Abstract
Purpose
To determine if changes in elastic properties of the lens capsule ex vivo with age contribute to the forces required to accommodate.
Methods
Post-mortem human (n=22; age average: 41±17years, range: 6–71 years) and cynomolgus monkey (n=19; age average: 7.7±1.8 years, range: 4.2–10 years) tissues including the lens, capsule, zonules, ciliary body, and sclera were mounted in an optomechanical lens stretching system. Starting at zero load, the sclera was symmetrically stretched to 2mm in 0.25 steps at a speed of 0.1mm.s−1. The load and lens diameter were measured at each step. The lens contents were removed through a mini-capsulorhexis. The stretching cycles were repeated on the empty capsular bag. The forces required to stretch the natural lens and empty bag were quantified as a function of age and compared.
Results
The force required to stretch the empty lens capsule was independent of age (human=2.6–34.9g/mm [25.2–342.7mN/mm], monkey=8.2–21.3g/mm [80.3–208.6mN/mm]). The ratio of the force required to stretch the empty lens capsule to the force required to stretch the natural lens decreases with age in human and monkey lenses (p=0.003, p=0.72, respectively).
Conclusions
The mechanical properties of the empty lens capsule assessed ex vivo in a lens stretcher remain constant with age, suggesting that the changes in elasticity of the lens capsule do not play a significant role in presbyopia. In young eyes, the lens capsule determines the force required to stretch the whole lens. The age-related increase in force required to stretch the lens is due to changes in the lens contents.
1. Introduction
The loss of accommodation with age is most likely due to optical and physical changes of the crystalline lens, lens capsule, ciliary muscle, and zonules. These changes commence at birth, but do not become symptomatic until around the age of 40. Lens and capsule-based theories of presbyopia assume that the contribution of age-related changes of the ciliary muscle and zonules are insignificant compared to the effect of changes in the lens and lens capsule1. These theories of presbyopia suggest that a main factor in the loss of accommodation is the decreased elasticity of both the lens matter and lens capsule with age. The loss of elasticity results in the inability of the lens capsule to mold the lens material, which is necessary for the lens shape changes during accommodation. Measuring the mechanical properties of the lens capsule, and their changes with age, is therefore vital to a more complete understanding of accommodation and presbyopia2.
The relative contribution of the lens matter and lens capsule in accommodation is also an important variable in the understanding of accommodation and presbyopia. Accommodation can only occur if the lens material is sufficiently pliable so that the lens capsule can mold it and subsequently change its shape3–6. In the young eye, the modulus of elasticity of the lens material is several orders of magnitude less than that of the lens capsule2,7–10. Therefore, the lens material is compliant with the molding pressure of the lens capsule11–14. With age, the lens modulus of elasticity increases, and the capsule can no longer change the shape of the lens. The onset of presbyopia may represent the point in an individual’s lifetime when Young’s modulus of the lens material exceeds that of the lens capsule13,14. Understanding the relative role of the lens and lens capsule in accommodation is also vital for the success of procedures designed to restore accommodation, such as lens refilling, which rely on the assumption that the loss of elasticity of the lens capsule with age does not have a significant effect on the loss of accommodative amplitude15–20.
The strength and elasticity of the lens capsule have been previously investigated using uniaxial stress-strain analysis7,21,22 or by stretching capsular rings2 or openings23–27. From these studies, it is known that the lens capsule becomes stiffer and looses extensibility with age. However, these uniaxial methodologies do not necessarily represent the physiological response of the lens capsule, as the accommodative mechanism involves the ciliary muscle, zonules, and lens capsule stretching circumferentially. To quantify the impact of the lens capsule on accommodation, the empty capsular bag should be stretched and this response should be compared to that of the entire lens plus capsule system.
The purpose of this study is to determine the forces required to stretch the empty lens capsule during simulated accommodation as a function of age using an optomechanical lens stretcher that was developed to simulate accommodation ex vivo28,29. The system was designed to measure lens and ciliary body diameters, load, and lens optical power as the dissected scleral tissue is stretched radially four millimeters in diameter. The lens stretcher also enables surgery to be performed directly on the mounted tissue, so the tissue can be measured in the natural state and after endocapsular surgery is performed. The results will be used to determine the relationship between the force required to stretch the natural lens and the empty lens capsule.
2. Materials and Methods
2.1 Experiments
Experiments were conducted on 22 donor globes from 22 humans (age average: 41±17 years, range: 6–71 years) and on 19 globes from 17 cynomolgus monkeys (macaca fascicularis, age average: 7.7±1.8 years, range: 4.2–10 years). The human and monkey globes arrived in sealed vials placed in styrofoam containers filled with ice. Upon arrival in the laboratory, the eyes were stored in the refrigerator at 4°C before they were used. Experiments were performed on monkey eyes less than two days postmortem (0.9±0.8 days) and on human eyes less than five days postmortem (2.6±1.0 days). The animal eyes were obtained after enucleation following approved institutional animal care guidelines. All animal experiments adhered to the ARVO Statement for the use of animals in research. All human eyes were obtained and used in compliance with the guidelines of the Declaration of Helsinki for research involving the use of human tissue.
