indeed, most experimental paradigms were based on recall (or recognition) of events collected before the scanning experiment, implying that these events might have been re-encoded in memory. The hippocampal activations detected might have been related to the retrieval of the more recent event, namely the pre-scan interview. Furthermore, most studies were conducted on young or middle-aged subjects in whom autobiographical memories are not very remote, as long retention intervals, over decades, cannot be examined (except for Ryan et al., 2001; Maguire et al., 2003b).
Moreover, growing evidence suggests that factors other than time-interval can influence the amount and laterality of hippocampal activity including recollective qualities and the age of subjects. First, hippocampal laterality can be influenced by the nature of the autobiographical memories recalled and the type of tasks used, not always specifically designed to promote remembering. Tasks engaging spatial or topographical memory, such as route recall or recognition tasks are unlikely to require retrieval of a specific event, as these tasks can be executed by accessing the general levels of autobiographical knowledge without retrieving the episodic details. Such studies have revealed unilateral hippocampal activations, the former revealing right-sided (Mayes et al., 2000) and the latter, left-sided (Maguire et al., 1999, 2000, 2001) activations. On the contrary, studies in which tasks were selected to emphasize re-living and re-experiencing of personal events, engaging the subjects in recollective experiences, often detected bilateral hippocampal activations (Ryan et al., 2001; Piefke et al., 2003; Piolino et al., 2004; Addis et al., 2004a; Gilboa et al., 2004; Greenberg et al., 2005). It appears that the retrieval of personally relevant autobiographical memories, rich in recollective qualities, requires both hippocampi, each participating in different aspects of recollection. Some results show that the left hippocampus would be particularly responsive to details or memory vividness (Addis et al., 2004a; Gilboa et al., 2004), while others suggest that the right hippocampus would be particularly responsive to the sense of reliving the encoding context (spatial, emotional) (Graham et al., 2003; Piolino et al., 2004; Gilboa et al., 2004; Steinvorth et al., 2006). Second, hippocampal laterality during autobiographical recall can also be influenced by the age of the subjects. Maguire et al. (2003b) detected left hippocampal activation regardless of age and additional right hippocampal activation in older (mean age 75 years old) compared to younger subjects during an autobiographical recognition task (see also Ryan et al., 2001). Additional recruitment of the right hippocampus might compensate the role of the left hippocampus.
Finally, hippocampal activity observed during retrieval could also reflect encoding processes and not the access per se to old memories (Persson and Nyberg, 2000; Buckner et al., 2001). Indeed, when old memories are recalled, they are encoded once again. More specifically, the MTT proposes that the retrieval of episodic memories reactivates pre-existing traces, while simultaneously creating new ones. Thus, activation in the hippocampus might be related to retrieval, as well as to re-encoding processes. On a related matter, the HIPER model (Hippocampal Encoding Retrieval, Lepage et al., 1998) predicts episodic encoding-related activity to be clustered in the anterior portion of the hippocampus, while retrieval-related activity would be located more in its posterior portion.
In this study, we used functional neuroimaging to address the issue of long-term memory consolidation and determine the cerebral structures required during strict episodic autobiographical recollection according to five time-intervals covering the whole lifespan of healthy older subjects. We were interested in assessing strictly episodic autobiographical memories, and not generic ones, avoiding biases present in previous studies. In particular, to avoid memories from being reactivated by subjects before the scanning session, close family members were interviewed to provide cues that would elicit personal memories. A fine-grained “episodic scale”, the remember or know paradigm (R/K, Gardiner, 1988, 2001) and behavioral measures critical in episodic memory were used to assess and characterize the episodic component of autobiographical memory (Piolino et al., 2004).
According to MTT, we expected a permanent activation of the MTL, whatever the time-interval during autobiographical retrieval. More specifically, with respect to previous neuroimaging findings showing that the retrieval of personally relevant autobiographical memories, rich in recollective qualities, is more likely to engage bilateral hippocampal activation, we predicted bilateral involvement of the hippocampus during recollection of strictly episodic events. On the contrary, with respect to the standard model, only the retrieval of memories from the recent time-periods would elicit a selective activation of the MTL. Moreover, to address the issue of encoding during the retrieval task, we were also interested in focussing on the anterior-posterior axis of the hippocampus taking into account, in particular, the HIPER model.
Materials and Methods
Subjects
Twelve right-handed healthy aged women (as measured by the Edinburgh Handedness Inventory; mean age ± SD = 67.17 ± 5.22 years; ranging from 60 to 75 years old) with no history of psychiatric or neurological disorder were recruited through a university, a retirement association or a newspaper advertisement. To obtain a homogeneous group, we recruited only women. Furthermore, it has been previously shown that gender differences are present both at the behavioral and neural level: behaviorally, women report more vivid memories and their memories contain more information, compared to men (MacDonald et al., 2000; Goddard et al., 2005); at the neural level, women show differential activation within the network subserving autobiographical memory, compared to men, notably in the dorsolateral prefrontal cortex and insula (Piefke et al., 2005; see review by Piefke and Fink, 2005). The study was approved by the Regional Ethics Committee and written informed consent was obtained from all subjects prior to participation in the study. Subjects had no abnormality on their T1-weighted high-resolution magnetic resonance imaging (MRI). The inclusion of subjects was also based on the absence of memory complaints, signs of depression (GDS or Geriatric Depression Scale; Yesavage et al., 1983; score less than 10), symptoms of dementia as assessed via the Mattis scale (Mattis, 1976; score greater than 135) and the MMSE (Mini-Mental Status Exam; Folstein et al., 1975; score greater than 26) and episodic memory deficits assessed by the Grober and Buschke test (Grober and Buschke, 1987). No medication known to impair memory was allowed. They had at least 8 years of education and were all living in their own homes. Moreover, their verbal abilities were assessed according to the 44-item Mill Hill test (French translation by Deltour, 1993), a multiple-choice synonym vocabulary test.
For the 12 subjects, the mean (± SD) total score (1) at the GDS was 4.83 (± 3.04) (maximum score = 30; pathological cut off > 10), (2) at the Mattis scale was 142.17 (± 1.34) (maximum score = 144; pathological cut off < 136), (3) at the Grober and Buschke test was 31.75 (± 5.36) for total free recall (maximum score = 48; normative data = 28.9 ± 6.8) and 11 (± 2.22) for the delayed free recall (maximum score = 16; normative data = 11 ± 2), (4) at the MMSE was 28.42 (± 1.38) (maximum score = 30; pathological cut off < 27) and (5) at the Mill Hill was 38.42 (± 1.38) (maximum score = 44; normative data = 37 ± 3). Based on the French normative data or pathological cut-off, each subject performed normally on episodic memory and other tests.
Task and Experimental Design
The protocol was divided in three sessions, the pre-scan interview (with a close family member), the scanning session and the post-scan interview.
Pre-scan interview
The first session was carried out with a close family member (mostly husbands and, in some cases, a sibling or a child) who was interviewed on the subject’s life-events (mean duration = 3.5 hours). He or she was asked to report autobiographical events for each of five time-periods covering the subject’s entire lifetime (P1: 0–17 years; P2: 18–30 years; P3: over 31 years old, except for the last 5 years; P4: last 5 years, except the last 12 months; P5: last 12 months). These five time-periods were chosen in order to explore the temporal distribution of autobiographical memories over the whole lifespan, distinguishing between childhood or adolescence (P1), young adulthood (P2), older adulthood (P3), relatively recent (P4) and very recent (P5) past (Piolino et al., 2003a, 2006, in press). He or she was instructed to report only specific context-rich personal events which, in his or her estimation constituted for the subject of the experiment (wife, sibling or mother) a personal relevant event. Family members were asked not to provide memories which his wife (mother or sibling) mentioned very often or had evoked recently. Only events that lasted less than a day, were unique, with a specific spatio-temporal context, not too emotionally extreme and not repeated over time were picked for the second session (i.e. scanning). When the family member gave less than five events, we provided indices of the concerned time-period which are pertinent for the age group (for example, “the day of my communion” or “a particular exam of my baccalaureate”). These cues were derived from a previous autobiographical memory experimental study, including older subjects (Piolino et al., 2002), which were particularly relevant for each age group. Altogether, we have used very few of these indices (i.e. 7 out of 300 cues). The family member was instructed to avoid talking about the aim of the study to his wife, sibling or mother, before the scanning session. In this way, subjects would have no prior knowledge of the aim of the experimental task they were to accomplish during scanning and, thus, prevented the possibility of reactivation of memories.
Scanning session
The scanning session was preceded by a training phase, and followed by a post-scan interview (debriefing). Personal sentence-cues were elaborated from the family member’s prior interview. Events were chosen following specific characteristics: they had occurred only once, had lasted less than a day, were personally relevant (i.e. the subject was personally implicated in the event) and possessed a specific locus in time and space (see above). Cues were visually presented in white on a black background, using Superlab software (3.0 version, Cedrus). Examples of cues, translated from French, are for P1, “dad falls down the slope”, for P2, “the wardrobe falls off the roof of the car”, for P3 “I spill the blueberry liqueur”, for P4, “my day at the Office’s Museum in Florence” and for P5, “the evening I went to see Gone with the Wind with my granddaughter”.
The scanning session consisted of five functional runs, each corresponding to one time-period and lasting four minutes. Each functional run was composed of five experimental and five control blocks. In the experimental condition, subjects were instructed to mentally relive personal episodes prompted by personally relevant sentence-cues, by “travelling back in time”, and remembering as much detail as possible (such as time, location, perceptions, feelings, scenery and people present). Subjects were aware of the fact that events would be presented according to five time-periods (each corresponding to a functional run): before each run, subjects were informed of the upcoming time-periods which were, however, not presented chronologically. A total of twenty-five sentence-cues were presented per subject, corresponding to the five different time-periods (see above) (e.g. five sentence-cues were proposed for each time-period). Subjects were asked to press on a button as soon as they gained access to the prompted event and try to re-experience and maintain the memory in mind until the end of the block. Each block, in which one sentence-cue was presented, lasted 24 seconds: the sentence-cue was presented for 5 seconds, followed by a black screen presented for 19 seconds during which subjects continued their mental retrieval. Subjects could start their mental evocation, after having pressed on the button, while the sentence-cue was still on the screen. We chose this memory retrieval duration based on our pilot study, carried out on 12 older subjects aged 66 to 82 years (mean age ± SD, 74.75 years ± 7.01), showing that it is an optimal time to access and fully retrieve personally rich events. Similarly, Graham et al. (2003) showed, in their pilot study, that subjects (mean age = 60.4 years; 42–68 years old) rarely produce specific details about a personal event during the first 4 to 5 seconds after cue presentation. They need more seconds to fully recollect their personal memories, retrieving a myriad of details.
