Abstract
Background
Mother-child human leukocyte antigen (HLA) concordance and maternal HLA homozygosity may increase the risk of vertical transmission of human immunodeficiency virus type 1 (HIV-1) risk by reducing infant immune responses.
Methods
We analyzed mother-child HLA concordance and maternal HLA homozygosity in a Kenyan perinatal cohort receiving antenatal zidovudine. HLA concordance was scored as the number of shared class I alleles, and relative risk estimates were adjusted for maternal HIV-1 load.
Results
Among 277 mother-infant pairs, HIV-1 transmission occurred in 58 infants (21%), with in utero transmission in 21 (36%), peripartum transmission in 26 (45%), and transmission via breast-feeding in 11 (19%). With increased concordance, we observed a significant increase in the risk of transmission overall (adjusted hazard ratio [aHR], 1.3 [95% confidence interval {CI}, 1.0–1.7]; P = .04), in utero (adjusted odds ratio, 1.72 [95% CI, 1.0 –1.7]; P = .04), and via breast-feeding (aHR, 1.6 [95% CI, 1.0 –2.5]; P = .04). Women with homozygosity had higher plasma HIV-1 RNA levels at 32 weeks of gestation (5.1 vs. 4.8 log10 copies/mL; P = .03) and an increased risk of transmission overall (aHR, 1.7 [95% CI, 1.1–2.7]; P = .03) and via breast-feeding (aHR, 5.8 [95% CI, 1.9 –17.7]; P = .002).
Conclusion
The risks of overall, in utero, and breast milk HIV-1 transmission increased with HLA concordance and homozygosity. The increased risk may be due to reduced alloimmunity or less diverse protective immune responses.
Vertical transmission of HIV-1 may occur during gestation, delivery, or breast-feeding when the fetus or infant is exposed to free virus or infected maternal cells. Despite probable exposure, HIV-1 acquisition rates in infants are relatively low, even in the absence of antiretroviral prophylaxis. Host factors such as maternal or infant genetics and immunity may influence the ability of a fetus or infant to avoid HIV-1 infection. These factors may be maternal or infant specific or may be shared because of similar HLA genes. An increased degree of HLA gene sharing between a mother and infant differentiates vertical transmission of HIV-1 from sexual transmission.
HLA class I and II loci are the most polymorphic genes known in humans [1]. HLA class I genes, located at the HLA-A, -B, and -C loci, encode molecules that differentially present endogenous viral peptides to CD8+ T lymphocytes. As a result of differential peptide binding, specific HLA molecules may influence susceptibility to HIV-1 infection and progression. Differential peptide binding leads to activation of cellular immune responses directed toward specific viral epitopes, exerting immune pressure on HIV-1 and resulting in viral mutations that increase the ability of HIV-1 to escape immune responses [2, 3]. For HLA alleles that an infant shares with his or her mother, the same mutations that allow HIV-1 to escape maternal responses will allow it to evade infant responses. Infants who share more alleles with their mother may also be at risk for more rapid HIV-1 disease progression if infected. Mother-child HLA discordance could be favorable by diversifying the immune response against maternal virus. HLA discordance might also protect against the perinatal transmission of viruses via infant alloimmune responses against HLA alloantigens expressed on maternally infected cells and, in the case of enveloped viruses, free virions. Rather than targeting viral antigens, the infant alloimmune response is directed at maternal cells expressing HLA antigens that are different from the infant’s own.
When a mother is homozygous for specific HLA alleles, the infant’s immune responses will also be less vigorous in proportion to the number of shared alleles. Infants of HLA homozygous mothers may be more susceptible to HIV-1 infection because of dampened alloimmune responses that lead to less rapid clearance of maternal cells, including those infected with HIV-1. Additionally, HLA homozygous women present less diverse arrays of viral peptides to CD8+ T cells, resulting in more rapid HIV-1 disease progression [4, 5]. Because HIV-1 disease progression influences the risk of transmission, HLA homozygous mothers may be more likely to transmit HIV-1 to their offspring.