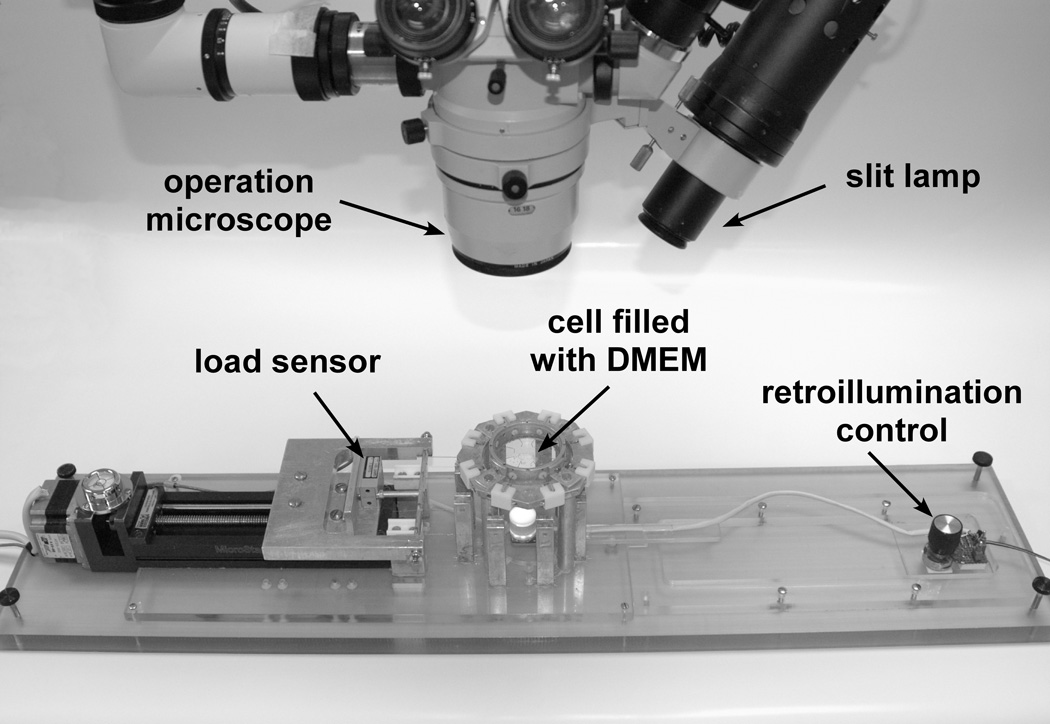
The tissue preparation protocol and optomechanical lens stretcher have been described in detail previously (Figure 1)29. The conjunctiva, adipose, and muscle tissues were removed from the globe to expose the scleral surface. Eight custom-made PMMA shoes designed to fit the external curvature of the globe were bonded with cyanoacrylate adhesive onto the anterior scleral surface to form a segmented ring covering the circumference of the globe. The posterior pole of the globe was then removed using surgical scissors. Excess vitreous was carefully removed, but care was taken to preserve the hyaloid membrane and anterior vitreous. The partially dissected sample was then placed in the tissue chamber of the lens stretcher. The chamber was filled with DMEM solution (Invitrogen, Carlsbad, CA), immersing the tissue, to maintain lens hydration to a physiological level30. Hooks were inserted into holes located in the shoes to connect each shoe to a 6-0 nylon monofilament suture that allowed the stretcher to pull on the tissue. The cornea and iris were excised and the sclera was sectioned between each shoe to produce eight independent segments for the stretching experiments.
Figure 1.
Ex vivo accommodation system.
Once the tissue was mounted in the stretcher and the dissection complete, the position of the motorized translation stage was adjusted using a joystick control until the strings stopped sagging and the recorded force began to increase. Due to differences in the diameter of the scleral shell of different globes, the strings are sometimes slack after the tissue is mounted. Jogging removes the slack of the lens-stretching system, but still leaves the lens-zonules-ciliary body-sclera system under zero tension. This position was chosen as the starting point for the stretching experiments. Once this position was determined, it remained the same for both natural lens and empty lens capsule experiments. The translation stage was programmed to move a total of 2mm in 0.25mm steps at a speed of 0.1mm.s−1, corresponding to a maximum 4mm diameter increase of the outer sclera. Preliminary results indicated that a 4mm increase in the diameter of the outer scleral ring was necessary to elicit lens diameter and power changes comparable to those found in vivo during accommodation. The translation stage was programmed to pause for 10 seconds at each of the displacement steps. At the end of the last step, the translation stage automatically returns to the initial position. The load was continuously recorded and a digital picture was taken at each step to measure the lens and ciliary body diameter changes. This stretching cycle was performed three times for each eye. After performing measurements on the natural lens, a mini-capsulorhexis31 no larger than 2mm was made using Utrata forceps in the peripheral lens capsule (Figure 2). The capsulorhexis size was measured in the horizontal and vertical directions by the surgeon. Any lenses with a capsulorhexis larger than 2mm were excluded from this study. The lens contents were then removed through this capsulorhexis by means of hydrodissection followed by aspiration or ultrasonic phacoemulsification so that only the empty capsular bag remained. The stretching cycle was then repeated for the empty lens capsular bag. The zero position of the motorized translation stage was the same as for the natural lens experiments. The lens and ciliary body diameters and load were also measured for the empty bag stretch cycle. All values presented are the average of the three repeated measurements.
Figure 2.
Close-up picture showing the size of the mini-capsulorhexis.
2.2 Data analysis
The stretching cycle for each experiment was programmed to begin when a trigger pulse was sent to the system. This trigger pulse was recorded along with the load data, so the beginning point of each cycle was easily distinguished. The beginning load value at this point was subtracted out to provide a common baseline for all experiments. The load value when the digital image was captured was superimposed on the image. This load value was used as the uncorrected load at that step. The final load value for each step was determined after subtracting out the baseline from the uncorrected load recorded at each step.
