Abstract
Background Numerous studies have documented a strong inverse association between cardiovascular disease and socioeconomic position (SEP). Several infections are associated with both cardiovascular disease and SEP; hence infection may form an important link between SEP and cardiovascular disease. This study examines whether seropositivity to cytomegalovirus (CMV), to herpes simplex virus type-1 (HSV-1), and/or to both pathogens mediates the relationship between SEP and cardiovascular disease history in a nationally representative sample of the United States.
Methods We conducted a cross-sectional study of subjects ≥45 years of age, who were tested for seropositivity to CMV, HSV-1 or both pathogens and assessed for cardiovascular disease history in the National Health and Nutrition Examination Survey III. Cardiovascular disease history was defined as history of stroke, heart attack and/or congestive heart failure and SEP as education level.
Results SEP was associated with CMV, HSV-1 and seropositivity to both pathogens. CMV seropositivity was associated with cardiovascular disease history even after adjusting for confounders as well as SEP. The odds of reporting a history of cardiovascular disease for those with less than a high school education compared with those with more than a high school education decreased by 7.7% after adjusting for CMV (Sobel mediation test for CMV, P = 0.0006). In contrast, neither seropositivity to HSV-1 nor to both pathogens was associated with cardiovascular disease history after adjusting for SEP.
Conclusions Persistent pathogens such as CMV infection may explain a portion of the relationship between SEP and cardiovascular disease in the United States. Further studies examining additional pathogens and sociobiological mechanisms are warranted.
Keywords: SEP, CMV, HSV-1, co-infection, cardiovascular disease, mediation
Introduction
The prevalence of cardiovascular disease has declined over the past 20 years.1–3 Nevertheless, marked differences in cardiovascular disease incidence across socioeconomic position (SEP) still persist.4–7 For example, poverty, education and income status have been linked to reported heart disease, ischaemic heart disease, hypertension and stroke in the United States4,5,8,9 Despite the established relationship between lower SEP and traditional cardiovascular disease risk factors such as smoking, physical inactivity, higher body mass index (BMI) (kg/m2) and higher levels of low-density lipoprotein cholesterol, the association between SEP and cardiovascular disease has not been fully explained in studies adjusting for traditional risk factors.7,8,10–12 For example, the percentage difference in the odds ratio (OR) for coronary heart disease comparing low vs high income categories after adjusting for smoking, alcohol, physical inactivity and BMI (kg/m2), was only reduced by 13% for men and 29% for women, compared with unadjusted models in a study using data from the Finnish Public Sector Study.7 While recent work has highlighted the fact that traditional cardiovascular disease risk factors likely explain the majority of absolute SEP differences in cardiovascular disease, novel risk factors such as infection, may help explain remaining absolute and relative differences in cardiovascular disease by SEP.13–16
There are several reasons why lifelong persistent viral infections such as cytomegalovirus (CMV) and Herpes Simplex Virus-1 (HSV-1) may represent a potential mechanism linking SEP and increased cardiovascular disease risk.17–20 First, animal models have shown that these life-long persistent pathogens may cause pathological damage to cardiovascular tissue by acting as pro-inflammatory stimuli or by directly invading the cardiovascular tissue which contributes to endothelial tissue damage in the vasculature.21–24 It has been hypothesized that these pathogen related pro-inflammatory mechanisms may contribute to cardiovascular disease in human populations.25–29 Although conclusions regarding the association between individual pathogens and cardiovascular disease in human populations have been conflicting,30 several studies examining the effect of co-infection with multiple pathogens on cardiovascular disease have identified consistent positive relationships between increased number of infections and cardiovascular disease.29,31–35 These data suggest that total pathogen burden may have an even greater impact on cardiovascular disease events than the effects of individual pathogens.
Other evidence supporting a potential link between SEP, infection and cardiovascular disease, includes the reported sociodemographic patterning of the implicated pathogens. CMV and HSV-1 are highly prevalent infections often contracted at a young age, with CMV and HSV-1 found in >64 and 72% of individuals 40 and older in the US, respectively.36–38 Cardiovascular disease is one of the most common chronic health conditions of ageing and has been conceptualized as a life-course process that includes multiple insults and alterations to the vascular system starting from childhood.39,40 Of note, there are overlapping demographic and SEP gradients in seropositivity to CMV and HSV-1 and cardiovascular disease risk, such that non-white race, and lower education and income are associated with greater likelihood of IgG seropositivity and also with cardiovascular disease risk.4,5,37,38,41–46
Last, persistent infections have been identified as contributing to an immune risk phenotype present in the elderly.47,48 For example, ageing populations infected with CMV experience large clonal expansion of CD8+ effector T-cells resulting in a reduction in ‘immunological space’ and a loss of response to foreign antigens other than CMV.49–52 Thus infection with CMV, the resulting oligoclonal expansion and inflammatory response may potentially influence the development of inflammation-related chronic conditions associated with ageing such as cognitive and physical impairment as well as cardiovascular disease.53–55
Therefore, the purpose of this study is to examine whether individual and/or combined seropositivity to CMV and HSV-1 partially mediates the relationship between SEP and cardiovascular disease in a US nationally representative sample.
