Abstract
Background
Colorectal cancer incidence was reduced among women assigned to active treatment in the Women's Health Initiative (WHI) estrogen plus progestin randomized trial, but the interpretation was obscured by an associated later stage of diagnosis. In contrast the estrogen-alone trial showed no incidence reduction or differential stage at diagnosis. Here, data from the WHI observational study are considered, in conjunction with colorectal cancer mortality data from the hormone therapy trials, in an attempt to clarify postmenopausal hormone therapy effects.
Participants and Methods
Postmenopausal women aged 50−79 at WHI enrollment. Estrogen-alone analyses include 21,552 and 10,739 women who were post-hysterectomy from the observational study and clinical trial respectively. Estrogen plus progestin analyses include 32,084 and 16,608 observational study and clinical trial women with uterus. Colorectal cancers were verified by central medical and pathology report review.
Results
Hazard ratios (95% confidence intervals) from the WHI observational study were 0.80 (0.53 to 1.20) for estrogen and 1.15 (0.74 to 1.79) for estrogen plus progestin, with respectively 168 and 175 women diagnosed with colorectal cancer. Delayed diagnosis with estrogen plus progestin is not evident in the observational study. No protective effect on colorectal cancer mortality in the estrogen plus progestin trial is seen over an 8-year intervention and follow-up period.
Conclusion
Hazard ratio patterns in the WHI clinical trial and observational study do not provide strong evidence of a clinically important colorectal cancer benefit with either estrogen-alone or estrogen plus progestin over 7−8 years of treatment and follow-up.
Keywords: cohort study, colorectal cancer, randomized controlled trial, postmenopausal hormone therapy
INTRODUCTION
Colorectal cancer was included in a ‘global index’ to summarize health benefits and risks in the WHI randomized controlled trials of daily 0.625 conjugated equine estrogens (CEE) versus placebo among 10,739 women who were post-hysterectomy, and this same estrogen preparation plus daily 2.5 mg of medroxyprogesterone acetate (CEE/MPA) among 16,608 postmenopausal women with a uterus (1, 2). The CEE/MPA trial was stopped early in 2002 when it was judged that overall health risks exceeded benefits (3). The (invasive) colorectal hazard ratio (HR) for the active treatment over a 5.6-year average intervention period was 0.56 with a 95% confidence interval (CI) of 0.38 to 0.81 (4). However, the interpretation of this finding was substantially obscured by the fact that ‘colorectal cancers in women who took estrogen plus progestin were diagnosed at a more advanced stage than those in women who took placebo’ (4). The CEE trial had health benefits and risks that were approximately balanced (5), but was also stopped early, in 2004, in part because of an elevation in stroke. The colorectal cancer HR over the 7.1-year average follow-up period in the CEE trial was 1.12 with 95% CI of 0.77 to 1.63, and there was no suggestion of an effect of CEE on diagnosis (6).
Observational studies have mostly reported an inverse association with colorectal cancer incidence for either estrogen or estrogen plus progestin (7-9), though some studies (10-12) have reported lower colorectal cancer incidence among users of estrogen plus progestin, but not among estrogen-alone users.
The WHI observational study (OS) provides an opportunity to further explore the effects of these preparations on colorectal cancer, and to compare both incidence associations, and tumor characteristics between the WHI clinical trial (CT) and OS, for CEE and CEE/MPA. The OS is a prospective cohort study among 93,676 postmenopausal women in the 50−79 year age range, who were drawn from the same populations as were CT women, with much commonality in protocol and procedures.
WHI investigators have compared cardiovascular disease (13, 14) and breast cancer (15, 16) effects between the CT and OS, for both CEE and CEE/MPA. Apparently discrepant findings for these outcomes could be explained mostly by taking suitable account of time from menopause to hormone therapy initiation, time since hormone therapy initiation, and applying standard confounding control procedures. Corresponding invasive colorectal cancer analyses are considered here. Additional analyses examine hazard ratios for subsets of colorectal cancer defined by local versus regional/distant spread, primary tumor size, or the presence of positive lymph nodes. Colorectal cancer mortality data are also examined during the intervention period in the CEE trial, and during both the intervention period and post-intervention follow-up period in the CEE/MPA trial.