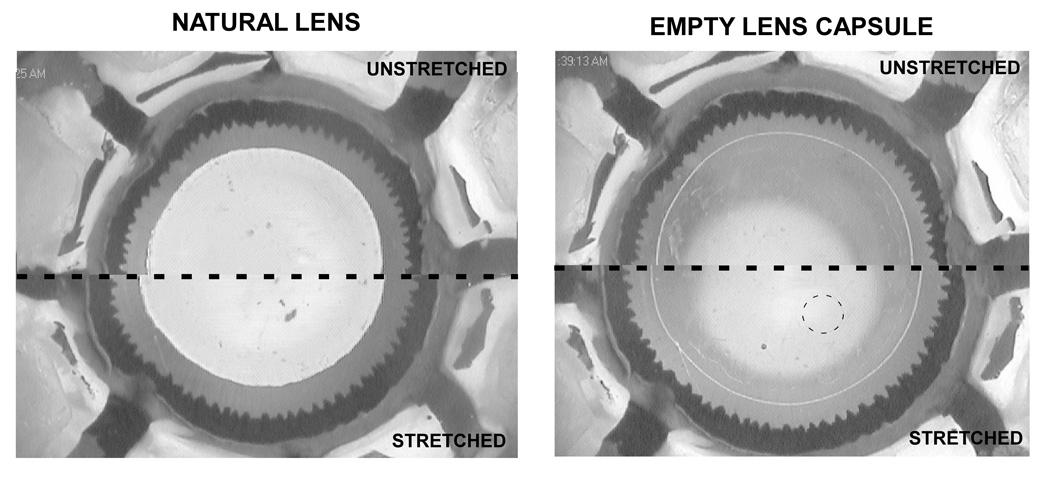
The horizontal and vertical diameters of the natural lens and empty lens capsule were measured from the digital pictures that were taken at each step of the stretching cycle (Figure 3) using image editing software (Canvas 8, ACD Systems, Inc., Miami, FL). The horizontal and vertical diameters were measured by drawing lines across the capsule on the picture using the software. A picture of a ruler with 1mm divisions was taken before the start of each experiment to determine the calibration factor for the particular magnification. The pixel resolution of these images in the plane of the lens was 37µm (Figure 4). The horizontal and vertical diameters found for each step were averaged to obtain a single average diameter value for that step.
Figure 3.
Digital pictures taken of a human natural lens (left) and empty lens capsule (right). The unstretched state is shown at the top, and the stretched state is shown at the bottom. The magnification is 10.6× for the left image and 10.9× for the right image. The dotted line highlights the size and location of the mini-capsulorhexis.
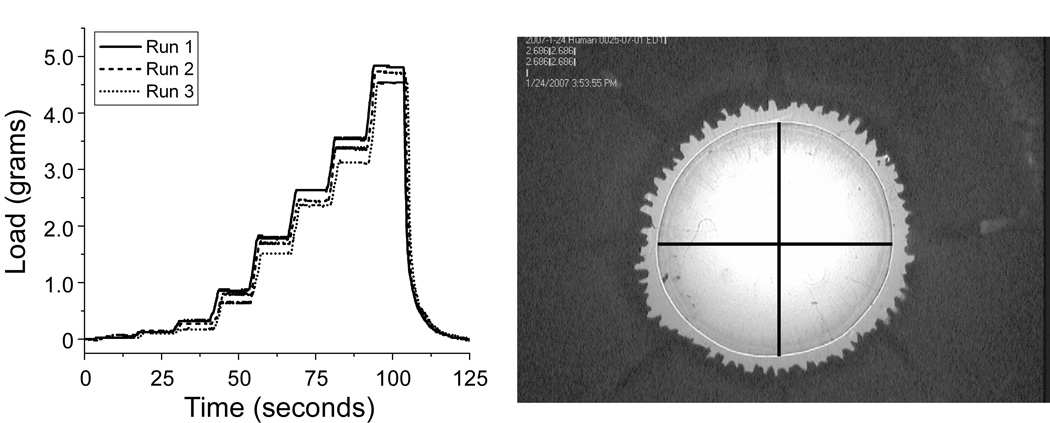
Figure 4.
Left, Graph obtained for the load recorded during each stretching cycle. Right, Digital picture taken for each step during the stretching cycle. The superimposed lines indicate how the capsular bag diameters were measured.
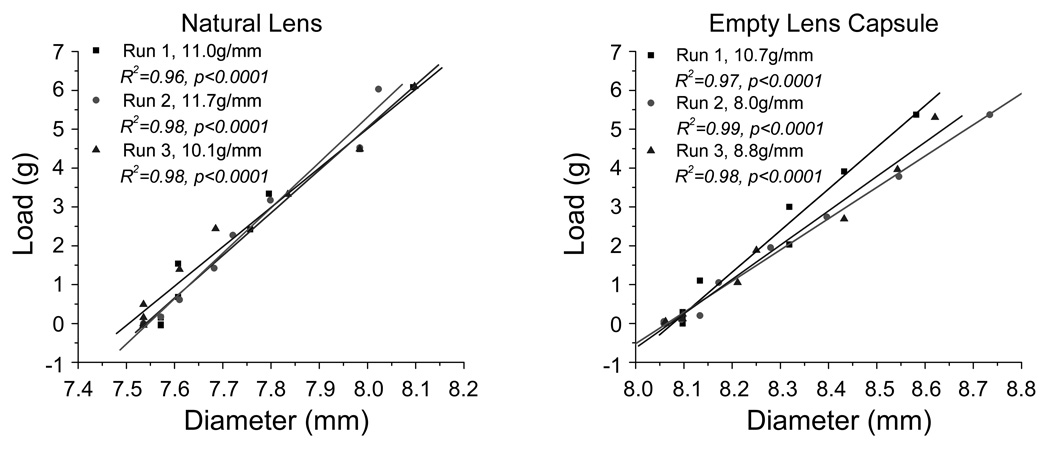
The load found at each step was graphed as a function of the corresponding diameter for all runs of both natural lens and empty lens capsular bag experiments. The force required to stretch was quantified as the slope of the load-diameter responses. The slope was found for all stretching cycles using linear regression analysis (Figure 5). In the case of paired eyes, the values obtained for the right and left eyes were averaged. In the empty lens capsule stretching experiments, the empty lens capsule diameter and load do not change for the first few stretching steps. These initial steps are required to remove the slack on the zonules resulting from the increase in diameter of the capsule after removal of the lens contents.