Methods
Study population
Data come from the National Health and Nutrition Examination Survey (NHANES) III (1988–1994); a population-based, multistage stratified probability survey which collects information on the health and nutrition of the United States, civilian, non-institutionalized population. The survey was carried out by the National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention, from 1988 to 1994 and is meant to be representative of the US population.
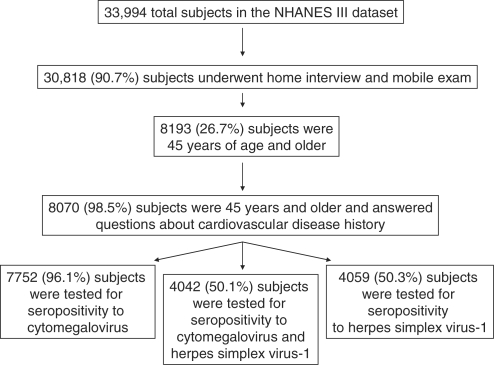
The derivation of our study sample is elucidated in Figure 1. Our study sample was first narrowed down by necessity to those that underwent the home interview and participated in the mobile examination (e.g. clinical examination), as data on demographics and cardiovascular disease history were elicited during the home exam and seropositivity testing was conducted among those that had sera drawn during the mobile examination. A total of 30 818 subjects (90.7%) participated among 33 994 in both the home interview and mobile examination. Those that did not undergo both the interview and the examination were older than those who participated in both components. Of the 30 818 subjects undergoing both components, we limited our study sample to those subjects that were 45 years of age and older [N = 8193 (26.7%)]. Of those 45 years of age and older, 8070 (98.5%) answered all three questions about history of cardiovascular disease. There were no differences according to age, gender, race, education level, smoking status or diabetes status between those that answered all three cardiovascular disease questions and those that did not among those 45 years of age and older.
Figure 1.
Derivation of study sample
Of the 8070 subjects that were 45 years of age or older and answered all cardiovascular disease history questions, 7752 (96.1%) were randomly selected to be tested for CMV seropositivity, 4059 (50.3%) for HSV-1 seropositivity and 4042 (50.1%) for seropositivity to both HSV-1 and CMV. Sera were collected from 95% of persons examined and sera samples for seropositivity testing were randomly selected, with sample size determined by various investigators making the request for each particular test. Although available sera from subjects were selected randomly for CMV and HSV-1 testing, by chance, available sera from non-Hispanic/blacks and from subjects of older age were less likely to be selected for CMV testing, available sera from non-Hispanic/blacks were less likely to be selected for HSV testing and available sera from subjects of younger age were less likely to be selected for testing for both pathogens.
Laboratory analyses
CMV specific IgG was measured by a commercially available Enzyme Linked Immunosorbent Assay (ELISA) (Quest International, Inc., Miami, FL). Sera with values near the ELISA cutoff were confirmed with a second ELISA assay (bioMerieux, Inc., Durham, NC). If the results from the first two tests disagreed, an Immunofluorescence Assay (IFA) (Bion International, Inc., Park Ridge, IL), was used and results from this test were provided as the final seropositivity test result. The sensitivity and specificity of these tests have been estimated to be 98 and 99%, respectively.38 HSV-1 seropositivity was assessed by solid-phase enzymatic immunodot assays using purified glycoprotein gG-1 of HSV-1 as the antigen.37 These immunodot assays have also been shown to have high sensitivity and specificity.56
Measures
Results from each of the CMV and HSV-1 IgG antibody tests were dichotomized as seronegative or seropositive based on the ELISA results. Seropositivity to both CMV and HSV-1 was dichotomized into seropositivity to 0–1 vs 2 pathogens. History of cardiovascular disease was recorded by asking whether a doctor ever told participants that they had congestive heart failure, a stroke or a heart attack. History of one or more of these conditions indicated a history of cardiovascular disease and the outcome was dichotomized as no history or history of one or more events.
SEP was measured as years of education and then categorized into completion of less than high school, high school or greater than high school education. The mean age of our sample was 61.6 years of age and consequently, many subjects may be in or nearing retirement, rendering income a less sensitive marker of life-course SEP.57,58 Therefore, we considered education level to be a more robust marker of lifetime SEP than current income for this study population and less subject to temporal ambiguity.
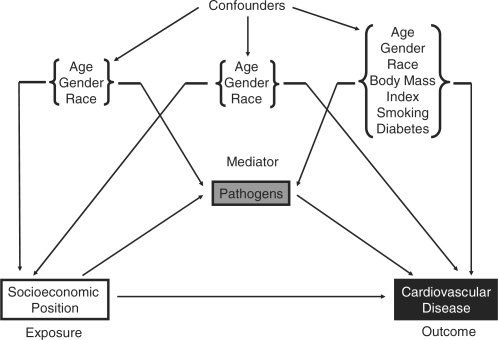
Covariates hypothesized to be potential confounders of the relationship between SEP and cardiovascular disease and between SEP and pathogens included age, gender and race (Figure 2).41–43 These same factors, along with BMI (kg/m2), smoking and diabetes were considered to be potential confounders of the relationship between each pathogen and cardiovascular disease because these factors have been shown to predict both risk for infection and for cardiovascular disease (Figure 2).20,43,59–63 Age was categorized into 45–54 years of age, 55–64 years of age and 65 years and older. Gender was dichotomized as female and male. Race/ethnicity was self-reported as non-Hispanic white, non-Hispanic black, Mexican-American or Other. BMI (kg/m2) was computed from weight and standing height using and categorized as BMI <25, 25 ≥ BMI < 30 and BMI ≥30. Smoking status was self-reported and categorized into never, past and current smoker. Diabetes was self-reported as whether a doctor ever informed subjects they had diabetes or not and dichotomized as reported or not reported.
