Abstract
Background
Continued high rates of HIV-1 transmission have fueled interest in the use of antiretrovirals to prevent infection. Attenuated infection with failure of tenofovir as prophylaxis has been reported in animal models. Here, we report a case of HIV-1 infection despite intermittent use of fixed-dose combination tenofovir and emtricitabine (FTC).
Methods
The patient was treated with tenofovir DF/FTC for reported repeated high-risk sexual exposures. After seroconversion, he was subjected to routine laboratory testing, CCR5 and HLA genotyping, and biopsy of gastrointestinal (GI) tissue. Resistance testing was performed both as bulk sequencing of plasma and cloning and sequencing of virus derived from plasma, peripheral blood mononuclear cells, and GI tissue.
Results
In this patient with no readily identifiable modifying host factors, acute HIV-1 infection with tenofovir DF/FTC—susceptible HIV-1 was associated with an attenuated clinical course, very low postseroconversion HIV-1 RNA levels, slow kinetics of seroconversion, and relative sparing of mucosal CD4+ T cells in the GI tract.
Conclusions
Despite the failure of tenofovir DF/FTC as prophylaxis, selection for drug-resistant transmission did not occur and the blunting of postinfection levels of viremia likely reduced the probability of subsequent forward transmissions during the acute phase. These results support continued investigations of the use of antiretrovirals as a means to reduce HIV-1 transmission.
Keywords: prophylaxis, tenofovir, emtricitabine, acute infection
Continued high rates of HIV-1 transmission have mandated vigorous interest in a variety of approaches to prevent new infections including the testing of topical microbicides and prophylactic vaccines. Though the ideal prophylactic agent would result in sterile immunity, it has been theorized that even in the face of infection, a reduction in levels of plasma viremia postinfection may result in reduced transmissions at both the individual and population level.1,2
Recent discouraging results in trials of microbicides and vaccines have further fueled interest in the use of antiretrovirals (ARVs) to prevent infection. ARV therapy has not only increased survival for HIV-infected patients3-5 but has also been used to prevent both vertical transmission6 and transmission due to occupational exposure.7
The demonstrated efficacy of tenofovir DF (TDF) in the treatment of HIV-1 infection,8 the lack of reported serious adverse events associated with its use,8 its long half-life,9,10 and the fact that HIV-1 strains resistant to TDF tend to be less replication competent than wild-type viruses11 make this ARV agent an ideal candidate for pre- and postexposure prophylaxis (PrEP and PEP).
Animal studies have provided evidence that PrEP and PEP with TDF alone, or in combination with emtricitabine (FTC), protect against simian immunodeficiency virus (SIV) and Simian HIV (SHIV) transmission.12-17 Furthermore, animals that become infected despite receiving TDF as PrEP or PEP showed delayed onset of viremia and seroconversion,14,17,18 demonstrating that even in the case of incomplete protection against infection, PrEP/PEP treatment may lead to attenuated acute infection. As transmission risk is a function of plasma viremia and acute infection represents a period during which transmission risk is high,19 such an effect may further act to prevent forward transmissions.
Here, we report a case of an individual infected with a drug-susceptible virus despite the intermittent use of fixed-dose combination TDF/FTC (Truvada; Gilead Sciences, Foster City, CA) as PrEP/PEP. As seen in animal models, the resultant infection displayed an attenuated course that could not be attributed to known modifying host factors, suggesting that despite inability to completely prevent infection, antiviral therapy may indeed modulate acute and early HIV-1 infection with resultant beneficial effects on preventing forward infections.
METHODS
Patient’s Clinical History
The patient is a 38-year-old man who has sex with men (MSM) who presented to the Aaron Diamond AIDS Research Center Primary Infection Program on February 8, 2007, for evaluation (Fig. 1) and was enrolled in a Rockefeller University Hospital Institutional Review Board—approved natural history study after giving written informed consent. Routine evaluations included detailed history and physical examination and determinations of plasma HIV-1 RNA levels using Roche Cobas Ultrasensitive Assay, version 1.5 (Branchburg, NJ), and T-cell subsets.
FIGURE 1.