Figure 5.
Load-diameter response of a cynomolgus monkey (7.4 years old) natural lens (left) and empty lens capsule (right). In the empty lens capsule stretching experiments, the empty lens capsule diameter and load do not change for the first few stretching steps. These initial steps are required to remove the slack on the zonules resulting from the increase in the lens capsule diameter after removal of the lens contents.
The load-empty bag diameter slopes found were graphed as a function of age for human and cynomolgus monkey whole lenses as well as lens capsules alone. The ratio of the force required to stretch the empty lens capsule to the force required to stretch the natural lens was also graphed as a function of age. This ratio was used to determine the relative contribution of the lens capsule and lens contents at different ages. These relationships were analyzed using linear regression analysis. The p-value of the regression analysis was used to evaluate statistical significance. The slope of the regression was considered to be significantly different from zero when the p-value was less than 0.05.
3. Results
There was a significant relationship between the unstretched empty lens capsule diameter and age (p=0.008) in humans only. There was no significant variation with age of the unstretched natural lens diameter in humans or the unstretched natural lens or empty bag diameter. The unstretched natural lens diameter was 9.1±0.5mm (range: 8.1–9.8mm) in human and 7.5±0.2mm (range: 7.0–7.9mm) in cynomolgus monkey. The unstretched empty bag diameter was 9.8±0.5mm (range: 8.4–10.5mm) in human and 8.1±0.3mm (range: 7.4–8.6mm) in cynomolgus monkey. Removal of the lens contents results in an 8% increase in lens capsule diameter, which is constant for both species and independent of age (Table 1).
Table1.
Natural lens and empty lens capsule unstretched diameters. The unstretched diameters and ratio of diameters were analyzed as a function of age using linear regression analysis.
| Species | n | Age (years) |
Unstretched Diameter (mm) | Ratio of Age Diameters |
|
|---|---|---|---|---|---|
| Natural | Empty Bag | ||||
| Cynomolgus Monkey | 19 | 7.7±1.8 (4.2–10) |
7.5±0.2 (7.0–7.9) |
8.1±0.3 (7.4–8.6) |
1.08±0.03 (1.02–1.15) |
| p-value | 0.82 | 0.90 | 0.65 | ||
| Human | 22 | 1±17 (6–71) |
9.1±0.5 (8.1–9.8) |
9.8±0.5 (9.3–10.5) |
1.08±0.05 (0.96–1.19 |
| p-value | 0.47 | 0.007* | 0.142 | ||
The p-value of the regression analysis was used to evaluate statistical significance. The slope of the regression was considered to be significantly different from zero when the p-value was less than 0.05. Statistically significant values are indicated by an asterisk (*). Removal of the lens contents results in a 8% increase in lens diameter, which is constant for both species and independent of age.
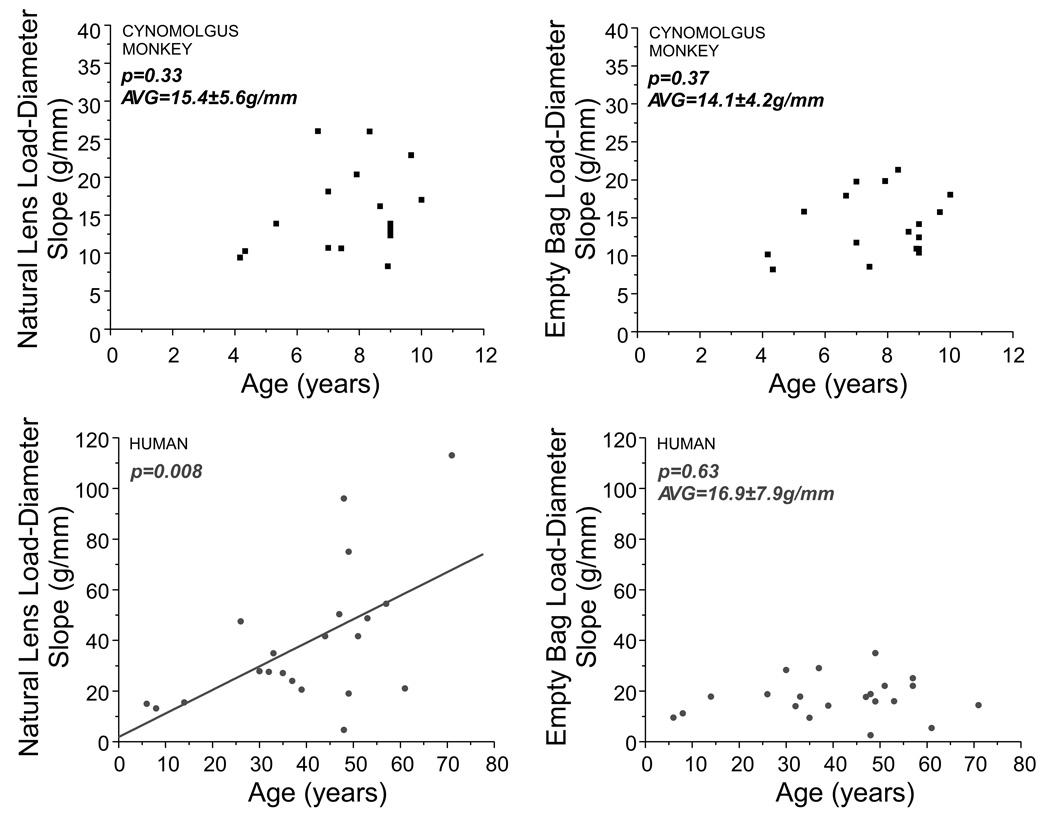
The load-natural lens diameter response significantly increases as a function of age in humans (p=0.008); however, this relationship was not significant in monkey lenses (p=0.33), most likely due to the small age range. There was no relationship between load-empty bag diameter response and age for humans or monkeys (Figure 6). The slope was 17.04±7.8g/mm (range: 2.6–34.9g/mm [25.2–342.7mN/mm]) in human and 14.1±4.2g/mm (range: 8.2–21.3g/mm [80.3–208.6mN/mm]) in cynomolgus monkey.