In the control condition, subjects were asked to detect the presence of two consecutive letters (“mb”) in pseudo-words of six letters (for example, “speugr” or “mbieha”) and were instructed to press on a button when “mb” was present in the pseudo-word (target word). The letters “mb” could be positioned at the beginning, in the middle or at the end of the target word. This low-level task was chosen as a baseline condition in order to control for reading operations, mental processing of visual cues and motor processing, common to both experimental and control tasks. Low-level baseline conditions (counting, reading, object visualisation) which involve lower strategic processes have already been used in previous studies on autobiographical memory to compare with the experimental condition (i.e. memory retrieval task; Mayes et al., 2000; Tsukiura et al., 2002; Steinvorth et al., 2006). Five pseudo-words were presented in each control block (1 second for cue presentation, followed by 3.8 seconds for the response; see Figure 1) and one out of the five was a target word. Thus, each control block lasted 24 seconds (i.e. 5 × (1 + 3.8) seconds). The position of target words was randomly intermixed across blocks. Thus, in both experimental and control blocks, cue presentation (e.g. reading) lasted five seconds. Of note, the order of the functional runs was randomly intermixed across subjects.
Figure 1.
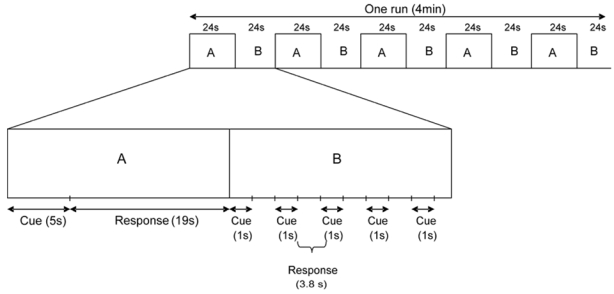
General organization of the protocol. A: Experimental blocks (mental evocation of a personal memory). B: Control blocks (attentional task).
Before the fMRI scan, subjects were familiarised with the tasks, in a training phase which lasted approximately 30 minutes, outside of the scanner. Each experimental block was followed by a control block, using the same design as described beforehand and giving the same instructions. To verify the subjects’ comprehension of the experimental task, they were asked to describe their thoughts, in detail, after each pair of experimental and control blocks. This procedure was repeated six times. The sentence-cues used in the training phase were different from the scanning session.
Post-scan interview
Following the scanning session, the debriefing took place (mean duration = 3 hours) in order to verify subjects’ engagement in the tasks and to identify the nature of the evocations retrieved during scanning, using a similar procedure as described previously (Piolino et al., 2004). Subjects were asked to retrieve each of the twenty-five memories evoked in the scanner, using the same sentence-cues, but this time aloud in order to check that their memories corresponded to the expected cued memories, that the elicited cues lead to recall of events in the indicated time-period and to assess the nature of their memories (i.e. specificity and detail). Episodicity was estimated 1) with “objective” measures, using a 5-point scale (AM and EM scores, see below); 2) with “subjective” measures, by collecting subjects’ ratings on ten analogical scales (see below).
The specificity of each evocation was measured by the investigators (objective measurement of episodicity) using a fine-grained five-point scale (0-1-2-3-4) rather similar to previous episodic scales used in healthy subjects and patients with cerebral diseases (Graham and Hodges, 1997; Kopelman et al., 1989; Piolino et al., 2002, 2003a,b, 2004,2006a,in press; Eustache et al., 2004). This scale takes into account the specificity of the content (single or repeated event), the spatiotemporal situation and the presence of details (perceptions, thoughts, feelings). A specific event, situated in time and space, with sensory details is given a score of 4. A specific event with few details, but situated in time and space, scores 3. A generic event (repeated or prolonged over time, situated in time and space) scores 2. A vague event (repeated or prolonged over time, not situated in time and space) scores 1. Absence of memory, or general information about a theme, scores 0 (see appendix 1). Two different total scores are obtained per period. First, the overall autobiographical score (AM: maximum score per period 4 × 5 = 20) includes all the memories (specific and generic) and corresponds to the classic episodic memory score used in the well-known Autobiographical Memory Interview (AMI: Kopelman et al., 1989). The AM score is expressed in terms of ratio (i.e. scores obtained for each of the five memories per time-period were summed and divided by the maximum score per time-period which is 20). Second, the strictly episodic score (EM: maximum score per period 4 × 5 = 20) includes only the number of specific and detailed memories scoring 4, using a more stringent criterion. EM is expressed in terms of ratio of strictly episodic memories per period (i.e. number of strictly episodic memories divided by the number of retrieved memories). Two independent experts (AV and PP) rated each memory recalled at debriefing and any difference of opinion between them was discussed until a consensus was reached.
Moreover, in order to specify the different aspects of recollective experience, subjects were asked to rate their memories on ten analogical scales (10 cm lines; subjective measurement of episodicity), well known to be crucially involved in episodic memories (Piolino et al., 2004), in terms of frequency of rehearsal, recency of the last recall before scanning, emotion during encoding and retrieval, state of consciousness and visual mental imagery (see appendix 2 to know which responses anchor which ends of each scale). More specially, several authors have demonstrated the influence of repetition on autobiographical memories (talking or thinking about the same memory) in the process of consolidation by reinforcing specific details and maintaining their accessibility with time interval (Conway and Dewhurst, 1995) or leading to a decontextualization or semantization of memories (Brewer, 1986; Linton, 1986, 1988, Cermak, 1984). We controlled for recent reactivation by evaluating if an event was recalled recently or not, using two analogical scales: (1) the last recall scale, to determine when each event was last recalled, (2) the frequency of rehearsal scale, to determine how frequently an event was rehearsed, prior to scanning. Thus, these scales intend to address the issue of any prior reactivation, especially for remote periods (i.e. to control that activation detected in the hippocampus, in particular for remote memories, was not due to a very recent recall of those memories by subjects). Emotion is also an important phenomenological characteristic of vivid and persistent autobiographical memories (Brewer, 1988; Dolan et al., 2000). Thus, subjects were asked to rate their memories on scales measuring emotional intensity and valence, both at encoding and retrieval (i.e. on the day of the experiment).
The autonoetic and noetic states of consciousness, which characterize episodic and semantic memory respectively, can be distinguished by the remember or know (R/K) paradigm. Thus, subjects were asked to rate their memories on a scale measuring the state of consciousness between the autonoetic and noetic states. The more the subject rates an event “R”, the more she thinks she is able to recollect the original event with the thoughts, feelings and perceptions present at acquisition: an “R response” reflects autonoetic consciousness. The more the subject rates an event “K”, the more she thinks she cannot explicitly remember the encoding source in details, eventhough the event feels familiar: a “K response” reflects noetic consciousness (Tulving, 1985; Gardiner et al., 1988; Gardiner and Java, 1993). Unlike the “knowing” state, the “remembering” state is characterized by phenomenal elements associated with the recall of specific events (visual images, sensations, feelings). Visual mental imagery increases the recall of specific details and the subjective sense of remembering (Dewhurst and Conway, 1994; Brewer, 1996; Rubin et al., 2003). Nigro and Neisser (1983) have distinguished two points of view in visual images: in the “field” perspective, which characterizes episodic recollection (Crawley and French, 2005; Piolino et al., 2006, in press), mental images represent the scene from the point of view from which it was originally experienced, while in the “observer” perspective, mental images represent the original scene as an external observer might have seen it. Thus, regarding the point of view, three choices were proposed: observer (0), field and observer (1) or field (2). Response times for each of the five memories per period were averaged across each time-period.
fMRI Data Acquisition
A blocked functional MRI design was used. Lying in the scanner, subjects viewed the display through mirror glasses and an active matrix video projector. Stimulus onset was synchronized with the acquisition of the first slice. Anatomical and functional MRIs were acquired on a General Electrics Signa 1.5 tesla MRI scanner (GE, BUC, France). First, a high-resolution T1-weighted MRI scan (T1-MRI) was acquired with a three-dimensional inversion recovery spoiled gradient echo sequence (matrix size = 256 × 256 × 128; slice thickness = 1.5 mm). Second, a proton density/T2-weighted MRI scan (PD-MRI, T2-MRI) was acquired with 32 axial slices covering the entire brain and the superior part of the cerebellum (slice thickness = 3.8 mm). Finally, functional images were acquired with echo planar imaging blood oxygen level dependent (BOLD) sequences (repetition time = 6 s, echo time = 60 ms, flip angle = 90°, matrix size = 64 × 64 × 32, 50 volumes, 3.8-mm-thick slices) covering the same field of view as the T2-MRI acquisition.
Functional Image Pre-processing
Functional images were processed and analysed using Statistical Parametric Mapping (SPM99; Wellcome Department of Cognitive Neurology, London, United Kingdom; http://www.fil.ion.ucl.ac.uk/spml). The first six volumes of the functional acquisition were discarded, allowing for signal stabilization, and differences in slice acquisition timing were corrected. Images were realigned to correct for interscan movement. The fourth volume of the run was considered as the reference functional volume (fMRI0). For registration of fMRI0 onto the stereotaxic Montreal Neurological Institute (MNI) template, rigid (fMRI0 onto T2-MRI and PD-MRI onto T1-MRI) and non-linear (T1-MRI onto the MNI template) registration matrices were computed and then combined. The registration of fMRI0 and T1-MRI volumes in the MNI space was thereafter visually checked with the MPI Tool software (Max-Planck Institute, Germany) and manually corrected when necessary. Then, each fMRI volume was registered onto the fMRI0 volume (SPM99) and resampled in the MNI space using the registration parameters calculated in the first procedure. Finally, data were spatially smoothed with an 8-mm3 FWHM Gaussian kernel, leading to an image smoothness of approximately 11 mm in the 3 directions.
Behavioral Data Analysis
A series of repeated measure analysis of variance (ANOVA) was applied on AM and EM scores and on scores obtained on each analogical scale (described above), followed by post hoc tests [protected least significant differences (PLSD) Fisher], to examine the influence of the time-period as within factor.
To better delineate what best characterized episodicity (i.e. memories scored 4, EM), we conducted stepwise regression analyses to study the relationships between the strictly episodic score (EM) and the ten analogical scales, the point of view and the response time, confounding all periods. Stepwise regression analyses are a special case of forward selection of predictive independent variables: in addition to the steps performed in the forward selection algorithm, all variables are tested if their contribution is significant after a new variable has been added. This may lead to elimination of an already selected variable, if this variable has become superfluous because of its relationship to the other variables. Thus, the stepwise method employs a combination of the procedures used in the forward entry and backward removal methods. We chose to include a restrictive criterion for the inclusion and exclusion of the variables, depending on the F values (3/2.996). Then, we carried out a multiple regression on the predictive variables selected by the stepwise regression, including tables to show the regression coefficient and associated significance probability of each variable.
fMRI Data Analysis
Statistical analysis was carried out in two stages, using SPM99 software. First, a fixed-effect (within-subject) model was applied to the time-series of each individual subject. After filtering (high-pass filter: 96 s), t-statistic maps were generated for (1) the contrasts period minus control (P minus C) generating five contrasts and (2) all possible combinations of subtraction (or comparisons) between remote and recent periods (for instance, [P1 - control] - [P5 - control] and the reverse).
Then, a second-level random effects analysis was conducted over contrast images obtained previously, applying the one sample t-test model of SPM99. Performing a second-level random effect analysis implies taking the between subject variability into account and extending inferences from the subjects to the overall population effects. First, a conjunction analysis was performed over the five P minus C contrasts to detect cerebral regions activated in common by all time-periods (height threshold: p < 0.001 uncorrected for multiple comparisons; extent threshold: k > 30 voxels), based on the recently proposed “valid conjunction inference with the minimum statistic” (Nichols et al., 2005). In this test, all the comparisons in the conjunction are individually significant, which corresponds to the valid test for a “logical AND” (i.e. allowing to assess brain areas activated by task A and by task B; in the present study, to assess brain areas activated in common by all five periods). Second, we analysed the direct comparisons between periods (height threshold: p < 0.001 uncorrected for multiple comparisons; extent threshold: k > 30 voxels). The resulting activation maps were superimposed onto the MNI template brain of SPM99.
