Abstract
Understanding of plant-pathogen co-evolution in natural systems continues to develop as new theories at the population and species level are increasingly informed by studies unraveling the molecular basis of interactions between individual plants and their pathogens. The next challenge lies in further integration of these approaches to develop a comprehensive picture of how life history traits of both players interact with the environment to shape evolutionary trajectories.
Advances in understanding host-pathogen co-evolutionary interactions requires integrating knowledge of the molecular basis of host resistance and pathogen virulence with studies of how polymorphism in genes controlling these characteristics affects disease in nature. This must be coupled with quantification of the roles of life history and environmental heterogeneity in the maintenance of such polymorphisms (1). Aspects of this broad challenge have been articulated in recent years (2,3), but major empirical and theoretical gaps remain. These include the impact of sexual recombination and short-term selection on variability in Avr gene sequences and pathogen virulence phenotypes, how sequence divergence and specific amino acid differences in Avr/R proteins affect host recognition, and the extent to which population and regional variation in host resistance influences the maintenance of pathogen population diversity. Answers to these questions may also help to resolve on-going debate regarding the role of co-evolutionary arms races versus balancing selection in shaping patterns of polymorphism in host resistance and pathogen virulence (4).
Here we briefly highlight recent studies of wild plant-pathogen associations, and illustrate their value as model co-evolutionary systems. Importantly, at a molecular level, plant defense systems have similarity to mammalian innate cellular immunity, and utilize analogous components to recognize pathogen-derived signals and induce defense responses (5–7). The evolutionary commonalities of animal and plant disease, and the scientific promise of this conceptual realization, have been previously highlighted (8,9). These characteristics, combined with the lack of ethical issues that constrain experimental manipulations in animal populations, make plant-based systems powerful models for quantifying the epidemiological impacts of genetic variation in host disease resistance.
Plant pathogens are ubiquitous – their demographic impacts are well recognized in agriculture and acknowledged in natural communities through the effects of iconic invasive diseases (e.g. sudden oak death, Phytophthora die-back). However, the epidemiology of wild plant-pathogen interactions is significantly under-studied relative to the dynamics of infectious disease in animal and human populations. Perhaps more critically, in both plant and animal systems, there has been little effort to directly investigate causal links between population genetic structure and disease dynamics, although studies involving agricultural pathogens provide insight into how disease pressure and host diversity may interact to determine rates of pathogen evolution (10,11). The lack of empirical evidence is surprising, given the potential for genetic variation to not only affect disease dynamics and prevalence, but also when or where new diseases emerge. Characterisation of ecological and evolutionary processes at spatio-temporal scales ranging from genes to populations to species is critical in this context.
Co-evolutionary biology has advanced beyond ecological and population genetics approaches that, on one hand, assumed detailed investigation of single populations could provide surrogate assessments for all populations, and on the other, that deterministic global models were adequate representations of real-world host-pathogen associations. The formal development of metapopulation theory (12,13) and the geographic mosaic theory of co-evolution (14) has given added impetus to the articulation of a new paradigm arising from the idea that understanding the forces driving co-evolutionary trajectories requires accounting for both within and among-population processes in space and time. In keeping with this perspective, spatial structure is increasingly viewed as integral to empirical studies of natural plant-pathogen interactions (15,16).
Simulation models further illustrate the importance of integrating studies across multiple, inter-connected populations, and undermine many premises underlying earlier thinking (e.g. the assumption of fitness costs associated with resistance)(17). While empirical studies are limited, available evidence is consistent with the idea that evolutionary trajectories of natural host-pathogen systems do not reflect the dynamics of ephemeral local populations, where the evolutionary consequences of random drift, extinction and re-colonization are magnified, and where selection can favor distinct resistance and virulence patterns even in adjacent populations (18). Indeed, environmental differences may create local variation in pathogen severity leading to hot and cold spots (14) of selection pressure. Intriguingly, recent evidence that infection may stimulate host recombination rates in subsequent generations suggests a mechanism whereby disease-prone populations may respond more rapidly than otherwise possible (19,20).
A major unanswered question regarding the evolution of wild plant-pathogen associations is the extent to which resistance and virulence depend on specific interactions between single genes (qualitative resistance) versus those in which resistance is determined by many genes, individually of minor effect (quantitative resistance). While in reality, most host-pathogen systems involve genes of both major and minor effect, the conceptual distinction between these has provided a powerful stimulus to theoretical models of host-pathogen interactions (21,22). Whether qualitative or quantitative resistance is more likely to provide hosts with the greatest selective advantages against disease organisms over time is unknown — does resistance confer high short-term fitness but a high probability of ultimate failure (gene-for-gene), or are fitness impacts more constantly present but largely restricted in magnitude? Resolution of this question depends on understanding the interaction between host and pathogen life history characters that affect reproduction, survival and dispersal and how they are mediated by environmental factors.