Figure 6.
Natural lens (left) and empty bag (right) load-diameter slopes as a function of age for cynomolgus monkey (top) and human (bottom) lenses. Only the human natural lens load-diameter response and age was statistically significant..
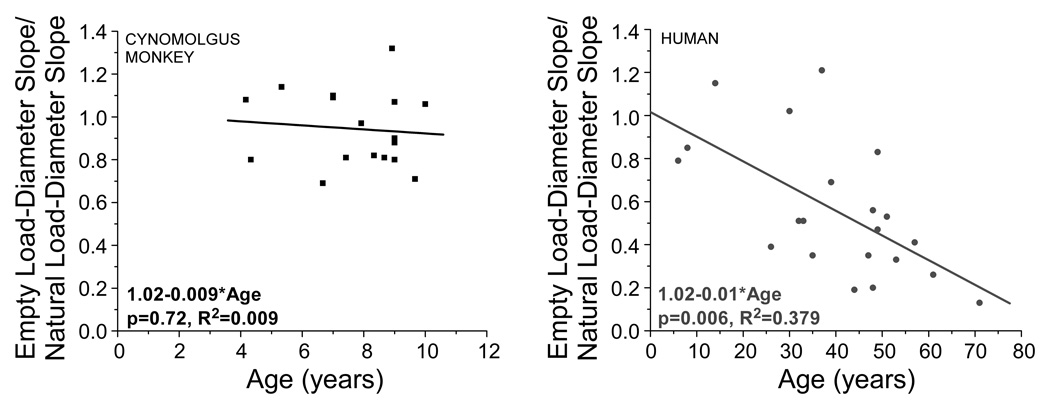
The ratio of the force required to stretch the empty lens capsule to the force required to stretch the natural lens decreases significantly with age (p=0.003; Figure 7) in human lenses. This ratio also decreases with age in cynomolgus monkey lenses, although it was not statistically significant (p=0.72). Statistical significance may not have been found due to the relatively small age range in the cynomolgus monkey lenses tested (1 monkey year=2–3 human years, Bito et al, 1982). In young human and monkey lenses, the ratio of the force required to stretch the empty lens capsule to the force required to stretch the natural lens is approximately 1.
Figure 7.
Empty bag load-diameter slope divided by natural lens load-diameter slope for cynomolgus monkey (left) and human (right) eyes. A linear regression shows statistical significance for human eyes (p=0.003), but not for monkey eyes (p=0.72).
4. Discussion
This study used an ex vivo accommodation system to measure the mechanical response of both the empty lens capsular bag and the natural lens.
The tissue preparation was designed to preserve the shape of the globe and the anatomical relationship between the accommodative components. In the current system, we do not have the capability to measure the angle of the ciliary body. However, the optical and biometrical changes we observed in the lens stretcher are similar to those reported in vivo29,33,34. The forces obtained are also in good agreement with predictions by finite element models33. The surgical preparation most likely does not greatly affect the results observed for the force transmission through the ciliary body and zonules. Since the lens density is only slightly higher than the density of the DMEM solution in which it is immersed, the relative weight of the natural lens in solution is small. The force exerted by gravity can be calculated using the Archimedes principle, and is approximately 0.03g. This load is more than one order of magnitude smaller than the changes measured during stretching. Therefore, the force transmission through the ciliary body and zonules should not be different for the natural lens and empty lens capsule due to differences in weight.
In both the natural lens and empty lens capsule experiments, the scleral shell was stretched 4mm in diameter. Preliminary results indicated that this degree of stretch was necessary to elicit lens diameter changes comparable to those found in vivo during accommodation. In vivo research found a 7.0±0.6% (monkeys)33 and 0–11.2% (humans)34 change in lens diameter, which compares to the 2–12% (monkey) and 0–7% (humans) found in the lens stretcher in the current study. Preliminary testing to determine an appropriate stretch range demonstrated the risk of tissue damage, including ciliary body detachment from the sclera segments, past a 4mm increase in the scleral shell diameter. The response of both the natural lens and empty lens capsule is linear throughout the 4mm stretch of the scleral shell (Figure 5). In all cases, the empty lens capsule total stretch was less than 10%. Increasing the amount of stretch could shift the experiments into the non-linear range of the stress-strain curve2.
The surgeon performed a mini-capsulorhexis31 in the peripheral of the lens capsule to remove the lens contents. The mini-capsulorhexis size was smaller than 2mm in all cases. This small size was chosen to preserve the integrity of the lens capsule as much as possible. Due to the small size and location of the capsulorhexis, we are essentially stretching the entire capsular bag, not a hoop or ring as was used in previous experiments on lens capsule mechanics2. The empty lens capsule response was analyzed as a function of capsulorhexis size, and it was found to be independent (p=0.393 humans and p=0.372 monkeys). This demonstrates that the use of a mini-capsulorhexis in these experiments had a minimal effect on the lens capsule response observed.
There was a significant relationship between the unstretched empty lens capsule diameter and age (p=0.008) in humans only. There was no significant variation with age of the unstretched natural lens diameter in humans or the unstretched natural lens or empty bag diameter. The unstretched (accommodated) natural lens diameters found in the lens stretcher in this study are comparable to those found for the in vivo accommodated state (monkey: 7.6–8.3mm in vivo33 versus 7.0–7.9mm in stretcher; human: 7.9–9.4mm in vivo34 versus 8.1–9.8mm in stretcher). The ratio of unstretched empty lens capsule diameter to unstretched natural lens diameter is constant for all ages and is the same for humans and monkeys. This result is important for the prediction of the proper intraocular implant size and volume to be implanted during cataract surgery.