Past studies have found that greater HLA concordance was associated with increased risk of vertical transmission; however, only a single study was conducted in a breast-feeding African cohort, and women did not receive antiretrovirals to prevent transmission [6, 7]. There are fewer data on maternal HLA homozygosity and transmission because studies of HLA homozygosity have focused on disease progression [4]. We built on these existing studies by analyzing the effect of mother-child HLA concordance and maternal HLA homozygosity on transmission risk among HIV-1—infected mothers receiving zidovudine in Kenya. In this cohort we were able to determine the timing of transmission. This enabled us to examine separately whether HLA concordance and maternal homozygosity increased the risk of HIV-1 acquisition in infants, including overall, in utero, and peripartum transmission and transmission via breast milk.
METHODS
Study setting and subjects
Study participants were recruited from antenatal clinics near Nairobi (as described elsewhere), began taking zidovudine twice daily at 34–36 weeks of pregnancy, and continued to do so according to a standard protocol [8, 9]. Written informed consent was obtained from all participants, and the study received ethical approval from the institutional review boards of the University of Washington and the University of Nairobi. Maternal blood was collected at 32 weeks of pregnancy to determine plasma HIV-1 RNA load. Plasma HIV-1 RNA levels were also measured at delivery. To determine HIV-1 status, infant blood was collected within 48 h of birth and at 1, 3, 6, 9, and 12 months of age.
HLA typing
DNA was extracted from maternal and infant peripheral blood mononuclear cells by use of Gentra Puregene reagents. High-resolution maternal HLA class I typing was conducted at the 4-digit level according to a sequence-specific oligonucleotide probe (SSOP) typing protocol developed by the 13th International Histocompatibility Workshop (http://www.ihwg.org/protocols/protocol.htm); alleles were determined by SSOP hybridization patterns after locus-specific polymerase chain reaction (PCR) amplification. Infant HLA class I typing was conducted at the 2-digit level with 4-digit typing performed when possible, using amplification refractory mutation system PCR with primers designed to characterize alleles expressed in East African cohorts [10]. HLA types are available from the authors on request.
Classification of HLA concordance and maternal homozygosity
HLA concordance was scored as the number of shared class I alleles. Because mothers and infants share at least 1 allele at each locus, pairs were matched at a minimum of 3 and a maximum of 6 alleles. Pairs were scored as having 2 matches at a locus if the mother was homozygous at that locus. Maternal homozygosity was scored as any versus none, according to whether the mother was homozygous at at least 1 class I locus.
Determination and timing of infant HIV-1 status
To ascertain infant HIV-1 status at birth and at each visit, PCR was used to detect HIV-1 gag DNA in filter paper blood specimens [11]. Infants were considered to be HIV-1 infected if 2 consecutive filter paper specimens were positive for HIV-1 DNA or if the result of the last available filter assay was positive. To determine more precisely the timing of infection, HIV-1 RNA levels were measured in plasma samples obtained before the first positive HIV-1 DNA filter paper by use of a Gen-Probe PCR assay sensitive for detection of HIV-1 subtypes A, C, and D, as described elsewhere [12]. Overall HIV-1 transmission included all infant infections before 1 year of age. In utero transmission was defined as infections occurring within 48 h of birth, and peripartum transmission was defined as infections occurring between 48 h after birth and 1 month of age [13]. Transmission via breast-feeding was defined as infections occurring between 1 month and 1 year of age and therefore did not include transmission via breast-feeding during the first postpartum month.
Data analysis
To analyze whether mother-infant HLA concordance and maternal homozygosity increased the risk of transmission overall and through breast-feeding, we used Cox proportional hazards regressions. Follow-up time was censored at the last visit at which an infant was found to be uninfected with HIV-1 or at 12 months, whichever was earlier. For breast milk transmission, we began calculating survival time after the first month of age and restricted the analyses to HIV-1—uninfected infants who were breast-fed after 1 month of age. Logistic regression was used to analyze in utero and peripartum HIV-1 infection. All analyses were specified a priori and were adjusted for log10 maternal plasma HIV-1 RNA load at 32 weeks of gestation.