Figure 2.
Potential confounders in the relationships between SEP, pathogens and cardiovascular disease
Statistical analyses
Statistical analyses were performed using SAS, version 9.1, with SAS-callable SUDAAN, version 9.0 (SAS Institute, Inc., Cary, NC). All analyses used appropriate weights and adjustments for strata and clustering used in the complex study design provided by the NCHS. Bivariate relationships between SEP and seropositivity to each pathogen as well as both pathogens and cardiovascular disease and potential confounders including age, gender, race, education level, smoking status and diabetes status were assessed. T-tests for difference in means and Wald chi-square tests for differences in proportions among demographic groupings were calculated. All significance tests were two-tailed.
Logistic regression was used to calculate ORs and 95% confidence intervals (CIs) and mediation was assessed by examining beta estimates generated from testing the following relational criteria established by Baron and Kenny:64,65 (i) Is SEP associated with cardiovascular disease history, even after controlling for confounders?; (ii) Is SEP is associated with seropositivity to each pathogen as well as both pathogens, even after controlling for confounders?; (iii) Is seropositivity to each pathogen and/or both pathogens a predictor of cardiovascular disease history after controlling for SEP and confounders?; and (iv) Is the effect of SEP on cardiovascular disease history attenuated after controlling for seropositivity to each pathogen and/or both pathogens as well as other confounders? If seropositivity to individual pathogens and/or both pathogens met the first three criteria and the association between SEP and cardiovascular disease history was reduced, but did not reach the null value, then seropositivity to individual pathogens and/or both pathogens was considered a partial mediator of the relationship. The Sobel test was used to calculate a critical ratio and a P-value to test whether the indirect effect of SEP on cardiovascular disease history explained by pathogen seropositivity is different from the null value.64,65
Last, we estimated the percentage of all cases of cardiovascular disease history among the study population that would not have occurred if CMV infection was eliminated,66 i.e. the attributable fraction (AF), using the following formula: AF = p(RR−1)/p(RR−1)−1.67 In this formula, p = the proportion seropositive for CMV and RR = the relative risk of CVD history, given CMV seropositivity.68 This formula was used after correcting the adjusted OR, using the following formula: RR = OR/(1−P0) + (P0 × OR), to obtain the appropriate risk ratio. In the formula, RR = the risk ratio, P0 = the incidence of CVD history in those seronegative to CMV, P1 = the incidence of CVD history in those seropositive for CMV and OR = the OR for CVD history comparing those seropositive for CMV to those that are seronegative.68 Given that the prevalence of cardiovascular disease history is generally >10% in this age cohort, the risk ratio is a better approximation of the relative risk than the OR and can be substituted in the above formula for the attributable fraction.67,68
Results
Weighted estimates of the bivariate relationships between covariates of interest and cardiovascular disease history for all 8070 subjects assessed for history of cardiovascular disease, age 45 and older are shown in Table 1. Of subjects assessed for cardiovascular disease, the population estimate for history of cardiovascular disease was 15.7%, and increased age, male gender, non-Hispanic black race, current smoking and self-reported diabetes (Table 1). Approximately 87.9% (N = 6811/7752) of those tested for CMV and 86.2% (N = 3499/4059) tested for HSV-1 were seropositive (data not shown). Of those tested for both CMV and HSV-1, 77.1% were seropositive to both pathogens, 19.0% were seropositive to one of the pathogens and 4.0% were seronegative to both (data not shown).
Table 1.
Demographic and clinical characteristics by cardiovascular disease history for subjects age 45 and older in NHANES III
| History of cardiovascular disease (N = 8070) |
|||
|---|---|---|---|
| No history | History | ||
| 6805 (84.30) | 1265 (15.70) | P-valuea | |
| Characteristics | |||
| Age | |||
| Mean ± SE | 60.62 ± 0.29 | 68.32 ± 0.49 | <0.0001 |
| Category (years), N (%) | |||
| 45–54 | 1839 (95.38) | 106 (4.62) | <0.0001 |
| 55–64 | 1838 (89.15) | 257 (10.85) | |
| ≥65 | 3128 (79.65) | 902 (20.35) | |
| Gender, N (%) | |||
| Female | 3619 (89.28) | 571 (10.72) | <0.0001 |
| Male | 3186 (85.32) | 694 (14.68) | |
| Race/Ethnicity, N (%) | |||
| Non-Hispanic White | 3594 (87.69) | 708 (12.31) | 0.0666 |
| Non-Hispanic Black | 1545 (84.65) | 291 (15.35) | |
| Mexican-American | 1406 (88.82) | 228 (11.18) | |
| Other | 260 (87.93) | 38 (12.07) | |
| BMI (kg/m2)b, N (%) | |||
| ≤24 | 2346 (88.90) | 407 (11.10) | 0.1014 |
| ≥25 to ≤30 | 2588 (87.18) | 499 (12.82) | |
| ≥30 | 1851 (86.15) | 348 (13.85) | |
| Smoking status, N (%) | |||
| Never | 3097 (89.71) | 500 (10.29) | 0.0003 |
| Former | 2275 (85.04) | 540 (14.96) | |
| Current | 1433 (87.21) | 225 (12.79) | |
| Diabetesc, N (%) | |||
| No | 6018 (89.18) | 959 (10.82) | <0.0001 |
| Yes | 779 (71.43) | 305 (28.57) | |
Cardiovascular disease history defined as history of stroke, congestive heart failure and/or heart attack. Diabetes defined as a doctor ever telling subjects they had diabetes. Total number for BMI was reduced by 31 subjects and for diabetes was reduced by 9 subjects, due to missing data.