Optimal anatomical localization of the significant activations was based on the MNI template brain of SPM99, as well as on Talairach coordinates, which were obtained using M. Brett’s linear transforms (see http://www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.html). All the coordinates listed in the sections below are SPM99 (MNI) coordinates.
In addition, a hypothesis-driven regions of interest (ROI) analysis of the hippocampal region (p < 0.05 corrected for multiple comparisons, small volume correction or SVC) was conducted to specifically assess the activity of this region during the five time-periods, based on a priori hypotheses regarding the hippocampus in autobiographical memory retrieval (Ryan et al., 2001; Maguire et al., 2001, 2003a, b; Piefke et al., 2003; Piolino et al., 2004; Gilboa et al., 2004; Addis et al., 2004; Greenberg et al., 2005). ROIs were delineated using MRIcro software (http://www.cla.sc.edu/psych/faculty/rorden/mricro.html). First, a single customized template was obtained by averaging the normalized and smoothed structural T1-MRI of each subject. Then, the boundaries of the right and left hippocampi were manually drawn, by a single rater, on the contiguous coronal slices of the customized template, using a mouse-driven cursor on each slice, from anterior to posterior. In order to ensure inter-rater reliability, a second independent rater traced the boundaries of the left and right hippocampi, from anterior to posterior, on the customized template (see below). We must mention that ROIs were traced by experts who have a good experience in delimiting the boundaries of both hippocampi (Chételat et al., 2003, 2005). We deliberately adopted a conservative approach not trying to include the whole hippocampus, but instead only the hippocampal formation. The hippocampal anatomical boundaries were derived from those used in Watson et al. (1997) to include the CA1 to CA4 sectors, the dentate gyrus, the subiculum complex, alveus and fimbria. Briefly, on anterior-most sections, the head of the hippocampus was separated from the adjacent amygdala by the inferior border of the temporal horn of the lateral ventricles of the alveus. The inferior margin of the hippocampus was outlined to include the subicular complex and the uncal cleft, but to exclude the parahippocampal gyrus, the crus of the fornix, and the isthmus of the cingulate gyrus. The tracing ended posteriorly in the section where the crus of the fornix departs from the lateral wall of the lateral ventricles. In addition of the right and left distinction, we also performed an antero-posterior separation to test models assigning a functional specialization of the hippocampus according to its antero-posterior axis (see introduction). The limit between the anterior and posterior portions of the hippocampus was chosen as corresponding to y = −24, on the Talaraich atlas (see also Krishnan et al., 2006). In total, these subdivisions generated four ROIs, corresponding to the right anterior (R-ant), left anterior (L-ant), right posterior (R-post) and left posterior (L-post) portions of the hippocampus. Of note, although the tracings of the hippocampi were conservative with respect to excluding non-hippocampal tissue, the ROI method is intrinsically more liberal statistically as the correction is for fewer multiple comparisons.
Two approaches were then used. First, we have conducted a ROI analysis with statistics corrected for multiple comparisons at p < 0.05, by applying Worsley et al.’s (1996) small volume correction (SVC) on the hippocampus, using the four ROIs obtained previously for each P minus C contrast. We also report results of the SVC analysis including all ROIs simultaneously. Second, in order to test the time-period effect more precisely, mean hippocampal activation values, corresponding to each time-period, were extracted using the “binary ROI analysis” of the fMRIroi SPM toolbox, with the appropriate ROI as binary image. These values correspond to the difference in BOLD activities between the experimental task and the control task, for each time-period. The statistical analysis of the data was processed with a repeated measure analysis of variance (ANOVA) to examine the influence of time-period (five modalities), hemispheric laterality (right vs. left), and antero-posterior axis (anterior vs. posterior) as within factors.
Both approaches (SVC and repeated measure ANOVA) were used on the new set of ROIs traced by the second independent rater. Since results using the ROIs traced by the first rater (see results) were confirmed by results obtained on the set of new ROIs (traced by the second independent rater), we report only results obtained on the first set of ROIs. Moreover, in all our analyses (conjunction, subtraction and ROI), the control task was subtracted from the experimental task each time. In this way, were subtracted, from the experimental task, processes common to both tasks (i.e. reading operations, mental processing of visual cues and motor processing).
Finally, we studied the relationships between the hippocampal activation (BOLD values) and the various attributes of episodic memory collected during the debriefing session. Stepwise regression analyses were conducted using BOLD values of the four ROIs of the hippocampus (i.e. right and left anterior and posterior portions) as dependent variables and the scores on each behavioral scale and response time as independent ones, confounding all periods. These regression analyses were performed in the same way as described in the section on the behavioral data analysis.
Results
Behavioral Results
Mean behavioral scores (± SD) as well as results from the post hoc analyses [PLSD Fisher tests] are presented in Table 1. Over the five time-periods, results indicate that memories did not differ in terms of specificity, whether it be for the overall autobiographical score (AM) or the strictly episodic score (EM). According to these objective measures of episodicity, this highlights that subjects retrieved strictly episodic memories for all time-periods, at the same extent.
Table 1.
Behavioral measures: comparisons between the five time-periods (mean ± S.D.)
| Behavioral measures | P1 | P2 | P3 | P4 | P5 | p |
|---|---|---|---|---|---|---|
| AM | 0.83 (± 0.17) | 0.92 (± 0.08) | 0.90 (± 0.11) | 0.91 (± 0.07) | 0.96 (± 0.06) | NS |
| EM | 0.62 (± 0.34) | 0.78 (± 0.20) | 0.72 (± 0.27) | 0.76 (± 0.22) | 0.80 (± 0.24) | NS |
| Frequency of rehearsal | 3.26 (± 2.04) | 4.73 (± 1.89) | 4.53 (± 1.04) | 5.07 (± 0.78) | 5.50 (± 1.99) | ** (1) |
| Last recall | 7.11 (± 2.69) | 6.22 (± 1.76) | 5.47 (± 2.11) | 3.26 (± 1.29) | 1.61 (± 0.89) | ‡ (2) |
| Emotion at encoding: | ||||||
| Intensity | 6.86 (± 1.58) | 7.99 (± 1.38) | 8.52 (± 1.30) | 7.51 (± 0.82) | 6.39 (± 1.67) | *** (3) |
| Valence | 4.15 (± 1.68) | 5.56 (± 2.39) | 5.74 (± 2.15) | 6.85 (± 1.42) | 6.89 (± 1.76) | ** (4) |
| Emotion at retrieval: | ||||||
| Intensity | 4.72 (± 2.19) | 5.50 (± 1.91) | 5.82 (± 2.37) | 6.32 (± 1.11) | 5.09 (± 1.59) | * (5) |
| Valence | 5.40 (± 1.83) | 6.40 (± 1.51) | 5.85 (± 1.43) | 6.94 (± 1.22) | 6.57 (± 1.27) | * (6) |
| State of consciousness | 8.11 (± 2.29) | 9.18 (± 1.05) | 9.23 (± 1.29) | 9.54 (± 0.68) | 9.38 (± 0.61) | * (1) |
| Mental strategy used | 9.06 (± 1.18) | 8.64 (± 1.42) | 9.29 (± 1.09) | 9.39 (± 0.69) | 8.65 (± 1.83) | NS |
| Number of mental visual images | 3.72 (± 2.74) | 4.39 (± 2.70) | 4.65 (± 2.40) | 4.59 (± 2.39) | 5.06 (± 2.12) | NS |
| Mental visual image quality | 7.78 (± 2.66) | 8.94 (± 1.41) | 8.99 (± 1.20) | 9.45 (± 0.75) | 9.13 (± 1.25) | * (1) |
| Point of view | 1.21 (± 0.79) | 1.39 (± 0.70) | 1.47 (± 0.55) | 1.47 (± 0.68) | 1.40 (± 0.59) | NS |
| Response times (ms) | 1702.64 (± 986.98) | 1588.53 (± 648.48) | 1679.81 (± 798.12) | 1479.36 (± 564.26) | 1748.43 (± 568.59) | |
p < 0.05
p < 0.01,
p < 0.001,
p < 0.0001.
(1) P1 < P2 = P3 = P4 = P5, (2) P1 = P2 = P3 > P4 > P5, (3) P1 < P2 = P3 > P4 > P5, (4) P1 < P3 = P4 = P5, (5) P1 < P3 = P4 > P5, (6) P1 < P4 = P5.
Abbreviations: AM = overall autobiographical score; EM = strictly episodic score; P1 = 0–17 years; P2 = 18–30 years; P3 = > 31 years old except for the last 5 years; P4 = last 5 years except the last 12 months; P5 = last 12 months.
A series of repeated measure analysis of variance (ANOVA) were applied on scores obtained on the ten analogical scales (see appendix 2), expressed in terms of total score per period. Means (± SD) for each scale and each retention interval are indicated. Numbers in parentheses indicate results of the post hoc analyses [PLSD Fisher tests] showing the effect time-period. Maximum values for each scale is 10 (except for AM, EM and point of view). AM is expressed in terms of ratio (i.e. scores obtained for each of the five memories per period were summed and divided by the maximum score per period which is 20): the higher the percentage, the more specific the memory. EM is also expressed in terms of ratio of strictly episodic memories per period (i.e. number of strictly episodic memories divided by the number of memories). For the point of view, the maximum score is two (0 = observer; 1 = field and observer; 2 = field).
Moreover, concerning subjects’ subjective ratings on the analogical scales, no time-interval effect was detected for the strategy used (massively visual), the number of images, the point of view (massively “field” perspective) and the response times. Subjects took a mean time of 1.64 seconds (mean response time for the whole group) to press on the button. This indicates that, on average, subjects took 1.64 seconds to read and access the cued event, while the rest of the time (i.e. approximately 24s minus 1.64s = 22.4s) was devoted to the mental evocation of the details constituting their memory and to their maintenance in mind. In terms of percentage, this means that subjects spent approximately 6.8% of the time allotted to access their memory and 93.3 % of the rest of the time to the maintenance of their memory in mind. This point clearly indicates that the following neuroimaging results will mainly depict cerebral regions recruited during strictly autobiographical memory (EM) evocation and maintenance, instead of access.
In contrast, significant differences among time-periods appeared for the frequency of rehearsal, last recall, emotional intensity and valence at encoding and retrieval, autonoetic consciousness and mental image quality. Results mainly indicate, first, that memories from the most remote time-period (P1) were less frequently rehearsed than memories from the other time-periods. Additionally, we examined the frequency of no prior rehearsals by calculating the percentage of events which were not rehearsed (i.e. events which scored 0 on the frequency of rehearsal scale, according to subjective ratings) for each time-period, across subjects. Results show that 20% of remote memories (from P1) and 14% of recent memories (from P5) were not rehearsed by subjects (10% for P2; 5 % for P3 and P4). Second, concerning the last recall, remote memories (from P1, P2 and P3) were recalled a longer time ago than recent memories (from P4 and P5) which strengthens the following neuroimaging results regarding the role of the hippocampus in the recall of memories from remote time-periods. Third, subjects retrieved memories from P1 with a slightly less autonoetic state of consciousness, although still autonoetic, compared to the other time-periods. Finally, the mental strategy used for retrieving memories from P1 was massively visual, but the quality of the mental images was slightly less clear, compared to the other time-periods. In addition, memories from P1 and P5 were rated lower in terms of emotional intensity (at encoding or retrieval).