There was no relationship between the force required to stretch the empty lens capsule and age, indicating that the lens capsule may retain the potential to produce accommodation if the lens would retain its ability to undergo accommodation. It has been suggested that the loss of accommodation with age is due partly to the inability of the lens capsule to apply molding pressure to the lens due to this loss of elasticity11–14. Previous research2,7 on ex vivo lens capsules during uniaxial stretching found that Young’s Modulus of elasticity of the lens capsule increases threefold with age2 We believe that this slight change is not sufficient to see changes in lens capsule behavior in the setting of the lens stretcher. Our previous study found that the force required to change the shape of the natural lens increases with age, but only about 4–5 times29. In contrast, previous research has shown that Young’s Modulus of lens elasticity changes several orders of magnitude with age9,10. This indicates that whole lens behavior, when together with the ciliary body and zonules as it is in the stretcher, is different than the excised state. In addition, differences in methodology alone could also explain why age-related changes in lens capsule behavior were not observed.
Previous research involved stretching excised lens capsular rings in one dimension2. In the current study, however, only a very small opening (approximately 1mm)31 was made in the lens capsule to remove the lens contents. Therefore, we were stretching a nearly intact lens capsule circumferentially. Other differences in methodology that most likely impacted the results include speed of loading and degree of stretch. Our results suggest that any changes in lens capsule mechanical properties are not significant factors in decreased accommodative ability, as the lens capsule retains its ability to stretch under the forces involved in focusing from near to far. The amount of force required to elicit a diameter change in the empty lens capsule is not dependent on age. The lens capsule of older individuals should still therefore have the ability to exert molding pressure on the lens material. Although there was no relationship between the force required to stretch the empty lens capsule and age, it should be noted that there was a high variability (47% in humans, and 30% in monkeys). This variability may be due to differences in the condition of the tissue due to different post-mortem times, donor age, and effects of tissue preparation. Overall the variability was smaller in monkey lenses, probably because these lenses were used within hours of enucleation and were from young monkeys. In older human lenses, the responses are generally found to be more variable, probably because the zonules and ciliary body are more fragile and variable in properties and architecture.
The ratio of the empty lens capsule to natural lens load-diameter slope decreased significantly with age in humans (p=0.006). This ratio is nearly 1.0 in young, pre-presbyopic humans and monkeys, indicating that the force applied by the ciliary muscle during accommodation is completely transferred from the lens capsule to the lens. The lens capsule, in turn, distributes this force to the pliable lens contents3,12,35,36. In presbyopic human eyes, however, the relative contribution of the lens capsule is less than 20%, meaning that the energy stored in the lens capsule is no longer sufficient to mold the lens material during accommodation. This finding indicates that it is changes in the lens material with age, and not the lens capsule, which are responsible for the increase in the force required to change the lens shape. In monkey eyes, the values and trends were similar to human eyes, but the age range was insufficient to make conclusions. Using the age comparison suggested by Bito et al37, all the monkeys used in this study are pre-presbyopic. This would explain the lack of age dependence observed in the monkeys. It would be expected that the ratio of empty lens capsule to natural lens load-diameter slope would always be less than 1, since the force to stretch the empty lens capsule should not be greater than the force to stretch the lens capsule plus the lens contents. In some cases, however, this ratio was nearly 1.2. This can most likely be attributed to the experimental variability as well as noise inherent to ex vivo experimentation.
Our experimental results demonstrate that there is a similarity between the ex vivo response of the empty lens capsule of both humans and monkeys. The load-diameter response of humans and monkeys are not statistically different (p=0.12), and the values are similar on average (16.9g/mm human compared to 14.1g/mm cynomolgus). The ratio of the force required to stretch the empty lens capsule to the force required to stretch the natural lens decreases in both human and cynomolgus lenses. The rate of this decrease is twice as fast for cynomolgus monkeys, which is most likely due to differences in growth rates between humans and monkeys. It would be expected that the empty lens capsule response of humans and cynomolgus monkeys correspond since the anterior and posterior lens capsule thickness of these two species are not statistically different38.
These results hold promise for techniques to restore accommodation, such as lens refilling and accommodating intraocular lenses, since the changes in lens capsule mechanical properties do not appear to significantly impact its ability to mold a lens material. The finding that the relative contribution of the lens capsule and lens contents plays a role in the onset of presbyopia also reveals the importance of the mechanical properties of the lens substitute used in lens refilling – the lens capsule must retain its role as a stress distributor for full accommodation to occur. Given a lens substitute with proper optical, mechanical, and material properties, the lens capsule will mold its shape during ciliary body contraction and relaxation.
In summary, the results of this study show that previously measured uniaxial mechanical changes in the lens capsule with age do not significantly affect its ability to undergo accommodation. At a young age, it is the capsule that determines the force to change the lens diameter, whereas it is the lens contents that determine the force in old eyes.
Table 2.
Summary of experimental results. The load-diameter slope and ratio of slopes was analyzed as a function of age using linear regression analysis.
| Species | n | Age (years) |
Load-Diameter Slope (g/mm) | Ratio of Slopes |
|
|---|---|---|---|---|---|
| Natural | Empty Bag | ||||
| Cynomolgus Monkey |
19 | 7.7±1.8 (4.2–10) |
15.4±5.6 (9.4–26.8) |
14.1±4.2 (8.2–21.3) |
1.02- 0.009*Age |
| p-value | 0.33 | 0.37 | 0.72 | ||
| Human | 22 | 41±17 (6–71) |
39.0±27.6 (4.6–113.1) |
17.04±7.8 (2.6–34.9) |
1.02- 0.01*Age |
| p-value | 0.008* | 0.63 | 0.003* | ||
The p-value of the regression analysis was used to evaluate statistical significance. The slope of the regression was considered to be significantly different from zero when the p-value was less than 0.05. Statistically significant values are indicated by an asterisk (*).