RESULTS
Cohort characteristics
HLA data were available for 277 mothers and 199 infants. The 199 pairs with known maternal and infant HLA types were included in the concordance analyses, and all 277 pairs with maternal HLA data were assessed in the maternal homozygosity analyses. At 32 weeks of gestation, the median maternal CD4+ cell count and plasma HIV-1 RNA load for the 277 mothers were 431 cells/μL (interquartile range [IQR], 295–608 cells/μL) and 4.7 log10 copies/mL (IQR, 4.2–5.3 log10 copies/mL), respectively. At delivery, the median maternal viral load was 4.1 log10 copies/mL (IQR, 3.5– 4.8 log10 copies/mL) and was strongly correlated with maternal viral load antenatally (Pearson’s correlation coefficient, 0.67).
Of the 277 infants, 212 (77%) were breast-fed, and 204 (74%) were breast-fed after 1 month of age. During the first year, the mean and median breast-feeding durations were 7.8 and 8.5 months, respectively, and the median breast milk viral load at the last visit during breast-feeding was 2.6 log10 copies/mL (IQR, 2.0 –3.5 log10 copies/mL). Overall, 58 infants (21%) were infected during 12 months of follow-up. Of these infections, 21 (36%) were detected within 48 h of birth and were classified as in utero infections, 26 (45%) were detected between birth and 1 month of age and were classified as peripartum infections, and 11 (19%) were detected between 1 and 12 months of age and were attributed to breast-feeding. During follow-up, 26 (9%) of the infants died, of whom 9 (35%) were not infected with HIV-1. Of the living infants, 21 (8%) were lost to follow-up.
Mother-child HLA concordance and risk of vertical transmission of HIV-1
Of 199 pairs with concordance data, 6 (3%) shared 6 alleles, 25 (12%) had 5 matches, 59 (30%) had 4 matches, and 109 (55%) had 3 matches. Overall, transmission occurred among 3 (50%) of the pairs with 6 matches, 8 (32%) with 5 matches, 13 (22%) with 4 matches, and 20 (18%) with 3 matches (figure 1). There was a 1.3-fold increased risk of transmission with each consecutive level of concordance from 3 to 6 matched alleles, before and after adjustment for maternal viral load (hazard ratio [HR], 1.37 [95% confidence interval {CI}, 1.01–1.86]; P = .04) (adjusted HR [aHR], 1.32 [95% CI, 1.01–1.73]; P = .04). Increased concordance was also significantly associated with in utero transmission risk after adjustment for maternal viral load (odds ratio [OR], 1.67 [95% CI, 0.99 –2.81]; P = .054) (adjusted OR [aOR], 1.72 [95% CI, 1.01–2.94]; P = .04).
Figure 1.
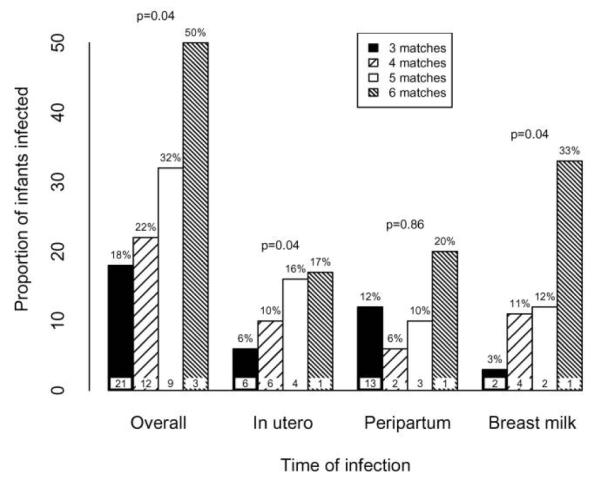
Proportion (%) of infants infected with HIV-1 for overall, in utero, peripartum, and breast-feeding transmission, according to the degree of mother-child HLA class concordance at the HLA-A, -B, and -C loci. Values in bars represent the nos. of infants in each concordance category who were infected during the specified period. P values were obtained using logistic and Cox regression models adjusted for maternal viral load. Cox regression was used to analyze overall and breast milk transmission, and logistic regression was used to analyze in utero and peripartum transmission.