Main results of the multiple regression performed on the predictive variables selected by the stepwise regression show that the last recall, the mental visual image quality and the autonoetic state of consciousness were the best predictors for the strictly episodic score (EM) (i.e. the more an event was recalled a long time ago, accompanied by clear mental visual images and was autonoetic, the more that event was episodic in nature; see Table 2).
Table 2.
Final multiple regression model performed on the predictive variables selected by the stepwise regression using the ten analogical scales, the point of view and the response time as independent variables, for the strictly episodic score (EM), confounding all periods. The table shows the regression coefficient (B) and associated significance probability (p) of each variable.
| Predictive independent variable | B coefficient | t | p |
|---|---|---|---|
| Intercept | 0.36 | 1.16 | 0.280 |
| Last recall | 0.12 | 6.31 | 0.000 |
| Mental visual image quality | 0.07 | 3.15 | 0.013 |
| State of consciousness | 0.09 | 2.36 | 0.045 |
The 12 independent variables: (1) frequency of rehearsal, (2) last recall, (3) emotional intensity at encoding, (4) emotional valence at encoding, (5) emotional intensity at retrieval, (6) emotional valence at retrieval, (7) state of consciousness, (8) mental strategy used, (9) number of mental visual images, (10) mental visual image quality, (11) point of view (12) and response time.
The debriefing session also helped to evaluate if subjects evoked the correct prompted event. Throughout the whole group, a total of nine events (“error” responses) out of three hundred (3 %) were not evoked by subjects: five events for P1, one for P2, P3, P4 and P5. Error responses correspond to events that subjects did not recall (i.e. events were not accessed) and not to the retrieval of wrong events. Thus, except for these rare exceptions, the sentence-cues elicited the retrieval of the correct prompted memory, in the indicated time-period.
fMRI Results
Conjunction Analysis
Relative to the control task (i.e. experimental minus control tasks), activations common to the five retention intervals involved a network including the left superior frontal gyrus, bilateral precuneus/posterior cingulate and lingual gyri, left angular gyrus (Table 3 and Figure 2) and, at p < 0.0015, the left hippocampus (MNI coordinates: x = −18, y = −16, z = −18). Comparing each period to the control task showed results similar to the conjunction analysis (data not shown). Furthermore, adding the response time (RT) as a covariate (i.e. controlling for the time subjects took to access their memories) did not change results either (data not shown). Results of the conjunction analysis performed on the reverse comparison, comparing the control task to the experimental task (i.e. control minus experimental tasks) yielded no hippocampal activation, using the same thresholds (p < 0.001 uncorrected, cluster-level k > 30 voxels).
Table 3.
Results of the conjunction analysis, specifying for each peak the Brodmann area(s) (BA), side, cluster size (k), Z score and t-value, and MNI coordinates (x y z) at p < 0.001 uncorrected, cluster-level k > 30 voxels.
| Regions | BA | Side | k | Z score (t-value) | x | y | z |
|---|---|---|---|---|---|---|---|
| Superior frontal gyrus | 6 | L | 93 | 4.60 (5.11) | −2 | 10 | 60 |
| Precuneus/posterior cingulate gyrus | 31/30/23 | L | 163 | 3.87 (4.16) | −8 | −56 | 10 |
| Lingual gyrus | 18/19 | L | 3.84 (4.14) | −6 | −52 | 0 | |
| Lingual gyrus | 18 | R | 76 | 3.76 (4.03) | 10 | −50 | 4 |
| Precuneus/posterior cingulate gyrus | 31/30/23 | R | 3.67 (3.92) | 10 | −54 | 14 | |
| Angular gyrus | 39 | L | 36 | 3.43 (3.64) | −48 | −74 | 28 |
| 3.43 (3.64) | −44 | −70 | 32 |
Figure 2.
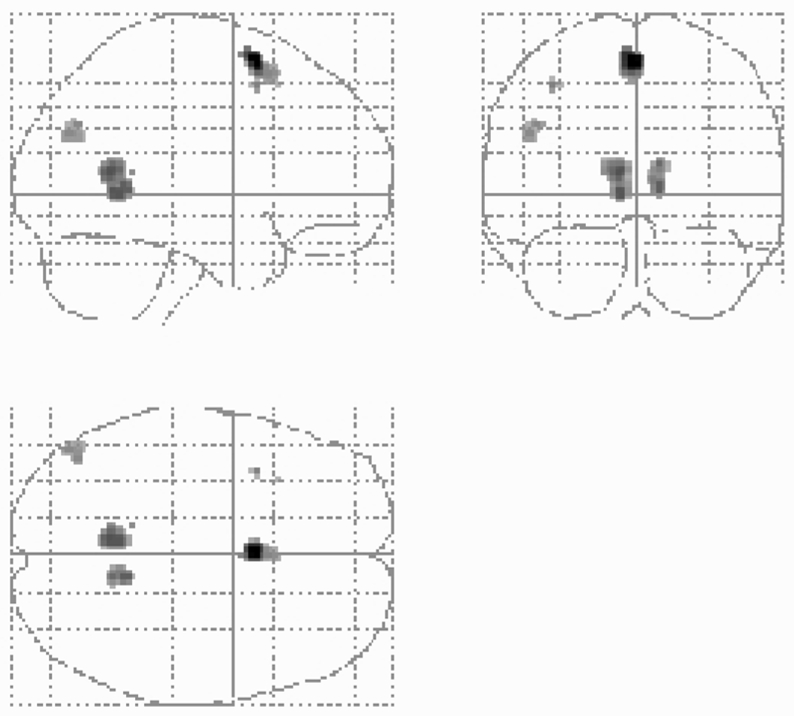
Statistical parametric maps showing the significant cerebral activations when subjects retrieve autobiographical memories regardless of the time-interval compared to the control condition (conjunction analysis). Stereotaxic coordinates are given on Table 3.
Subtraction Analyses
Subtraction analyses showed no differences between each pairwise period contrasts, except between P5 compared to P1 (mainly higher activation of the bilateral precuneus for P5) and between P2 compared to P3 (higher activation of the right temporal pole and gyrus for P2). Results are presented on Table 4 and Figure 3A. The reversed comparisons (i.e. contrasts P1 minus P5 and P3 minus P2) did not show any differences of activation, suggesting that there is no additional significantly active region in P1 compared to P5 and in P3 compared to P2.
Table 4.
Significant results of all SPM contrasts (i.e. for example, when comparing P5 to P1, the contrast computed is equivalent to [P5 - control] - [P1 - control]), showing for each peak the Brodmann area(s) (BA), side, cluster size (k), Z score and t-value, and MNI coordinates (x y z) at p < 0.001 uncorrected, cluster-level k > 30 voxels.
| Region | BA | Side | k | Z score (t-value) | x | y | z |
|---|---|---|---|---|---|---|---|
| P5-P1 | |||||||
| Precuneus | 7 | L | 146 | 4.28 (4.41) | −12 | −64 | 36 |
| Precuneus | 7 | L | 3.53 (3.59) | −8 | −68 | 24 | |
| Middle cingulate gyrus | 23 | L | 52 | 4.24 (4.36) | −6 | −22 | 30 |
| Caudate | R | 36 | 3.79 (3.87) | 22 | 24 | 2 | |
| Postcentral gyrus | L | 40 | 3.59 (3.67) | −64 | −12 | 20 | |
| Precuneus | 7 | R | 132 | 3.56 (3.63) | 12 | −64 | 40 |
| 3.54 (3.47) | 10 | −68 | 28 | ||||
| 3.38 (3.44) | 10 | −78 | 36 | ||||
| P2-P3 | |||||||
| Superior temporal pole | 38 | R | 189 | 4.60 (4.75) | 48 | 16 | −10 |
| Middle temporal gyrus | 21 | R | 3.95 (4.05) | 54 | 4 | −18 | |
| Superior temporal gyrus | 38 | R | 3.86 (3.95) | 52 | 14 | −24 | |
Abbreviations: P1 = 0–17 years; P2 = 18–30 years; P3 = over 31 years old, except for the last 5 years; P5 = last 12 months.
Figure 3.
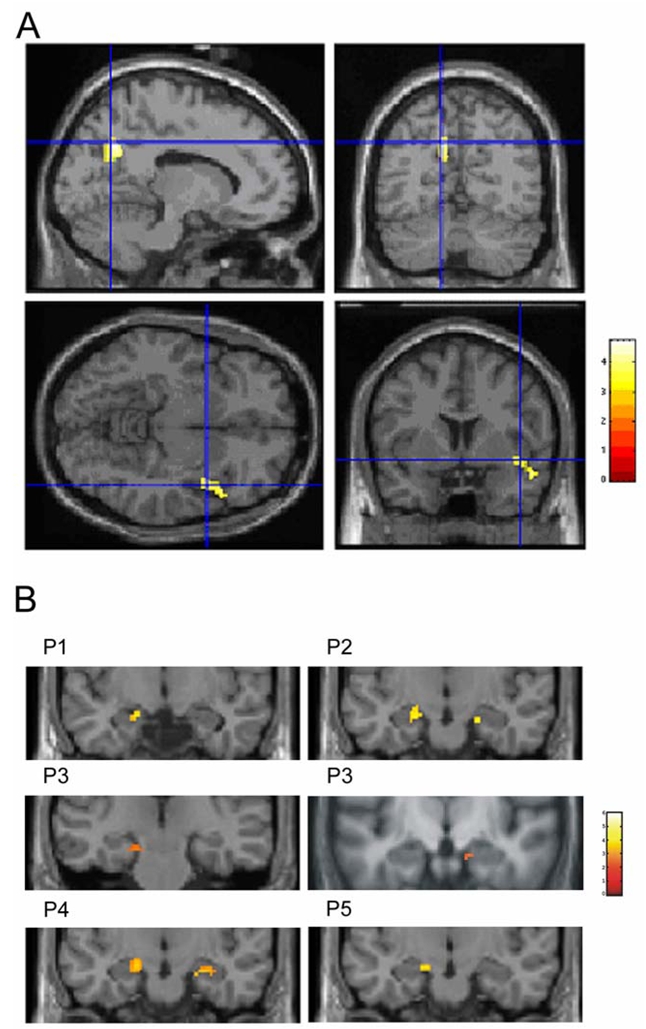
A: Images depicting results of the subtraction analyses. Top left and right: sagittal (right) and coronal (left) planes indicate activation of the bilateral precuneus when comparing memories retrieved from P5 to memories from P1 (crosshair indicates MNI coordinates: x = −12, y = −64, z = 36). Bottom left and right: transverse (right) and coronal (left) planes show activation of the right superior/middle temporal lobe when comparing memories retrieved from P2 to memories from P3 (crosshair indicates MNI coordinates: x = 48, y = 16, z = −10). Colour scale: voxel Z score values. Cross-hairs indicate MNI coordinates.