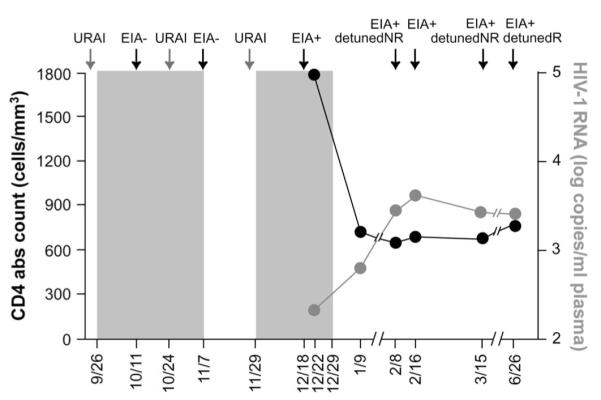
Patient’s clinical history. The graph shows the periods of time when the patient assumed tenofovir/FTC preventive therapy (gray areas), he reported URAI and his clinical test results. The patient underwent repeated diagnostic tests: EIA, low-sensitivity EIA (detuned, NR = nonreactive, R = reactive), and HIV-1 RNA test. During the course of observation, viral loads and CD4 cell counts were monitored periodically. URAI, unprotected receptive anal intercourse.
Virologic Studies
The phenotype and genotype of the patient’s plasma-derived virus were determined using the PhenoSense assay (Monogram Biosciences, San Francisco, CA) and the HIV-1 protease and reverse transcriptase (RT) genotyping kit TruGene HIV-1 (Bayer HealthCare, Tarrytown, NY), respectively. The sequence analysis of HIV-1 RT reading frames was performed also by cloning and sequencing the RT gene of viruses derived from plasma, PBMC, and gastrointestinal (GI) tissue. Briefly, total DNA was extracted from PBMC and total RNA was extracted from plasma and GI tissue and reverse transcribed. DNA and cDNA were amplified by nested polymerase chain reaction (PCR) with the following sets of primers: forward 5′-CACTTTAAATTTTCCMATTAGTCCTATT-3′, reverse 5′-AGGAGTCTTTCCCCATATTACTATGCTTTC-3′ and subsequently forward 5′-ACTGTACCAGTAAAATTAAAGCCAGG-3′, reverse 5′-TCTGTATRTCATTGACAGTCCAGC-3′. The PCR products were cloned using the Zero Blunt TOPO PCR Cloning Kit (Invitrogen, Carlsbad, CA) and sequenced with SP6 and T7 primers. Finally, allele-specific PCRs for M184V and K65R were performed on patient’s plasma as per published methods.20
Viral tropism was determined by genotyping of the third variable loop of the envelope gene and employing the X4/R5 algorithm from the WebPSSM coreceptor prediction program (Mullins Lab, University of Washington). Total DNA was extracted from PBMC and amplified by nested PCR with the following sets of primers: forward 5′-GGCATCTCCTATGGCAGGAAGAAGC-3′, reverse 5′-AATTAGCCCTTCCAGTCCCCCCTTT-3′ and subsequently forward 5′-GAAAGAGCAGAAGACAGTGGMAATG-3′, reverse 5′-TACYTTTTGACCACTTGCCMCCCAT-3′.
Host Factor Determinations
CCR5 genotyping was performed by PCR using the primers 5′-GTGGTGGCTGTGTTTGCGTCTCT-3′ and 5′-CAGCCCTGTGCCTCTTCTTCTCAT-3′. The PCR product was analyzed by electrophoresis on a 3% agarose gel and identified according to its size (CCR5 and CCR5Δ32 amplicons 241 and 209 bp, respectively).
Class I HLA genotyping was performed in the Laboratory of Experimental Immunology of the National Cancer Institute at Frederick (Frederick, MD). We performed genotyping of the HLA-A,-B, and-C loci using the sequencebased typing method. Amplicons of ∼1 kb that include exons 2 and 3 genomic regions were amplified with locus-specific primers. Amplicons were cleaned with Ampure (APN 000132, Agencourt Biosource Corporation, Beverly, MA) in a Biomek FX Laboratory Automation Workstation (Beckman Coulter, Inc, Fullerton, CA) and sequenced with ABI Prism BigDye Terminator v1.1 Cycle Sequencing Kits (PN 4336776, Applied Biosystems, Foster City, CA) following the vendor’s protocols. Sequence reactions were cleaned with CleanSeq (APN 000196, Agencourt Biosource Corporation) in a Biomek FX Laboratory Automation Workstation and run on the ABI 3730XL DNA analyzer (Applied Biosystems). Sequences were analyzed with the “Assign 400 ATF” software (Conexio Genomics, Western Australia) to determine the genotype.