5. Acknowledgements
Grant support: NIH EY14225; NSF Graduate Student Fellowship (NMZ); NIH Predoctoral Fellowship 5F31EY15395 (DB); Advanced Medical Optics, Inc.; Florida Lions Eye Bank; Vision Cooperative Research Centre, Sydney, New South Wales, Australia, supported by the Australian Federal Government through the Cooperative Research Centres Programme; NIH center grant P30-EY014801; Research to Prevent Blindness.; Henri and Flore Lesieur Foundation (JMP).
Norma Kenyon, PhD, and Dora Berman-Weinberg, PhD, of the Diabetic Research Institute and Linda Waterman, DVM and Daniel Rothen, DVM, of the Division of Veterinary Resources of the University of Miami gave scientific support. Izuru Nose and William Lee of the Ophthalmic Biophysics Center of Bascom Palmer Eye Institute gave technical support. Donor human eyes were provided by the Florida Lions Eye Bank, Lions Eye Bank of Oregon, Lions Medical Eye Bank (Norfolk, VA), Lions Eye Institute for Transplantation and Research Inc. (Tampa, FL), Illinois Eye Bank, Alabama Eye Bank, Old Dominion Eye Foundation Inc. (Richmond, VA), North Carolina Eye Bank, Utah Lions Eye Bank, and the North West Lions Eye Bank (Seattle, WA).
Footnotes
The authors do not have any proprietary or financial interest in any of the devices presented. Presented in part at the Association for Research in Vision and Ophthalmology 2007 meeting
References
- 1.Werner LP, Werner L, Pandey SK, Apple DJ. Physiology of Accommodation and Presbyopia. In: Amar Agarwal, editor. Presbyopia: A Surgical Textbook. Thorofare, NJ: Slack Incorporated; 2002. [Google Scholar]
- 2.Krag S, Olsen T, Andreassen TT. Biomechanical characteristics of the human anterior lens capsule in relation to age. Investigative Ophthalmology and Visual Science. 1997;38:357–363. [PubMed] [Google Scholar]
- 3.Fincham EF. The mechanism of accommodation. British. Journal of. Ophthalmology. 1937;Monograph VIII:7–80. [Google Scholar]
- 4.Kessler J. Experiments in refilling the lens. Arch Ophthalmol. 1964;71:412–417. doi: 10.1001/archopht.1964.00970010428021. [DOI] [PubMed] [Google Scholar]
- 5.Kessler J. Refilling the rabbit lens. Further experiments. Arch Ophthalmol. 1966;76(4):596–598. doi: 10.1001/archopht.1966.03850010598021. [DOI] [PubMed] [Google Scholar]
- 6.Glasser A, Kaufman PL. Accommodation and Presbyopia. In: Kaufman PL, Alm A, editors. Adler’s Physiology of the Eye, Clinical Application. 10th ed. St Louis, MO: Mosby; 2003. pp. 197–233. [Google Scholar]
- 7.Fisher RF. Elastic constants of the human lens capsule. Journal of Physiology. 1969;201:1–19. doi: 10.1113/jphysiol.1969.sp008739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher RF. The elastic constants of the human lens. Journal of Physiology. 1971;212:147–180. doi: 10.1113/jphysiol.1971.sp009315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heys KR, Cram SL, Truscott RJW. Massive increase in the stiffness of the human lens nucleus with age: the basis for presbyopia? Molecular Vision. 2004;10:956–963. [PubMed] [Google Scholar]
- 10.Weeber HA, Eckert G, Soergel F, Meyer CH, Pechhold W, van der Heijde RGL. Dynamic mechanical properties of human lenses. Experimental Eye Research. 2005;80:425–434. doi: 10.1016/j.exer.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Tscherning M. Le mecanisme de l'accommodation. Annales d’Oculistique. 1904;131:168–179. [Google Scholar]
- 12.Fincham The changes in the form of the crystalline lens in accommodation. Transactions of the Optical Society. 1925;26(5) [Google Scholar]
- 13.Fisher RF. The significance of the shape of the lens and capsular energy changes in accommodation. Journal of Physiology. 1969;201:21–47. doi: 10.1113/jphysiol.1969.sp008740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilmartin B. The aetiology of presbyopia: a summary of the role of lenticular and extralenticular structures. Ophthalmic and Physiological Optics. 1995;15(5):431–437. [PubMed] [Google Scholar]
- 15.Parel JM, Gelender H, Trefers WF, Norton EWD. Phaco-Ersatz: cataract surgery designed to preserve accommodation. Graefe’s Archives of Clinical and Experimental Ophthalmology. 1986;224:165–173. doi: 10.1007/BF02141492. [DOI] [PubMed] [Google Scholar]
- 16.Hettlich HJ, Lucke K, Asiyo-Vogel MN, Schulte M, Vogel A. Lens refilling and endocapsular polymerisation of an injectable intraocular lens: In vitro and in vivo study of potential risks and benefits. Journal of Cataract and Refractive Surgery. 1994;20:115–123. doi: 10.1016/s0886-3350(13)80149-6. [DOI] [PubMed] [Google Scholar]
- 17.Nishi O, Nishi K. Accommodation amplitude after lens refilling with injectable silicone by sealing the capsule with a plug in primates. Archives of Ophthalmology. 1998;116(10):1358–1361. doi: 10.1001/archopht.116.10.1358. [DOI] [PubMed] [Google Scholar]
- 18.Koopmans SA, Terwee T, Barkhof J, Haitjema HJ, Kooijman AC. Polymer refilling of presbyopic human lenses in vitro restores the ability to undergo accommodative changes. Investigative Ophthalmology and Visual Science. 2003;44(1):250–257. doi: 10.1167/iovs.02-0256. [DOI] [PubMed] [Google Scholar]