B: Images depicting results of the small volume correction (SVC) analysis centered on the hippocampus. Coronal planes indicate left hippocampal activation for P1 (−16, −12, −16) and P5 (−14, −10, −16) and bilateral hippocampal activation for P2 (34, −38, −6 and −18, −12, −16), P3 (16, −12, −20 and −18, −18, −18) and P4 (24, −14, −20 and −16, −12, −14). Colour scale: voxel Z score values.
ROI Analysis
The SVC procedure detected significant hippocampal peaks of activation for all time-periods (bilateral for P2, P3 and P4; left-lateralized for P1 and P5; Table 5, Figure 3B). Of note, results of the SVC analysis including all ROIs simultaneously show the same results: left hippocampal activation for each time-period with additional right hippocampal activation for the intermediate time-periods (P2, P3 and P4). The three-way ANOVA (laterality x antero-posterior axis x period) performed on the mean hippocampal values within each ROI revealed a significant main effect of the antero-posterior axis (anterior, 0.168 ± 0.270; posterior, 0.060 ± 0.179; F(1, 11) = 28.9, p = 0.0002). Main effects of laterality (right, 0.121 ± 0.244; left, 0.107 ± 0.226; F(1, 11) < 1, p = 0.43) and period (P1, 0.066 ± 0.204; P2, 0.135 ± 0.261; P3, 0.131 ± 0.246; P4, 0.164 ± 0.287; P5, 0.072 ± 0.144; F(1, 11) < 1, p = 0.56) did not reach statistical significance (see Figure 4). There was no interaction between factors.
Table 5.
Results of the SVC approach depicting significant peak activations in the hippocampus for each P - C contrast (i.e. period minus control).
| Period | Region | Cluster | Voxel | MNI coordinates | ||||
|---|---|---|---|---|---|---|---|---|
| Pcorr | k | p FWE | Z score | x | y | z | ||
| Period 1 | L-ant | 0.006 | 39 | 0.001 | 4.36 | −16 | −12 | −16 |
| Period 2 | R-ant | 0.010 | 27 | 0.006 | 3.88 | 24 | −14 | −20 |
| R-post | 0.015 | 13 | 0.000 | 4.49 | 34 | −38 | −6 | |
| L-ant | 0.007 | 33 | 0.021 | 3.47 | −18 | −12 | −16 | |
| Period 3 | R-ant | 0.031 | 6 | 0.023 | 3.44 | 16 | −12 | −20 |
| L-ant | 0.020 | 13 | 0.014 | 3.60 | −18 | −18 | −18 | |
| L-post | 0.029 | 5 | 0.032 | 3.27 | −30 | −26 | −12 | |
| 0.043 | 1 | 0.044 | 3.16 | −16 | −42 | 8 | ||
| 0.043 | 1 | 0.048 | 3.13 | −12 | −40 | 6 | ||
| Period 4 | R-ant | 0.003 | 54 | 0.000 | 4.61 | 24 | −14 | −20 |
| 0.013 | 3.63 | 32 | −10 | −20 | ||||
| 0.033 | 3.32 | 36 | −16 | −14 | ||||
| R-post | 0.037 | 1 | 0.044 | 3.10 | 14 | −38 | 2 | |
| L-ant | 0.009 | 28 | 0.004 | 3.94 | −16 | −12 | −14 | |
| Period 5 | L-ant | 0.037 | 4 | 0.025 | 3.42 | −14 | −10 | −16 |
| 0.050 | 1 | 0.055 | 3.13 | −20 | −18 | −20 | ||
Abbreviations: L-ant = left anterior hippocampus; R-ant = right anterior hippocampus; L-post = left posterior hippocampus; R-post = right posterior hippocampus.
Figure 4.
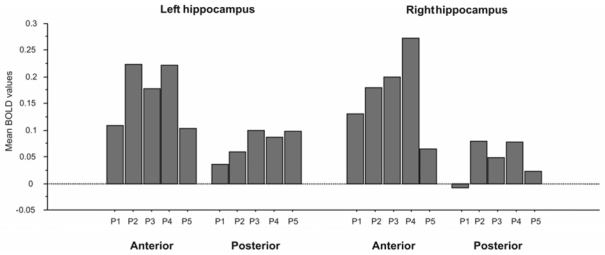
Results of the three-way ANOVA performed on the mean hippocampal values of BOLD activation, within each ROI, according to time-period, laterality and antero-posterior axis.
Abbreviations: P1 = 0–17 years; P2 = 18–30 years; P3 = over 31 years old, except for the last 5 years; P4 = last 5 years, except the last 12 months; P5 = last 12 months.
Main results of the multiple regression performed on the predictive variables selected by the stepwise regression showed that the various attributes of episodic autobiographical memory were good predictors of hippocampal activation (Table 6). Firstly, specificity and number of details, as measured by the EM score, and the different attributes of mental visual imagery (mental visual strategy, mental image quality, number of images and field point of view) were good predictors of right hippocampal activation. Secondly, emotional positive valence and intensity were good predictors of both left and right hippocampal activation, irrespective of the antero-posterior axis. Finally, a low frequency of rehearsal and an autonoetic state of consciousness were good predictors of anterior bilateral hippocampal activation.
Table 6.
Final multiple regression model performed on the predictive variables selected by the stepwise regression using BOLD measures of the four hippocampal ROIs as dependent variables and scores on the ten analogical scales, the point of view and the response time as independent variables, all periods combined. The table shows the regression coefficient (B) and associated significance probability (p) of each variable.
| Dependent variable | Predictive independent variable | B coefficient | T | p |
|---|---|---|---|---|
| L-ant | Intercept | 0.84 | 2.95 | 0.021 |
| Emotional valence at retrieval | 0.11 | 5.18 | 0.001 | |
| Emotional intensity at retrieval | 0.03 | 2.88 | 0.023 | |
| Frequency of rehearsal (−) | 0.05 | 3.93 | 0.005 | |
| State of consciousness | 0.05 | 2.67 | 0.031 | |
| L-post | Intercept | 1.09 | 5.14 | 0.000 |
| Emotional valence at retrieval | 0.13 | 5.25 | 0.053 | |
| Emotional intensity at retrieval | 0.03 | 2.23 | 0.000 | |
| R-ant | Intercept | −0.22 | −1.63 | 0.163 |
| Frequency of rehearsal (−) | −0.09 | −8.15 | 0.000 | |
| State of consciousness | 0.08 | 5.25 | 0.003 | |
| EM score | 0.46 | 5.34 | 0.003 | |
| Emotional valence at encoding | 0.07 | 5.17 | 0.003 | |
| Point of view | 0.08 | 3.56 | 0.016 | |
| Number of mental visual images | 0.01 | 2.35 | 0.065 | |
| R-post | Intercept | −1.20 | −16.60 | 0.000 |
| EM score | 0.35 | 22.49 | 0.000 | |
| Emotional valence at encoding | 0.07 | 19.28 | 0.000 | |
| Frequency of rehearsal (−) | −0.03 | −13.81 | 0.000 | |
| Mental strategy used | 0.05 | 10.34 | 0.000 | |
| Emotional intensity at retrieval | 0.02 | 7.71 | 0.001 | |
| Point of view | 0.02 | 3.56 | 0.023 | |
| Number of mental visual images | 0.00 | 3.05 | 0.038 | |
Abbreviations: EM = strictly episodic score; df = degrees of freedom; L-ant = left anterior hippocampus; R-ant = right anterior hippocampus; L-post = left posterior hippocampus; R-post = right posterior hippocampus.
We added a minus (−) to indicate negative relation with predictors.
Discussion
We examined the cerebral structures implicated in the retrieval of autobiographical memories according to five time-periods covering the whole lifespan of healthy aged subjects with a methodology enabling a strict control of the nature of the events evoked. Firstly, the main neuroimaging data showed that a network including, in particular, the left superior frontal gyrus, the bilateral precuneus/posterior cingulate and lingual gyri, and the left hippocampus, was commonly active for all time-periods, although subtle differences appeared when subtracting certain time-periods to others. Secondly, the ROI analysis revealed that the hippocampus was active whatever the time-period during the retrieval of specific autobiographical memories as confirmed by the behavioral data obtained at debriefing. These results will now be discussed in the light of recent concepts of autobiographical recollection and according to their relevance with models of long-term memory consolidation.
Behavioral Data
Based upon an experimental investigation preventing prior re-activation, using tight criteria for episodicity and an extensive checking procedure, the behavioral results confirm that subjects were engaged in episodic recollection during scanning whatever the time-period. Indeed, for all time-periods, memories were characterized by specificity, as measured by the AM and EM scores, which represent objective measures of episodicity. Moreover, subjective measures of episodicity, based on subjects’ ratings on the analogical scales, showed that memories were retrieved with a massively visual mental strategy and an overall autonoetic state of consciousness, whatever the time-period, attesting to their episodic nature (Brewer, 1996; Conway, 2001; Tulving, 2001).
Additional analyses helped to determine what best characterized episodicity in our study. Results of the regression analyses showed that the last recall was the best predictor for the strict episodic score (EM) suggesting that the more an event was reminded very recently, the less the memory was very detailed. Interestingly, results on the last recall scale indicate that the more memories were remote, the less they were reminded or recalled recently (see Table 1). Additionally, examination of the frequency of no prior rehearsals (see behavioral results) shows that many remote memories were not rehearsed by subjects prior to scanning. Thus, the fact that remote memories were specific and detailed was not due to the fact that they were recalled recently, but probably because they kept th eir episodic nature through time. Indeed, for remote memories to be retained and preserved over retention intervals as long as fifty to sixty years indicates that they probably constitute, for the subjects, highly self-defining memories which resist the effects of time. Otherwise, the quality of the mental visual images and the autonoetic state of consciousness were also good predictors of the EM score. This result is not surprising as autonoetic consciousness refers to the mental state in which one can consciously recollect the details such as images, emotions and perceptions, present during the original encoding context (Wheeler et al., 1997; Tulving, 2002). Detailed memories are, indeed, often accompanied by strong imagery reports (Brewer, 1988, 1996; Greenberg and Rubin, 2003).
Although memories from the five retention intervals were well matched on numerous characteristics including objective ratings, memories from the most remote time-period (P1) showed subtle differences compared to the other periods, on some of the subjective recollective ratings. They were less rehearsed, emotionally less intense, slightly less autonoetic, although still recalled with an autonoetic state of consciousness, and image quality was slightly less clear, although the mental strategy used by subjects, for this time-period, was still massively visual. These features of very remote memories have already been reported in previous neuroimaging studies (Niki and Luo, 2002; Piefke et al., 2003). However, memories from P1 were not significantly different from memories of the other time-periods in terms of specificity, as measured by the objective AM and EM scores of episodicity, confirming previous findings which have demonstrated the existence of some old episodic memories, even in older subjects (Piolino et al., 2002, 2006). The objective AM and EM scores might actually tap a different aspect of episodicity than the subjective recollective ratings. Indeed, one might need to reach only a certain level or threshold of episodicity, in order for an event to be episodic. Then, above the threshold, there are graduations or modulations on certain episodic qualities (Piolino et al., 2006). Thus, our results show that all memories reached the limit of episodicity and were all specific, but some memories showed different modulations on certain episodic qualities.