aP-value based on an independent samples t-test or chi-square tests for differences in proportions.
bN = 8039.
cN = 8061.
The association between education level and cardiovascular disease is shown in Table 2. Among those assessed for cardiovascular disease, those that completed high school or less than high school compared with those with more than a high school education had greater odds of cardiovascular disease history, adjusting for confounders such as age, gender and race. The associations between education level and seropositivity to each pathogen as well as both pathogens are shown in Table 3. Less education was also associated with increased odds of seropositivity to CMV, HSV-1 and both pathogens compared with those with the highest level of education, even after controlling for confounders such as age, gender and race. Table 4 shows the associations between seropositivity to CMV, HSV-1 and both pathogens and cardiovascular disease history, controlling for education and confounders including age, gender and race. We did not control for BMI (kg/m2), diabetes or smoking in this relationship because despite being hypothesized as potential confounders, none of these covariates were associated with seropositivity and furthermore BMI (kg/m2) was not associated with CVD history among the unexposed in our study sample. After controlling for education in addition to confounders, CMV was the only pathogen that remained associated with cardiovascular disease history (Table 4). The association between education and cardiovascular disease history, after controlling for CMV seropositivity is shown in Table 5. The association between education and cardiovascular disease history was attenuated by 7.7% after controlling for confounders and CMV seropositivity. The Sobel test statistic for mediation was 4.0, P = 0.0006, suggesting that the indirect influence of education on cardiovascular disease as mediated by CMV seropositivity is different from the null value. Last, the attributable fraction for the percentage of CVD prevalence attributable to CMV seropositivity was 0.40.
Table 2.
Association between SEP and cardiovascular disease history in the NHANES III
| History of cardiovascular disease |
|||
|---|---|---|---|
| Model 1a OR (95% CI) | Model 2b OR (95% CI) | ||
| Education | |||
| Greater than high school | 1.0 | 1.0 | |
| High school | 1.20 (0.91–1.59) | 1.20 (0.93–1.55) | |
| Less than high school | 2.33 (1.89–2.87) | 1.83 (1.49–2.26) | |
Cardiovascular disease history defined as history of stroke, congestive heart failure and/or heart attack. This table shows the odds of cardiovascular disease history for those with high school and those with less than high school education compared with those with greater than high school education. The total N for models 1 and 2 was reduced by 56 subjects due to missing data on education.
aModel 1 unadjusted (N = 8014).
bModel 2 adjusted for age, gender and race (N = 8014).
Table 3.
Association between SEP and pathogens in NHANES III
| Seropositivity |
|||
|---|---|---|---|
| Cytomegalovirusa OR (95% CI) | HSV-1b OR (95% CI) | Number of pathogensa OR (95% CI) | |
| Education | |||
| Greater than high school | 1.0 | 1.0 | 1.0 |
| High school | 1.71 (1.37–2.14) | 1.45 (1.00–2.10) | 1.82 (1.44–2.30) |
| Less than high school | 3.22 (2.52–4.11) | 3.32 (2.22–4.95) | 3.95 (2.81–5.55) |
Number of pathogens was dichotomized as seropositivity to 0–1 pathogens vs 2 pathogens. This table shows the odds of seropositivity to CMV and HSV-1 vs seronegativity and odds of seropositivity to 2 pathogens vs 0–1 pathogens, for those with high school and those with less than high school education compared with those with greater than high school education. The total N was reduced by 53 subjects for the model with CMV and by 24 subjects for both the models with HSV-1 and number of pathogens due to missing data on education.
aModel with CMV (N = 7699) and number of pathogens (N = 4035) adjusted for age, gender and race.
bModel with HSV-1 adjusted for age and race (N = 4018).
Table 4.
Association between pathogen seropositivity and history of cardiovascular disease, controlling for SEP
| History of cardiovascular disease OR (95% CI) | |
|---|---|
| Cytomegalovirusa | |
| Seronegative | 1.0 |
| Seropositive | 1.75 (1.21–2.54) |
| Herpes Simplex Virus-1b | |
| Seronegative | 1.0 |
| Seropositive | 1.37 (0.87–2.18) |
| Number of pathogensa | |
| 0–1 | 1.0 |
| 2 | 1.51 (0.99–2.31) |
Cardiovascular disease history defined as history of stroke, congestive heart failure and/or heart attack. Number of pathogens defined as seropositivity to 0–1 pathogens vs 2 pathogens. This table shows the odds of cardiovascular disease history for those seropositive vs seronegative for CMV and HSV-1 and for seropositivity to 2 pathogens vs 0–1. The total N for the model with CMV was reduced by 53 subjects and for the models with HSV-1 and number of pathogens was reduced by 24 subjects due to missing data on education.
aModel with CMV (N = 7699) and total number of pathogens (N = 4018) adjusted for age, gender, race and education.
bModel with HSV-1 adjusted for age, race and education (N = 4035).
Table 5.
Mediation of the association between SEP and cardiovascular history, by CMV seropositivity in the NHANES III
|
History of cardiovascular disease |
||||
|---|---|---|---|---|
| Model 1a OR (95% CI) | Model 2b OR (95% CI) | Sobel test statistic | P-value | |
| Education | ||||
| Greater than high school | 1.0 | 1.0 | ||
| High school | 1.20 (0.93–1.55) | 1.16 (0.91–1.50) | 4.00 | 0.0006 |
| Less than high school | 1.83 (1.49–2.26) | 1.69 (1.38–2.08) | ||
Cardiovascular disease history defined as history of stroke, congestive heart failure and/or heart attack. This table shows the odds of cardiovascular disease history for those with high school and less than high school education compared with those with greater than high school education, after controlling for CMV seropositivity. The total N for models 1 and 2 was reduced by 53 subjects due to missing data on education.
aModel 1 adjusted for age, gender and race (N = 7699).
bModel 2 adjusted for age, gender, race and CMV seropositivity (N = 7699).
Discussion
This study examined whether seropositivity to CMV, HSV-1 and/or both pathogens partially mediates the relationship between SEP and cardiovascular disease in a nationally representative sample of US adults. The results of our study suggest a consistent cross-sectional association between education and seropositivity for CMV and HSV-1, as well as both pathogens, even after controlling for age, gender and race. CMV seropositivity was associated with cardiovascular disease history after controlling for age, gender, race and education level. In addition, CMV seropositivity partially mediated the relationship between SEP and cardiovascular disease history, reducing the relative SEP inequality in history of cardiovascular disease by 7.7%. In contrast, neither seropositivity to HSV-1 nor to both pathogens was associated with cardiovascular disease history after controlling for confounders and SEP. These findings suggest a potential role for CMV in the SEP and cardiovascular disease relationship.
Although the direction of the association between HSV-1 seropositivity as well as seropositivity to both pathogens and cardiovascular disease history in this study is consistent with the hypothesis that seropositivity increases odds of cardiovascular disease history, after adjusting for confounders, the 95% CI for the ORs contained the null value. It is possible that the smaller sample sizes available for assessing these associations and the complex survey design lowered the precision of the estimates for these outcomes compared with CMV making it difficult to detect an association. We conducted a post hoc power analysis using the methods described by Hsieh et al.69 for logistic regression. The logistic regression of CMV on CVD with our sample size of 7699 observations provided us with 99% power at the 0.05 significance level to detect an OR = 1.75 or larger. In contrast, the logistic regression of HSV on CVD with a sample size of 4035 observations provided only 37% power at a 0.05 significance level to detect an OR = 1.37 or larger. For both pathogens together, we had a power of 65% to detect an OR = 1.51 or greater. Thus, we were underpowered to detect smaller ORs for the relationship between HSV and CVD in comparison with CMV.
Nevertheless, the lack of association between HSV-1 seropositivity and cardiovascular disease is consistent with conflicting results of studies assessing the role of HSV-1 and chronic inflammation-mediated conditions, including incident cardiovascular disease.19,53,55,70 While HSV-1 may not play a direct role in the development of cardiovascular disease, it may be correlated with the presence of other pathogens related to cardiovascular disease processes, such as CMV, which might explain why HSV-1 sometimes appears to be associated with inflammation-related disease outcomes and other times does not.
There are several plausible biologic reasons why CMV might play a more important role in cardiovascular disease than other pathogens. CMV is more likely to directly invade the vasculature than HSV-1 and serves to accelerate atherosclerosis by causing cell proliferation and increased expression of oxidized low density lipoprotein scavenger receptors on macrophages.71–73 As a result, macrophages can transition to foam cells and form fatty streaks in the intimal lining of the coronary arteries, thereby accelerating atherosclerotic processes.25–27,29,73 Moreover, CMV has been associated with immunosenescence, a process in which cell-mediated immunity wanes, clonal T-cell expansion occurs and as a result, there is an increase in inflammatory mediators such as IFN-γ and TNF-α which may also contribute to endothelial tissue damage and the atherosclerotic process.50,74–76 For these reasons, CMV may play a unique role in the pathogenesis of cardiovascular disease, explaining the stronger association to history of cardiovascular disease than HSV-1 observed in this study.
The mechanisms accounting for higher CMV seroprevalence among those of lower SEP are beginning to be explored, and may be due to earlier and/or increased exposure or increased likelihood of transmission due to adverse living conditions such as overcrowding.20 It is also possible that lower SEP increases susceptibility to primary infection through nutritional deficiencies or stress-related immune dysfunction.77–79 Earlier exposure to and increased reactivation of CMV over the lifetime among low SEP individuals may result in higher levels of circulating inflammatory molecules implicated in cardiovascular disease in adulthood.
To our knowledge, this is the first study to examine the potential pathways between SEP, CMV, HSV-1 and history of cardiovascular disease in a population-based representative sample of the US. An earlier study by Steptoe et al.80 assessed whether seropositivity to CMV, HSV-1, and Chlamydia pneumoniae individually as well as combined pathogen burden mediated the relationship between grade of employment and various ‘risk factors’ for coronary heart disease. Their results showed that grade of employment was associated with CMV seropositivity and combined pathogen burden. In addition, they found that combined pathogen burden was associated with greater BMI (kg/m2), higher waist/hip ratio, increased prevalence of diabetes and higher systolic and diastolic blood pressure.80 In contrast to our study, the purpose of their study was to examine whether pathogen burden mediated the relationship between grade of employment and cardiovascular disease ‘risk factors’ rather than cardiovascular disease events. Moreover, they did not examine whether CMV seropositivity in particular was related to each cardiovascular disease ‘risk factor’ so it is not possible to compare their results directly to our findings. We add to this earlier study by investigating the relationship between SEP, individual pathogens, co-infection with both CMV and HSV-1, and cardiovascular disease event history. Further research on the mechanisms by which each of these pathogens may influence cardiovascular disease, including potential pathogen specific effects on traditional risk factors for cardiovascular disease is warranted.
Despite the fact that the proportion of the relationship between SEP and cardiovascular disease, explained by CMV seropositivity may be relatively small compared with that explained by traditional risk factors (13–29%),7 if CMV were to be identified as causally related to CVD, it may be possible to eliminate ∼40% of cardiovascular disease prevalence as calculated by the attributable fraction.67 A major assumption of the attributable fraction is that the exposure disease relationship is causal.81 Indeed, this is still a highly debated topic in the CMV and CVD literature and most research supports an indirect effect of CMV on CVD via inflammation and autoimmunity rather than direct pathological damage from infection.25 Therefore, it is possible that CMV is merely associated with CVD but not causally related to CVD. Another caveat of the attributable fraction is the need for an intervention that would actually lead to the immediate abatement of CMV bringing the level of exposure to that in the unexposed group.81
In addition, CMV would need to be an independent causal factor not related to other risk factors for CVD, so that one could legitimately alter CMV exposure without any effect on the distribution of other risk factors for CVD.81 In the case of CMV, it is realistic to conceive that we could attain ‘unexposed’ status among those currently exposed through primary prevention measures such as vaccination without necessarily altering other conventional CVD risk factors, such as smoking and diabetes. Development of a vaccine for CMV has been considered a top priority of the institute of medicine.82 However, CMV vaccines have been under development for the past 30 years and have yet to be used in practice because the target population is unclear and there have been difficulties in specifying immunological components and endpoints.82,83 A few vaccination studies of mothers and children have been conducted and future studies should focus on following vaccinated and unvaccinated cohorts for incidence of CVD.84 CMV vaccination presents itself as a strong candidate for intervention if it is ultimately found to be causally related to CVD.
However, vaccination should not be the only focus since it may be possible to garner larger reductions in CMV by shifting macro-level determinants of exposure and susceptibility, such as reducing poverty, psychosocial stress, substandard housing and crowding. Indeed, the force of CMV infection (e.g. instantaneous per capita rate of CMV acquisition) has been found to be three times higher in lower income vs higher income households in the US.85 The independent exposure assumption is an inherent limitation of the attributable fraction since it often necessitates a proximate focus on intervention targets to the detriment of macro-level determinants that shape the overall population distribution of disease.81 Also, the attributable fraction should be considered along with the extent of the exposure since a very broad exposure definition can increase the attributable fraction.81,86 Using data from NHANES, earlier research has shown that the basic reproductive rate is 1.7, indicating that on average an infected person transmits CMV to almost two susceptible individuals.85 CMV is highly prevalent among participants in our nationally representative study population, therefore the prevalence is likely to result in a large attributable fraction compared with less prevalent exposures.86 Finally, depending on the combinations of risk factors examined, it is possible for the attributable risk to sum to >100%.87
This study was cross-sectional and therefore caution should be taken when making temporal inferences. IgG antibodies may reflect past infection that occurred as recently as a month ago to many years previous and measures of IgM antibodies are necessary for distinguishing between recent and past infection.88 Nevertheless, nationally representative studies have shown that the majority of primary CMV and HSV-1 infections occur in childhood, suggesting that CMV infection is likely to biologically precede cardiovascular disease events in the majority of cases.37,38 For example, the average age of first heart attack is 66 years, while the seroprevalence of CMV infection among persons ages 30–40 is already 54.3% and over 80% by the 6th decade of life.1,38 Therefore, the proportion of individuals acquiring CMV infection subsequent to their first cardiovascular disease event would be quite small. Future prospective studies following individuals without cardiovascular disease history at baseline can help to further minimize this potential bias.
Temporal ambiguity is also a concern when examining the relationship between confounders of interest and CVD history. For example, in our study sample, BMI was not associated with CVD history. This lack of association may be generated because those experiencing a CVD event may then lose weight in order to reduce their risk of recurrence.89–91 Therefore, a measurement of BMI taken at baseline in those followed longitudinally would serve to better estimate the relationship between current BMI and future CVD events than the cross-sectional measure of BMI used in this study. In addition, when considering the relationship between smoking status and CVD history, the highest prevalence of CVD history is among former smokers, again suggesting that those with a CVD event may modify their smoking behaviour. Hence, the association between baseline measurement of smoking status and future CVD events would better serve to demonstrate this relationship. We chose not to control for BMI, diabetes or smoking because none of these covariates were associated with CMV seropositivity and BMI was not associated with CVD history among the unexposed, in our study sample. Although these covariates did not appear to be confounders we explored how adjustment might influence the results we observed here. Controlling for these covariates had very little effect on the ORs and CIs.
In our study, we chose to use education level since it best represents a life-course measure of SEP and likely provides an indication of SEP prior to the occurrence of a CVD event and prior to entering retirement. Since a large proportion of our samples are likely to be in or soon to enter retirement, we did not feel that current income or measures of wealth were representative of subject SEP before undergoing CVD events. In fact, the use of current income or wealth as a measure of SEP could lead to temporal ambiguity in our conclusions because the occurrence of CVD history would influence future income and/or accumulation of wealth. Although we feel that using education level was the most appropriate choice of SEP marker for our study population, we do acknowledge that education level may not adequately represent the economic returns garnered across different races or genders.58 This potential measurement error in the exposure could bias the results of our study, albeit in the direction of the null.92
Another limitation of our study is that the outcome of CVD history is self-reported by subjects in our study. Therefore our outcome may be vulnerable to differential misclassification bias if certain subjects are more or less likely to accurately recall their CVD history. In terms of the relationship between SEP and CVD history, it is plausible that subjects of lower SEP are less likely to seek medical care for health conditions and therefore may be less likely to report CVD history biasing the results towards the null.93,94 It is highly unlikely that history of CVD would be differentially misclassified according to seropositive status since CMV is not clinically diagnosed unless an individual experiences a serious CMV condition, such as transplant or HIV-associated CMV disease, which is a rare occurrence in our study age population.95
Also of concern in our study is the potential for participation bias. Ninety per cent of those that underwent the household interview also participated in the mobile examination. The 10% that did not participate in the examination were more likely to be older. Since subjects that participated in both the interview and the mobile examination tended to be younger, this may have led to an underestimation of the prevalence of CVD history in the population. However, there were no differences according to age, gender, race, education level, smoking status or diabetes status between those that answered all three cardiovascular disease questions and those that did not, among those 45 years of age and older, suggesting no impact of participation bias on our outcome of interest.
Last, although available sera samples were selected randomly for seropositivity testing, by chance, serum from non-Hispanic/Blacks and from subjects of older age were less likely to be selected for CMV testing. Similarly, by chance available sera from non-Hispanic/blacks were less likely to be selected for HSV testing and available sera from subjects of younger age were less likely to be selected for testing for both pathogens. Thus, our analyses may be subject to some selection bias due to chance differences in the random sampling of pathogens in the available serum samples. For example, non-Hispanic/Blacks have both a higher risk for cardiovascular disease96 and a higher prevalence of CMV and HSV38,97 and therefore the chance differences in the selection of these subjects for pathogen testing may have biased our results towards the null.
More work is needed to understand whether social gradients in CMV and other infections result from increased exposure to infection, greater susceptibility, increased reactivation over the life course or a combination of these factors. In addition, future studies could benefit from examining the association between SEP and antibody titres to these herpesviruses, as increased antibody titre may be a better indication of socioeconomic stress-mediated immunological changes and therefore more closely associated with cardiovascular disease.20,79,98–103 Moreover, it is possible that antibody titres may be a more sensitive measure of the degree to which reactivation or repeated infection is occurring as an adult, and therefore a better marker of immune system alterations including increased cardiovascular disease-related inflammatory processes.99,104
Herpesvirus infections have been correlated with increased levels of pro-inflammatory cytokines.17,18,25,105 Thus, measurement of CRP and IL-6 levels along with seropositivity and/or antibody titre, might serve to better elucidate the mechanisms by which CMV mediates these pathways.17–19 Cytokines were not assayed in NHANES III to test their potential relationship to CMV antibody levels in our sample. Testing of C-reactive protein (CRP) data in NHANES III preceded high-sensitivity assay techniques, resulting in 60% of the CRP values in our sample falling below the detectable threshold, limiting its usefulness in our analyses.
Conclusion
CMV seropositivity partially mediates the relationship between SEP and cardiovascular disease history in this sample. Neither infection with HSV-1 nor both pathogens, however, mediated this relationship. This study is consistent with earlier studies showing that CMV is related to cardiovascular disease outcomes such as atherosclerosis, restenosis and future risk of myocardial infarction.34,106,107 The proportion of the association between SEP and cardiovascular disease explained by CMV seropositivity is less than that observed for traditional risk factors. Nevertheless, if the relationship observed in this sample between CMV infection and cardiovascular disease prevalence is found to be causal, the attributable fraction suggests that a moderate proportion of cardiovascular disease prevalence could be eliminated if CMV were to be prevented. Therefore, the clinical implications of this study indicate that interventions targeting prevention of primary CMV infection, through vaccinations or improving socioeconomic related living conditions may ultimately decrease the impact of CMV on cardiovascular disease among ageing populations in the United States.
Funding
University of Michigan-Rackham Graduate School, Rackham Merit Fellowship (A.M.S.); University of Michigan-Medical School, Institute of Gerontology, Pepper Center Research Career Development Core (to A.E.A.); Robert Wood Johnson Health and Society Scholars Program, University of Michigan (to J.B.D.). Additional funding was provided by National Institutes of Health (R21 NR011181-01 to A.E.A. and J.B.D.); Centers for Integrative Approach to Health Disparities (P60 MD002249 to AE.A.).
Acknowledgements
We would like to acknowledge Steven Heeringa, PhD, University of Michigan, Population Studies Center, Survey Research Center, who assisted with analysis of complex survey data.
Conflict of interest: None declared.
KEY MESSAGES.
Several infections have been associated with both CVD and SEP.
This study examines whether seropositivity to CMV, to HSV-1 and/or to both pathogens mediates the relationship between SEP and CVD history in a nationally representative sample of the United States.
Results show that SEP was associated with CMV, HSV-1 and seropositivity to both pathogens; however, neither seropositivity to HSV-1 nor to both pathogens was associated with CVD history after adjusting for SEP.
Persistent pathogens such as CMV infection may explain a portion of the relationship between SEP and CVD in the United States.
References
- 1.American Heart Association. Heart Disease Stroke Stat—2007. Update. 2007. [Google Scholar]
- 2.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 3.National Institutes of Health. National Heart, Lung, and Blood Institute. Incidence and Prevalence: 2007 Chart Book on Cardiovascular and Lung Diseases. 2007. [Google Scholar]
- 4.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88(4 Pt 1):1973–98. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- 5.Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol. 2006;16:91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 6.Ljung R, Hallqvist J. Socioeconomic position, clustering of risk factors, and the risk of myocardial infarction. Am J Public Health. 2007;97:1927–28. doi: 10.2105/AJPH.2007.119248. ; author reply 1928–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kivimaki M, Lawlor DA, Davey Smith G, et al. Socioeconomic position, co-occurrence of behavior-related risk factors, and coronary heart disease: the Finnish Public Sector study. Am J Public Health. 2007;97:874–79. doi: 10.2105/AJPH.2005.078691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mensah G, Mokdad A, Ford E, Greenlund K, Croft J. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–41. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 9.Ljung R, Hallqvist J. Accumulation of adverse socioeconomic position over the entire life course and the risk of myocardial infarction among men and women: results from the Stockholm Heart Epidemiology Program (SHEEP) J Epidemiol Community Health. 2006;60:1080–84. doi: 10.1136/jech.2006.047670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winkleby MA, Kraemer HC, Ahn DK, Varady AN. Ethnic and socioeconomic differences in cardiovascular disease risk factors: findings for women from the Third National Health and Nutrition Examination Survey, 1988–1994. JAMA. 1998;280:356–62. doi: 10.1001/jama.280.4.356. [DOI] [PubMed] [Google Scholar]
- 11.Lawlor DA, Ebrahim S, Davey Smith G. Adverse socioeconomic position across the lifecourse increases coronary heart disease risk cumulatively: findings from the British women's heart and health study. J Epidemiol Community Health. 2005;59:785–93. doi: 10.1136/jech.2004.029991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Syme SL, Balfour JL. Explaining inequalities in coronary heart disease. Lancet. 1997;350:231–32. doi: 10.1016/S0140-6736(05)62220-9. [DOI] [PubMed] [Google Scholar]
- 13.Khang YH, Lynch JW, Jung-Choi K, Cho HJ. Explaining age-specific inequalities in mortality from all causes, cardiovascular disease and ischaemic heart disease among South Korean male public servants: relative and absolute perspectives. Heart. 2008;94:75–82. doi: 10.1136/hrt.2007.117747. [DOI] [PubMed] [Google Scholar]
- 14.Harald K, Koskinen S, Jousilahti P, Torppa J, Vartiainen E, Salomaa V. Changes in traditional risk factors no longer explain time trends in cardiovascular mortality and its socioeconomic differences. J Epidemiol Community Health. 2008;62:251–57. doi: 10.1136/jech.2007.060707. [DOI] [PubMed] [Google Scholar]
- 15.Laaksonen M, Talala K, Martelin T, et al. Health behaviours as explanations for educational level differences in cardiovascular and all-cause mortality: a follow-up of 60 000 men and women over 23 years. Eur J Public Health. 2008;18:38–43. doi: 10.1093/eurpub/ckm051. [DOI] [PubMed] [Google Scholar]
- 16.Lynch J, Davey Smith G, Harper S, Bainbridge K. Explaining the social gradient in coronary heart disease: comparing relative and absolute risk approaches. J Epidemiol Community Health. 2006;60:436–41. doi: 10.1136/jech.2005.041350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53:747–54. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- 18.Soderberg-Naucler C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J Intern Med. 2006;259:219–46. doi: 10.1111/j.1365-2796.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- 19.Aiello AE, Haan M, