Abstract
Our previous studies have shown that the activation of the transient receptor potential vanilloid type 1 (TRPV1) expressed in the renal pelvis leads to an increase in ipsilateral afferent renal nerve activity (ARNA) and contralateral renal excretory function, but the molecular mechanisms of TRPV1 action are largely unknown. This study tests the hypothesis that activation of receptors of neurokinin 1 (NK1) or calcitonin gene-related peptide (CGRP) by endogenously released substance P (SP) or CGRP following TRPV1 activation, respectively, governs TRPV1-induced increases in ARNA and renal excretory function. Capsaicin (CAP, 0.04, 0.4, 4nM), a selective TRPV1 agonist, administrated into the renal pelvis dose-dependently increased ARNA. CAP (4nM)-induced increases in ipsilateral ARNA or contralateral urine flow rate (Uflow) and urinary sodium excretion (UNa) were abolished by capsazepine (CAPZ), a selective TRPV1 antagonist, RP67580, or L703,606, selective NK1 antagonists, but not by CGRP8-37, a selective CGRP receptor antagonist. Both SP (7.4nM) and CGRP (0.13μM) increased ARNA, Uflow, or UNa, and increases in these parameters induced by CGRP but not SP were abolished by CAPZ. CAP at 4nM perfused into the renal pelvis caused the release of SP and CGRP, which was blocked by CAPZ but not by RP67580, L703,606, or CGRP8-37. Immunofluorescence results showed that NK1 receptors were expressed in sensory neurons in dorsal root ganglion (DRG) and sensory nerve fibers innervating the renal pelvis. Taken together, our data indicate that NK1 activation induced by SP release upon TRPV1 activation governs TRPV1 function and a TRPV1-dependent mechanism is operant in CGRP action.
INTRODUCTION
The transient receptor potential vanilloid type 1 (TRPV1) channel is a non-selective cation channel that can be activated by capsaicin (CAP), noxious heat, lipid metabolites, and protons (Guo et al., 1999; Julius and Basbaum, 2001; Klionsky et al., 2006). Our previous data show that activation of TRPV1 by CAP perfused into the unilateral renal pelvis leads to bilateral diuresis and natriuresis via a dual renorenal reflex, and this effect is abolished after ipsilateral renal denervation (Zhu et al., 2005). Moreover, hypertonic saline perfused into the renal pelvis causes increases in ipsilateral afferent renal nerve activity (ARNA) and contralateral renal excretory function by activation of TRPV1 and neurokinin 1 (NK1) receptors (Zhu et al., 2007). These data indicate that TRPV1-positive sensory nerves innervating the renal pelvis play an important role in regulating ARNA and maintaining sodium and water homeostasis, but the mechanism by whichTRPV1 activation induces elevated ARNA is largely unknown.
Activation of TRPV1 expressed in sensory nerves of unmyelinated C-fibers or thinly myelinated Aδ-fibers causes release of a variety of sensory neuropeptides, including substance P (SP) and calcitonin gene-related peptide (CGRP). SP and CGRP are co-localized in renal pelvis sensory nerves and may be totally depleted after CAP treatment (Hua et al., 1987). CGRP perfused into the renal pelvis causes an increase in ARNA, which is blocked by a NK1 receptor antagonist (Gontijo et al., 1999). While these studies demonstrate a relation between CGRP and SP, their contribution to TRPV1 action is unknown.
SP perfused into the renal pelvis increases ispilateral ARNA, contralateral urine flow and urine sodium excretion, which is abolished by NK1 receptor antagonists (Kopp and Smith, 1991; Lindberg and Dolata, 1993). Increases in renal pelvis pressure or bradykinin perfused into the renal pelvis cause an increase in ARNA, which is also blocked by the NK1 receptor antagonist CP-96,345, or abolished when SP release is blocked (Kopp et al., 2000; Kopp and Smith, 1993). While these results show a relation between increased renal pelvis pressure, bradykinin, and NK1 activation by SP, the role of NK1 receptors in TRPV1-induced increases in ipsilateral ARNA and contralateral renal function is unknown. Thus, the goal of the present study was to define the molecular mechanisms of TRPV1-induced increases in ARNA and the relation between TRPV1, NK1 and CGRP receptors.
METHODS
All experiments were approved by the Institutional Animal Care and Use Committee of Michigan State University. Male Wistar rats weighing 273±5 g (Charles River Laboratories; Wilmington, MA) were housed in the animal facility for 1 week before used in the experiments.
Surgical Procedures
Anesthesia was induced via intraperitoneally administered pentobarbital sodium at 50 mg·kg−1 and maintained with an intravenous infusion of 10 mg·kg−1hr−1 at 50 μl·min−1. Polyethylene catheters (PE50) were placed in the left jugular vein for infusion of pentobarbital sodium, and in the left carotid artery to measure mean arterial pressure (MAP) with a Statham 231D pressure transducer coupled to a Gould 2400s recorder (Gould Instrument Systems, Valley View, Ohio, USA). Two catheters (PE-50) were inserted into both of the ureters with their tips in the renal pelvis via midline incision for urine collection. A MD-2000 microdialysis tube (ID 0.18/OD 0.22 mm, BASi, West Lafayette, IN 47906, USA) was placed inside the PE-50 catheter with its tip extending 1–2mm out of the PE50 catheter for perfusion drugs at 20 μl·min−1, a rate that did not change renal pelvis pressure (Zhu et al., 2005; Zhu et al., 2007).
For the ARNA recording experiments, the renal nerves were isolated at the angle between the abdominal aorta and the renal artery via a left flank incision with the use of a stereoscopic dissecting microscope. The nerves were placed on the bipolar stainless steel electrode to record multifiber nerve activity. The electrode was connected to a high-impedance probe (HIP-511, Grass Instruments). The signals were amplified x20,000, filtered with a high-frequency cutoff at 1,000Hz and a low-frequency cutoff at 100Hz by a Grass model P511 AC Amplifier and recorded by Gould 2400s recorder (Grould Instrument System, Valley View, Ohio, USA). After the renal nerve activity was verified using its pulse synchronous rhythmicity with the heartbeat, the nerves were sectioned and the distal part was placed on the electrode for ARNA recording. The electrode was fixed to the renal nerve with Kwik-Cast & Kwik-Sil (World Precision Instruments, Sarasota, Florida, USA). The renal nerve activity was transformed into voltage integration. The post-mortem renal nerve activity recorded as background of renal nerve activity was subtracted from all values. ARNA is expressed in percent of its basal value (Ma et al., 2002a; Ma et al., 2002b; Zhu et al., 2007).
Left renal pelvis perfusion was performed after surgery. The rats were stabilized for 1.5 hours before the experiment started. The drugs were perfused into the renal pelvis in two 3-minute periods, i.e., capsazepine (CAPZ, 0.4mM, Calbiochem, San Diego, California, USA) (Zhu et al., 2005), an antagonist of TRPV1; RP67580 (0.2mM, Tocris) (Zhu et al., 2005) and L-703, 606 (10μM, Sigma), antagonists of NK1 receptors; or CGRP8-37 (0.32μM, American Peptide), an antagonist of CGRP receptors, were perfused within the first 3–minute period, and CAP, SP or CGRP (0.13μM, Sigma) with or without the antagonists within the second 3–minute period following right after the first 3-minute period. In the case when one of the drugs above was perfused alone (either agonists or antagonists alone), the other period was perfused with the vehicle. Urine samples were collected for three 10-minute periods, i. e. 10 mins before the beginning of the first 3-min period as baseline; 10 mins starting at the beginning of the second 3-min period; and 10 mins following right after the second 10 min period. Urine flow rate (Uflow) and urinary sodium excretion (UNa) was determined in the samples collected from the contralateral kidney. Urinary sodium excretion was measured as described previously using a flame photometer (Model IL 943, Instrumentation Laboratory), and Uflow and UNa were expressed per gram of kidney weight per minute (μmol/min/g) (Zhu et al., 2005; Zhu et al., 2007).
Renal Pelvis SP and CGRP Release
To measure the release of SP and CGRP from the renal pelvis, 10μM thiorphan (Sigma), an endopeptidase inhibitor, was perfused into the renal pelvis to reduce the catabolism of SP and CGRP (Ma et al., 2002a). The samples were collected in three 10-min periods in animals that the ARNA was recorded, and stored at −70°C for the following analysis of SP and CGRP levels (Ma et al., 2002a).
Radioimmunoassay
The urine samples from the left kidney were purified and analyzed by radioimmunoassay (rat RIA kits; Peninsula Laboratories Inc, San Carlos, CA, USA) as described previously for the measurement of SP and CGRP release. The concentration of CGRP and SP was normalized by the kidney weight (Wang and Wang, 2004).
Immunofluorescence
Dorsal root ganglion (DRG from both sides, T8-L4) and the renal pelvis were collected from control rats and frozen in OCT and stored at −70°C. The tissue sections were fixed with formalin before the experiment. They were first incubated in PBS contained 5% bovine serum albumin (BSA) for 30min at room temperature to block nonspecific binding, then the sections were incubated with rabbit anti-NK1 receptor antiserum (1:50, Sigma) with 2% BSA at 4°C overnight. The negative control sections were incubated with 5% BSA instead of anti-NK1 receptor antiserum at 4°C overnight. After being rinsed three times for 10 min, the sections were incubated with donkey-anti-rabbit CY2-labeled IgG (1:500, Jackson Immunoresearch) for 1 hour in room temperature. The sections were washed and viewed under microscope after incubation (Aline Boer et al., 2005; Wang and Wang, 2004).
Statistical analysis
All values were expressed as means±SE. The differences among groups were analyzed using one-way ANOVA followed by the Tukey-Kramer multiple comparison tests. Comparisons of MAP before and after administration of drugs were performed by the use of a paired t-test. Differences were considered statistically significant at p < 0.05.
RESULTS
Body weight was not different among groups (data no shown). Moreover, the MAP was not different between basal, treatment, and recovery periods in each group or among groups (Tab. 1).
Table 1.
Mean arterial pressure (mmHg) during basal, treatment, and recovery periods from each group
| Basal | Treatment | Recovery | |
|---|---|---|---|
| CAP | 119 ± 6 | 118 ± 7 | 118 ± 6 |
| CAPZ+CAP | 125 ± 2 | 125 ± 2 | 124 ± 2 |
| RP67580+CAP | 124 ± 3 | 124 ± 2 | 121 ± 2 |
| L703, 606+CAP | 121 ± 6 | 122 ± 5 | 125 ± 5 |
| CGRP8-37+CAP | 124 ± 3 | 122 ± 3 | 120 ± 4 |
| SP | 121 ± 5 | 120 ± 5 | 118 ± 4 |
| CAPZ+SP | 120 ± 4 | 120 ± 3 | 119 ± 4 |
| CGRP | 117 ± 5 | 118 ± 6 | 118 ± 5 |
| CAPZ+CGRP | 120 ± 4 | 123 ± 4 | 120 ± 4 |
Value was expressed as mean ± SE, n=5–7 in each group
CAP, Capsaicin CAPZ, Capsazepine, TRPV1 antagonist
RP67580, NK1 antagonist L703,606, NK1 antagonist
CGRP8-37, CGRP receptors antagonist SP, Substance P
CGRP, Calcitonin gene-related peptide
Change of Contralateral Uflow, UNa and Ipsilateral ARNA from Each Group
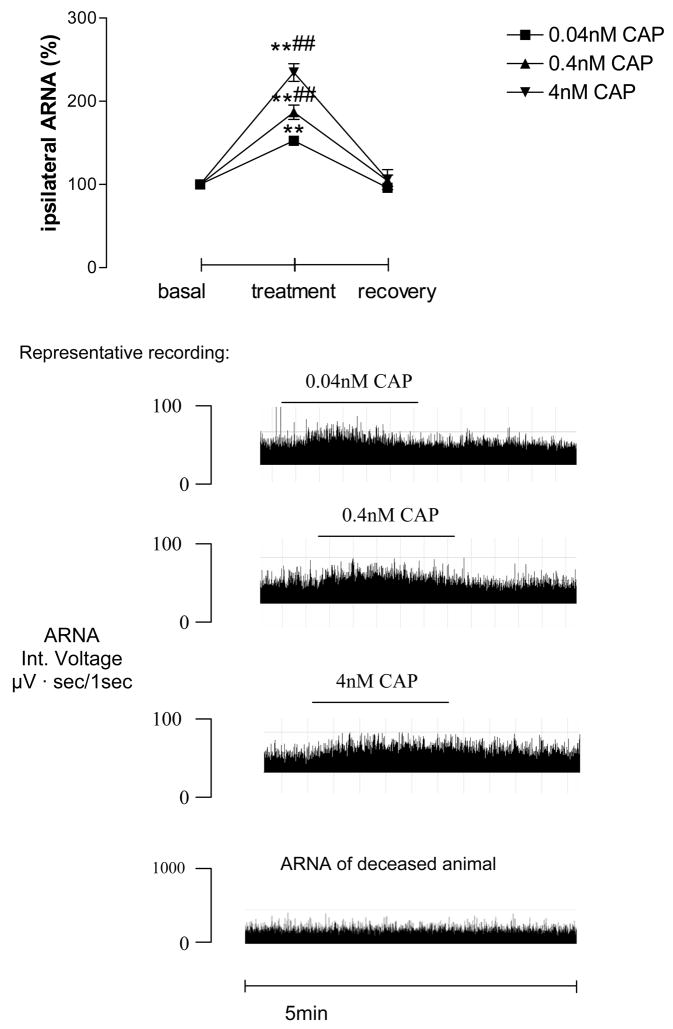
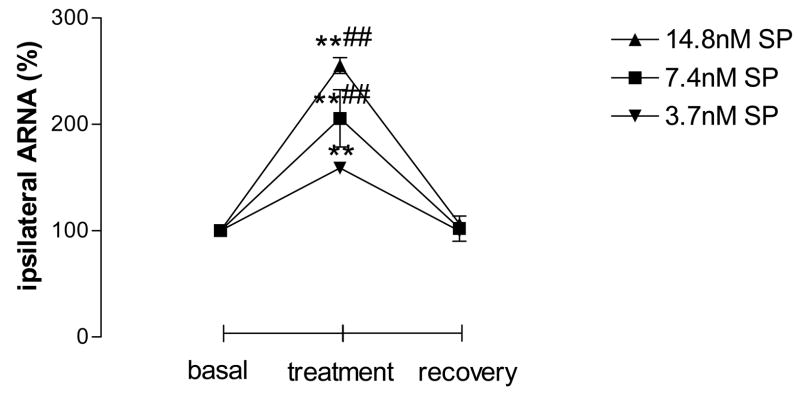
While CAP was perfused into the left renal pelvis at concentrations of 0.04nM, 0.4nM and 4nM, ipsilateral ARNA was increased dose-dependently (Fig. 1). No difference existed in the basal and recovery values among different groups. CAP at 4nM was chosen to be used in the following experiments unless otherwise indicated. SP at 3.7, 7.4 and 14.8nM also increases ipsilateral ARNA dose-dependently, which occurred immediately after SP applications (Fig. 2). There was no difference in the basal and recovery values among groups. SP at 7.4nM was used in the following experiments unless otherwise indicated.
Figure 1.
Ipsilateral afferent renal nerve activity (ARNA) induced by capsaicin (CAP) perfused into the left renal pelvis (n=5–6 in each group). CAP was given at 0.04nM, 0.4nM and 4nM into the left renal pelvis, and reprehensive recording at each dose is shown. ** vs basal value and ## vs 0.04nM CAP-treated group, ** p<0.01, ## p<0.01.
Figure 2.
Ipsilateral ARNA induced by different concentration of substance P (SP) given via left renal pelvis perfusion (n=5–6 in each group). SP was given at 3.7nM, 7.4nM and 14.8nM into the left renal pelvis. ** vs basal value and ## vs 0.04nM CAP-treated group, ** p<0.01, ## p<0.01.
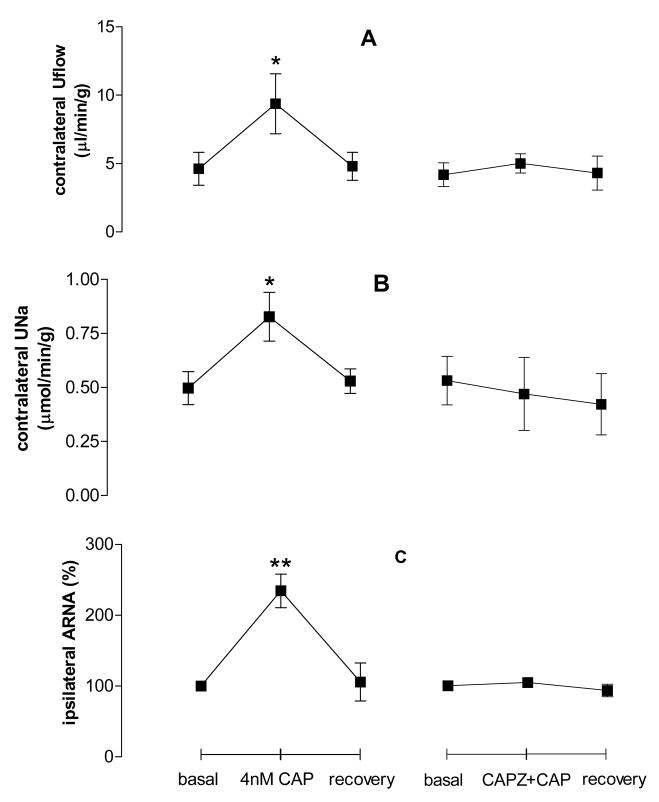
CAP at 4nM perfused into the left renal pelvis led to increases of contralateral Uflow, UNa and ipsilateral ARNA. The increase of Uflow, UNa and ARNA was abolished when CAPZ was pre-perfused into the left renal pelvis (Fig. 3), indicating that TRPV1 activation increases Uflow, UNa, and ARNA.