GI Tissue Studies
After obtaining written informed consent, endoscopic biopsies were obtained from the colon from macroscopically normal mucosa and were processed for flow cytometry as previously published.21
RESULTS
The patient is an MSM and reported unprotected receptive anal intercourse (URAI) with multiple partners on September 24, 2006, and September 25, 2006. TDF/FTC was initiated as PEP on September 26, 2006. Due to repeated exposures at the end of initial 4 weeks (October 24, 2006) of PEP, the course was extended and continued for an additional 2 weeks until November 7, 2006. On November 28, 2006, patient reported unprotected receptive anal intercourse with multiple partners, and TDF/FTC was again started on November 29, 2006, without serologic or HIV-1 RNA testing and therapy continued through December 29, 2006. Patient remained asymptomatic throughout and was found to have seroconverted on December 18, 2006. Critically, the patient insisted that there were no additional sexual contacts between November 7, 2006, and November 28, 2006—the period during which he remained off TDF/FTC.
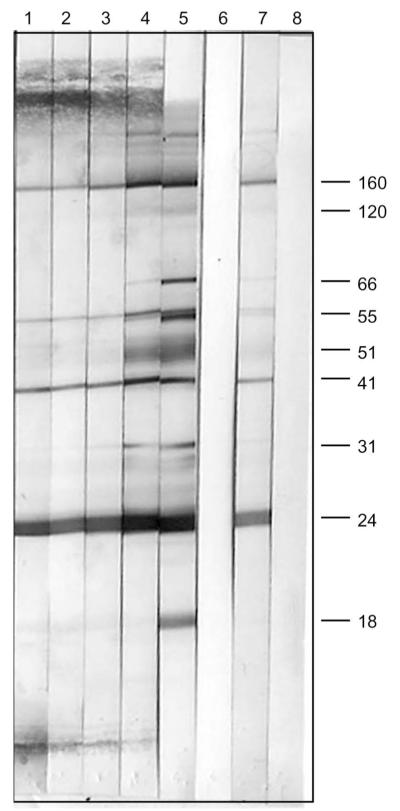
At presentation, the patient did not report any symptoms that could be consistent with acute HIV-1 infection.22 He had nonreactive serologic tests between October 11, 2006, and November 7, 2006, 17 and 44 days after the first reported exposure and 14 days after the second reported event (Fig. 1). A positive enzyme-linked immunoabsorbent (EIA) test, confirmed by Western blot with viral bands reportedly observed at p24, p40, p51, and gp160, was documented on December 18, 2006, while on TDF/FTC, 20, 56, and 84 days after the 3 reported episodes of high-risk sexual exposures, respectively. On February 8, 2007, 52 days after the first positive serology, the EIA remained positive and a detuned EIA was nonreactive with an optical density (OD) of 0.005. A subsequent EIA was reactive on February 16, 2007. The detuned EIA remained nonreactive with an OD of 0.133 on March 15, 2007, and was reactive by June 26, 2007, with an OD of 2.304. Serial Western blots over the 5-month period are shown in Figure 2 demonstrating increasing intensity of bands at gp160, p55, and p24 and the gradual appearance of bands at gp120, p51, and p31, consistent with the slowly evolving serologic response to HIV-1.
FIGURE 2.
Serial Western blots—Lane 1: February 8, 2007, Lane 2: February 16, 2007, Lane 3: March 15, 2007, Lane 4: June 26, 2007, Lane 5: kit positive control, Lane 6: kit negative control, Lane 7: in-house positive control, and Lane 8: in-house negative control.
Despite the recent infection and seroconversion, plasma HIV-1 RNA levels were 213 copies per milliliter while on TDF/FTC on December 22, 2006, and 647 copies per milliliter on January 9, 2007, 11 days after terminating TDF/FTC. Subsequent HIV-1 RNA levels and CD4 cell counts are shown (Fig. 1).
Resistance Testing
The PhenoSense assay indicated that the patient’s virus was pansusceptible to all ARV agents. Consensus sequence analysis of plasma-derived HIV-1 and 41 clones from plasma, 25 from PBMC, and 18 from GI tract—associated lymphoid tissue HIV-1 RNA were uniform (Fig. 3) and failed to reveal amino acid substitutions known to confer resistance to TDF (K65R, K70E)23,24 or FTC (M184V/I).25,26 These data were confirmed by allele-specific PCR which failed to detect point mutations associated with TDF or FTC resistance. The T215D amino acid substitution in reverse transcriptase was seen in all clones and the plasma consensus sequence consistent with a thymidine analogue resistant revertant that is not associated with reduced susceptibility to either TDF or FTC.