MATERIALS AND METHODS
Study Cohorts
The design of the WHI clinical trial and observational study has been presented (17), and overall clinical trial findings have been recently summarized (18). All women were postmenopausal, in the age range 50−79, and without a medical condition likely to result in death within three years, at the time of enrollment. Women with a personal history of breast cancer were excluded from the hormone therapy trials. Characteristics of the OS cohort have been described (19).
OS women were included in the CEE component of this analysis if they were post-hysterectomy and either taking the same daily 0.625 mg CEE preparation as studied in the CT or not using any hormone therapy at the time of enrollment. Women included were also required to have known values for a list of potential confounding factors. Women with a personal history of breast cancer at baseline, or without a mammogram in the two-year period prior to enrollment, were also excluded to correspond with CT exclusionary criteria, giving a subcohort of 21,552 OS women including 10,582 baseline CEE users, and 10,970 non-users. A total of 32,084 OS women with uterus were included using these same criteria in the corresponding CEE/MPA component of this analysis, including 6756 women who were using the same daily CEE/MPA combination as studied in the CT, and 25,328 non-users.
Information on lifetime hormone use was obtained at baseline from CT and OS women by trained interviewers, assisted by structured questionnaires and charts displaying colored photographs of various hormone preparations.
Follow-up
Clinical outcomes were reported semi-annually in the CT and annually in the OS (20). Medical records documentation of initial self-reports were obtained and diagnoses were confirmed by physician adjudicators. All colorectal cancer cases were centrally reviewed and classified using SEER program guidelines (21). Information on adherence to study hormone pills was obtained semi-annually in the CT, and information on hormone therapy use was updated annually by questionnaire in the OS.
Statistical Analysis
Statistical methods and variable definitions are similar to previous reports of this type (13-16), for other clinical outcomes. Briefly, follow-up in the hormone therapy trials was included through the end of the respective intervention periods, while OS subcohorts were followed through December 15, 2004 for CEE analyses, and through February 28, 2003 for CEE/MPA analyses to give respective average follow-up periods of 7.1 and 5.5 years, similar to the CT. Women in the HT trials were required to obtain mammograms annually, or study pills were withheld. Toward ensuring comparable exposure to the medical care system, follow-up times for women were censored at the first instance of being more than two years from most recent mammogram, in both the CT and OS.
Hazard ratio estimation for colorectal cancer incidence was based on Cox regression (22), with time from WHI enrollment as the basic time variable. The baseline hazard rate was stratified on age at enrollment in 5-year intervals and on a personal history of colorectal cancer at enrollment (yes vs. no) in both CT and OS analyses. CT analyses also stratify on WHI dietary modification trial randomization (intervention, control, or not randomized). OS analyses stratify also on prior postmenopausal hormone therapy (no versus hormone therapy prior to enrollment for non-users at baseline, or prior to the beginning of the ongoing hormone therapy episode for baseline hormone therapy users, with a usage gap of one year or longer defining a new hormone therapy episode), and include baseline colorectal cancer risk factors, as listed below, in the hazard ratio regression model for confounding control (with separate regression coefficients for prior hormone therapy users and non-users). Because of the random allocation, these factors were not included in the HR model in the CT, but randomization into the calcium and vitamin D clinical trial component (active, versus placebo or not randomized) was included as a time-dependent regression variable.
Hazard ratios among adherent women were estimated using these same modeling procedures, with follow-up times censored six months after a change from baseline in hormone therapy status. For a non-user, a status change involved the initiation of any hormone therapy. For a baseline user a status change involved either HT discontinuation, or a change to another HT preparation.
Colorectal cancer mortality data and all-cause mortality data were also considered through the end of the active intervention periods for both clinical trials and for the CEE/MPA trial also through the end of a subsequent CT follow-up period ending 3/31/05. These analyses also used Cox models, with baseline hazard ratios stratified as in previous trial reports (3-6).
Nominal 95% CIs are presented for hazard ratios, and all significance levels (p-values) are two-sided.
RESULTS
Table 1 shows age-adjusted colorectal cancer incidence rates in the CT and OS cohorts, according to hormone therapy group, and prior use of hormone therapy for both CEE and CEE/MPA. Age-adjusted incidence rates do not vary strongly among the non-user groups according to uterine status, or prior hormone therapy use, but tend to be somewhat lower in the OS than in the CT.
Table 1.