Figure 3.
Effect of CAP without or with capsazepine (CAPZ) given into the left renal pelvis on contralateral urine flow rate (Uflow, Fig. 3A), contralateral urine sodium excretion (UNa, Fig. 3B) and ipsilateral ARNA (Fig. 3C, n=5–6 in each group). ** vs basal value of each group, ** p<0.01.
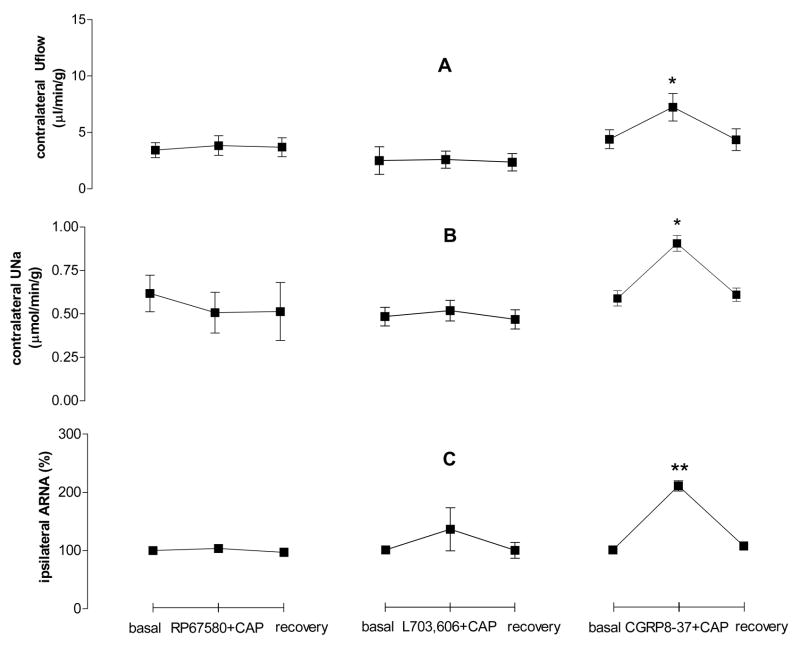
To evaluate the effect of NK1 and CGRP receptor antagonists on CAP-induced increases in contralateral Uflow, UNa, and ipsilateral ARNA, RP67580, L703,606 or CGRP8-37 was pre-perfused into the left renal pelvis. Both of the two NK1 antagonists, RP67580 and L703,606, abolished the increase of contralateral Uflow, UNa and ipsilateral ARNA induced by CAP, while the CGRP receptor antagonist, CGRP8-37, did not alter the CAP effect (Fig. 4). RP67580 and L703,606 also blocked the increase of SP-induced increase in ARNA (from 206±12% to 108 ± 10%, and from 206±12% to 115 ± 13%, respectively, p<0.01). These results indicate that CAP action is NK1 but not CGRP receptors-dependent.
Figure 4.
Effect of RP67580, L703, 606 and CGRP8-37 on contralateral urine flow rate (Uflow, Fig. 4A), contralateral urine sodium excretion (UNa, Fig. 4B) and ipsilateral ARNA (Fig. 4C) induced by CAP given into the left renal pelvis (n=5–6 in each group). ** vs basal value of each group, ** p<0.01.
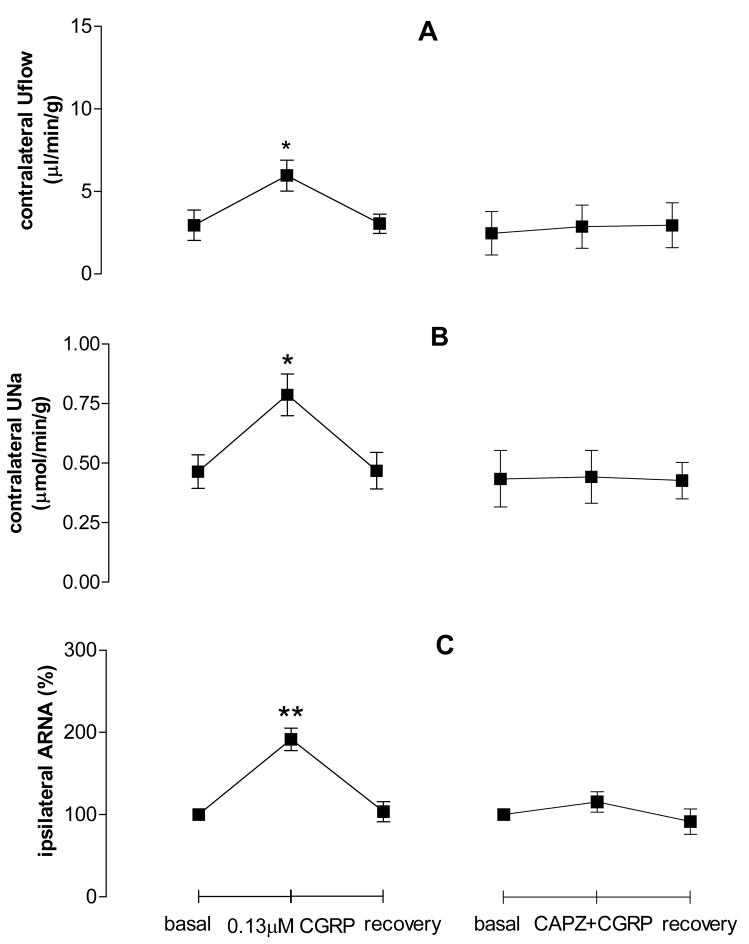
CGRP perfused into the left renal pelvis led to increases of contralateral Uflow, UNa and ipsilateral ARNA, which were delayed by about 100 seconds (Fig. 5). CGRP-induced increases of contralateral Uflow, UNa and ipsilateral ARNA were abolished by CAPZ pre-perfused into the left renal pelvis (Fig. 5), indicating that TRPV1 mediates CGRP action.
