Abstract
Background
Spirituality has been suggested to be associated with positive health, but potential biological mediators have not been well characterized.
Purpose and Methods
The present study examined, in a population based sample of middle-aged and older adults, the potential relationship between spirituality and patterns of cardiac autonomic control, which may have health significance. Measures of parasympathetic (high-frequency heart rate variability) and sympathetic (pre-ejection period) cardiac control were obtained from a representative sample of 229 participants. Participants completed questionnaires to assess spirituality (closeness to and satisfactory relation with God). Personality, demographic, anthropometric, health behavior, and health status information was also obtained. A series of multivariate regression models was used to examine the relations between spirituality, the autonomic measures, and two derived indexes-- cardiac autonomic balance (CAB, reflecting parasympathetic to sympathetic balance) and cardiac autonomic regulation (CAR, reflecting total autonomic control).
Results
Spirituality, net of demographics or other variables, was found to be associated with enhanced parasympathetic as well as sympathetic cardiac control (yielding a higher CAR); but was not associated with CAB. Although the number of cases was small (N=11), both spirituality and CAR were significant negative predictors of the prior occurrence of a myocardial infarction.
Conclusions
In a population based sample, spirituality appears to be associated with a specific pattern of cardiac autonomic regulation, characterized by a high level of cardiac autonomic control, irrespective of the relative contribution of the two autonomic branches. This pattern of autonomic control may have health significance.
Keywords: spirituality, sympathetic, parasympathetic pre-ejection period, heart rate variability, cardiac control, autonomic balance, myocardial infarction
INTRODUCTION
Cardiovascular disease is the leading cause of mortality and morbidity in the United States, and more than 1 million Americans suffer a myocardial infarction each year (National Center for Health Statistics, see 1), and psychosomatic factors increasingly appear to play an important role in the development of cardiovascular pathology. The relationship between psychological states and processes and health has long been studied, and the distinguished physiologist Walter Cannon speculated on a specific physiological mechanism that may underlie what he termed “Voodoo” death (2). More recently, a hyper-sympathetic state associated with psychological stress has been implicated in what has been variously termed takosubo cardiomyopathy, myocardial stunning, human stress cardiomyopathy, or broken heart syndrome (3). These and other developments have led to the expansion of the field of neurocardiology and the recent establishment of the Society of Heart Brain Medicine (see 4). It is now clear that a wide variety of psychological and behavioral variables may powerfully impact autonomic control and even distinct patterns of sympathetic and parasympathetic regulation (5,6). Consequently, it is important to clarify the specific role of psychological variables in health and disease. Although the concept of stress has figured prominently in the prior literature, a more recent focus has been on resilience and potential mediators of positive health impact (e.g., 7).
In this regard, although limitations and caveats exist (8), there is now a growing literature suggesting that religiosity and/or spirituality may be associated with positive health outcomes, especially cardiovascular health (9-15). Although many of these studies are cross-sectional and correlational, making causal linkages difficult to establish, there are ample behavioral, physiological, endocrinological, and immunological pathways through which spirituality and religiosity might impact health (12,16-19). Moreover, longitudinal/prospective, experimental and clinical intervention studies do suggest some causal linkages between spirituality or spiritual practices and autonomic cardiovascular control and health (13,15,20).
Religious service attendance is the aspect of religiosity that has most often been examined in relation to health outcomes, and has been shown to predict a lower risk of cardiovascular and coronary heart disease mortality in well-designed prospective studies (21, 22,23). Religious service attendance, however, is a global index that does not illuminate what it is about religiosity that might explain its association with health outcomes (24). Accordingly, researchers have employed more specific measures of religious and spiritual beliefs and experiences and have shown physical and/or mental health benefits associated with subjective assessments of religious support (25), religious coping (26), religious orientation (27), and perceived closeness to God (28).
This latter aspect of religiosity/spirituality, closeness to God, implies a relationship with God, a relationship that may have its roots in sociality (16) and may therefore be usefully studied in that context. The evolutionary motive of the social human species to form and maintain attachments, interpersonal relationships, and collectives, which is in part genetically determined (29), contributes to the capacity for humanity and spirituality with which people are born (30). Moreover, the human tendency to form social connections extends beyond kin and includes real and imagined others (31). The feeling of social connectedness and purpose that comes from a relationship with a higher being or God is a potent component of what we refer to as “spirituality” (as distinguished from religiosity, which we will use to refer to more objective measures such as church service attendance; 24). The conceptualization of spirituality as a relationship between an individual and God links this work with an extensive scientific literature on the effects of interpersonal connections on physiology, health, and well-being (32,33,34,35,36).
Over a century ago, William James pointed to the need for psychophysical theory concerning the potential relation between spiritual values and “determinate sorts of physiological change” (37). Some potential mediators of relations between spirituality and cardiovascular health, such as smoking (19), diet (38) or other health-related behaviors (39), are transparent and may not require theoretical explication. Others, however, may benefit from a more theoretical grounding and conceptual modeling of the relevant physiological dimensions that underlie health relationships. This is the case for possible autonomic nervous system mediators of potential relations between spirituality and cardiovascular regulation and health.
Certain spiritual or meditation practices have been reported to be associated with increased parasympathetic and decreased sympathetic cardiac control (40,41). Such a pattern of autonomic control could be a potential mediator of spirituality and health outcomes. High sympathetic cardiac control, for example, is a known risk factor for myocardial infarction and for survival thereafter (42-46), and a hyper-sympathetic state appears to underlie human stress cardiomyopathy (47). Indeed, drugs that block sympathetic actions (primarily beta adrenergic blockers) are a common treatment strategy after myocardial infarction (46). In contrast, the parasympathetic system exerts antifibrillary actions (42), and low parasympathetic activity is a predictor of negative outcomes after myocardial infarction (43,47).
From these considerations, a dimension of autonomic control that may be relevant to cardiovascular health is the relative balance between sympathetic and parasympathetic cardiac regulation. The autonomic balance model has a long history. Eppinger and Hess (48) proposed that individuals are constitutionally disposed toward a predominance of either sympathetic (sympathicotonia) or parasympathetic (vagotonia) control, and that these physiological predilections may bias toward distinct psychosomatic disorders (e.g.,, hypertension and asthma, respectively). Wenger (49) subsequently confirmed aspects of the Eppinger and Hess findings, but their proposed autonomic balance metric (Ā), was characterized by a continuous, normal distribution rather than a dichotomous categorical variable.
The autonomic balance model continues to be represented in the contemporary literature, both as an individual difference characteristic, and as a predictor of health outcomes. A current example is the proposed autonomic balance metric derived from measures of heart rate variability (50,51). Specifically, high frequency (HF) heart rate variability, in the respiratory frequency band, provides a relatively pure index of parasympathetic cardiac control, whereas low frequency (LF) variability reflects a combination of sympathetic and parasympathetic influences (see 52-54). Based on this and other empirical findings, Malliani and colleges suggest that a ratio of LF to HF variability indexes the relative autonomic balance, along a continuum from sympathetic to parasympathetic predominance (50,51). Although this metric has been challenged on both conceptual and empirical grounds (55), it continues to be widely employed as a metric of sympathovagal balance.