Age-adjusted incidence rates of, and numbers of women developing, invasive colorectal cancer in the WHI hormone trials and in corresponding observational study subcohorts, according to prior use of postmenopausal hormone therapy (HT) and hysterectomy status.
| Without Uterus at Enrollment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical Trial | Observational Study | ||||||||
| Prior HT* |
No |
Yes |
No |
Yes |
|||||
| Placebo |
CEE† |
Placebo |
CEE |
Non-User |
CEE |
Non-User |
CEE |
||
| Number of women | 2770 | 2769 | 2659 | 2541 | 6541 | 8677 | 4429 | 1905 | |
| Average age (yrs) | 63.8 | 63.6 | 63.4 | 63.6 | 65.1 | 63.4 | 65.7 | 64.2 | |
| Incidence rate† | 1.52 | 1.65 | 1.21 | 1.47 | 1.31 | 0.73 | 0.86 | 1.66 | |
| Number of cases | 30 | 32 | 23 | 26 | 65 | 46 | 33 | 24 | |
| With Uterus at Enrollment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical Trial | Observational Study | ||||||||
| Prior HT* | No |
Yes |
No |
Yes |
|||||
| Placebo |
CEE/MPA† |
Placebo |
CEE/MPA |
Non-User |
CEE/MPA |
Non-User |
CEE/MPA |
||
| Number of women | 6020 | 6277 | 2082 | 2229 | 19668 | 5710 | 5660 | 1046 | |
| Average age (yrs) | 63.4 | 63.4 | 63.0 | 62.6 | 64.7 | 61.0 | 64.8 | 64.3 | |
| Incidence rate‡ | 1.64 | 1.00 | 1.53 | 0.70 | 0.97 | 1.08 | 1.06 | 0.72 | |
| Number of cases | 55 | 35 | 17 | 8 | 109 | 27 | 35 | 4 | |
Prior HT is defined relative to WHI enrollment in the clinical trial and non-user group in the OS, and defined relative to the beginning of the ongoing hormone therapy episode at enrollment in the OS user groups.
CEE, conjugated equine estrogens; CEE/MPA, conjugated equine estrogens plus medroxyprogesterone acetate.
Incidence rate per 1000 person years, adjusted to the 5-year age distribution in the respective clinical trials.
Table 2 shows invasive colorectal cancer hazard ratio estimates for CEE and CEE/MPA both from the CT as previously reported, and from the OS. The HR (95% CI) for CEE from the OS is 0.80 (0.53, 1.20) with 168 colorectal cancer cases, while that for CEE/MPA from the OS is 1.15 (0.74, 1.79) with 175 cases. Hence, OS data provide little evidence overall for a colorectal cancer association with either CEE or CEE/MPA. Potential confounding factors in OS analyses are listed in a Table 2 footnote.
Table 2.
Numbers of women diagnosed with colorectal cancer, hazard ratio (HR) estimates and 95% confidence intervals (CIs) from the WHI postmenopausal hormone therapy trial and observational study for conjugated equine estrogen (CEE) and for CEE plus medroxyprogesterone acetate (MPA).
| CEE |
Non-User |
HR* |
95% CI |
CEE/MPA |
Non-User |
HR* |
95% CI |
|
|---|---|---|---|---|---|---|---|---|
| Clinical Trial | 58 | 53 | 1.12 | 0.77 − 1.63 | 43 | 72 | 0.56 | 0.38 − 0.81 |
| Observational Study | 70 | 98 | 0.80 | 0.53 − 1.20 | 31 | 144 | 1.15 | 0.74 − 1.79 |
HRs in the clinical trial are from a Cox regression stratified by age group at enrollment, dietary modification trial randomization, and prior colorectal cancer, with assignment to the calcium and vitamin D trial as a time-dependent covariate. HRs in the observational study from a Cox regression model stratified by age group at enrollment, prior colorectal cancer, and prior postmenopausal hormone therapy, and adjusted for age (linear), body mass index, education, cigarette smoking, alcohol consumption, bilateral oophorectomy, type and duration of prior hormone therapy, family history of colorectal cancer, waist circumference, height, history of polyp removal, dietary selenium intake, non-steroidal anti-inflammatory drug use, and prior oral contraceptive use. HR regression coefficients were estimated separately for prior postmenopausal therapy users and non-users.