Of pairs with concordance data, 125 infants were uninfected at 1 month of age and were breast-fed. Among these breast-feeding pairs, concordance was associated with HIV-1 transmission via breast-feeding in the unadjusted analysis and after adjustment for HIV-1 load at 32 weeks of gestation (HR, 1.94 [95% CI, 1.09 –3.48]; P = .02) (aHR, 1.59 [95% CI, 1.02–2.47]; P = .04). On the basis of this model, pairs with 4 matches experienced a 1.6-fold increased risk of transmission relative to pairs with 3 matches, pairs with 5 matches experienced a 2.6-fold increased risk, and pairs with 6 matches experienced a 4.1-fold increased risk. There was not a significant increase in peripartum transmission risk by HLA concordance.
The degrees of mother-infant concordance at the HLA-A, -B, and -C loci were evaluated separately to determine whether individual loci were associated with increased transmission risk in a model adjusted for maternal HIV-1 load. Transmission overall occurred among 12 (35%) of the pairs sharing 2 HLA-B alleles, whereas 32 infants (19%) were infected when only 1 HLA-B allele was shared (HR, 1.74 [95% CI, 0.93–3.27]; P = .08) (aHR, 1.96 [95% CI, 1.11–3.47]; P = .02) (table 1). In utero transmission occurred in 6 (18%) of the pairs who shared 2 HLA-B alleles and 11 (7%) of the pairs who shared a single HLA-B allele (OR, 3.00 [95% CI, 1.02– 8.80]; P = .045) (aOR, 3.28 [95% CI, 1.07–10.01]; P = .04). After adjustment for A and C locus concordance in addition to maternal viral load, there was still a trend toward increased overall transmission risk with B locus concordance (aHR, 1.94 [95% CI, 0.97–3.89]; P = .06). However, there were no differences in the risk of peripartum or breast-feeding transmission according to HLA-B concordance, and there were no significant associations between concordance at the HLA-A and -C loci and transmission risk at any time point.
Table 1.
Influence of HLA-A, -B, and -C loci concordance separately on overall HIV-1 transmission risk at any time before 1 year of age.
| HLA locus |
No. (%) infected with |
HR (95% CI) from |
||
|---|---|---|---|---|
| 2 matches at locus |
1 match at locus |
Models for each locus separatelya |
Multivariate modelb |
|
| A | 10 (26) | 34 (21) | 1.33 (0.70–2.54) | 1.26 (0.65–2.45) |
| B | 12 (35) | 32 (19) | 1.96 (1.11–3.47) | 1.94 (0.97–3.89) |
| C | 16 (29) | 28 (19) | 1.34 (0.77–2.36) | 0.98 (0.49–1.96) |
Hazard ratios (HR) and 95% confidence intervals (CIs) for the influence of HLA concordance at class I loci separately after adjusting for maternal viral load at 32 weeks of gestation, using a distinct Cox regression model for each locus.
Adjusted HRs and 95% CIs from a multivariate model that included HLA-A, -B, and -C concordance as well as maternal viral load.
Maternal HLA homozygosity association with vertical HIV-1 transmission
Two hundred twenty-nine (83%) of the mothers were heterozygous at the HLA-A, -B, and -C loci, and 48 (17%) were homozygous at 1 or more loci. At 32 weeks of gestation and at delivery, mothers with homozygosity at 1 or more loci had higher HIV-1 RNA loads than did mothers who were completely heterozygous. The median HIV-1 RNA level at 32 weeks of gestation was 4.8 log10 copies/mL (IQR, 4.2–5.3 log10 copies/mL) among heterozygous mothers and 5.1 log10 copies/mL (IQR, 4.4 –5.4 log10 copies/mL) among those who were homozygous at 1 or more loci (P = .03). A similar relationship was observed between HLA homozygosity and HIV-1 RNA levels at delivery (4.3 vs. 4.0 log10 copies/mL; P = .03).