Although there may well be individual differences in the relative contributions of sympathetic and parasympathetic branches, it is not at all clear that sympathovagal balance is a physiologically regulated dimension. Moreover, despite powerful homeostatic controls over cardiovascular parameters such as blood pressure, it is clear that even regulated dimensions are not characterized by fixed, invariant levels. Rather, it is alterations in these dimensions (blood pressure, heart rate, myocardial contractility, etc) that permit an adaptive cardiovascular response to perturbations such as orthostatic stress, exercise, or fight/flight responses. This pattern of regulatory flexibility has been termed allostatic (56,57) or allodynamic (6) regulation, and is conceptualized as a means of achieving “stability through change” (58 p 631). According to this view, the critical dimension of autonomic regulation may be not so much autonomic balance, but autonomic flexibility, or regulatory capacity that permits an organism to adaptively deal with changing demands.
In this regard, diminished HF heart rate variability, reflective of lower parasympathetic cardiac control, has been reported to be a significant risk stratifier for recovery following myocardial infarction (54,59-62) and is also a predictor of hypertension after controlling for age and other risk factors (63). However, it is also the case that diminished low frequency variability (which includes sympathetic contributions) may be an equivalent or superior risk stratifier (54,60-62) and is also predictive of the development of hypertension (64). These findings suggest that the relevant predictive authority may derive not so much from a change in autonomic balance, but from an overall reduction in autonomic flexibility or variability. This is in keeping with the suggestion that autonomic irregularity, rather than a regulatory fixedness, may be the more relevant parameter in cardiovascular health (65). In this regard, Hemingway et al. (66) report that low heart rate variability may be a critical mediator of the relation between low social status and increased cardiac risk. Moreover, these authors find that diminished LF power is a potent component of metabolic syndrome, which in turn is a cardiac risk factor.
Low overall heart rate variability is not linked just with cardiovascular disorders, but has been reported to be a risk factor for all cause mortality and morbidity (67,68). Moreover, reduced “autonomic flexibility”, reflected by low heart rate variability, has been reported in conditions as disparate as anxiety and dyspepsia (69-72). These findings raise an additional or an alternative possibility to the sympathovagal balance model. A relevant health determinant may be the overall regulatory capacity, which supports flexible adjustments in the face of adaptive challenges.
The present study examines the links between a specific aspect of spirituality, namely satisfaction with the God relationship, and autonomic control in a population based sample (73), from the standpoint of two models. The first is the autonomic balance model, associated with a regulated autonomic end-point (narrow range of variability), and the other being the autonomic regulation model, associated with a regulatory capacity (wide range of variability). The present study employs two metrics to evaluate this contrast. Pre-ejection period (PEP) is employed as a measure of sympathetic cardiac control (74-76), and high frequency heart rate variability (HF) is employed as a metric of parasympathetic control (51-53). Cardiac Autonomic Balance (CAB) is operationalized as the difference between the (normalized) sympathetic and parasympathetic measures, and overall Cardiac Autonomic Regulation (CAR) is taken as the sum of sympathetic and parasympathetic cardiac controls.
METHOD
Study Population
Data for this study were collected in years 1-3 of the Chicago Health, Aging and Social Relations Study, a longitudinal, population based study of persons born between 1935 and 1952. The target population was non-Hispanic Caucasian, African American, and non-Black Latino American persons between the ages of 50 an 68 living in Cook County, IL, who were English-speaking and sufficiently ambulatory to come to the University of Chicago for a daylong visit to the laboratory. The sample was selected using a multistage probability design in which African Americans and Latino Americans were over sampled and gender equality maintained. Data for individual participants were averaged over the three year period, to increase reliability. In cases where data points were missing from one or two years, the participant’s score was based on the available data. Across variables, 60-83% of the participant’s had scores for all three years, 88-89% had scores for at least two years. The final sample size was 229.
Procedure
Participants arrived at the laboratory between 8 a.m. and 9 a.m. They provided informed consent and then began a day of assessments that included standard psychological surveys, interviews, lunch, and a cardiovascular protocol.
Cardiovascular activity was measured prior to lunch for all participants. Experimenters attached sensors for electrocardiograph, impedance cardiograph, and blood pressure recording. Participants were then seated in a comfortable padded chair. During a 15-min adaptation period, participants completed questionnaires while experimenters established good signal quality. Participants then sat quietly for an additional 5 min prior to recording baseline cardiovascular activity (4 min).
Cardiovascular Measures
Primary cardiovascular measures of sympathetic and parasympathetic cardiac control, respectively, were pre-ejection period (PEP) and high frequency (0.15-0.4 Hz) heart rate variability (HF). PEP, derived from impedance cardiography, is commonly used as a measure of sympathetic cardiac control (74,75,76). HF heart rate variability is a rhythmical fluctuation of heart rate in the respiratory frequency band (respiratory sinus arrhythmia), and has been shown to be a relatively pure index of parasympathetic control (see 52,53,54).
The electrocardiogram was obtained using the standard lead II configuration. The impedance cardiogram was obtained using the standard tetrapolar electrode system and procedures described elsewhere (76). The electrocardiogram and basal thoracic impedance (Z0) were measured using a Biopac MP100 system (ECG100 and EB1100 modules, respectively; Biopac Systems, Inc., Santa Barbara, CA). The electrocardiogram and Z0 were digitized at 1000 Hz.
Custom software (Mindware, Gahanna, OH) was used to generate the dZ/dt waveform necessary to obtain impedance-derived measures (i.e., PEP). The same software was used to verify, edit, and summarize cardiovascular data. For each subject, electrocardiograph and impedance data were ensemble averaged for each minute to produce estimates of the PEP. PEP was quantified as the time interval in milliseconds from the onset of the electrocardiogram Q wave to the B point of the dZ/dt wave (76). Minute by minute means were then averaged over the 4 min baseline period.
HF heart rate variability was derived by spectral analysis (Fast Fourier Transform; Mindware, Gahanna, OH) of the interbeat interval series derived from the electrocardiogram, following procedures specified by Berntson et al. (53). Briefly, the RR interval series was time sampled at 4 Hz (with interpolation) to yield an equal interval time series. This time series was detrended (2nd order polynomial), end-tapered, and submitted to a Fast Fourier Transform. HF spectral power was then integrated over the respiratory frequency band (0.15-0.4 Hz). Respiratory measures were also obtained to ensure that the respiratory rates were within the analytical band. If respiratory rates fell below the HF cutoff, data from that minute were excluded from analysis. This was an issue in only two cases and, for each, resulted in a single minute (of 4) being excluded. Further preliminary analyses were pursued to ensure that respiratory parameters did not co-vary with, and potentially bias, experimental variables. Neither respiratory frequency nor respiratory depth was correlated with any of the major variables (spirituality, CAB or CAR; for all correlations, rs < .10, ps > .15).
Two measures of autonomic control were derived from HF and PEP. An index of autonomic balance, Cardiac Autonomic Balance (CAB), was derived as the difference between normalized values of parasympathetic control (HF) and sympathetic control (PEP). A metric of overall Cardiac Autonomic Regulation (CAR) was derived as the sum of the normalized values of HF and PEP. Normalization of values was necessary because of the wide differences in means and scaling among the HF and PEP measures. Normalization was accomplished by transforming values to z-scores, so all normalized values are expressed in standard deviations relative to the population means. In addition, because increased sympathetic control is associated with shortened PEP values, PEP was multiplied by -1 (-PEP), in order to invert the relationship to a positive association. Consequently, Cardiac Autonomic Balance (CAB) = HFz - (-PEPz), and Cardiac Autonomic Regulation (CAR) = (HFz + (-PEPz)).