Additional joint analyses of the CT and OS data were carried out to provide more detailed HR comparisons. Most hormone therapy users in the OS were some years into their ongoing hormone therapy episode at WHI enrollment, and the OS mostly contributes HR information well after therapy initiation. Hence, separate HRs were calculated for 0−2, 2−5, and ≥5 years from hormone therapy initiation. Table 3 shows results of these analyses, which also included product term between hormone therapy and cohort (CT vs. OS) to quantitatively judge overall HR agreement between the two sources. Under this statistical model the hormone therapy HRs in the OS are restricted to differ from those in the CT by a simple multiplicative factor, for which an estimate and 95% confidence interval are shown in the final row of the left side of Table 3. This ratio of HR in the OS to HR in the CT would be close to unity if HRs from the two sources agree, but note that CEE/MPA HRs in the OS are estimated to be 81% higher than in the CT, while CEE HRs in the OS are estimated to be 37% lower than in the CT, though neither ratio is significantly different from one. The right side of Table 3 shows corresponding analyses among women who were adherent to their baseline hormone therapy group designation, by censoring the follow-up time six months following a change from baseline hormone therapy status. Among adherent women, HRs do not agree closely between the CT and OS for either hormone therapy preparation.
Table 3.
Colorectal cancer hazard ratio estimates for CEE and CEE/MPA from combined analysis of WHI hormone therapy trial and observational study data.
| Without non-adherence censoring | With non-adherence censoring | |||||||
|---|---|---|---|---|---|---|---|---|
| Years from Hormone Therapy | CEE |
CEE/MPA |
CEE |
CEE/MPA |
||||
| Initiation |
HR* |
95% CI |
HR* |
95% CI |
HR* |
95% CI |
HR* |
95% CI |
| No prior hormone therapy | ||||||||
| < 2 | 1.03 | 0.43 − 2.47 | 0.90 | 0.44 − 1.83 | 1.10 | 0.45 − 2.67 | 0.89 | 0.39 − 2.04 |
| 2 − 5 | 1.20 | 0.47 − 3.06 | 0.62 | 0.35 − 1.09 | 1.45 | 0.42 − 5.06 | 0.73 | 0.35 − 1.54 |
| > 5 | 0.98 | 0.47 − 2.03 | 0.62 | 0.27 − 1.46 | 1.83 | 0.51 − 6.50 | 0.65 | 0.22 − 1.88 |
| Prior hormone therapy | ||||||||
| < 2 | 0.78 | 0.27 − 2.21 | 0.54 | 0.13 − 2.19 | 0.91 | 0.31 − 2.68 | 0.60 | 0.15 − 2.47 |
| 2 − 5 | 0.81 | 0.35 − 1.90 | 0.32 | 0.09 − 1.15 | 0.42 | 0.11 − 1.57 | 0.31 | 0.07 − 1.46 |
| > 5 | 2.46 | 1.12 − 5.38 | 0.60 | 0.19 − 1.86 | 3.98 | 1.06 − 4.86 | 0.84 | 0.20 − 3.55 |
| Ratio of HR in OS to HR in CT | 0.63 | 0.30 − 1.34 | 1.81 | 0.82 − 4.00 | 0.35 | 0.10 − 1.23 | 1.62 | 0.61 − 4.33 |
From Cox regression analyses with stratification and adjustment variables as in Table 2 footnotes, based on combined clinical trial and observational study analyses that include an interaction between hormone therapy HR and cohort (CT vs. OS) that produces the ratio of hormone therapy HR in the OS to that in the CT shown at the bottom of the Table.
Additional analyses extended the Table 3 analyses by including an interaction term between hormone therapy and baseline age in the log-hazard ratio. For CEE, a modest increase in HR with age could be detected (p=0.02) with the CEE HR increased by a factor of 1.19 (95% CI of 1.03 to 1.37) for each 5-year increment in age. This interaction was also significant (p=0.02) among adherent women, with the CEE HR increased by 1.23 (95% CI of 1.03 to 1.47) for each 5-year age increment. The corresponding hormone therapy by age interaction was not significant for CEE/MPA, but in the same direction with HR of 1.09 (95% CI of 0.84 to 1.42) without adherence restriction and with HR of 1.15 (95% CI of 0.85 to 1.55) among adherent women, for a 5-year age increment. We also examined the possibility of an interaction of hormone therapy HRs with time from menopause to first use of hormone therapy, but found little evidence of such dependency for CEE (p=0.15), or CEE/MPA (p=0.87) without adherence restriction, or for CEE (p=0.29) or CEE/MPA (p=0.54) among adherent women. Additional analyses of this type with focus on women who initiate CEE or CEE/MPA soon after the menopause can be found in (23) for a range of clinical outcomes, including colorectal cancer.