Similarly, although memories from the most recent period (P5) were actually rated as episodic (AM and EM scores) as the other periods, they were rated as less intense emotionally at encoding. These memories were qualitatively different from other memories as they were composed, in part, of non-emotional events that had happened very recently (past days, weeks or months). According to Conway (2001), although such recent memories are episodic in nature, they are unlikely to be retained, unless they are highly goal-specific and rehearsed. Altogether, there were no differences across time-periods in terms of specificity and presence of details as measured by the AM and EM scores (objective measures of episodicity), suggesting that all memories passed the threshold of episodicity. Then, above this threshold, some memories (e.g. from P1 and P5) showed graduations on certain episodic qualities as measured by the recollective ratings (subjective measures).
Imaging Data
Cerebral Network Underlying Autobiographical Memory Retrieval
The neuroimaging data showed that regardless of the time-interval, the retrieval of autobiographical memories involved a network comprising regions which have previously been found to be implicated in autobiographical recollection (see reviews by Cabeza and Nyberg, 2000; Maguire, 2001; Gilboa, 2004; Moscovitch et al., 2005): the left superior frontal gyrus (BA 6; involved in verbal working memory tasks; Conway et al., 1999; Piefke et al., 2003; Piolino et al., 2004), the bilateral lingual gyrus (active in encoding and recall of complex visual stimuli; Gilboa et al., 2004), the left angular gyrus (BA 39, or temporo-parietal junction; implicated in imagery and spatial processing in the context of episodic memory; Conway et al., 1999; Maguire et al., 1999, 2000, 2003a, b; Gilboa et al., 2004;Addis et al, 2004a; Levine et al., 2004; Greenberg et al., 2005) and the bilateral precuneus/posterior cingulate gyrus (strongly engaged in visual mental imagery; e.g. Gilboa et al., 2004; see Fletcher et al., 1995; Cabeza and Nyberg, 2000). A role of the posterior cingulate region in episodic memory retrieval has already been established (Chételat et al., 2003; Shannon et al., 2004; for reviews see Cabeza and Nyberg, 2000). Neuroimaging studies suggest a role of the posterior cingulate cortex and the adjacent precuneus in integrating self-referential stimuli (for example, familiar names) in the autobiographical context of a person (Fink et al., 1996; Maddock et al., 2001; for review, see Northoff et al., 2004). We report activations bilaterally in posterior sites. This pattern of activation fits nicely with that proposed by Conway and Pleydell-Pearce (2000) in their cognitive-motivational model of autobiographical memory which posits that sensory-perceptual episodic details are stored in bilateral occipito-parietal networks. The left hippocampal involvement whatever memory remoteness will be discussed, in more details, hereafter.
Differential Activations According to the Remoteness of Autobiographical Memories
Specific activations were found when subtracting particular periods to others. Increased activation in the bilateral precuneus (BA 7/31) was observed for the most recent period (P5) compared to the most remote period (P1), in line with results reported by other authors (Tsukiura et al., 2002; Niki and Luo, 2002). Tsukiura et al. (2002) detected activation in the left precuneus during the retrieval of autobiographical memories from the recent past compared to memories from childhood. Niki and Luo (2002) also detected activation of the precuneus (BA 7) when recalling recent memories compared to remote memories, bilaterally. Previous functional imaging studies suggest a role for the precuneus in mental imagery during episodic memory retrieval (Fletcher et al., 1995; Shallice et al., 1994). Thus, retrieval of very remote memories from P1 appears to engage lower visual imagery processing than recent memories in keeping with the behavioral data.
Additional right superior temporal pole and superior/middle temporal gyrus activations involved in semantic memory were detected for the recall of memories from P2 compared to memories from the intermediate time-period (P3). Fink et al. (1996) found right lateral (superior and middle) temporal lobe and temporal pole activations when comparing personal with impersonal memory conditions and suggested their importance in autobiographical ecphory. Indeed, memories from this remote time-period (P2), termed the “reminiscence bump” by Rubin et al. (1986), correspond to memories encoded during adolescence and young adulthood which are particularly well remembered by subjects over 40 years old and resist the effects of time. They consist of first-time experiences (Robinson, 1992), important and emotional events (such as marriage or children’s birth), known to be especially vivid memories for older subjects (for review, see Rubin and Schulkind, 1997; Piolino et al., 2002, 2006). Interestingly, this result points to the role of semantic knowledge in accessing episodic memories from the reminiscence bump which are particularly critical in sense of identity (Conway and Pleydell-Pearce, 2000).
Hippocampal Involvement in the Retrieval of Autobiographical Memories
The conjunction analysis revealed left hippocampal activation for all time-periods and the ROI analysis, using SVC, confirmed this result: significant left hippocampal activation was detected for all time-periods and an additional significant right hippocampal activation was detected for the intermediate periods (P2, P3 and P4). Further analyses based on a three-way ANOVA (laterality x antero-posterior axis x period) confirmed a permanent involvement of the hippocampus whatever the time-period. By subtracting the control task from the experimental task in all our analyses (conjunction, subtraction and ROI), we controlled for processes common to both tasks (i.e. reading operations, mental processing of visual cues and motor processing). Thus, presumably, processes concerning only the experimental task, in particular, memory retrieval per se, were detected in the experimental minus control contrasts. In consequence, the hippocampal activation detected in the experimental task is probably due to the nature of the memory task, as there is no memory retrieval in the control task.
Overall, the present data challenge the standard model of memory consolidation and support the multiple-trace model, instead (see introduction), confirming previous findings showing a permanent involvement of the hippocampus in the retrieval of recent and remote autobiographical memories with no reactivation of the memories before scanning (Mayes et al., 2000; Piolino et al., 2004; Gilboa et al., 2004; Steinvorth et al., 2006; some subjects for Ryan et al., 2001), as well as studies examining the recency effect using parametric modulation analyses (Maguire et al., 2001 and Addis et al., 2004), albeit these studies both fail to examine long retention intervals given the age of the subjects.
Our behavioral data bring us strong insights on the relationships between hippocampal activation and the various attributes of episodic memory. Behavioral results showed that episodicity of memories explains the permanent hippocampal activation for all time-periods, independently of a recent reactivation. Then, according to the time-period, memories presented subtle differences on certain recollective qualities (see above) which might explain the unilateral involvement of the hippocampus for the most remote and recent time-periods. We can posit that right hippocampal activation associated to left hippocampal activation reflects the retrieval of specific memories particularly richer on certain recollective qualities, which is the case for memories of the intermediate time-periods. Indeed, recent neuroimaging studies have detected bilateral hippocampal activation in subjects engaged in the retrieval of specific autobiographical memories rated high in terms of richness of detail, vividness, emotionality, re-experiencing (Ryan et al., 2001; Piefke et al., 2003; Piolino et al., 2004; Gilboa et al., 2004; Addis et al., 2004; Greenberg et al., 2005; Steinvorth et al., 2006) or personal significance (Addis et al., 2004).
Further argument was provided by the regression analyses which showed that the EM score and the various behavioral scales were predictive of hippocampal activation. Indeed, hippocampal activation appeared particularly sensitive to specificity (EM which, on our scale of episodicity, takes into account the presence of phenomenological details), particularly so on the right. Hippocampal activation was also predicted by recollective qualities, such as the different attributes of mental visual imagery (i.e. retrieval strategy used, number of images, field point of view) and activation was also lateralized to the right. In our study, the task was specially designed to engage subjects in the re-experiencing or reliving of affective and perceptual details of the encoding episode. Accordingly, the subjects used a primarily visual strategy to gain access and relive the spatial context of the personal events. This aspect could have contributed to our results concerning the right hippocampal activation since this region seems particularly involved in spatial memory (Burgess et al., 2002). Otherwise, results of the regression analyses also showed that emotional positive valence and intensity of autobiographical memories was not lateralized, predicting both right and left hippocampal activation, concordant with recent findings (Vandekerckhove et al., 2005). Similarly, Piefke et al. (2003) detected bilateral activation in the entorhinal cortex when retrieving positive autobiographical memories, compared to negative ones. Thus, the earlier view of hemispheric cerebral specialization for positive and negative emotional experience (valence model of emotion, Davidson, 1992; Lee et al., 2004; see also Markowitsch et al., 2003) may have to be modified suggesting that a more bi-hemispheric distributed neural network supports the multimodal emotional components of autobiographical memories. Interestingly, our results confirm previous data showing enhanced bilateral hippocampal activation during conscious re-experiencing (remembering) compared to retrieval accompanied only by a feeling of familiarity (knowing) (Eldridge et al., 2000; Wheeler and Buckner, 2004).
The effect of aging on brain activity might also be a factor to take into account in our results concerning the bilaterality of hippocampal activation. To date, only two studies on autobiographical memory have been conducted on older subjects and both detected bilateral activation (instead of left-sided activation in young subjects) during the autobiographical memory task compared to the control task (Ryan et al., 2001; Maguire et al., 2003b). Maguire et al. (2003b) suggested that a hemispheric asymmetry reduction in older adults (the HAROLD model, Cabeza, 2002) could account for the bilateral involvement of the hippocampus. Yet, several authors have detected bilateral hippocampal involvement in young subjects during the retrieval of episodic autobiographical memories (Piefke et al., 2003; Piolino et al., 2004; Addis et al., 2004a), which challenges the idea of a bilateral involvement of the hippocampus due to age. Thus, in our study, we do not believe that bilateral hippocampal activations observed in the group of subjects was only attributable to their age.
Moreover, the ROI analysis based on a three-way ANOVA clearly showed that the involvement of the hippocampus concerned both anterior and posterior parts during autobiographical recollection, whatever the time-period, although more importantly in its anterior than in its posterior part. In light of the HIPER model (see introduction, Lepage et al., 1998), the posterior hippocampal activation would reflect retrieval processes which concerned memories from the five time-periods. The anterior hippocampal activation would reflect simultaneous encoding processes, accompanying the retrieval. We controlled for basic encoding-related processes in all our analyses since the control task was subtracted from the experimental task, both likely to yield encoding-related processes. Yet, one might argue that the encoding processes engaged by the control task are likely to be much less complex than those engaged by the experimental task and, thus, may not fully control for re-encoding. Hence, hippocampal activation detected in our experimental task could be attributable to the task itself (i.e. memory retrieval; Buckner et al., 2001), as well as to complex encoding-related processes, not present in the control task, which accompany episodic memory retrieval processes regardless of their remoteness, as suggested by MTT (see introduction). Interpretations based on the HIPER model must, however, be modulated (Schacter and Wagner, 1999), suggesting that further work is needed to clarify this model.
Unlike other authors (Piefke et al., 2003; Piolino et al., 2004; Gilboa et al., 2004), we did not detect differential antero-posterior activation of the hippocampus according to memory remoteness. Gilboa et al. (2004) showed that the retrieval of remote memories, after presentation of familiar (i.e. personal) photos, was associated with distributed activation along the antero-posterior axis of the hippocampus, while activations associated with the retrieval of recent memories were clustered in its anterior portion. Their use of a re-encoding control task (imagine scenarios associated with unfamiliar photos) served as a control for encoding-related processes of complex visual scenes (common to both their experimental and control conditions), as encoding of information, especially in the case of processing of complex visual scenes, might yield hippocampal activation (Stern et al., 1996; Stark and Squire, 2001). Yet, this hypothesis is unlikely, in our study, since cues were not complex visual scenes, but consisted of short sentences. Although using a re-encoding control condition, Gilboa et al. (2004) still detected anterior hippocampal activation for both recent and remote memories. Overall, they suggest that the retrieval of context-rich autobiographical memories invariably involves the whole hippocampus, consistent with our findings.