- 19.Koopmans SA, Terwee T, Glasser A, Wendt M, Vilupuru AS, van Kooten TG, Norrby S, Haitjema HJ, Kooijman AC. Accommodative lens refilling in rhesus monkeys. Investigative Ophthalmology and Visual Science. 2006;47(7):2976–2984. doi: 10.1167/iovs.05-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norrby S, Koopmans S, Terwee T. Artificial crystalline lens. Ophthalmology Clinics of North America. 2006;19(1):143–146. doi: 10.1016/j.ohc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Yang X, Zou L, Binrong M, Dong D, Dai H, Lu X. Tensile strength of lens capsules in eye-bank eyes. Journal of Cataract and Refractive Surgery. 1998;24(4):543–546. doi: 10.1016/s0886-3350(98)80299-x. [DOI] [PubMed] [Google Scholar]
- 22.Danielsen CC. Tensile mechanical and creep properties of Descemet membrane and lens capsule. Experimental Eye Research. 2004;79(3):343–350. doi: 10.1016/j.exer.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Assia EI, Apple DJ, Barden A, Tsai JC, Castaneda VE, Hoggatt JS. An experimental study comparing various anterior capsulectomy techniques. Archives of Ophthalmology. 1991;109(5):642–647. doi: 10.1001/archopht.1991.01080050056028. [DOI] [PubMed] [Google Scholar]
- 24.Morgan JE, Ellingham RB, Young RD, Trmal GJ. The mechanical properties of the human lens capsule following capsulorhexis or radiofrequency diathermy capsulotomy. Archives of Ophthalmology. 1996;114:1110–1115. doi: 10.1001/archopht.1996.01100140312010. [DOI] [PubMed] [Google Scholar]
- 25.Wood MG, Schelonka LP. A porcine model predicts that a can-opener capsulotomy can be done safely in pediatric patients. Journal of American Association for Pediatric Ophthalmology and Strabismus. 1999;3(6):356–362. doi: 10.1016/s1091-8531(99)70045-5. [DOI] [PubMed] [Google Scholar]
- 26.Andreo LK, Wilson ME, Apple DJ. Elastic properties and scanning electron microscopic appearance of manual continuous curvilinear capsulorhexis and vitrectorhexis in an animal model of pediatric cataract. Journal of Cataract and Refractive Surgery. 1999;25(4):534–539. doi: 10.1016/s0886-3350(99)80051-0. [DOI] [PubMed] [Google Scholar]
- 27.Parel JM, Ziebarth NM, Denham D, Fernandez V, Manns F, Lamar P, Rosen A, Ho A, Erickson P. Assessment of the strength of mini-capsulorhexes. Journal of Cataract and Refractive Surgery. 2006;32:1366–1373. doi: 10.1016/j.jcrs.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Parel JM, Fernandez V, Billotte C, Denham D, Lamar PD, Rosen A, Ho A, Kenyon N, Collins BR, Erickson P. Accommodation stress-strain relation in human and non-human primate eyes ex-vivo. Investigative Ophthalmology and Visual Science. 2002;43 ARVO E-Abstract 406. [Google Scholar]
- 29.Manns F, Parel JM, Denham D, Billotte C, Ziebarth N, Borja D, Fernandez V, Aly M, Arrieta E, Ho A, Holden B. Optomechanical response of human and monkey lenses in a lens stretcher. Investigative Ophthalmology and Visual Science. 2007;48(7):3260–3268. doi: 10.1167/iovs.06-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Augusteyn RC, Rosen AM, Borja D, Ziebarth NM, Parel JM. Biometry of primate lenses during immersion in preservation media. Molecular Vision. 2006;12:740–747. [PubMed] [Google Scholar]
- 31.Tahi H, Fantes F, Hamaoui M, Parel J-M. Small peripheral anterior continuous curvilinear capsulorhexis. Journal of Cataract and Refractive Surgery. 1999;25:744–747. doi: 10.1016/s0886-3350(99)00041-3. [DOI] [PubMed] [Google Scholar]
- 32.Hermans EA, Dubbelman M, van der Heijde GL, Heethaar RM. Change in the accommodative force on the lens of the human eye with age. Vision Research. 2008;49:119–126. doi: 10.1016/j.visres.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 33.Glasser A, Wendt M, Ostrin L. Accommodative changes in lens diameter in rhesus monkeys. Investigative Ophthalmology and Visual Science. 2006;47:278–286. doi: 10.1167/iovs.05-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strenk SA, Semmlow JL, Strenk LM, Munoz P, Gronlund-Jacob J, DeMarco JK. Age-related changes in human ciliary muscle and lens: a Magnetic Resonance Imaging study. Investigative Ophthalmology and Visual Science. 1999;40:1162–1169. [PubMed] [Google Scholar]
- 35.Koretz J, Handelman GH. Model of the accommodative mechanism in the human eye. Vision Research. 1982;22:917–922. doi: 10.1016/0042-6989(82)90028-1. [DOI] [PubMed] [Google Scholar]
- 36.Fincham EF. An experiment on the influence of tension upon the form of the crystalline lens. Transactions of the Optical Society of the United Kingdom. 1936;56:138–147. [Google Scholar]
- 37.Bito LZ, DeRousseau CJ, Kaufman PL, Bito JW. Age-dependent loss of accommodative amplitude in rhesus monkeys: an animal model for presbyopia. Investigative Ophthalmology and Visual Science. 1982:23–31. [PubMed] [Google Scholar]
- 38.Ziebarth N, Manns F, Uhlhorn S, Venkatraman A, Parel JM. Non-contact optical measurement of lens capsule thickness in human, monkey, and rabbit postmortem eyes. Investigative Ophthalmology and Visual Science. 2005;46:1690–1697. doi: 10.1167/iovs.05-0039. [DOI] [PubMed] [Google Scholar]