Figure 5.
Effect of calcitonin gene-related peptide (CGRP) with or without CAPZ on contralateral urine flow rate (Uflow, Fig. 6A), contralateral urine sodium excretion (UNa, Fig. 6B) and ipsilateral ARNA (Fig. 6C) induced by CAP given into the left renal pelvis (n=5–6 in each group). ** vs basal value of each group, ** p<0.01.
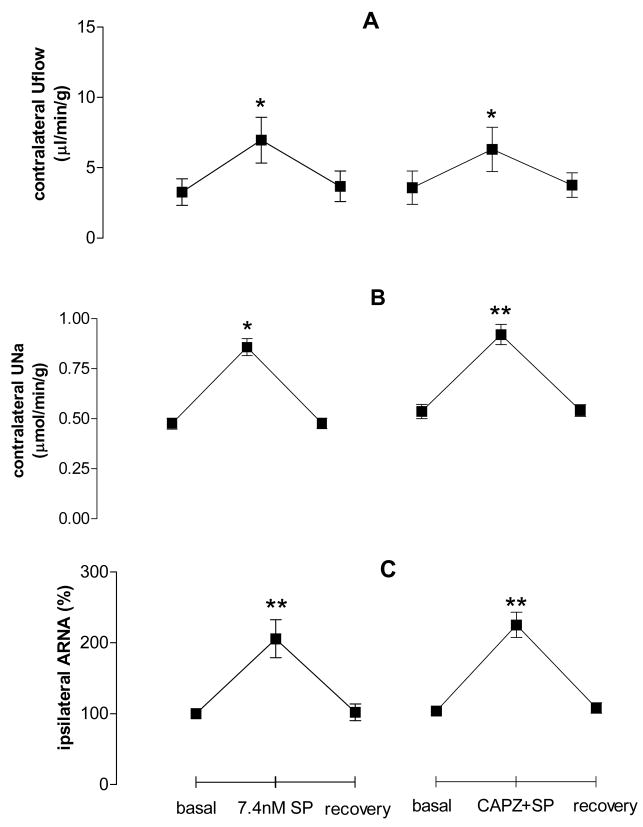
To determine the effect of the TRPV1 antagonist, CAPZ, on SP-induced increases in contralateral Uflow, UNa and ipsilateral ARNA, SP with or without CAPZ was perfused into the left renal pelvis. Different from the effect of CAPZ on CGRP-induced responses, CAPZ pre-perfused into the left renal pelvis did not alter SP-induced increases in contralateral Uflow, UNa and ipsilateral ARNA (Fig. 6), indicting that SP action is not TRPV1-dependent.
Figure 6.
Effect of SP with or without CAPZ on contralateral urine flow rate (Uflow, Fig. 5A), contralateral urine sodium excretion (UNa, Fig. 5B) and ipsilateral ARNA (Fig. 5C) induced by CAP given into the left renal pelvis (n=5–7 in each group). ** vs basal value of each group, ** p<0.01.
Level of SP and CGRP Release in Urine from the Left Renal Pelvis
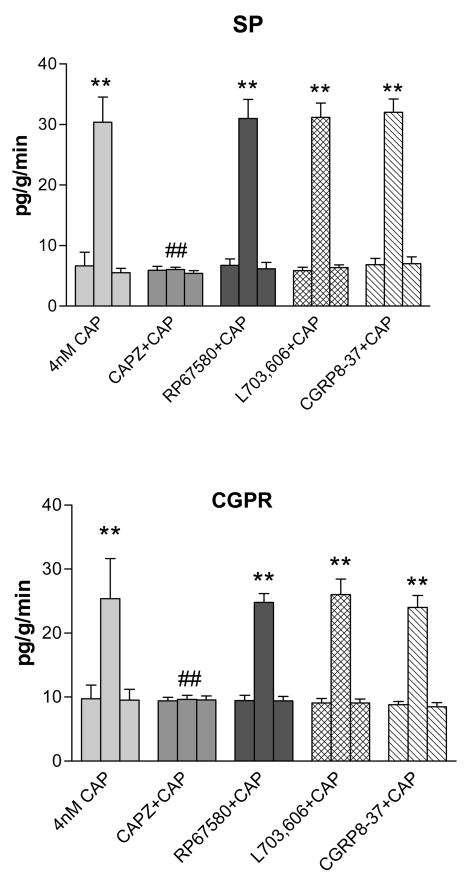
Radioimmunoassay was used to measure the level of SP and CGRP release into urine from the left kidneys when different drugs were given via the left renal pelvis. CAP at 4nM perfused into the left renal pelvis increased the release of SP and CGRP (Fig. 7). The increase was blocked when CAPZ was pre-perfused into the left renal pelvis. In contrast, NK1 antagonists, RP67580 or L703, 606, did not change the SP and CGRP release caused by CAP. Neither did CGRP antagonist CGRP8-37 (Fig. 7).
Figure 7.