In conclusion, we conducted an experimental procedure designed to assess strictly episodic autobiographical memories, preventing subjects from re-activating their memories before the scanning session, using a strict checking procedure. To the best of our knowledge, this study is the first to provide evidence of a hippocampal involvement over five time-periods covering the entire lifetime of older subjects, during the retrieval of episodic autobiographical memories. Our data provide new insights that question the standard model of memory consolidation and support the alternative MTT regarding the episodic nature of the stored information.
Acknowledgments
The authors wish to thank Nicolas Delcroix, Marc Joliot, Franck Lamberton, Bernard Mazoyer, Emmanuel Mellet, Laurent Petit and Nathalie Tzourio-Mazoyer for their help. We are also grateful to the family members who participated in the pre-scan interviews. A.V. is supported by the Association France Alzheimer’s fellowships for Young Researchers.
Appendix 1
Specificity scoring chart of event recall task performed after scans (Piolino et al., 2002).
| 4 | Specific event (isolated, situated in time and space) with details (unfolding of actions, thoughts, perceptions, images, etc) |
| 3 | Specific event (isolated, situated in time and space) with few details |
| 2 | Generic event (repeated or prolonged over time, situated in time and space) |
| 1 | Vague event (repeated or prolonged over time, not situated in time and space) |
| 0 | Absence of memory or general information about a theme |
Appendix 2
Ten analogical scales (10 cm lines) were used to specify the different aspects of recollective experience in terms of:
Frequency of rehearsal (0 = never to 10 = very frequent),
Last recall (0 = today to 10 = over 10 years ago),
Emotional intensity at encoding (0 = no emotion to 10 = very strong emotion),
Emotional valence at encoding (0 = very negative to 10 = very positive),
Emotional intensity at retrieval in the scanner (0 = no emotion to 10 = very strong emotion),
Emotional valence in the scanner (0 = very negative to 10 = very positive),
State of consciousness (0 = knowing to 10 = remembering).
Mental strategy used (0 = verbal to 10 = visual),
Number of mental visual images (0 = no images to 10 = over 10 images),
Mental visual image quality (0 = very blurry to 10 = very clear).
References
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004a;14:752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP. Characterizing spatial and temporal features of autobiographical memory retrieval networks: a partial least squares approach. Neuroimage. 2004b;23:1460–1471. doi: 10.1016/j.neuroimage.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Bayley PJ, Squire LR. Failure to acquire new semantic knowledge in patients with large medial temporal lobe lesions. Hippocampus. 2005;15:273–280. doi: 10.1002/hipo.20057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower GH. How might emotions affect learning? In: Christianson SA, editor. The handbook of emotion and memory: Research and theory. Hillsdale: Lawrence Erlbaum; 1992. pp. 3–31. [Google Scholar]
- Brewer W. What is autobiographical memory? In: Rubin DC, editor. Autobiographical Memory. Cambridge: Cambridge University Press; 1986. pp. 25–49. [Google Scholar]
- Brewer W. Memory for randomly sampled autobiographical events. In: Neisser U, Winograd E, editors. Remembering reconsidered: ecological and traditional approaches to the study of memory. Cambridge: Cambridge University Press; 1988. pp. 21–90. [Google Scholar]
- Brewer W. What is recollective memory? In: Rubin DC, editor. Remembering our past: studies in autobiographical memory. Cambridge: Cambridge University Press; 1996. pp. 19–66. [Google Scholar]
- Buckner RL, Wheeler ME, Sheridan MA. Encoding processes during retrieval tasks. J Cogn Neurosci. 2001;13:406–415. doi: 10.1162/08989290151137430. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cermak LS. The episodic semantic distinction in amnesia. In: Squire LR, Butters N, editors. The Neuropsychology of Memory. New York: The Guilford Press; 1984. pp. 55–62. [Google Scholar]
- Chételat G, Desgranges B, de la Sayette V, Viader F, Berkouk K, Landeau B, Lalevee C, Le Doze F, Dupuy B, Hannequin D, Baron JC, Eustache F. Dissociating atrophy and hypometabolism impact on episodic memory in mild cognitive impairment. Brain. 2003;126:1955–1967. doi: 10.1093/brain/awg196. [DOI] [PubMed] [Google Scholar]
- Chételat G, Landeau B, Eustache F, Mezenge F, Viader F, de la Sayette V, Desgranges B, Baron JC. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 2005;27:934–46. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Shallice T, Chan D, Fox N, Scahill R, Harrison G, Stevens J, Rudge P. Long-term retrograde amnesia... the crucial role of the hippocampus. Neuropsychologia. 2001;39:151–172. doi: 10.1016/s0028-3932(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Conway MA, Dewhurst SA. Remembering, familiarity, and source monitoring. Q J Exp Psychol A. 1995;48:125–140. doi: 10.1080/14640749508401380. [DOI] [PubMed] [Google Scholar]
- Conway MA, Turk DJ, Miller SL, Logan J, Nebes RD, Meltzer CC, Becker JT. A positron emission tomography (PET) study of autobiographical memory retrieval. Memory. 1999;7:679–702. doi: 10.1080/096582199387805. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychol Rev. 2000;107:261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Conway MA. Sensory-perceptual episodic memory and its context: autobiographical memory. Philos Trans R Soc Lond B Biol Sci. 2001;356:1375–1384. doi: 10.1098/rstb.2001.0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW, Whitecross SE, Sharpe H. Brain imaging autobiographical memory. Psychol Learn Motiv: Adv Res Theory. 2002;41:229–263. [Google Scholar]
- Crawley SE, French CC. Field and observer viewpoint in remember/know memories of personal childhood events. Memory. 2005;13:673–681. doi: 10.1080/09658210444000296. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain Cogn. 1992;20:125–51. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Deltour JJ. Braine-le-Château: Éditions l’application des techniques modernes. 1993. Échelle de vocabulaire de Mill Hill de JC Raven. Adaptation française et normes européennes du Mill Hill et du Standard Progressive Matrices de Raven. [Google Scholar]
- Denkova E, Botzung A, Scheiber C, Manning L. Implicit emotion during recollection of past events: a nonverbal fMRI study. Brain Res. 2006;1078:143–50. doi: 10.1016/j.brainres.2006.01.061. [DOI] [PubMed] [Google Scholar]
- Dewhurst SA, Conway MA. Pictures, images, and recollective experience. J Exp Psychol Learn Mem Cogn. 1994;20:1088–1098. doi: 10.1037//0278-7393.20.5.1088. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Lane R, Chua P, Fletcher P. Dissociable temporal lobe activations during emotional episodic memory retrieval. Neuroimage. 2000;11:203–209. doi: 10.1006/nimg.2000.0538. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3:1149–52. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Eustache F, Piolino P, Giffard B, Viader F, De La Sayette V, Baron JC, Desgranges B. ‘In the course of time’: a PET study of the cerebral substrates of autobiographical amnesia in Alzheimer’s disease. Brain. 2004;127:1549–1560. doi: 10.1093/brain/awh166. [DOI] [PubMed] [Google Scholar]
- Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD. Cerebral representation of one’s own past: neural networks involved in autobiographical memory. J Neurosci. 1996;16:4275–4282. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ. The mind’s eye-precuneus activation in memory related imagery. Neuroimage. 1995;2:195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fujii T, Moscovitch M, Nadel L. Memory consolidation, retrograde amnesia, and the temporal lobe. In: Boller F, Grafman J, editors. The handbook of neuropsychology. Vol. 4. Amsterdam: Elsevier; 2000. pp. 223–250. [Google Scholar]
- Gadian DG, Aicardi J, Watkins KE, Porter DA, Mishkin M, Vargha-Khadem F. Developmental amnesia associated with early hypoxic-ischaemic injury. Brain. 2000;123:499–507. doi: 10.1093/brain/123.3.499. [DOI] [PubMed] [Google Scholar]
- Gardiner JM. Functional aspects of recollective experience. Mem Cognit. 1988;16(4):309–13. doi: 10.3758/bf03197041. [DOI] [PubMed] [Google Scholar]
- Gardiner JM, Java RI. Recognizing and remembering. In: Collins AF, Gathercole SE, Conway MA, Morris PE, editors. Theories of memory. Hove: Lawrence Erlbaum Associates Ltd; 1993. pp. 163–188. [Google Scholar]
- Gardiner JM. Episodic memory and autonoetic consciousness: a first-person approach. Philos Trans R Soc Lond B Biol Sci. 2001;356:1351–1361. doi: 10.1098/rstb.2001.0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events. Cereb Cortex. 2004;14:1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Gilboa A. Autobiographical and episodic memory-one and the same? Evidence from prefrontal activation in neuroimaging studies. Neuropsychologia. 2004;42:1336–1349. doi: 10.1016/j.neuropsychologia.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Goddard L, Pring L, Felmingham N. The effects of cue modality on the quality of personal memories retrieved. Memory. 2005;13:79–86. doi: 10.1080/09658210344000594. [DOI] [PubMed] [Google Scholar]
- Graham KS, Hodges JR. Differentiating the roles of the hippocampal complex and the neocortex in long-term memory storage: evidence from the study of semantic dementia and Alzheimer’s disease. Neuropsychology. 1997;11:77–89. doi: 10.1037//0894-4105.11.1.77. [DOI] [PubMed] [Google Scholar]
- Graham KS, Lee AC, Brett M, Patterson K. The neural basis of autobiographical and semantic memory: new evidence from three PET studies. Cogn Affect Behav Neurosci. 2003;3:234–254. doi: 10.3758/cabn.3.3.234. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Rubin DC. The neuropsychology of autobiographical memory. Cortex. 2003;39:687–728. doi: 10.1016/s0010-9452(08)70860-8. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Rice HJ, Cooper JJ, Cabeza R, Rubin DC, Labar KS. Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia. 2005;43:659–674. doi: 10.1016/j.neuropsychologia.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Grober E, Buschke H. Genuine memory deficits in dementia. Dev Neuropsychol. 1987;3:13–36. [Google Scholar]
- Guillery B, Desgranges B, Katis S, de la Sayette V, Viader F, Eustache F. Semantic acquisition without memories: evidence from transient global amnesia. Neuroreport. 2001;12:3865–3869. doi: 10.1097/00001756-200112040-00052. [DOI] [PubMed] [Google Scholar]
- Haist F, Bowden Gore J, Mao H. Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nat Neurosci. 2001;4:1139–1145. doi: 10.1038/nn739. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Ryan L, Schnyer DM, Nadel L. An fMRI study of episodic memory: retrieval of object, spatial, and temporal information. Behav Neurosci. 2004;118:885–896. doi: 10.1037/0735-7044.118.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman MD, Wilson BA, Baddeley AD. The autobiographical memory interview: a new assessment of autobiographical and personal semantic memory in amnesic patients. J Clin Exp Neuropsychol. 1989;11:724–744. doi: 10.1080/01688638908400928. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Slavin MJ, Tran TT, Doraiswamy PM, Petrella JR. Accuracy of spatial normalization of the hippocampus: Implications for fMRI research in memory disorders. Neuroimage. 2006;31(2):560–71. doi: 10.1016/j.neuroimage.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Lee GP, Meador KJ, Loring DW, Allison JD, Brown WS, Paul LK, Pillai JJ, Lavin TB. Neural substrates of emotion as revealed by functional magnetic resonance imaging. Cogn Behav Neurol. 2004;17:9–17. doi: 10.1097/00146965-200403000-00002. [DOI] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: the HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Levine B, Turner GR, Tisserand D, Hevenor SJ, Graham SJ, McIntosh AR. The functional neuroanatomy of episodic and semantic autobiographical remembering: a prospective functional MRI study. J Cogn Neurosci. 2004;16:1633–1646. doi: 10.1162/0898929042568587. [DOI] [PubMed] [Google Scholar]