FIGURE 3.
Alignment (A) and phylogenetic tree (B) of viral RT deduced amino acid sequences. The sequences were obtained by cloning the RT gene from viruses derived from plasma, PBMC, and GI tissue.
Viral Tropism
We sequenced the viral envelope and used a 105-bp segment incorporating the third variable loop to predict the tropism of the infecting viral population, and not surprisingly R5 tropism was predicted. Interestingly, the crown or antigenic tip of the loop contained an unusual amino acid, APGK, which though reported27 is not known to be associated with enhanced neutralizability.
Host Factors
The determination of the patient’s CCR5 genotype indicated homozygosity for the wild-type allele. The class I HLA type of the patient is as follows: HLA-A*0201/A*3002, B*1501/B*1801, C*0304/C*0501. Though this evaluation is limited, there are no clear host factors identified, that is, HLA B57, 27, or CCR5δ32 heterozygosity, to readily explain the attenuated acute infection observed.
GI Tract Data
Flow cytometry analysis revealed that the percentage of mucosal CD4+ T cells in the patient (33%) was intermediate between the percentage observed on average in patients with acute infection and in uninfected subjects (16% and 56%, respectively).21 Furthermore, the percentage of memory (CD45R0+) CD4+ and CD8+ T cells expressing the activation marker HLA-DR in the GI tissue of the patient (18% and 30%, respectively) was intermediate between the percentage observed in uninfected subjects (11.3% and 19.8%, respectively) and acutely infected patients (29.0% and 47.6%, respectively).28
DISCUSSION
As the HIV-1 pandemic enters its 26th year, we are faced with continued high rates of new infections despite efforts to modify behaviors, and the prospect of an effective vaccine remains distant. Though microbicide research has clearly advanced, recent failures of clinical trials are reported29 and this preventative mode has questionable applicability to MSM transmission. As such, the use of antiviral drugs as prophylaxis to stem the current tide would seem to be a reasonable and attractive option.
Here, we have presented a case report of a patient who becomes infected with drug-susceptible HIV-1 despite intermittent prophylaxis with TDF/FTC. Failure of antiviral drugs used as prophylaxis in animal models has been associated with transmission of drug-susceptible SIV and SHIV, delayed seroconversion, and reduced levels of plasma viremia. As is generally the case, documenting such in newly HIV-1—infected individuals is far more difficult. However, based on the absence of symptoms, slow evolution of a serologic response, documented low levels of plasma viremia, high levels of circulating CD4+ T cells at the time of seroconversion, and relative sparing of the GI mucosa, we conclude that the infection seems attenuated. Furthermore, an extensive analysis of plasma, PBMC, and GI tissue all reveal the infecting viral population to be relatively homogenous and both TDF and FTC susceptible.
The patient’s history describes 3 discrete potential dates of infection. The first documented positive serology was distant from the first exposure (84 days), 54 days from the second, and quite proximal to the third exposure (20 days). Given the very protracted evolution of a serologic response as documented from the Western blot patterns and the very low OD results obtained when using the detuned enzyme-linked immunosorbent assay nearly 2 months after the first reactive serology and the absence of symptoms of acute infection, a clear date of infection is nearly impossible to discern. However, the pace at which antigen-driven serologic responses evolved postinfection was clearly slowed by the use of TDF/FTC, and we believe that this is due to blunting of the burst of plasma viremia generally associated with acute infection—as demonstrated by the plasma viral load levels of 213 and 647 copies per milliliter most proximal to the time of infection during and shortly after TDF/FTC therapy.
The number and severity of symptoms are generally indicative of high viral loads during acute infection.22 That the acute infection was attenuated is further supported by the lack of symptoms. Furthermore, the peripheral CD4+ T-cell count of 1781 cells per cubic millimeter at the time of first reactive serology and the relative sparing of the GI mucosa in the months after infection are also consistent with an attenuated acute infection. Indeed, at the time of biopsy, this patient had approximately 2-fold the %CD4+ T cells, 33% compared with the mean value of 16% in our previously published cohort. Even after the cessation of antiviral therapy, this patient continued to demonstrate modest viremia and well-preserved peripheral CD4+ T-cell counts—whether this reflects a benefit of antiviral therapy or other factors remain unclear. However, as has been seen in the SIV/SHIV macaque model, this patient exhibits delayed seroconversion and persistently modest levels of plasma viremia.