To better understand suggested HR differences between the CT and OS, and hormone therapy effects more generally, the analyses of Table 2 were extended by calculating HRs separately according to metastatic spread, primary tumor size, and the presence of one or more positive lymph nodes. Hazard ratio estimates and 95% CIs for related tumor subtypes are shown in Figure 1 for each preparation, separately for the CT and OS. The previously-noted (4) deficit of early stage tumors with CEE/MPA in the CT is not evident in the OS. In contrast, there appears to be some deficit of more advanced tumors with CEE in the OS that, as previously noted (6), is not evident in the CT.
Figure 1.
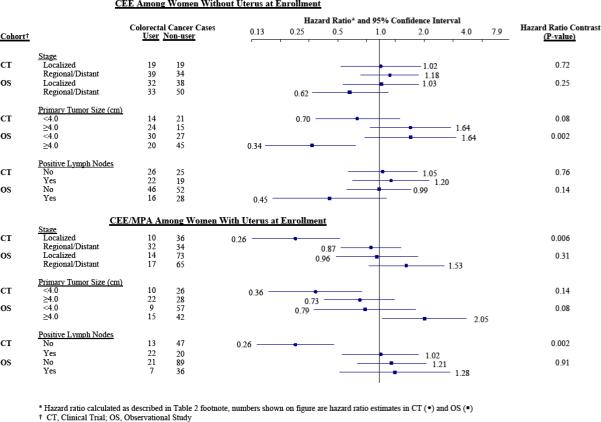
Colorectal cancer hazard ratios and 95% confidence intervals from WHI clinical trial and observational study for conjugated equine estrogen (CEE) and for CEE plus medroxyprogesterone acetate (MPA), according to three aspects of extent of disease at diagnosis.
Colorectal cancer mortality data were considered to examine whether the lower incidence in the CT for women assigned to CEE/MPA translated to reduced colorectal cancer mortality. Through the end of the active intervention period (July 7, 2002) there were 10 colorectal cancer deaths in each of the CEE/MPA and placebo groups, giving a colorectal cancer mortality HR (95% CI) of 0.95 (0.40 to 2.28) and logrank p-value of 0.91. Participating women were followed systematically through March 31, 2005 (24) by which time there were 18 colorectal cancer deaths in the CEE/MPA group and 17 in the placebo group, with HR (95% CI) of 1.00 (0.51 to 1.94) with logrank p=1.00. Among the 115 women diagnosed with colorectal cancer during the intervention phase of the CEE/MPA trial, 12 had died in the CEE/MPA group and 11 in the placebo group by the end of the intervention period, giving a total mortality HR (95% CI) for CEE/MPA of 1.64 (0.70 to 3.83) with p=0.25. Among the 182 women diagnosed with colorectal cancer through March 31, 2005, there were 23 deaths in the CEE/MPA group and 21 in the placebo group, giving a total mortality HR (95% CI) of 1.54 (0.82 to 2.87) and p=0.18.
Corresponding colorectal cancer mortality data from the CEE trial were also considered. Through the end of the intervention period (2/29/2004) there were 16 colorectal cancer deaths in the active arm and 17 in the placebo, with corresponding HR (95% CI) of 0.99 (0.50 to 1.96) and logrank p-value of 0.99. Among 111 women diagnosed with colorectal cancer, there were 16 deaths in each of the intervention groups, with all-cause mortality HR (95% CI) of 0.75 (0.34 to 1.70) and logrank p-value of 0.49.
DISCUSSION
The data analyses presented here were undertaken to further the interpretation of a reduced colorectal cancer incidence with CEE/MPA, and lack of evidence of any CEE effect on colorectal cancer incidence in the WHI clinical trial. The CEE/MPA finding was obscured (4) by a later stage diagnosis in the active treatment versus the placebo group, allowing the possibility that the treatment itself, or some aspect of the trial protocol, led to a delayed colorectal cancer diagnosis in the CEE/MPA group. This concern is heightened by the WHI Observational Study findings herein presented, that provide no suggestion of a lower risk among women using the same CEE/MPA preparation as studied in the clinical trial compared to non-users of postmenopausal hormones, and little suggestion of a different extent of disease at diagnosis between CEE/MPA users and non-users. Furthermore, the reduced incidence in the active treatment group in the CEE/MPA trial is shown here to have not led to any suggestion of colorectal cancer mortality benefit during an average 8-year intervention and follow-up period. It is important to note, however, that an even longer time period may be required to observe a mortality benefit from an actual reduction in the incidence of small, localized colorectal cancers.