Level of SP and CGRP released into urine from left kidney before, during and after treatment. ** vs basal value of each group, ** p<0.01. ## vs 4nM CAP-treated group, ## p<0.01
Staining of the NK1 Receptor in DRG and the Renal Pelvis nerve fibers
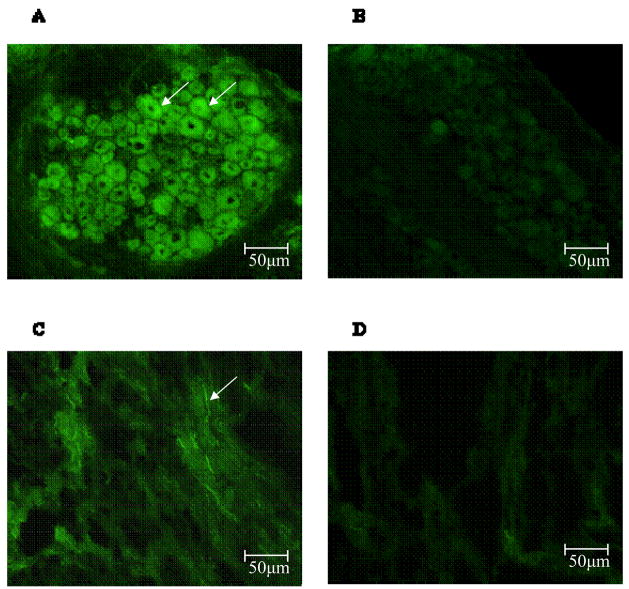
To determine whether there are NK1 receptors expressed in DRG and the renal pelvis nerve fibers, immunofluorescence was performed. In DRG, immunoreactivity of NK1 receptor was seen in DRG neurons (Fig. 8A). In the renal pelvis, NK1 receptor positive nerve fibers were found (Fig. 8C).
Figure 8.
Immunofluorscence staining of neurokinin 1 (NK1) receptor in DRG and the renal pelvis. Staining of NK1 receptors (arrows) in DRG (Fig. 8A) and in the renal pelvis in green (Fig. 8C) is shown. The negative controls in which no anti-NK1 receptor antiserum was used for DRG (Fig. 8B) and the renal pelvis (Fig. 8D) are also shown.
DISCUSSION
TRPV1 expresses primarily in sensory nerves of unmyelinated C-fibers or thinly myelinated Aδ-fibers (Guo et al., 1999). Activation of TRPV1 leads to release of various neuropeptides, including SP and CGRP, both of which may increase ARNA (Gontijo et al., 1999; Guo et al., 1999; Kopp and Smith, 1991). The goal of the present study was to examine whether there was cross-talk between TRPV1, NK1 and CGRP receptors. We have previously shown that CAP perfused into the renal pelvis leads to an increase in diuresis and natriuresis, which can be blocked by the TRPV1 antagonist, CAPZ, perfused into the renal pelvis or by ipsilateral denervation (Zhu et al., 2005). Furthermore, hypertonic saline-induced increases in ipsilateral ARNA and contralateral renal excretory function are mediated by activation of TRPV1 and NK1 receptors (Zhu et al., 2007). These data indicate that activation of TRPV1-positive sensory nerves innervating the renal pelvis leads to increases in ipsilateral ARNA and enhances contralateral renal excretory function. However, the role of endogenously released CGRP and SP in TRPV1-mediated actions is unknown.
It has been shown that CGRP administered into the renal pelvis increases ARNA, which is blocked by the CGRP receptor antagonist, CGRP8-37 (Gontijo and Kopp, 1999). However, the CGRP receptor antagonist is not effective in blocking the increase of ipsilateral ARNA, contralateral Uflow and UNa induced by increases in renal pelvis pressure or by NaCl or KCl perfused into the renal pelvis (Gontijo and Kopp, 1999). Consistently, we have shown in the present experiment that the increase in ipsilateral ARNA, contralateral Uflow and UNa induced by activation of TRPV1 is not blocked by the CGRP receptor antagonist, indicating that TRPV1, activated by altered physical- or chemical-environments in the renal pelvis, may function independently of CGRP receptor activation. Indeed, we have shown that TRPV1, when activated by hypertonic saline perfused into the renal pelvis, mediates hypertonic saline induced natriuresis and diuresis (Zhu et al., 2007). Intriguingly, in contrast to CGRP-independent effects of TRPV1, the increases of ipsilateral ARNA, contralateral Uflow and UNa induced by CGRP perfused into the renal pelvis are blocked by the TRPV1 antagonist, CAPZ. These data indicate that CGRP-induced increases in ipsilateral ARNA and contralateral renal excretion function depend on the activation of TRPV1. It has been shown that CGRP receptor activation activates phospholipase C (PLC), which involves the Galphaq/11 subunit of G-proteins (Drissi et al., 1998). CAP can also activate PLC in TRPV-1 positive cells and the activation of phosphoinositedes leads to TRPV1 activation (Lukacs et al., 2007). Thus, the cross-talk between CGRP receptors and TRPV1 channels may mediate by PLC activation.
Increases in the renal pelvis pressure may cause an increase in ARNA, which can be blocked by the NK1 receptor antagonist, CP-96,345 (Kopp and Smith, 1993). Increases in renal pelvis pressure lead to increases of ARNA and the release of prostaglandin E2 and SP in the ipsilateral renal pelvis (Kopp and Smith, 1993). Increases in ARNA are abolished when the release of prostaglandin E2 and SP is blocked (Kopp et al., 1996). This evidence indicates that the increase of ARNA induced by increase of the renal pelvis pressure depends on SP release and its activation of the NK1 receptor (Kopp et al., 1996; Kopp and Smith, 1993). Moreover, bradykinin perfused into the renal pelvis causes an increase in ARNA, and this response also depends on SP release and its activation of the NK1 receptor (Kopp et al., 2000). Our results show that CAP-induced increases in ipsilateral ARNA, contralateral Uflow and UNa are blocked by the NK1 receptor antagonists RP67580 and L703,606. These data indicate that CAP activation of ARNA depends on activation of the NK1 receptor following SP release induced by CAP activation of TRPV1.