As an ancillary analysis, we also derived low frequency (LF) heart rate variability (0.05-0.15 Hz). Both sympathetic and parasympathetic branches contribute to variability at this frequency, but the LF/HF ratio has been proposed as a measure of sympathovagal balance (50,51). Although this metric has been challenged on both conceptual and empirical grounds (53,55), it continues to be employed as a metric of sympathovagal balance. For completeness, we also include it here.
Religiosity, Spirituality and Psychological Measures
Religiosity
Religious service attendance was represented by 5 categories ranging from 0 (never) to 4 (more than once a week). Attendance was averaged across all three years.
Religious Well Being (RWB)
Religious well-being scores were derived from the relevant 10 items of the 20-item Spiritual Well-Being Scale (77). Religious well-being is represented by items such as “I believe that God loves me and cares about me” and “My relationship with God helps me not to feel lonely.” Participants were asked to rate the extent of their agreement with each item on a Likert scale ranging from 1 (strongly agree) to 6 (strongly disagree). Negatively worded items were reverse-scored and responses summed to generate a Religious Well Being score. See Paloutzian and Ellison (77) for scale design and psychometric properties. Religious Well being scores were averaged across all three years.
Multi-Dimensional Relationship Satisfaction
This is a self-report questionnaire we designed to assess, on a common metric, participants’ ratings of their relationship with (1) the single most important person in their life, (2) the single most important group to which they belong, (3) their pet or pets, and (4) God. Within each relationship category, participants are asked to what extent (a) they feel this relationship is satisfying, and (b) this relationship effectively serves as a refuge, a source of safety, security, and consolation, and as a shelter from danger or trouble. Responses were made on a Likert scale that ranged from 0 (not at all) to 7 (very much). Only those who responded affirmatively to a belief in God were asked to respond to the follow-up questions for that item. The means of the responses within each relationship category were used to create measures of person satisfaction, group satisfaction, pet satisfaction, and satisfaction with God.
Aggregate measure of spirituality
Because the measure of Religious Well Being was highly correlated with Satisfaction with God (r(226) = .84, p = <.001), we aggregated these two measures by summing the z-score transforms of each for an Aggregate Spirituality (AS) measure.
Big “Three” (Big3)
To examine personality characteristics that may mediate potential relations between spirituality and other variables, participants also completed three of the Big5 personality scales (78). This is a self-report questionnaire in which participants are asked to rate how accurately each of 100 trait words describes how they see themselves at the present using a 9-point Likert scale that ranges from 1 (extremely inaccurate) to 9 (extremely accurate). The 100-items represent five personality subscales consisting of 10 positive and 10 negative items each. A 60-item version of this scale was used to assess Surgency (Extraversion), Agreeableness, and Emotional Stability (Neuroticism). Scores on these subscales were computed by reverse scoring the negative items (items phrased so that a low score indicates a high endorsement of the item) in each factor and then finding the mean item response for all 20 items. This yields subscale scores with ranges of 1 to 9; scores above 5 mean that the subject viewed the positive aspects of the factor as being more accurate in descriptions of herself/himself while a score below five means the negative aspects were viewed as more descriptive.
Psychosocial characteristics
Standardized questionnaires for loneliness (revised UCLA Loneliness Scale; 79), perceived stress (Perceived Stress Scale; 80), social support (Interpersonal Support Evaluation List; 81), hostility (Cook Medley Hostility Scale; 82), and depressive symptoms (Center for Epidemiological Studies Depression Survey; 83) were used to measure psychosocial characteristics that might be confounded with spirituality and account for an association between spirituality and autonomic cardiac control.
Covariates
Demographic covariates were gender, ethnicity, age, education (years of schooling), and household income. Household income was reported in 12 categories ranging from less than 5,000 to more than 200,000; to achieve a more continuous distribution, we used the log-transformed median of each category in analyses. Missing values for education (6 subjects) and household income (13 subjects) were replaced with means from the corresponding gender by ethnic group combination.
Body mass index, calculated as weight in kg/(height in m) 2, served as a covariate in analyses of cardiovascular and health status variables. Forty percent of participants were on vasoactive medications, 5% were on volume active medications, and an additional 11% were on both types of medication. The likelihood of being on cardiovascular medications did not differ as a function of spirituality (r(225) = -.008, p = .91). However, spirituality may influence participants’ likelihood of complying with medication regimens. Holding cardiovascular medications constant permits an assessment of the independent effects of the spirituality factors. All reported effects were not changed by the addition of any covariates.
Health behaviors, including smoking (yes/no), exercise (some/none), and having visited a physician within the past year (yes/no), were included to examine whether these health behaviors account for associations between spirituality and autonomic as well as health outcomes. Total endorsement of adverse health conditions (diabetes, arthritis, Alzheimer’s, stroke, myocardial infarction, cancer, and HIV) were also tabulated to provide an overall index of health status.
Data Analysis
A set of linear regression models was used to test associations between spirituality and the cardiac autonomic measures, and whether these associations withstood statistical control for demographic characteristics (i.e. age, gender, ethnicity), psychosocial characteristics, health status, medications, health behaviors, and personality traits known or likely to influence the outcome measures. Logit regression was employed for the analysis of myocardial infarction, which was coded either as a yes or no. All continuous predictor variables were standardized to a mean of zero and a standard deviation of 1 in order to represent potentially substantive individual differences in these characteristics.
Path analysis was employed to evaluate potential mediation effects according to the general methods outlined by Baron and Kenny (84) and MacKinnon et al. (85). Specifically, a bootstrapping procedure was employed to obtain estimates and confidence intervals for indirect effects (86).
RESULTS
Participant Characteristics
Demographic and other characteristics of the participants and the sample are illustrated in Table 1. Table 2 shows the spirituality scores and autonomic parameters by age and gender, as well as for participants with and without a prior myocardial infarction. Over 95% of the sample professed a belief in God, and religious preferences were divided among Protestants (38.4%), Roman Catholics (43.7%), other (Jewish, Orthodox, other; 10%), and no preference (7.9%).
Table 1.
Participant Characteristics at Baseline (Means ± SEM or percentages)
| Overall (n =229) | Males (n = 109) | Female (n = 120) | |
|---|---|---|---|
| Age | 57.43 ± (.29) | 57.53 ± (.46) | 57.34 ± (.39) |
| Income | 67,122 ± (3829) | 77,875 ± (6025) | 57,501 ± ($4700) |
| Education | 13.27 ± (.21) | 13.16 ± (.31) | 13.37 ± (.28) |
| Married/Cohabitating | 140 (61%) | 82 (75%) | 58 (48%) |
| Body Mass Index | 31.50 ± (.46) | 31.17± (.61) | 31.81 ± (.69) |
| Caucasians | 82 (36%) | 39 (36%) | 43 (36%) |
| African Americans | 81 (36%) | 37 (34%) | 44 (37%) |
| Hispanics | 66 (28%) | 33 (30%) | 33 (28%) |
Table 2.