Maternal HLA homozygosity increased HIV-1 transmission risk overall (figure 2). Transmission occurred at some time during the 12 months of follow-up among 41 pairs (18%) who were heterozygous at the HLA-A, -B, and -C loci, compared with 17 pairs (35%) in whom the mother was homozygous at 1 or more loci. In unadjusted analyses, infants of homozygous mothers experienced an ∼2-fold increased risk of overall transmission compared with infants of heterozygous mothers (HR, 1.9 [95% CI, 1.1–3.2]; P = .02). This association remained after adjustment for maternal plasma viral load at 32 weeks of gestation (aHR, 1.7 [95% CI, 1.1–2.8]; P = .03).
Figure 2.
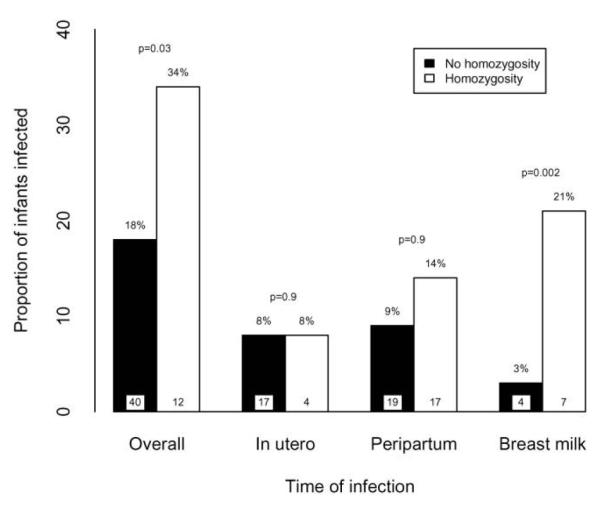
Proportions (%) of infants infected with HIV-1 for overall, in utero, peripartum, and breast-feeding transmission, according to the degree of maternal HLA class I homozygosity at the HLA-A, -B, and -C loci. Values in bars represent the nos. of infants in each homozygosity category who were infected during the specified period. P values were obtained using logistic and Cox regression models adjusted for maternal viral load. Cox regression was used to analyze overall and breast milk transmission, and logistic regression was used to analyze in utero and peripartum transmission.
Mothers who were homozygous at any HLA locus also more frequently transmitted HIV-1 to their infants via breast-feeding. Five (4%) of the heterozygous mothers transmitted HIV-1 to their infants, compared with 6 (21%) of the homozygous mothers. In the unadjusted analysis, the risk of transmission among homozygous mothers was increased >7—fold (HR, 7.4 [95% CI, 2.2–24.9]; P = .001). This association remained after adjustment for maternal plasma viral load at 32 weeks of gestation (aHR, 5.6 [95% CI, 1.8 –17.1]; P = .002).
When specific loci were evaluated, overall transmission was more frequent among mothers who were homozygous at the HLA-B or -C loci. HLA-B homozygosity was associated with a 1.8-fold increased transmission risk overall in unadjusted and adjusted analyses (HR, 1.9 [95% CI, 0.98 –3.78]; P = .06) (aHR, 1.8 [95% CI, 1.01–3.2]; P = .04). Homozygosity at the HLA-C locus was also associated with a 1.7-fold increased overall transmission risk in unadjusted and adjusted analyses (HR, 2.0 [95% CI, 1.12–3.57] P = .02) (aHR, 1.7 [95% CI, 1.04 –2.9]; P = .04). HLA-A homozygosity was not significantly associated with overall transmission. In a single model that included homozygosity at the HLA-A, -B, and -C loci separately, no significant associations were observed for transmission at any time.
DISCUSSION
In this cohort of breast-feeding women and their infants, mother-child HLA concordance was associated with increased risks of overall, in utero, and breast milk HIV-1 transmission. Additionally, maternal homozygosity was associated with the risk of transmission overall and via breast-feeding. The effects of HLA concordance on transmission appeared to derive predominantly from the HLA-B locus, but this is difficult to confirm with our data.
Increased susceptibility to vertical HIV-1 transmission due to mother-child HLA concordance has several possible biological mechanisms. Infants whose HLA is the same as their mothers may be less able to recognize HIV-1 that has evolved to evade maternal immune responses via HLA-mediated selection. Concordance might also decrease the likelihood of infant alloimmune responses against maternally derived lymphocytes. Among mother-infant pairs with more concordance, HLA molecules on the surface of HIV-1—infected or -uninfected maternal cells will be recognized as “self” by cytotoxic T lymphocytes or NK cells and will be less likely to be destroyed. Infant alloimmune responses may also target maternal HLA molecules incorporated into the HIV-1 envelope when virus buds from host cells [14]. Thus, increased concordance could restrict T cell destruction of cell-free HIV-1, in addition to reducing destruction of HIV-1—infected maternal cells.