It is interesting to speculate on reasons for later stage diagnoses with CEE/MPA in the CT, but not in the OS. One possible difference is that colorectal tumors among CEE/MPA users in the OS tended to be diagnosed many years following treatment initiation, compared to mostly within the first few years of use in the CT. Hence, a limited-time response of colorectal tissue to CEE/MPA initiation having potential to impede the detection of small tumors, could affect CT and OS findings differentially. However, we see little evidence of time trends in HRs in either the CT or OS, though numbers of colorectal cancer events is small for this type of analysis (data not shown). Another possibility relates to vaginal bleeding: Women assigned to CEE/MPA in the WHI trial experienced persistent vaginal bleeding to a greater extent than expected, and followed a protocol designed to manage bleeding while allowing them to continue with study hormones to the extent practical. We reanalyzed the CEE/MPA trial data while including an interaction term between randomization assignment and vaginal bleeding as a time-dependent variable. The colorectal cancer HR for CEE/MPA among women with bleeding was 0.54 with a 95% CI of 0.27 to 1.10, while that for women without bleeding was 0.57 with 95% CI of 0.38 to 0.86, so that this trial feature does not help to explain any diagnostic delay in the CEE/MPA trial. Hence, in summary, collective WHI data suggest that either the observed lower incidence was due to a comparatively delayed colorectal cancer detection in the CEE/MPA group perhaps as a result of attributing symptoms to hormone therapy use, in spite of intervention blinding, resulting in delayed evaluation; or simply as a chance occurrence. Alternatively, CEE/MPA results could reflect an actual reduction in localized, small tumors that apparently do not imply a colorectal cancer mortality benefit over an average 8-year intervention and follow-up period.
The CEE clinical trial did not suggest an effect on colorectal cancer incidence or on diagnosis (6). The OS also does not suggest an effect of CEE on incidence overall, though there is some evidence for a deficit of larger, more advanced tumors at diagnosis among women using CEE. This is the direction of bias that would be expected if hormone therapy users in the community are under greater health surveillance than non-users. Efforts to control such bias, here through imposing mammography utilization requirements prior to and after WHI enrollment, may not be sufficient for complete avoidance of bias from this source. The CEE trial does not provide evidence of any effect on colorectal cancer mortality over its 7.1-year average follow-up period. Hence, our summary interpretation is that collective WHI data provide little evidence for an effect of CEE on colorectal cancer incidence.
In summary, hazard ratio patterns in the WHI clinical trial and observational study do not provide strong evidence of a clinically important colorectal cancer benefit with CEE or CEE/MPA over an average 7 to 8 year treatment and follow-up period.
ACKNOWLEDGMENTS
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings. Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Aleksandar Rajkovic; (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (Brown University, Providence, RI) Charles B. Eaton; (Emory University, Atlanta, GA) Lawrence Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Lisa Martin; (Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Yvonne Michael; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Lauren Nathan; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) J. David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O'Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Mara Vitolins; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Michael Simon.
Women's Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
FUNDING
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health, U. S. Department of Health and Human Services [contracts N01WH22110, 24152, 32100−2, 32105−6, 32108−9, 32111−13, 32115, 32118−19, 32122, 42107−26, 42129−32, and 44221]. Clinical Trials Registration: ClinicalTrials.gov identifier: NCT00000611. Dr. Prentice's work was partially supported by grant CA53996 from the National Cancer Institute. Decisions concerning study design, data collection and analysis, interpretation of the results, the preparation of the manuscript, or the decision to submit the manuscript for publication resided with committees comprised of WHI investigators that included NHLBI representatives.