We performed extensive sequence analyses of plasma, PBMC, and GI tract—associated HIV-1 RNA. Though we cannot rule out the possibility that a drug-resistant virus established infection and reverted to susceptible by both phenotype and genotype, the homogenous viral populations, negative results using the highly sensitive allele-specific PCR, and failure to detect archived drug-resistant virus in PBMC would suggest that this was not the case. That TDF/FTC failed to prevent infection with a drug-susceptible virus could be explained by various factors including intermittent dosing, failure to complete a 4-week course of prophylactic therapy after the second reported high-risk exposure, and the possibility that despite history to the contrary, the patient did engage in high-risk behaviors during the 22-day period off TDF/FTC between the second and third reported high-risk sexual encounters. It is encouraging however that despite intermittent therapy, there was no selection for infection with a drug-resistant virus. It is important to emphasize that this case report reflects “real-world” use of antiviral drugs to prevent infection. It is likely that even in the best of circumstances, adherence will be intermittent, patients will interrupt therapy for periods of time and will likely stop and restart therapy from time to time based on behaviors and perceived risk as was the case here.
Given the nature of attenuated clinical course and laboratory studies, we searched for obvious host factors known to attenuate HIV-1 disease progression. The patient is not heterozygous for the CCR5Δ32. HLA class I alleles did not reveal either B57 or B27. We realize that this is a minimal exploration into host factors that may modify acute HIV-1 infection and subsequent clinical course, nevertheless, this does support our contention that antiviral therapy did contribute significantly to the observed picture.
We suggest that even if prophylactic antiviral treatment failed in preventing infection, it may have dramatically reduced levels of viral replication postinfection, avoiding the extensive CD4+ cell depletion that usually accompanies acute infection and possibly enabling an adequate immune response to develop and partially control the infection after the withdrawal of the drug.
This finding could have critical relevance on a population level. As it is believed that HIV-1 transmission events occur predominantly during the acute phase of infection as a result of very high plasma viral loads, any intervention that could blunt peak viremia and reduce postinfection levels of circulating virus could have a very beneficial effect on the subsequent spread of infection. We believe that the low postinfection levels of viremia observed in this case likely reduced the probability of subsequent forward transmissions. Hence, these results echo that which has been seen in animal models and strongly support continued investigations of the use of antiviral agents as a means to reduce HIV-1 transmission not only for the individual but also for the population at highest risk.
ACKNOWLEDGMENTS
We would like to thank the New York City Department of Health and Mental Hygiene for performing the enzyme-linked immunoabsorbent and Western blots contained in the manuscript, Wendy Chen for assistance with graphics, and the Nursing Staff at the Rockefeller University Hospital for clinical assistance.
Supported by funding from the National Institutes of Health RO1-AI47033, and the Acute Infection and Early Disease Research Program, AI41534, and the Clinical and Translational Science Award UL1 RR024143.
REFERENCES
- 1.Davenport MP, Ribeiro RM, Chao DL, et al. Predicting the impact of a nonsterilizing vaccine against human immunodeficiency virus. J Virol. 2004;78:11340–11351. doi: 10.1128/JVI.78.20.11340-11351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta SB, Jacobson LP, Margolick JB, et al. Estimating the benefit of an HIV-1 vaccine that reduces viral load set point. J Infect Dis. 2007;195:546–550. doi: 10.1086/510909. [DOI] [PubMed] [Google Scholar]
- 3.Mocroft A, Gill MJ, Davidson W, et al. Predictors of a viral response and subsequent virological treatment failure in patients with HIV starting a protease inhibitor. AIDS. 1998;12:2161–2167. doi: 10.1097/00002030-199816000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Sterne JA, Hernan MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366:378–384. doi: 10.1016/S0140-6736(05)67022-5. [DOI] [PubMed] [Google Scholar]
- 5.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 6.Suksomboon N, Poolsup N, Ket-Aim S. Systematic review of the efficacy of antiretroviral therapies for reducing the risk of mother-to-child transmission of HIV infection. J Clin Pharm Ther. 2007;32:293–311. doi: 10.1111/j.1365-2710.2007.00825.x. [DOI] [PubMed] [Google Scholar]