SP is a neuropeptide found in sensory nerves (Guo et al., 1999). SP given via renal pelvis perfusion causes an increase in ipsilateral ARNA and contralateral excretion function (Kopp and Smith, 1991; Ma et al., 2002b). Inhibition of Na-K-ATPase which plays a role in maintaining membrane resting potential sensitizes renal mechanoreceptors activated by increases of renal pelvis pressure (Kopp et al., 1994). In contrast, the Na+ channel blocker, amiloride, given into the renal pelvis reduces the increase of ARNA triggered by increases in renal pelvis pressure or by SP administrated into the renal pelvis (Kopp et al., 1998). SP produces a slow depolarization in respiratory pacemaker and non-pacemaker neurons, and the depolarization is induced by activating a low-threshold TTX-insensitive cation channel that is mostly selective for Na+ (Peäna and Ramirez, 2004). In cultured rat cortex neurons, a voltage-dependent sodium current activated by SP can be blocked by NK1 receptor antagonists (Caeser et al., 1993). Unlike Na-K-ATPase inhibitors or Na channel blockers, our results indicated that the TRPV1 antagonist did not affect the increase of ipsilateral ARNA and contralateral excretion function induced by SP perfused into the renal pelvis, albeit the NK1 receptor antagonists abolish the increases in ARNA and renal excretory function caused by CAP. Taken together, these data indicate that the TRPV1-induced increases in ARNA and contralateral renal excretion function depend on SP-induced activation of NK1, i.e. NK1 activation by endogenously released SP governs TRPV1-mediated action. Furthermore, SP-induced increases in ARNA and renal function might be mediated by the activation of Na+ channels.
Our data show that CAP-induced CGRP and SP release from sensory nerves in ipsilateral renal pelvis was blocked by CAPZ pre-perfused into the renal pelvis, indicating a TRPV1-dependent mechanism. Although NK1 receptor antagonists given into the left renal pelvis did not block the release of CGRP and SP triggered by CAP, they did block CAP-induced increases in ipsilateral ARNA and contralateral renal excretion function. Neither CGRP and SP release nor ARNA and renal excretion function induced by CAP was alter by the CGRP receptor antagonist. Taken together, our data indicates that CAP-induced increases in ipsilateral ARNA and contralateral Uflow and UNa depend on activation of NK1 receptors following SP release.
In conclusion, these experiments show that activation of NK1 by endogenously released SP mediates TRPV1-induced increases in ipsilateral ARNA and contralateral renal excretion function, indicating that NK1 controls TRPV1 function. Furthermore, TRPV1 governs CGRP- but not SP-induced ipsilateral ARNA and contralateral renal excretion function, indicating a TRPV1-dependent mechanism of CGRP action.
Consistent with previous findings (Chen and Hoover, 1995), our immunofluorescence results show that NK1 receptors are located in both DRG neurons and the renal pelvic nerves, albeit specific cell localization is needed to be determined in the future. It has been shown that the reduction of NK1 receptors expressed in the renal pelvis might contribute to the impaired renorenal reflex in cirrhotic rat (Ma et al., 2002b). The decrease in functional NK1 receptors in the renal pelvis also plays a role in the impaired ARNA after renal ischemia (Ma et al., 2002c). Co-localization of the NK1 receptor, CGRP and SP is found in DRG neurons, and the impaired ARNA in SHR rats may be related to the changes of SP and CGRP and/or to the decrease in NK1 receptors in DRG neurons (Aline Boer et al., 2005). Moreover, impaired expression and function of TRPV1 have been found in Dahl salt sensitive hypertensive rats, which may contribute to the development of salt-induced increases in blood pressure in this strain (Wang and Wang, 2006). Taken together, the data of the present studies regarding the cross-talk between TRPV1, NK1 and CGRP receptors raises the possibility that impairment or dysfunction in these components may impair renal function and may contribute to disease state such as hypertension.
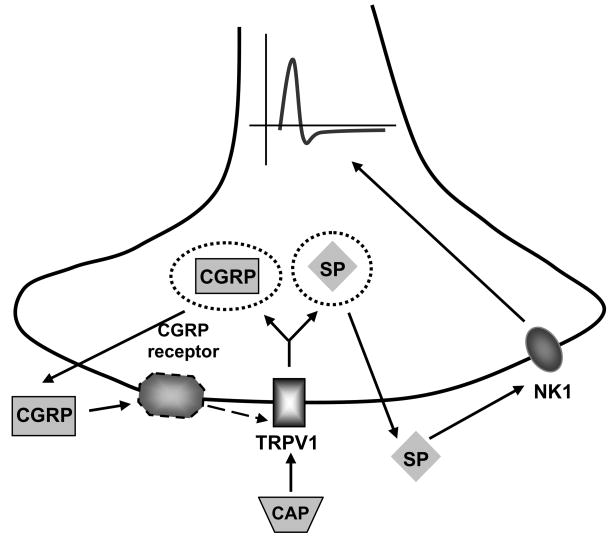
Figure 9.
The figure shows a putative scheme for the possible pathways studies in the present study. Activation of TRPV1 causes the release of the neuropeptides, including CGRP and SP. SP subsequently activates NK1 receptors located in sensory nerves and evokes ARNA. In contrast, CGRP-induced increases in ARNA depend on TRPV1 activation. It is unknown whether all the components are expressed in the same cell, and the scheme is a simplified version that intends to depict possible crosstalk between TRPV1, CGRP and NK1 receptors.
Acknowledgments
This study was supported in part by National Institutes of Health (grants HL-57853, HL-73287, and DK67620) and a grant from The Michigan Economic Development Corporation.