Our observation that mother-child HLA concordance was associated with increased HIV-1 transmission risk is consistent with previous findings and adds to them by demonstrating that concordance is also associated with a significantly greater risk of breast milk HIV-1 transmission. A study of 125 mother-child pairs in Kenya without antiretroviral prophylaxis found that each additional concordant class I allele was associated with a 2.6-fold increased risk of perinatal infection [6]. A United States— based study in which mothers and infants were given zidovudine found a 4-fold increased transmission risk before 6 weeks of age among pairs who were completely concordant at 1 or more class I loci compared with pairs who shared only 3 alleles [7]. Neither of these studies found an association between HLA concordance and HIV-1 transmission via breast-feeding.
One strength of the present study is that the sampling schedule permitted more precise estimation of the timing of infection. Thus, infections could be classified as having been in utero, peripartum, or breast milk. The only other study of whether HLA concordance influences breast milk transmission classified infections occurring before 6 months of age as early and infections occurring after 6 months as being transmitted through breast-feeding. In our sample, nearly 50% of infections occurring through breast-feeding happened before the age of 6 months. These would have been classified as early infections by the criteria of MacDonald et al. and may have increased the likelihood of observing an association between concordance and early transmission rather than breast-feeding [6]. Another difference that may have increased our power to detect an association is that both of the previous studies used serologic HLA typing, whereas we used molecular-based typing, which is more specific. Serologically defined HLA alleles are broad, whereas many alleles that can be identified only by molecular techniques have distinct epitope-binding motifs.
In the present study, mothers with HLA homozygosity had higher plasma HIV-1 RNA loads, and any maternal homozygosity was associated with an increased risk of HIV-1 transmission overall and via breast-feeding. We are not aware of any studies that have specifically focused on HLA homozygosity and HIV-1 transmission. Previous studies have examined HLA homozygosity as it relates to HIV-1 disease progression [4, 5]. Carrington et al. [4] found that homozygosity at any HLA class I locus was associated with more rapid disease progression. One mechanism through which maternal homozygosity might increase vertical transmission risk is accelerated HIV-1 progression and higher viral loads in homozygous mothers. However, we observed a strong association between maternal HLA homozygosity and HIV-1 transmission overall and through breast-feeding, even after adjusting for maternal HIV-1 load, which suggests that the effect of maternal HLA homozygosity on transmission risk is not due only to its effect on maternal disease progression. Because this association is independent of viral load antenatally, another mechanism for the influence of maternal HIV-1 homozygosity on vertical transmission risk must be considered. It is possible that HIV-1—infected cells of homozygous mothers elicit a weaker alloimmune response in their infants, which in turn may lead to increased survival of maternal HIV-1—infected cells and increased risk of transmission.
In addition to associations between concordance at the HLA-A, -B, and -C loci together, there were associations with overall and in utero transmission for the HLA-B locus individually and with overall transmission after adjustment for A and C locus concordance. These results suggest that associations observed when concordance was classified according to the class I loci considered together may have been driven by the B locus. The importance of HLA-B alleles in HIV-1 transmission and disease progression has been demonstrated in past studies. First, a study of HIV-1—discordant couples in Zambia found that increased concordance at the HLA-B locus was associated with a 2-fold increased transmission risk [15]. In another study, Kiepiela et al. demonstrated a dominant influence of HLA-B alleles on CD8+ T cell responses against HIV-1 [16].