- 7.Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337:1485–1490. doi: 10.1056/NEJM199711203372101. [DOI] [PubMed] [Google Scholar]
- 8.Schooley RT, Ruane P, Myers RA, et al. Tenofovir DF in antiretroviral-experienced patients: results from a 48-week, randomized, double-blind study. AIDS. 2002;16:1257–1263. doi: 10.1097/00002030-200206140-00008. [DOI] [PubMed] [Google Scholar]
- 9.Barditch-Crovo P, Deeks SG, Collier A, et al. Phase i/ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2001;45:2733–2739. doi: 10.1128/AAC.45.10.2733-2739.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deeks SG, Barditch-Crovo P, Lietman PS, et al. Safety, pharmacokinetics, and antiretroviral activity of intravenous 9-[2-(R)-(phosphonomethoxy) propyl]adenine, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults. Antimicrob Agents Chemother. 1998;42:2380–2384. doi: 10.1128/aac.42.9.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber J, Chakraborty B, Weberova J, et al. Diminished replicative fitness of primary human immunodeficiency virus type 1 isolates harboring the K65R mutation. J Clin Microbiol. 2005;43:1395–1400. doi: 10.1128/JCM.43.3.1395-1400.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Lerma J, Qari S, Jackson E, et al. Prevention of Rectal SHIV Transmission in Macaques by Tenofovir/FTC Combination. 13th Conference on Retroviruses and Opportunistic Infections; Denver, CO. February 5—February 8, 2006; Abstract 32LB. [Google Scholar]
- 13.Subbarao S, Otten RA, Ramos A, et al. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J Infect Dis. 2006;194:904–911. doi: 10.1086/507306. [DOI] [PubMed] [Google Scholar]
- 14.Tsai CC, Emau P, Follis KE, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265–4273. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai CC, Follis KE, Sabo A, et al. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 16.Van Rompay KK, McChesney MB, Aguirre NL, et al. Two low doses of tenofovir protect newborn macaques against oral simian immunodeficiency virus infection. J Infect Dis. 2001;184:429–438. doi: 10.1086/322781. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Lerma JG, Otten RA, Qari SH, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008;5:e28. doi: 10.1371/journal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Rompay KK, Dailey PJ, Tarara RP, et al. Early short-term 9-[2-(R)-(phosphonomethoxy)propyl]adenine treatment favorably alters the subsequent disease course in simian immunodeficiency virus-infected newborn rhesus macaques. J Virol. 1999;73:2947–2955. doi: 10.1128/jvi.73.4.2947-2955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JA, Li JF, Wei X, et al. Simple PCR assays improve the sensitivity of HIV-1 subtype B drug resistance testing and allow linking of resistance mutations. PLoS ONE. 2007;2:e638. doi: 10.1371/journal.pone.0000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn JO, Walker BD. Acute human immunodeficiency virus type 1 infection. N Engl J Med. 1998;339:33–39. doi: 10.1056/NEJM199807023390107. [DOI] [PubMed] [Google Scholar]
- 23.Wainberg MA, Miller MD, Quan Y, et al. In vitro selection and characterization of HIV-1 with reduced susceptibility to PMPA. Antivir Ther. 1999;4:87–94. doi: 10.1177/135965359900400205. [DOI] [PubMed] [Google Scholar]
- 24.XIV International Drug Resistance Workshop; Quebec City, Quebec. June 7—June 11, 2005; Abstract 92. [Google Scholar]
- 25.Schinazi RF, Lloyd RM, Jr, Nguyen MH, et al. Characterization of human immunodeficiency viruses resistant to oxathiolane-cytosine nucleosides. Antimicrob Agents Chemother. 1993;37:875–881. doi: 10.1128/aac.37.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tisdale M, Kemp SD, Parry NR, et al. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc Natl Acad Sci USA. 1993;90:5653–5656. doi: 10.1073/pnas.90.12.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segal DM, Shapshak P, Zhang BT, et al. Novel tetrameric tip motifs (APGK and VPGK) in the V3 loop of HIV type 1 envelope sequences in blood and brain from two injection drug users in Miami, Florida. AIDS Res Hum Retroviruses. 1997;13:1643–1646. doi: 10.1089/aid.1997.13.1643. [DOI] [PubMed] [Google Scholar]
- 28.Mehandru S, Poles MA, Tenner-Racz K, et al. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 2006;3:e484. doi: 10.1371/journal.pmed.0030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen J. AIDS research. Microbicide fails to protect against HIV. Science. 2008;319:1026–1027. doi: 10.1126/science.319.5866.1026b. [DOI] [PubMed] [Google Scholar]