Autonomic and religious values across age, gender, and presence/absence of a prior myocardial infarction
| Mean ± (SEM) | RSA | PEP | CAR | AR |
|---|---|---|---|---|
| Age 50 - 57 (n = 66) | 5.11 ± (.11) | 102.29 ± (1.43) | .12 ± (.10) | - .14 ± (.18) |
| Age 58 - 68 (n = 90) | 4.86 ± (.11) | 103.68 ± (1.34) | - .12 ± (.08) | .14 ± (.17) |
| Males (n = 109) | 4.81 ± (.11) | 104.95 ± (1.57) | -.22 ± (.10) | -.52 ± (.19) |
| 50 - 57 (n = 52) | 4.77 ± (.15) | 103.08 ± (2.49) | -.18 ± (.14) | - .52 ± (.32) |
| 58 - 68 (n = 57) | 4.86 ± (.16) | 106.83 ± (1.96) | -.26 ± (.12) | - .52 ± (.25) |
| Females (n = 120) | 5.15 ± (.11) | 101.53 ± (1.18) | .20 ± (.08) | .52 ± (.14) |
| 50 - 57 (n = 63) | 5.42 ± (.14) | 101.56 ± (1.63) | .36 ± (.12) | .25 ± (.19) |
| 58 -68 (n = 57) | 4.88 ± (.15) | 100.51 ± (1.73) | .04 ± (.12) | .79 ± (.18) |
| MI (n = 11) | 4.09 ± (.37) | 117.5 ± (4.58) | -1.02 ± (.16) | -1.43 ± (.81) |
| No-MI (n = 218) | 5.03 ± (.08) | 103.4 ± (.98) | .04 ± (.06) | .60 ± (.12) |
Spirituality Predicts Cardiac Autonomic Regulatory Capacity
Initial analyses examined the relationship between spirituality and two general models of autonomic cardiac control, the regulatory capacity model (CAR) and the autonomic balance model (CAB). CAR (Cardiac Autonomic Regulation) provides an aggregate index of total or summed autonomic control across autonomic branches [HFz +(-PEPz)]. CAB (Cardiac Autonomic Balance) indexes a classical model of autonomic balance expressed along a continuum extending from parasympathetic to sympathetic dominance [(HFz - (-PEPz]).
CAR
The distribution of CAR scores across age and gender is illustrated in Figure 1. A significant correlation was observed between the spirituality index (AS) and CAR (r (219) = .25, p = <.001)). To examine this relation further, demographic and other covariates were held constant in linear regression models predicting CAR. Independent of age, gender, ethnicity and cardiovascular medications, AS retained significant associations with CAR, accounting for an additional 4% of the variance in this index (B = .10; Beta = .21; t (192) = 2.87, p = .005; overall model R2 = .14, F(1,192) = 3.83, p = < .001; effect size = .04).
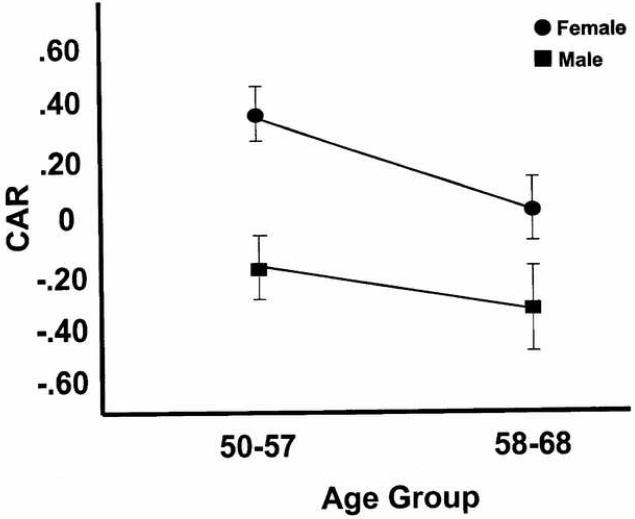
Figure 1.
Cardiac Autonomic Regulation (CAR), as a function of age (median split) and gender. Overall, females show higher CAR scores than men, and these decrease for both genders with age.
Spirituality remained a significant predictor of CAR, even after holding constant body mass index, blood pressure, cardiovascular medications, health status, and health behaviors. It also continued to predict CAR after entry of the Big 3 personality variables [Surgency (mean = 5.74 ± .94 SD), Agreeableness (7.03 ± .80), and Emotional Stability (5.58 ± .90)] into the equation. The predictive relationship between spirituality and CAR also was not attributable to potential associated psychosocial characteristics including loneliness (35.82 ± 9.01), perceived stress (12.81 ± 5.51), social support (12.81 ± 1.92), hostility (17.14 ± 7.34), or depression (10.06 ± 7.83). None of these latter variables were significantly correlated with CAR and the relationship between spirituality and autonomic regulation survived prior entry of each of these variables into the regression equation. Similarly, the relationship between spirituality and autonomic regulation was not attributable to a general satisfaction with personal relationships (as measured by the Multi-Dimensional Relationship Satisfaction scale).
As expected, spirituality was correlated with religious service attendance (r(225) = .51, p = <.001)) and religious service attendance was also correlated with CAR (r(223)=.15, p = .03). The measure of spirituality, however, appeared to reflect more of a psychological state, rather than the behavioral manifestation, as religious service attendance did not significantly predict CAR in a regression analysis holding demographics and body mass index constant (R2 change = .01, p = .13).
CAB
In contrast, spirituality was not a significant predictor of CAB. We also evaluated another metric, the LF/HF ratio, which has also been suggested to be a marker of autonomic balance (50,51). Although there was a significant correlation between spirituality and the LF/HF ratio (r(219) = .23, p = .001), spirituality was not a significant predictor when demographics and body mass index were held constant.
Spirituality Predicts Autonomic Cardiac Control
Parasympathetic cardiac control (HF)
The aggregate index of spirituality was positively correlated with parasympathetic cardiac control as indexed by HF (r (222) = .151, p = .02)). Regression analyses further revealed that spirituality was a significant predictor of HF, net of demographic variables, accounting for an additional 2% of the variance in HF (B = .09; Beta = .21; t(222) = 2.06, p = .04; effect size = .02).
Spirituality continued to predict HF after other autonomic (PEP, blood pressure) and personality (Big 3) variables were entered into the equation. Similarly, spirituality continued to significantly predict HF after holding constant health behaviors, body mass index, health status and cardiovascular medications.
Sympathetic cardiac control (PEP)
Spirituality was also positively correlated with sympathetic cardiac control indexed (inversely) by PEP (r (222) = -.18, p = .009)). Consistent with the findings for CAR, this suggests that spirituality may be associated with increased autonomic cardiac control regardless of its parasympathetic or sympathetic source. Regression analyses further revealed that spirituality was a significant predictor of PEP, net of demographic variables, accounting for an additional 2% of the variance in PEP (B = -1.12; Beta = -.14; t (195) = 1.98, p = .05; effect size = .02).
Spirituality continued to predict PEP after other autonomic measures (HF, blood pressure), psychosocial characteristics, and personality (Big 3) variables were entered into the equation. Similarly, spirituality continued to significantly predict PEP after holding constant health behaviors, body mass index, health status, and cardiovascular medications. The distributions of HF and PEP scores across age and gender are illustrated in Figure 2.