REFERENCES
- 1.Anderson GL, Kooperberg C, Geller N, Rossouw JE, Pettinger M, Prentice RL. Monitoring and reporting in the Women's Health Initiative randomized hormone therapy trials. Clinical Trials. 2007;4:207–17. doi: 10.1177/1740774507079252. [DOI] [PubMed] [Google Scholar]
- 2.Wittes J, Barrett-Connor E, Braunwald E, et al. Monitoring the randomized trials of the Women's Health Initiative: the experience of the Data and Safety Monitoring Board. Clin Trials. 2007;4:218–34. doi: 10.1177/1740774507079439. [DOI] [PubMed] [Google Scholar]
- 3.Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 4.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. for the Women's Health Initiative Investigators Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350:991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 5.Anderson GL, Limacher M, Assaf AR, et al. for the Women's Health Initiative Steering Committee Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 6.Ritenbaugh C, Stanford JL, Wu L, et al. Conjugated equine estrogens and colorectal cancer incidence and survival: the Women's Health Initiative randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2008;17:2609–18. doi: 10.1158/1055-9965.EPI-08-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbert-Croteau N. A meta-analysis of hormone replacement therapy and colon cancer in women. Cancer Epidemiol Biomarkers Prev. 1998;7:653–9. [PubMed] [Google Scholar]
- 8.Janne PA, Mayer R. Chemoprevention of colorectal cancer. N Engl J Med. 2000;342:196008. doi: 10.1056/NEJM200006293422606. [DOI] [PubMed] [Google Scholar]
- 9.Grodstein F, Newcomb PA, Stampfer MJ. Postmenopausal hormone therapy and the risk of colorectal cancer: a review and meta-analysis. Am J Med. 1999;106:574–82. doi: 10.1016/s0002-9343(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 10.Newcomb PA, Zheng Y, Chia VM, et al. Estrogen plus progestin use, microsatellite instability and the risk of colorectal cancer in women. Cancer Res. 2007;67:7534–9. doi: 10.1158/0008-5472.CAN-06-4275. [DOI] [PubMed] [Google Scholar]
- 11.Persson I, Yuen J, Bergkvist L, Schairer C. Cancer incidence and mortality in women receiving estrogen and estrogen-progestin replacement therapy—longterm follow-up of a Swedish cohort. Int J Cancer. 1996;67:327–32. doi: 10.1002/(SICI)1097-0215(19960729)67:3<327::AID-IJC4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 12.Tannen RL, Weiner MG, Xie D, Barnhart K. Estrogen affects postmenopausal women differently than estrogen plus progestin replacement therapy. Hum Reprod. 2007;22:1769–77. doi: 10.1093/humrep/dem031. [DOI] [PubMed] [Google Scholar]
- 13.Prentice RL, Langer R, Stefanick ML, et al. for the Women's Health Initiative Investigators Combined postmenopausal hormone therapy and cardiovascular disease: toward resolving the discrepancy between observational studies and the Women's Health Initiative clinical trial. Am J Epidemiol. 2005;162:404–14. doi: 10.1093/aje/kwi223. [DOI] [PubMed] [Google Scholar]
- 14.Prentice RL, Langer RD, Stefanick ML, et al. for the Women's Health Initiative Investigators Combined analysis of Women's Health Initiative observational and clinical trial data on postmenopausal hormone treatment and cardiovascular disease. Am J Epidemiol. 2006;163:589–99. doi: 10.1093/aje/kwj079. [DOI] [PubMed] [Google Scholar]
- 15.Prentice RL, Chlebowski RT, Stefanick ML, et al. Estrogen plus progestin therapy and breast cancer in recently postmenopausal women. Am J Epidemiol. 2008;167:1207–16. doi: 10.1093/aje/kwn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prentice RL, Chlebowski RT, Stefanick ML, et al. Conjugated equine estrogens and breast cancer risk in the Women's Health Initiative clinical trial and observational study. Am J Epidemiol. 2008;167:1407–15. doi: 10.1093/aje/kwn090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Women's Health Initiative Study Group Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 18.Prentice RL, Anderson GL. The Women's Health Initiative: Lessons learned. Annu Rev Public Health. 2008;29:131–50. doi: 10.1146/annurev.publhealth.29.020907.090947. [DOI] [PubMed] [Google Scholar]
- 19.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: Baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–21. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 20.Curb JD, McTiernan A, Heckbert SR, et al. WHI Morbidity and Mortality Committee Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13:S122–8. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute [March 28, 2005];About SEER. Available at http://www.seer.cancer.gov/.
- 22.Cox DR. Regression models and life tables (with discussion). J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 23.Prentice RL, Manson JE, Langer RD, et al. Benefits and risks of postmenopausal hormone therapy when initiated soon after the menopause. AJE; 2008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heiss G, Wallace R, Anderson GL, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299:1036–45. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]