Abbreviations
- TRPV1
transient receptor potential vanilloid type 1
- ARNA
afferent renal nerve activity
- NK1
neurokinin 1
- CGRP
calcitonin gene-related peptide
- SP
substance P
- CAP
capsaicin
- CAPZ
capsazepine
- Uflow
urine flow rate
- UNa
urinary sodium excretion
- DRG
dorsal root gangilion
- MAP
mean arterial pressure
- RIA
radioimmunoassay
- BSA
bovine serum albumin
- PLC
phospholipase C
References
- Aline Boer P, Ueno M, Sant’ana JS, Saad MJ, Gontijo JA. Expression and localization of NK(1)R, substance P and CGRP are altered in dorsal root ganglia neurons of spontaneously hypertensive rats (SHR) Brain Res Mol Brain Res. 2005;138:35–44. doi: 10.1016/j.molbrainres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Caeser M, Seabrook GR, Kemp JA. Block of voltage-dependent sodium currents by the substance P receptor antagonist (+/−)-CP-96,345 in neurones cultured from rat cortex. Br J Pharmacol. 1993;109:918–924. doi: 10.1111/j.1476-5381.1993.tb13708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hoover DB. Autoradiographic localization of NK1 and NK3 tachykinin receptors in rat kidney. Peptides. 1995;16:673–681. doi: 10.1016/0196-9781(95)00027-h. [DOI] [PubMed] [Google Scholar]
- Drissi H, Lasmoles F, Le Mellay V, Marie PJ, Lieberherr M. Activation of phospholipase C-beta1 via Galphaq/11 during calcium mobilization by calcitonin gene-related peptide. J Biol chem. 1998;273:20168–20174. doi: 10.1074/jbc.273.32.20168. [DOI] [PubMed] [Google Scholar]
- Gontijo JA, Kopp UC. Activation of renal pelvic chemoreceptors in rats: role of calcitonin gene-related peptide receptors. Acta Physiol Scand. 1999;166:159–165. doi: 10.1046/j.1365-201x.1999.00540.x. [DOI] [PubMed] [Google Scholar]
- Gontijo JR, Smith LA, Kopp UC. CGRP activates renal pelvic substance P receptors by retarding substance P metabolism. Hypertension. 1999;33:493–498. doi: 10.1161/01.hyp.33.1.493. [DOI] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Hua XY, Theodorsson-Norheim E, Lundberg JM, Kinn AC, Hèokfelt T, Cuello AC. Co-localization of tachykinins and calcitonin gene-related peptide in capsaicin-sensitive afferents in relation to motility effects on the human ureter in vitro. Neuroscience. 1987;23:693–703. [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Klionsky L, Tamir R, Holzinger B, Bi X, Talvenheimo J, Kim H, Martin F, Louis JC, Treanor JJ, Gavva NR. A polyclonal antibody to the prepore loop of transient receptor potential vanilloid type 1 blocks channel activation. J Pharmacol Exp Ther. 2006;319:192–198. doi: 10.1124/jpet.106.108092. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Farley DM, Cicha MZ, Smith LA. Activation of renal mechanosensitive neurons involves bradykinin, protein kinase C, PGE(2), and substance P. Am J Physiol Regul Integr Comp Physiol. 2000;278:R937–946. doi: 10.1152/ajpregu.2000.278.4.R937. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Farley DM, Smith LA. Renal sensory receptor activation causes prostaglandin-dependent release of substance P. Am J Physiol. 1996;270:R720–727. doi: 10.1152/ajpregu.1996.270.4.R720. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Matsushita K, Sigmund RD, Smith LA, Watanabe S, Stokes JB. Amiloride-sensitive Na+ channels in pelvic uroepithelium involved in renal sensory receptor activation. Am J Physiol. 1998;275:R1780–1792. doi: 10.1152/ajpregu.1998.275.6.R1780. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Smith LA. Inhibitory renorenal reflexes: a role for substance P or other capsaicin-sensitive neurons. Am J Physiol. 1991;260:R232–239. doi: 10.1152/ajpregu.1991.260.1.R232. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Smith LA. Effects of the substance P receptor antagonist CP-96,345 on renal sensory receptor activation. Am J Physiol. 1993;264:R647–653. doi: 10.1152/ajpregu.1993.264.3.R647. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Smith LA, Pence AL. Na(+)-K(+)-ATPase inhibition sensitizes renal mechanoreceptors activated by increases in renal pelvic pressure. Am J Physiol. 1994;267:R1109–1117. doi: 10.1152/ajpregu.1994.267.4.R1109. [DOI] [PubMed] [Google Scholar]
- Lindberg S, Dolata J. NK1 receptors mediate the increase in mucociliary activity produced by tachykinins. Eur J Pharmacol. 1993;231:375–380. doi: 10.1016/0014-2999(93)90113-v. [DOI] [PubMed] [Google Scholar]
- Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J Neurosci. 2007;27:7070–7080. doi: 10.1523/JNEUROSCI.1866-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma MC, Huang HS, Chen CF. Impaired renal sensory responses after unilateral ureteral obstruction in the rat. J Am Soc Nephrol. 2002a;13:1008–1016. doi: 10.1681/ASN.V1341008. [DOI] [PubMed] [Google Scholar]
- Ma MC, Huang HS, Chien CT, Wu MS, Chen CF. Temporal decrease in renal sensory responses in rats after chronic ligation of the bile duct. Am J Physiol Renal physiol. 2002b;283:F164–172. doi: 10.1152/ajprenal.00231.2001. [DOI] [PubMed] [Google Scholar]
- Ma MC, Huang HS, Wu MS, Chien CT, Chen CF. Impaired renal sensory responses after renal ischemia in the rat. J Am Soc Nephrol. 2002c;13:1872–1883. doi: 10.1097/01.asn.0000022009.44473.56. [DOI] [PubMed] [Google Scholar]
- Peäna F, Ramirez JM. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J Neurosci. 2004;24:7549–7556. doi: 10.1523/JNEUROSCI.1871-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang DH. Prevention of endothelin-1-induced increases in blood pressure: role of endogenous CGRP. Am J Physiol Heart Circ Physiol. 2004;287:H1868–1874. doi: 10.1152/ajpheart.00241.2004. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang DH. A novel mechanism contributing to development of Dahl salt-sensitive hypertension: role of the transient receptor potential vanilloid type 1. Hypertension. 2006;47:609–614. doi: 10.1161/01.HYP.0000197390.10412.c4. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Wang Y, Wang DH. Diuresis and natriuresis caused by activation of VR1-positive sensory nerves in renal pelvis of rats. Hypertension. 2005;46:992–997. doi: 10.1161/01.HYP.0000174603.27383.67. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Xie C, Wang DH. TRPV1-mediated diuresis and natriuresis induced by hypertonic saline perfusion of the renal pelvis. Am J Nephrol. 2007;27:530–537. doi: 10.1159/000107665. [DOI] [PubMed] [Google Scholar]