Our observation that HLA concordance and maternal homozygosity were associated with in utero and breast milk transmission but not with peripartum transmission might be explained by the roles played by cell-associated and cell-free virus in transmitting HIV-1 from mother to child. It is not well established whether cell-free or cell-associated virus is more influential in vertical HIV-1 transmission. However, in vitro data suggest that in utero transmission is caused more by cell-associated virus than by cell-free virus [17-19]. Additionally, cell-associated virus may be more readily transmitted through breast-feeding than cell-free virus, whereas intrapartum transmission may be caused by both types of virus [20, 21]. Thus, HLA concordance and homozygosity might have stronger associations with increased risks of in utero and breast milk transmission to the extent that alloimmune responses act primarily on cell-associated virus. The role played by HLA concordance in increasing vertical transmission of cell-associated virus via breast-feeding is further supported by findings that HLA concordance increased the risk of vertical transmission of human T cell lymphotrophic virus type 1, which is transmitted only via cell-to-cell interaction [22]. This adds to a growing body of evidence indicating that cell-associated HIV-1 is critically important for in utero and breast milk transmission [17-21].
There were several limitations to our study. First, because maternal and infant HLA typing was conducted in different laboratories, there is a possibility of measurement error when concordance scores are computed. Measurement error of this kind would most likely decrease the indicated number of shared alleles, biasing our results toward the null. Hence, the true effect of concordance on transmission could be even greater than the effects observed here. Second, because a limited number of transmission events occurred through breast-feeding, these analyses could have been underpowered. Third, our ability to determine the timing of infections, although very good compared with earlier studies, was nevertheless less than perfect. Late in utero infections and early infections via breast-feeding may have been misclassified as intrapartum infections because of the inability to differentiate the specific route of transmission in these cases.
In conclusion, mother-child HLA concordance and maternal HLA homozygosity increased the risk of HIV-1 infection in infants for overall, in utero, and breast-feeding transmission. When HLA-B concordance was considered individually, there is evidence that it increased the risk of in utero and overall transmission. These data are consistent with evidence in the literature indicating that HLA-mediated immune responses are important drivers of HIV-1 selection and HLA diversity, and they support the hypothesis that the HLA-B locus is particularly important. Our results also suggest a role for alloimmune responses in vertical transmission of HIV-1, which is intriguing because similar studies have indicated that concordance and alloimmune responses may also alter the risk of heterosexual HIV-1 transmission [14]. Further study of the role played by HLA class I and II and cellular alloimmune responses in both vertical and heterosexual HIV-1 transmission is warranted.
Acknowledgments
Financial support: US National Institute of Child Health and Development (grant HD23412) and National Institutes of Health (NIH) (grants K23 HD41879 to C.F. and KO1 TW06080 to B.L.P.). J.M. was a scholar in the AIDS International Training and Research Program, which is supported by the NIH/Fogarty International Center (grant D43 TW000007).
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Parham P, Ohta T. Population biology of antigen presentation by MHC class I molecules. Science. 1996;272:67–74. doi: 10.1126/science.272.5258.67. [DOI] [PubMed] [Google Scholar]
- 2.McMichael A, Klenerman P. HIV/AIDS: HLA leaves its footprints on HIV. Science. 2002;296:1410–1. doi: 10.1126/science.1072492. [DOI] [PubMed] [Google Scholar]
- 3.Moore CB, John M, James IR, Christiansen FT, Witt CS, Mallal SA. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science. 2002;296:1439–43. doi: 10.1126/science.1069660. [DOI] [PubMed] [Google Scholar]
- 4.Carrington M, Nelson GW, Martin MP, et al. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science. 1999;283:1748–52. doi: 10.1126/science.283.5408.1748. [DOI] [PubMed] [Google Scholar]