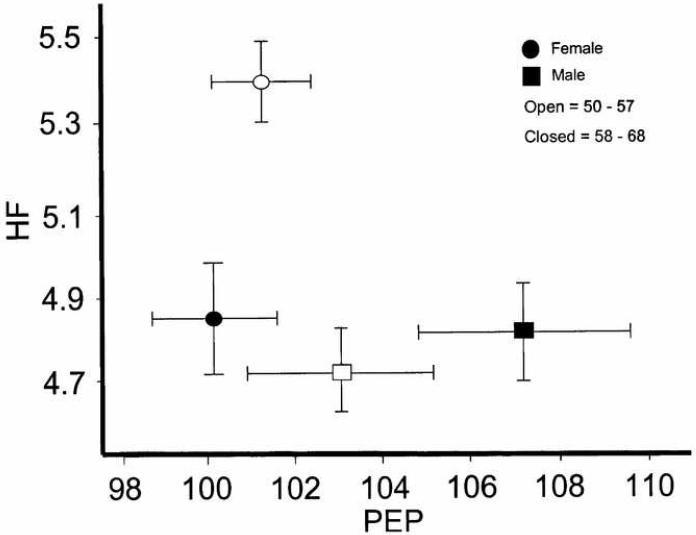
Figure 2.
Distribution of the high frequency heart rate variability (HF) and for pre-ejection period (PEP) acreoss age (median split) and gender. HF is in units of msec2 (natural log of the integral spectral power in the respiratory frequency band), and PEP is in units of msec. Females have generally higher HF values (reflecting paraympathetic cardiac control) than males, and these values tended to decrease with age. Females also had lower PEP values (indexing sympathetic cardiac control) than men, and these values also tended to decrease with age.
A Prior Myocardial Infarction is Associated with Patterns of Autonomic Cardiac Control
Eleven of 229 participants reported having had a heart attack or myocardial infarction (7 of 109 males, 4 of 120 females). A significant negative (point biserial) correlation was observed between spirituality and myocardial infarction (r (226) = -.14, p = .035). A logit regression was used to predict previous occurrence of myocardial infarction from spirituality, net of demographics. Spirituality was found to be a significant predictor of myocardial infarction, with demographics (age, gender, marital status, education and income) and BMI held constant. For every one unit increase in AS, the odds of a myocardial infarction decreased by a factor of .62 (B = -.48, Wald = 5.21, p = .02, 95% CI .41-.94). Spirituality continued to significantly predict myocardial infarction after holding constant church attendance, psychosocial characteristics, health behaviors, body mass index, health status, and cardiovascular medications. The interpretation of this relationship is unclear, however, as the occurrence of a myocardial infarction may have impacted spirituality. We will return to this issue below.
CAR
Overall cardiac autonomic control was also negatively correlated with the prior occurrence of a heart attack, r(229) = -.26, p < .001). A logit regression revealed that CAR was a significant negative predictor of myocardial infarction, with demographics (age, gender, marital status, education and income) and body mass index held constant. For every one unit increase in CAR, the odds of a myocardial infarction decreased by a factor of .25 (B = -.1.37, Wald = 5.63, p = .02, 95% CI .08-.79).
The significance of this result held even after controlling for other autonomic variables constant (blood pressure, PEP and HF), although HF and PEP did show independent predictive relationship with myocardial infarction (see below). Similarly, CAR continued to significantly predict myocardial infarction after holding constant health behaviors, body mass index, health status, and cardiovascular medications.
HF and PEP
Correlational analysis revealed the expected relations between myocardial infarction and sympathetic and parasympathetic control. Sympathetic cardiac control (as indexed by (-)PEP) was positively related to the prior incidence of a heart attack (r(226) = .19, p = .004), whereas parasympathetic cardiac control (as indexed by HF) was negatively associated with the prior occurrence of a heart attack (r(226) = -.14, p = .04). Although higher sympathetic control may be a significant predictor of a prior myocardial infarction, the finding of a negative relation between CAR and myocardial infarction, suggests that high sympathetic activity may be buffered by parasympathetic control.
In contrast to CAR, neither CAB nor the LF/HF ratio was a significant predictor of a prior myocardial infarction.
Path Analysis
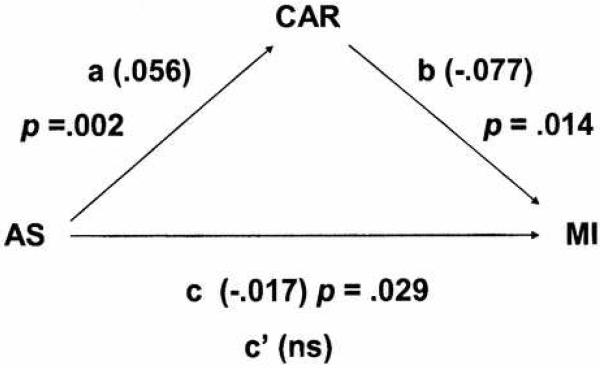
Because spirituality is predictive of CAR and both spirituality and CAR are predictive of myocardial infarction, the potential role of CAR as a mediator of the relation between spirituality and myocardial infarction was evaluated by path analysis. As illustrated in Figure 3, both spirituality and CAR were significantly predictive of the prior occurrence of myocardial infarction. As is apparent in the figure, however, the direct effect (c’) of spirituality was no longer significant after the effects of CAR were accounted for. This suggests that the pattern of autonomic control may be a mediator of the relation between spirituality and cardiac health.
Figure 3.
Path analysis of mediation. Spirituality (AS) was significantly predictive of both cardiac autonomic regulation (CAR) and prior myocardial infarction. CAR was also predictive of myocardial infarction and may be a mediator of the relationship between spirituality and infarction, as spirituality was no longer predictive of myocardial infarction (c’) after the effects of CAR were accounted for. Values in parenthesis are the coefficients.
DISCUSSION
The present study found that a measure of spirituality can predict important dimensions of autonomic regulation, in a middle-age and older population based sample. Specifically, spirituality is associated with an increase in overall cardiac regulatory capacity (Cardiac Autonomic Regulation, or CAR), but not autonomic balance as indexed by Cardiac Autonomic Balance (CAB) or the LF/HF ratio. The predictive relationship between spirituality and CAR held after demographics (age, gender, education, marital status, income) and health behaviors (smoking, exercise, physician visits) were accounted for. The underlying psychophysiological mediators between spirituality and patterns of autonomic regulation have yet to be determined, but the relation between spirituality and CAR was not simply related to a personality variable indexed by the Big 3. Nor was it attributable to other psychosocial characteristics including loneliness, perceived stress, social support, hostility, depression, or general satisfaction with others. Although church attendance has been widely studied in the literature on religiosity and health, this behavioral measure did not predict CAR in the present study. Rather, there appears to be something more specific to the perceived relationship with God. This is consistent with findings in the literature relating social factors with health-related outcomes and showing that relationship perceptions are more important than objective social realities in explaining their effects (87,88). Clearly, individuals attend religious services for a variety of reasons, so it is not surprising that the mental representation of one’s relationship with God is more proximal than religious behavior to health-related outcomes.