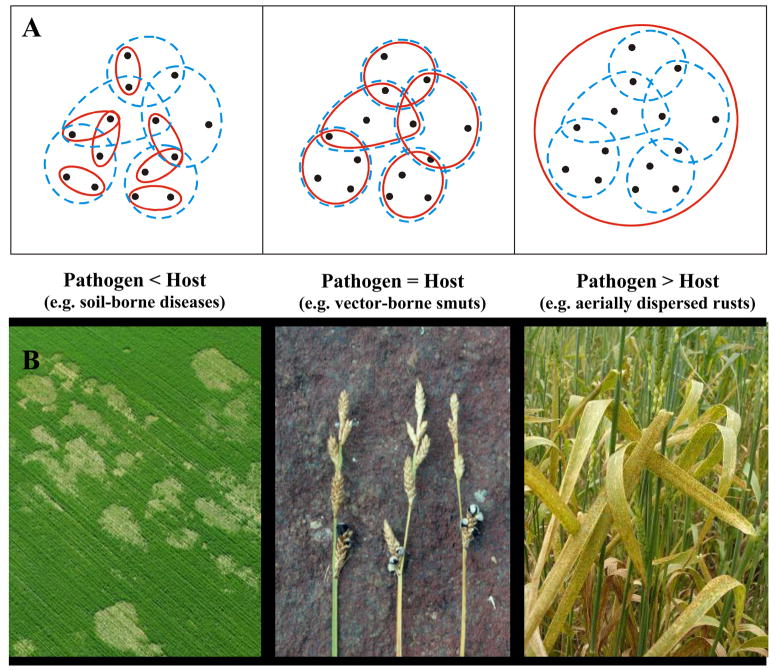
More generally, the role of life history (e.g. reproductive system, host range, pathogen dispersal mechanisms) in influencing the timing, severity and selective impact of disease has received little concerted focus in natural plant-pathogen interactions despite demonstration that comparative analysis of such data can provide valuable insights into the evolution of animal-parasite systems (23) and a means of assessing the durability of resistance in crops (i.e. following commercial adoption of a resistant cultivar, the period of time before the evolution of new pathotypes that can parasitize the resistant cultivar) (24). In plant-pathogen associations, even characters as simple as mode of pathogen impact or dispersal distance suggest a rich repertoire of possible interactions. Thus, pathogen dispersal envelopes may be substantially smaller than, may equate with, or may exceed that of their host [Fig. 1]. Variation in such traits is likely to result in different evolutionary trajectories as direct effects on contact patterns and disease incidence lead to longer-term impacts on the maintenance of genetic variation (25) and patterns of local adaptation (26).
Fig 1.
A) Examples of different host and pathogen dispersal strategies (redrawn from 29) that determine spatial interactions (dashed and solid lines represent the relative scale of host and pathogen movement respectively); B) specific host-pathogen associations that fit these dispersal scenarios while simultaneously representing pathogens that (left to right): kill hosts outright (Fusarium), castrate hosts (Anthracoidea fischeri on Carex mackenziei), and debilitate hosts (Puccinia striformiis on Triticum avestinum).
Environmental variation generates further complexity in life-history interactions. Heuristic models suggest that particular combinations of environmental conditions impose a series of selective sieves, the nature and intensity of which may be determined by specific plant and pathogen life-history features (27). For example, harsh environments that promote plant survival via seed or dormant root-stock, impose selective pressure on foliar pathogens, the intensity of which depends on particular traits. Pathogens that can survive saprophytically, or have specialised resting stages, or refugia on other hosts are subject to much smaller population fluctuations than those without such adaptations. These contrasting situations may generate marked differences in the relative importance of drift, migration, extinction and re-colonization, thus promoting distinctly different evolutionary trajectories. To understand the evolutionary drivers of resistance and virulence we therefore need a theoretical framework for investigating the interplay between physical environment and host and pathogen life-histories.
In summary, progress in understanding host-pathogen evolutionary dynamics in nature needs characterization of processes occurring at many spatio-temporal scales, including genes and cells, within host individuals, and within and among host and pathogen populations. To achieve this requires integration of molecular approaches currently being focused on agricultural pathogens, with population and species level studies. For example, cloning and sequencing of specific avirulence (Avr) genes in Melampsora lini found evidence for functional changes in the coding regions of targeted Avr genes that occurred almost exclusively via non-synonymous mutations (28). These observations provide strong independent evidence for the operation of selection on these genes. These results, coupled with population-level studies of L. marginale resistance structure, are generating a picture of the evolutionary dynamics of selection on specific Avr genes. Insights provided by the Linum system will be greatly strengthened by application of approaches developed to investigate interactions between Arabidopsis and its pathogens where, for example, molecular studies have demonstrated significant resistance costs (29). However, the need now is to place this work into a real-world ecological genetics context involving studies of interacting suites of host and pathogen populations.
The scientific rewards from comprehensive research programs such as described above include greater fundamental understanding of the natural world as well as immediate practical benefits. For weed biocontrol, population models incorporating parasitic mode and host longevity have been used to predict pathogen characteristics most likely to lead to initial rapid host population declines, as well as relative effectiveness in preventing subsequent population recovery (30). Similar approaches could estimate threats posed by specific pathogens to conservation and population restoration, or the dangers of deliberate translocation of species to new habitats where they may serve as inoculum reservoirs for vulnerable host species. Finally, in forestry and agricultural situations where plant genetic diversity is still significant, deeper understanding of the complex array of factors affecting host-pathogen co-evolution could ensure efficient targeting of control methods.
These examples underscore the need for a more generally predictive science of coevolutionary biology (14,31) which can account for human impacts on all levels of biological organization (e.g. fragmentation of natural systems, exotic introductions), and in novel ways (genetically modified organisms, introduction of new resistance genes into crops, antibiotics). Integrated investigations of coevolution in host-microbe interactions will ultimately increase our ability to predict the long-term consequences of different types of human intervention on disease.
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
References and Notes
- 1.Barrett LG, et al. TREE. 2008;23:678. [Google Scholar]
- 2.Tibayrenc M. Intern J Parasitol. 1998;28:85. doi: 10.1016/s0020-7519(97)00180-x. [DOI] [PubMed] [Google Scholar]
- 3.Real LA, et al. PNAS USA. 2005;102:12107. [Google Scholar]
- 4.Wichmann G, et al. Appl Environ Microbiol. 2005;71:2418. doi: 10.1128/AEM.71.5.2418-2432.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dangl JL, Jones JDG. Nature. 2001;411:826. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- 6.Staskawicz BJ, et al. Science. 2001;292:2285. doi: 10.1126/science.1062013. [DOI] [PubMed] [Google Scholar]
- 7.Holt BF, Hubert DA, Dangl JL. Curr Opin Immunol. 2003;15:20. doi: 10.1016/s0952-7915(02)00014-6. [DOI] [PubMed] [Google Scholar]
- 8.Rahme LG, et al. PNAS USA. 2000;97:8815. [Google Scholar]
- 9.Keen N, et al. PNAS USA. 2000;97:8752. [Google Scholar]
- 10.Chin KM, Wolfe MS. Plant Pathol. 1984;33:535. [Google Scholar]
- 11.Lannou C, et al. Plant Pathol. 2005;54:699. [Google Scholar]
- 12.Gilpin M, Hanski I, editors. Metapopulation Dynamics: Empirical and Theoretical Investigations. Harcourt Brace Jovanovich: London, UK; 1991. [Google Scholar]
- 13.Hanski I. Metapopulation Ecology. Oxford Univ. Press: Oxford, UK; 1999. [Google Scholar]
- 14.Thompson JN. The Geographic Mosaic of Coevolution. Chicago Univ. Press: Chicago, IL; 2005. [Google Scholar]
- 15.Thrall PH, Burdon JJ. Science. 2003;299:1735. doi: 10.1126/science.1080070. [DOI] [PubMed] [Google Scholar]
- 16.Laine AL. J Ecol. 2004;92:990. [Google Scholar]
- 17.Damgaard C. J Theor Biol. 1999;201:1. doi: 10.1006/jtbi.1999.1007. [DOI] [PubMed] [Google Scholar]
- 18.Thrall PH, Burdon JJ, Young A. J Ecol. 2001;89:736. [Google Scholar]
- 19.Boyko A, et al. Nucleic Acids Res. 2007;35:1714. doi: 10.1093/nar/gkm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molinier J, et al. Nature. 2006;442:1046. doi: 10.1038/nature05022. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal A, Lively CM. Evol Ecol Res. 2002;4:79. [Google Scholar]
- 22.Nuismer S. Evolution. 2006;60:24. [PubMed] [Google Scholar]
- 23.Lockhart AB, Thrall PH, Antonovics J. Biol Rev Camb Phil Soc. 1996;71:415. doi: 10.1111/j.1469-185x.1996.tb01281.x. [DOI] [PubMed] [Google Scholar]
- 24.McDonald BA, Linde C. Euphytica. 2002;124:163. [Google Scholar]
- 25.Thrall PH, Burdon JJ. Plant Pathol. 2002;51:169. [Google Scholar]
- 26.Gandon S, et al. Proc Roy Soc London B. 1996;263:1003. [Google Scholar]
- 27.Burdon JJ, et al. Oikos. 1996;76:411. [Google Scholar]
- 28.Dodds PN, et al. PNAS USA. 2006;103:8888. [Google Scholar]
- 29.Tian D, et al. Nature. 2003;423:74. doi: 10.1038/nature01588. [DOI] [PubMed] [Google Scholar]
- 30.Thrall PH, Burdon JJ. Weed Technol. 2004;18:1269. [Google Scholar]
- 31.Thrall PH, et al. TREE. 2007;22:120. [Google Scholar]
- 32.Thrall PH, Burdon JJ. J Ecol. 1997;85:743. doi: 10.1111/j.1365-2745.2010.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.This work was supported by CSIRO and NIH grant 5R01GM074265–01A2.