- 5.Tang J, Costello C, Keet IP, et al. HLA class I homozygosity accelerates disease progression in human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 1999;15:317–24. doi: 10.1089/088922299311277. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald KS, Embree J, Njenga S, et al. Mother-child class I HLA concordance increases perinatal human immunodeficiency virus type 1 transmission. J Infect Dis. 1998;177:551–6. doi: 10.1086/514243. [DOI] [PubMed] [Google Scholar]
- 7.Polycarpou A, Ntais C, Korber BT, et al. Association between maternal and infant class I and II HLA alleles and of their concordance with the risk of perinatal HIV type 1 transmission. AIDS Res Hum Retroviruses. 2002;18:741–6. doi: 10.1089/08892220260139477. [DOI] [PubMed] [Google Scholar]
- 8.Farquhar C, Rowland-Jones S, Mbori-Ngacha D, et al. Human leukocyte antigen (HLA) B*18 and protection against mother-to-child HIV type 1 transmission. AIDS Res Hum Retroviruses. 2004;20:692–7. doi: 10.1089/0889222041524616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thaineua V, Sirinirund P, Tanbanjong A, Lallemant M, Soucat A, Lamboray JL. From research to practice: use of short course zidovudine to prevent mother-to-child HIV transmission in the context of routine health care in northern Thailand. Southeast Asian J Trop Med Public Health. 1998;29:429–42. [PubMed] [Google Scholar]
- 10.Bird TG, Kaul R, Rostron T, et al. HLA typing in a Kenyan cohort identifies novel class I alleles that restrict cytotoxic T-cell responses to local HIV-1 clades. AIDS. 2002;16:1899–904. doi: 10.1097/00002030-200209270-00006. [DOI] [PubMed] [Google Scholar]
- 11.Panteleeff DD, John G, Nduati R, et al. Rapid method for screening dried blood samples on filter paper for human immunodeficiency virus type 1 DNA. J Clin Microbiol. 1999;37:350–3. doi: 10.1128/jcm.37.2.350-353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emery S, Bodrug S, Richardson BA, et al. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J Clin Microbiol. 2000;38:2688–95. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryson YJ, Luzuriaga K, Sullivan JL, Wara DW. Proposed definitions for in utero versus intrapartum transmission of HIV-1. N Engl J Med. 1992;327:1246–7. doi: 10.1056/NEJM199210223271718. [DOI] [PubMed] [Google Scholar]
- 14.Arthur LO, Bess JW, Jr, Sowder RC, II, et al. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science. 1992;258:1935–8. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- 15.Dorak MT, Tang J, Penman-Aguilar A, et al. Transmission of HIV-1 and HLA-B allele-sharing within serodiscordant heterosexual Zambian couples. Lancet. 2004;363:2137–9. doi: 10.1016/S0140-6736(04)16505-7. [DOI] [PubMed] [Google Scholar]
- 16.Kiepiela P, Leslie AJ, Honeyborne I, et al. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature. 2004;432:769–75. doi: 10.1038/nature03113. [DOI] [PubMed] [Google Scholar]
- 17.Lagaye S, Derrien M, Menu E, et al. Cell-to-cell contact results in a selective translocation of maternal human immunodeficiency virus type 1 quasispecies across a trophoblastic barrier by both transcytosis and infection. J Virol. 2001;75:4780–91. doi: 10.1128/JVI.75.10.4780-4791.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth WW, Zuberi JA, Stringer HG, Jr, Davidson SK, Bond VC. Examination of HIV type 1 variants in mother-child pairs. AIDS Res Hum Retroviruses. 1996;12:925–30. doi: 10.1089/aid.1996.12.925. [DOI] [PubMed] [Google Scholar]
- 19.Scarlatti G, Leitner T, Hodara V, et al. Neutralizing antibodies and viral characteristics in mother-to-child transmission of HIV-1. AIDS. 1993;7(Suppl 2):S45–8. doi: 10.1097/00002030-199311002-00010. [DOI] [PubMed] [Google Scholar]
- 20.Koulinska IN, Villamor E, Chaplin B, et al. Transmission of cell-free and cell-associated HIV-1 through breast-feeding. J Acquir Immune Defic Syndr. 2006;41:93–9. doi: 10.1097/01.qai.0000179424.19413.24. [DOI] [PubMed] [Google Scholar]
- 21.Rousseau CM, Nduati RW, Richardson BA, et al. Association of levels of HIV-1—infected breast milk cells and risk of mother-to-child transmission. J Infect Dis. 2004;190:1880–8. doi: 10.1086/425076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biggar RJ, Ng J, Kim N, et al. Human leukocyte antigen concordance and the transmission risk via breast-feeding of human T cell lymphotropic virus type I. J Infect Dis. 2006;193:277–82. doi: 10.1086/498910. [DOI] [PubMed] [Google Scholar]