The relation between spirituality and CAR derived in part from a positive relationship between spirituality and parasympathetic control, as indexed by HF heart rate variability. It was also partially attributable to a positive relationship between spirituality and sympathetic cardiac control (as evidenced by PEP). Thus, spirituality is associated with enhanced cardiac regulation, regardless of its sympathetic or parasympathetic origin. Given sympathetic and parasympathetic control of the heart decreases with age, the present findings may indicate a protective effect of spirituality on the neuroregulatory control of the heart.
While high parasympathetic activity is generally considered to reflect a positive health state or reduced health risk (54,60,63,72,89), high sympathetic activity can be a clear health risk factor (42-46). High parasympathetic control, however, may serve to buffer the health consequences of sympathetic activity. This is in keeping with the observations that high overall heart rate variability including high LF heart rate variability (which includes a sympathetic contribution) is a predictor of positive outcome after myocardial infarction (54,60-62). According to this view, high overall heart rate variability may index a high cardiac regulatory capacity (in both sympathetic and parasympathetic branches), which may have positive health benefits. In contrast, as noted above, low overall heart rate variability has been reported to be a risk factor for all cause mortality and morbidity (67,68).
In this regard, the measure of cardiac regulatory capacity, CAR was negatively associated with the prior incidence of myocardial infarction, and that association continued after holding constant demographics, body mass index, health behaviors (smoking, exercise, alcohol use, physician visits). As expected, PEP (reflecting high sympathetic cardiac control) was a positive predictor of the prior occurrence of a myocardial infarction, but accounted for less than half of the variance accounted for by CAR. The negative relationship between HF and myocardial infarction also likely contributed to the predictive power of CAR. However, CAR continued as a significant predictor of myocardial infarction even after HF and PEP were held constant in the regression analysis. This indicates that the CAR metric may reflect a physiological state that is more relevant to health than the independent sympathetic or parasympathetic controls, or the autonomic balance between these controls as indexed by CAB (or LF/HF ratio). Because the highest values of CAR were associated with high sympathetic control (which predicts myocardial infarction), the associated high parasympathetic control may buffer the sympathetic risk factor.
In summary, spirituality was found to be associated with particular patterns of autonomic control and regulation. Specifically, spirituality predicts a high level of cardiac autonomic regulatory capacity, rather than a position along an autonomic balance continuum. This pattern of autonomic regulation may be a mediator of health effects of spirituality or religiosity, as high regulatory capacity was negatively associated with the prior occurrence of a myocardial infarction. Because this was a cross sectional study, the causal relations between spirituality, CAR and myocardial infarction have yet to be determined. As the Chicago Health, Aging, and Social Relations study continues, a longitudinal analysis may provide some insight as to whether spirituality antedates the autonomic patterns observed, and whether the autonomic patterns antedate and predict the subsequent occurrence of an insult. Additional studies will also be necessary to identify the specific links between spirituality and the autonomic regulatory patterns—that is, how spirituality gets under the skin.
REFERENCES
- (1).Thom T, editor. Morbidity and Mortality: 2004 Chart Book on Cardiovascular, Lung and Blood Diseases. National Heart, Lung, and Blood Institute; Bethesda: 2004. [Google Scholar]
- (2).Cannon WB. “Voodoo” death. American Anthropologist. 1942;44:169–181. doi: 10.2105/ajph.92.10.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Wittstein IS. The broken heart syndrome. Cleveland Clinic Journal of Medicine. 1996;74(Suppl 1):S17–22. doi: 10.3949/ccjm.74.suppl_1.s17. [DOI] [PubMed] [Google Scholar]
- (4).Samuels MA. “Voodoo” death revisited: The modern lessons of neuroscariology. Cleveland Clinic Journal of Medicine. 2007;74(Suppl 1):S8–16. doi: 10.3949/ccjm.74.suppl_1.s8. [DOI] [PubMed] [Google Scholar]
- (5).Berntson GG, Cacioppo JT, Quigley KS. Autonomic Determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review. 1991;98:459–487. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- (6).Berntson GG, Cacioppo JT. Integrative physiology: Homeostasis, allostasis and the orchestration of systemic physiology. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 3rd edition Cambridge University Press; Cambridge, UK: 2007. pp. 433–452. [Google Scholar]
- (7).Maier SF, Amat J, Baratta MV, Paul E, Watkins LR. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues in Clinical Neuroscience. 2006;8(4):397–406. doi: 10.31887/DCNS.2006.8.4/smaier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Freedman O, Orenstein S, Boston P, Amour T, Seely J, Mount BM. Spirituality, religion, and health: a critical appraisal of the Larson reports. Annals of Royal College of Physicians and Surgeons of Canada. 2002;35:90–3. [PubMed] [Google Scholar]
- (9).Koenig HG. Religion and medicine IV: religion, physical health, and clinical implications. International Journal of Psychiatry in Medicine. 2001;31:321–36. doi: 10.2190/X28K-GDAY-75QV-G69N. [DOI] [PubMed] [Google Scholar]
- (10).Levin JS, Chatters LM. Religion, health, and psychological well-being in older adults: findings from three national surveys. Journal of Aging and Health. 1998;10:504–31. doi: 10.1177/089826439801000406. [DOI] [PubMed] [Google Scholar]
- (11).Matthews DA, McCullough ME, Larson DB, Koenig HG, Swyers JP, Milano MG. Religious commitment and health status: a review of the research and implications for family medicine. Archives of Family Medicine. 1998;7:118–24. doi: 10.1001/archfami.7.2.118. [DOI] [PubMed] [Google Scholar]
- (12).Miller WR, Thoresen CE. Spirituality, religion, and health. An emerging research field. American Psychologist. 2003;58:24–35. doi: 10.1037/0003-066x.58.1.24. [DOI] [PubMed] [Google Scholar]
- (13).Seeman TE, Dubin LF, Seeman M. Religiosity/spirituality and health. A critical review of the evidence for biological pathways. American Psychologist. 2003;58:53–63. doi: 10.1037/0003-066x.58.1.53. [DOI] [PubMed] [Google Scholar]
- (14).Tartaro J, Luecken LJ, Gunn HE. Exploring heart and soul: effects of religiosity/spirituality and gender on blood pressure and cortisol stress responses. Journal of Health Psychology. 2005;10:753–66. doi: 10.1177/1359105305057311. [DOI] [PubMed] [Google Scholar]
- (15).Weaver AJ, Koenig HG. Religion, spirituality, and their relevance to medicine: an update. American family Physician. 2006;73:1336–7. [PubMed] [Google Scholar]
- (16).Cacioppo JT, Hawkley LC, Rickett EM, Masi CM. Sociality, spirituality, and meaning-making: Chicago health, aging, and social relations study (CHASRS) Review of General Psychology. 2005;9:143–155. [Google Scholar]