- Linton M. Ways of searching and the contents of memory. In: Rubin DC, editor. Autobiographical Memory. Cambridge: Cambridge University Press; 1986. pp. 50–67. [Google Scholar]
- Linton M. The maintenance of knowledge: some long-term specific and generic changes. In: Gruneberg MM, Morris PE, Sykes RN, editors. Practical aspects of memory. London: Wiley; 1988. pp. 378–384. [Google Scholar]
- MacDonald S, Uesiliana K, Hayne H. Cross-cultural and gender differences in childhood amnesia. Memory. 2000;8:365–376. doi: 10.1080/09658210050156822. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–676. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ. Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus. 1999;9:54–61. doi: 10.1002/(SICI)1098-1063(1999)9:1<54::AID-HIPO6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ, Buchel C. Patterns of hippocampal-cortical interaction dissociate temporal lobe memory subsystems. Hippocampus. 2000;10:475–482. doi: 10.1002/1098-1063(2000)10:4<475::AID-HIPO14>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Henson RN, Mummery CJ, Frith CD. Activity in prefrontal cortex, not hippocampus, varies parametrically with the increasing remoteness of memories. Neuroreport. 2001;12:441–444. doi: 10.1097/00001756-200103050-00004. [DOI] [PubMed] [Google Scholar]
- Maguire EA. Neuroimaging studies of autobiographical event memory. Philos Trans R Soc Lond B Biol Sci. 2001;356:1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Lateral asymmetry in the hippocampal response to the remoteness of autobiographical memories. J Neurosci. 2003a;23:5302–5307. doi: 10.1523/JNEUROSCI.23-12-05302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain. 2003b;126:1511–1523. doi: 10.1093/brain/awg157. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Phil Trans R Soc Lond B. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ. Differential contribution of the right and left amygdale to affective information processing. Behav Neurol. 1988;11:233–244. doi: 10.1155/1999/180434. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Vandekerckhovel MM, Lanfermann H, Russ MO. Engagement of lateral and medial prefrontal areas in the ecphory of sad and happy autobiographical memories. Cortex. 2003;39:643–665. doi: 10.1016/s0010-9452(08)70858-x. [DOI] [PubMed] [Google Scholar]
- Mattis S. Mental status examination for organic mental symptoms in elderly patients. In: Bellak L, Karatsu TB, editors. Geriatric psychiatry: a handbook for psychiatrists and primary care physicians. New York: Grune et Stratton; 1976. pp. 77–121. [Google Scholar]
- Mayes AR, Mackay CE, Montaldi D, Downest JJ, Singh KD, Norberts N. Does retrieving decades-old spatial memories activate the medial temporal lobes less than retrieving recently acquired spatial memories ? NeuroImage. 2000;11:S421. [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, McAndrews MP, Levine B, Black S, Winocur G, Nadel L. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. J Anat. 2005;207:35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre JM. TraceLink: a model of amnesia and consolidation of memory. Hippocampus. 1996;6:675–684. doi: 10.1002/(SICI)1098-1063(1996)6:6<675::AID-HIPO10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. The hippocampal complex and long-term memory revisited. Trends Cogn Sci. 2001;5:228–230. doi: 10.1016/s1364-6613(00)01664-8. [DOI] [PubMed] [Google Scholar]
- Nadel L, Hardt O. The spatial brain. Neuropsychology. 2004;18:473–476. doi: 10.1037/0894-4105.18.3.473. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nigro G, Neisser U. Point of view in personal memories. Cogn Psychol. 1983;15:467–482. [Google Scholar]
- Niki K, Luo J. An fMRI study on the time-limited role of the medial temporal lobe in long-term topographical autobiographic memory. J Cogn Neurosci. 2002;14:500–507. doi: 10.1162/089892902317362010. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, Kawashima R, Fukuda H, Itoh M, Yamadori A. Thinking of the future and past: the roles of the frontal pole and the medial temporal lobes. Neuroimage. 2003;19:1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- Persson J, Nyberg L. Conjunction analysis of cortical activations common to encoding and retrieval. Microsc Res Tech. 2000;51:39–44. doi: 10.1002/1097-0029(20001001)51:1<39::AID-JEMT4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126:650–668. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- Piefke M, Fink GR. Recollections of one’s own past: the effects of aging and gender on the neural mechanisms of episodic autobiographical memory. Anat Embryol. 2005;210:497–512. doi: 10.1007/s00429-005-0038-0. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Markowitsch HJ, Fink GR. Gender differences in the functional neuroanatomy of emotional episodic autobiographical memory. Hum Brain Mapp. 2005;24:313–324. doi: 10.1002/hbm.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Benali K, Eustache F. Episodic and semantic remote autobiographical memory in ageing. Memory. 2002;10:239–257. doi: 10.1080/09658210143000353. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Belliard S, Matuszewski V, Lalevée C, De la Sayette V, Eustache F. Autobiographical memory and autonoetic consciousness: triple dissociation in neurodegenerative diseases. Brain. 2003a;126:2203–2219. doi: 10.1093/brain/awg222. [DOI] [PubMed] [Google Scholar]
- Piolino P, Belliard S, Desgranges B, Perron M, Eustache F. Autobiograhical memory and autonoetic consciousness in a case of semantic dementia. Cognitive Neuropsychology. 2003b;20:619–639. doi: 10.1080/02643290242000899. [DOI] [PubMed] [Google Scholar]
- Piolino P, Giffard-Quillon G, Desgranges B, Chételat G, Baron JC, Eustache F. Re-experiencing old memories via hippocampus: a PET study of autobiographical memory. Neuroimage. 2004;22:1371–1383. doi: 10.1016/j.neuroimage.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Clarys D, Guillery-Girard B, Taconnat L, Isingrini M, Eustache F. Autobiographical memory, autonoetic consciousness and self-perspective in aging. Psychology and Aging. 2006;21:510–25. doi: 10.1037/0882-7974.21.3.510. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Manning L, North P, Jokic C, Eustache F. Autobiographical memory, the sense of recollection and executive functions after severe traumatic brain injury. Cortex. doi: 10.1016/s0010-9452(08)70474-x. (in press) [DOI] [PubMed] [Google Scholar]
- Reed JM, Squire LR. Retrograde amnesia for facts and events: findings from four new cases. J Neurosci. 1998;18:3943–3954. doi: 10.1523/JNEUROSCI.18-10-03943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekkas PV, Constable RT. Evidence that autobiographic memory retrieval does not become independent of the hippocampus: an fMRI study contrasting very recent with remote events. J Cogn Neurosci. 2005;12:1950–61. doi: 10.1162/089892905775008652. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JA. First experiences memories: context and functions in personal histories. In: Conway MA, Rubin DC, Spinnler H, Wagenaar WA, editors. Theoretical perspectives on autobiographical memory. Dordrecht: Kluwer Academic Publishers; 1992. pp. 223–240. [Google Scholar]
- Rosenbaum RS, Winocur G, Moscovitch M. New views on old memories: reevaluating the role of the hippocampal complex. Behav Brain Res. 2001;127:183–197. doi: 10.1016/s0166-4328(01)00363-1. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Wetzler SE, Nebes RD. Autobiographical memory across the lifespan. In: Rubin DC, editor. Autobiographical Memory. Cambridge: Cambridge University Press; 1986. pp. 202–221. [Google Scholar]
- Rubin DC, Schulkind MD. Distribution of important and word-cued autobiographical memories in 20-, 35-, 70-year-old adults. Psychology and Aging. 1997;12:524–535. doi: 10.1037//0882-7974.12.3.524. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Schrauf RW, Greenberg DL. Belief and recollection of autobiographical memories. Mem Cognit. 2003;31:887–901. doi: 10.3758/bf03196443. [DOI] [PubMed] [Google Scholar]
- Ryan L, Nadel L, Keil K, Putnam K, Schnyer D, Trouard T, Moscovitch M. Hippocampal complex and retrieval of recent and very remote autobiographical memories: evidence from functional magnetic resonance imaging in neurologically intact people. Hippocampus. 2001;11:707–714. doi: 10.1002/hipo.1086. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest non-traditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci. 2004;24:10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidke K, Vollmer H. Retrograde amnesia and semantic memory deficits. Neuropsychologia. 1997;35:505–518. doi: 10.1016/s0028-3932(96)00109-1. [DOI] [PubMed] [Google Scholar]
- Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiack RSJ, Dolan R. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994;121:561–579. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinvorth S, Corkin S, Halgren E. Ecphory of autobiographical memories: an fMRI study of recent and remote memory retrieval. Neuroimage. 2006;30:285–98. doi: 10.1016/j.neuroimage.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. A co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Tsukiura T, Fujii T, Okuda J, Ohtake H, Kawashima R, Itoh M, Fukuda H, Yamadori A. Time-dependent contribution of the hippocampal complex when remembering the past: a PET study. Neuroreport. 2002;13:2319–2323. doi: 10.1097/00001756-200212030-00030. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and Semantic Memory. In: Tulving E, editor. Organization of Memory. New York: Academic Press; 1972. [Google Scholar]
- Tulving E. How many memory systems are there? Am Psychol. 1985;40:385–398. [Google Scholar]
- Tulving E, Schacter DL, McLachlan DR, Moscovitch M. Priming of semantic autobiographical knowledge: a case study of retrograde amnesia. Brain Cogn. 1988;8:3–20. doi: 10.1016/0278-2626(88)90035-8. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic memory and common sense: how far apart? Philos Trans R Soc Lond B Biol Sci. 2001;356:1505–1515. doi: 10.1098/rstb.2001.0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove MM, Markowitsch HJ, Mertens M, Woermann FG. Bi-hemispheric engagement in the retrieval of autobiographical episodes. Behav Neurol. 2005;16:203–210. doi: 10.1155/2005/460745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Watson G, Clifford RJ, Fernando C. Volumetric magnetic resonance imaging: clinical applications and contributions to the understanding of temporal lobe epilepsy. Neurol Rev. 1997;54:1521–1531. doi: 10.1001/archneur.1997.00550240071015. [DOI] [PubMed] [Google Scholar]
- Wheeler MA. Episodic memory and autonoetic awareness. In: Tulving E, Craik FIM, editors. Oxford handbook of memory. New York: Oxford; 1999. [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: the frontal lobes and autonoetic consciousness. Psychol Bull. 1997;121:331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey MB, Leir O. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]