- (17).King DE, Mainous AG, 3rd, Steyer TE, Pearson W. The relationship between attendance at religious services and cardiovascular inflammatory markers. International Journal of Psychiatry in Medicine. 2001;31:415–25. doi: 10.2190/F4MP-KLYE-VED4-3LDD. [DOI] [PubMed] [Google Scholar]
- (18).Powell LH, Shahabi L, Thoresen CE. Religion and spirituality: Linkages to physical health. American Psychologist. 2003;58:36–52. doi: 10.1037/0003-066x.58.1.36. [DOI] [PubMed] [Google Scholar]
- (19).Whooley MA, Boyd AL, Gardin JM, Williams DR. Religious involvement and cigarette smoking in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Archives of Internal Medicine. 2002;162:1604–10. doi: 10.1001/archinte.162.14.1604. [DOI] [PubMed] [Google Scholar]
- (20).Bernardi L, Sleight P, Bandinelli G, Cencetti S, Fattorini L, Wdowczyc-Szulc J, Lagi A. Effect of rosary prayer and yoga mantras on autonomic cardiovascular rhythms: comparative study. British Medical Journal. 2001;323:1446–9. doi: 10.1136/bmj.323.7327.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Goldbourt U, Yaari S, Medalie JH. Factors predictive of long-term coronary heart disease mortality among 10,059 male Israeli civil servants and municipal employees. Cardiology. 1993;82:100–121. doi: 10.1159/000175862. [DOI] [PubMed] [Google Scholar]
- (22).Hummer RA, Rogers RG, Nam CB, Ellison CG. Religious involvement and U.S. adult mortality. Demography. 1999;36:273–285. [PubMed] [Google Scholar]
- (23).Oman D, Kurata JH, Strawbridge WJ, Cohen RD. Religious attendance and cause of death over 31 years. International Journal of Psychiatry in Medicine. 2002;32:69–89. doi: 10.2190/RJY7-CRR1-HCW5-XVEG. [DOI] [PubMed] [Google Scholar]
- (24).Hill PC, Pargament KI. Advances in the conceptualization and measurement of religion and spirituality: Implications for physical and mental health research. American Psychologist. 2003;58:64–74. doi: 10.1037/0003-066x.58.1.64. [DOI] [PubMed] [Google Scholar]
- (25).Krause N, Ellison CG, Wulff KM. Church-based support, negative interaction, and well-being. Journal for the Scientific Study of Religion. 1993;37:725–741. [Google Scholar]
- (26).Pargament KI. The Psychology of Religion and Coping: Theory, Research, Practice. Guilford Press; New York: 1997. [Google Scholar]
- (27).Payne IR, Bergin AE, Bielema KA, Jenkins PH. Review of religion and mental health: Prevention and the enhancement of psychosocial functioning. Prevention in Human Services. 1991;2:11–40. [Google Scholar]
- (28).Kass JD, Friedman R, Lesserman J, Zuttermeister P, Benson H. Health outcomes and a new index of spiritual experiences. Journal for the Scientific Study of Religion. 1991;30:203–211. [Google Scholar]
- (29).Boomsma DI, Willemsen G, Hawkley LC, Cacioppo JT. Genetic and environmental contributions to loneliness in adults: The Netherlands Twin Register Study. Behavior Genetics. 2005;35:745–752. doi: 10.1007/s10519-005-6040-8. [DOI] [PubMed] [Google Scholar]
- (30).Cacioppo JT, Hawkley LC, Ernst JM, Burleson MH, Berntson GG, Nouriani B, Spiegel D. Loneliness within a nomological net: An evolutionary perspective. Journal of Research in Personality. 2006;40:1054–1085. [Google Scholar]
- (31).Epley N, Waytz A, Cacioppo JT. On seeing human: A three-factor theory of anthropomorphism. Psychological Review. doi: 10.1037/0033-295X.114.4.864. in press. [DOI] [PubMed] [Google Scholar]
- (32).Horsten M, Ericson M, Perski A, Wamala SP, Schenck-Gustafsson K, Orth-Gomér K. Psychosocial factors and heart rate variability in healthy women. Psychosomatic Medicine. 1999;61:49–57. doi: 10.1097/00006842-199901000-00009. [DOI] [PubMed] [Google Scholar]
- (33).House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;24:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- (34).Pollner M. Divine relations, social relations, and well-being. Journal of Health and Social Behavior. 1989;30:92–104. [PubMed] [Google Scholar]
- (35).Seeman TE. Health promoting effects of friends and family on health outcomes in older adults. American Journal of Health Promotion. 2000;14:362–370. doi: 10.4278/0890-1171-14.6.362. [DOI] [PubMed] [Google Scholar]
- (36).Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: A review with emphasis on underlying mechanisms and implications for health. Psychological Bulletin. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- (37).James W. The varieties of religious experience. Random House; New York: 1996. [Google Scholar]
- (38).Fraser GE. A comparison of first event coronary heart disease rates in two contrasting California populations. Journal of Nutrition, Health and Aging. 2005;9:53–8. [PubMed] [Google Scholar]
- (39).Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. Journal of the American College of Cardiology. 2005;45:637–51. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- (40).Udupa K, Madanmohan, Bhavanani AB, Vijayalakshmi P, Krishnamurthy N. Effect of pranayam training on cardiac function in normal young volunteers. Indian Journal of Physiology and Pharmacology. 2003;47:27–33. [PubMed] [Google Scholar]
- (41).Vempati RP, Telles S. Yoga-based guided relaxation reduces sympathetic activity judged from baseline levels. Psychological Reports. 2002;90:487–94. doi: 10.2466/pr0.2002.90.2.487. [DOI] [PubMed] [Google Scholar]
- (42).Airaksinen KE. Autonomic mechanisms and sudden death after abrupt coronary occlusion. Annals of Medicine. 1999;31:240–5. doi: 10.3109/07853899908995886. [DOI] [PubMed] [Google Scholar]
- (43).Billman GE. A comprehensive review and analysis of 25 years of data from an in vivo canine model of sudden cardiac death: implications for future anti-arrhythmic drug development. Pharmacology & Therapeutics. 2006a;111:808–35. doi: 10.1016/j.pharmthera.2006.01.002. [DOI] [PubMed] [Google Scholar]
- (44).Billman GE. Heart rate response to onset of exercise: evidence for enhanced cardiac sympathetic activity in animals susceptible to ventricular fibrillation. American Journal of Physiology: Heart and Circulation Physiology. 2006b;291:H429–35. doi: 10.1152/ajpheart.00020.2006. [DOI] [PubMed] [Google Scholar]
- (45).Schwartz P, La Rovere M, Vanoli E. Autonomic nervous system and sudden cardiac death: experimental basis and clinical observations for post-myocardial infarction risk stratification. Circulation. 1992;85(Suppl1):177–91. [PubMed] [Google Scholar]
- (46).Hohnloser SH. Ventricular arrhythmias: antiadrenergic therapy for the patient with coronary artery disease. Journal of Cardiovascular Pharmacology and Therapeutics. 2005;10(Suppl 1):S23–31. doi: 10.1177/10742484050100i404. [DOI] [PubMed] [Google Scholar]
- (47).Manfrini O, Pizzi C, Trere D, Fontana F, Bugiardini R. Parasympathetic failure and risk of subsequent coronary events in unstable angina and non-ST-segment elevation myocardial infarction. European Heart Journal. 2003;24:1560–6. doi: 10.1016/s0195-668x(03)00345-2. [DOI] [PubMed] [Google Scholar]
- (48).Eppinger H, Hess L. Vagotonia. Nervous & Mental Disease Publishing Company; New York: 1915. Mental and Nervous Disease Monograph. No 20. [Google Scholar]
- (49).Wenger MA. Studies of autonomic balance: a summary. Psychophysiology. 1966;2:173–86. doi: 10.1111/j.1469-8986.1966.tb02641.x. [DOI] [PubMed] [Google Scholar]
- (50).Malliani A. Heart rate variability: from bench to bedside. European Journal of Internal Medicine. 2005;16:12–20. doi: 10.1016/j.ejim.2004.06.016. [DOI] [PubMed] [Google Scholar]
- (51).Malliani A, Montano N. Heart rate variability as a clinical tool. Italian Heart Journal. 2002;3:439–45. [PubMed] [Google Scholar]
- (52).Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30:183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- (53).Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- (54).Malik M, Task Force of the European Society of Cardiology. the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- (55).Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation. 1997;96:3224–32. doi: 10.1161/01.cir.96.9.3224. [DOI] [PubMed] [Google Scholar]
- (56).McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones & Behavior. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- (57).Sterling P, Eyer J. Biological basis of stress-related mortality. Social Science and Medicine. 1981;15:3–42. doi: 10.1016/0271-5384(81)90061-2. [DOI] [PubMed] [Google Scholar]
- (58).Sterling P, Eyer J. In: Handbook of Life Stress, Cognition and Health. Fisher S, Reason J, editors. J Wiley Ltd.; New York: 1988. p. 631. [Google Scholar]
- (59).Balanescu S, Corlan AD, Dorobantu M, Gherasim L. Prognostic value of heart rate variability after acute myocardial infarction. Medical Science Monitor. 2004;10:CR307–15. [PubMed] [Google Scholar]
- (60).Kleiger RE, Stein PK, Bigger JT., Jr Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiology. 2005;10:88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events: the Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- (62).Liao D, Cai J, Rosamond WD, Barnes RW, Hutchinson RG, Whitsel EA, Rautaharju P, Heiss G. Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study: the ARIC Study: Atherosclerosis Risk in Communities Study. American Journal of Epidemiology. 1997;145:696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- (63).Masi CM, Hawkley LC, Rickett EM, Cacioppo JT. Respiratory sinus arrhythmia and diseases of aging: Obesity,diabetes mellitus, and hypertension. Biological Psychology. 2007;74:212–223. doi: 10.1016/j.biopsycho.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Singh JP, Larson MG, Tsuji H, Evans JC, O’Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension. 1998;32:293–7. doi: 10.1161/01.hyp.32.2.293. [DOI] [PubMed] [Google Scholar]
- (65).Aubert AE, Ramaekers D. Neurocardiology: the benefits of irregularity. The basics of methodology, physiology and current clinical applications. Acta Cardiologica. 1999;54:107–20. [PubMed] [Google Scholar]
- (66).Hemingway H, Shipley M, Brunner E, Britton A, Malik M, Marmot M. Does autonomic function link social position to coronary risk? The Whitehall II study. Circulation. 2005;111:3071–7. doi: 10.1161/CIRCULATIONAHA.104.497347. [DOI] [PubMed] [Google Scholar]
- (67).Dekker JM, Schouten EG, Klootwijk P, Pool J, Swenne CA, Kromhout D. Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men: the Zutphen Study. American Journal of Epidemiology. 1997;145:899–908. doi: 10.1093/oxfordjournals.aje.a009049. [DOI] [PubMed] [Google Scholar]
- (68).Gang Y, Malik M. Heart rate variability analysis in general medicine. Indian Pacing and Electrophysiology Journal. 2003;3:34–40. [PMC free article] [PubMed] [Google Scholar]
- (69).Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology. 2007;74:185–99. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- (70).Friedman BH, Thayer JF. Anxiety and autonomic flexibility: a cardiovascular approach. Biological Psychology. 1998;49:303–23. doi: 10.1016/s0301-0511(98)00051-9. [DOI] [PubMed] [Google Scholar]
- (71).Hoehn-Saric R, McLeod DR. Anxiety and arousal: physiological changes and their perception. Journal of Affective Disorders. 2000;61:217–24. doi: 10.1016/s0165-0327(00)00339-6. [DOI] [PubMed] [Google Scholar]
- (72).Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Annals N Y Academy of Sciences. 2006;1088:361–72. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- (73).Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, Thisted R. Loneliness as a specific risk factor for depressive symptoms: Cross sectional and longitudinal analyses. Psychology of Aging. 2006;21:140–151. doi: 10.1037/0882-7974.21.1.140. [DOI] [PubMed] [Google Scholar]
- (74).Berntson GG, Cacioppo JT, Binkley PF, Uchino BN, Quigley KS, Fieldstone A. Autonomic cardiac control: III. Psychological stress and cardiac response in autonomic space as revealed by pharmacological blockades. Psychophysiology. 1994;31:599–608. doi: 10.1111/j.1469-8986.1994.tb02352.x. [DOI] [PubMed] [Google Scholar]
- (75).Cacioppo JT, Berntson GG, Binkley PF, Quigley KS, Uchino BN, Fieldstone A. Autonomic cardiac control. II. Basal response, noninvasive indices, and autonomic space as revealed by autonomic blockades. Psychophysiology. 1994;31:586–598. doi: 10.1111/j.1469-8986.1994.tb02351.x. [DOI] [PubMed] [Google Scholar]
- (76).Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- (77).Paloutzian RF, Ellison CW. Loneliness, spiritual well-being, and quality of life. In: Peplau LA, Periman D, editors. Loneliness: A sourcebook of current theory, research, and therapy. Wiley; New York: 1982. pp. 224–237. [Google Scholar]
- (78).Goldberg LR. The development of markers for the BigFive factor structure. Psychological Assessment. 1992;4:26–42. [Google Scholar]
- (79).Russell D, Peplau LA, Cutrona CE. The revised UCLA Loneliness Scale: Concurrent and discriminant validity evidence. Journal of Personality and Social Psychology. 1980;39:472–480. doi: 10.1037//0022-3514.39.3.472. [DOI] [PubMed] [Google Scholar]
- (80).Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- (81).Cohen S, Hoberman HM. Positive events and social supports as buffers of life change stress. Journal of Applied Social Psychology. 1983;13:99–125. [Google Scholar]
- (82).Cook WW, Medley DM. Proposed hostility and Pharisaic-virtue scales for the MMPI. Applied Psychology. 1954;38:414–418. [Google Scholar]
- (83).Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- (84).Baron RM, Kenny DA. The moderator-mediator distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- (85).MacKinnon DP, Warsi G, Dwyer JH. A simulation study of mediated effect measures. Multivariate Behavioral Research. 1995;30:41–62. doi: 10.1207/s15327906mbr3001_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- (87).Hawkley LC, Burleson MH, Berntson GG, Cacioppo JT. Loneliness in everyday life: Cardiovascular activity, psychosocial context, and health behaviors. Journal of Personality & Social Psychology. 2003;85:105–120. doi: 10.1037/0022-3514.85.1.105. [DOI] [PubMed] [Google Scholar]
- (88).Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: A review with emphasis on underlying mechanisms and implications for health. Psychological Bulletin. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- (89).Porges SW. A phylogenetic journey through the vague and ambiguous Xth cranial nerve: a commentary on contemporary heart rate variability research. Biological Psychology. 2007;74:301–7. doi: 10.1016/j.biopsycho.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]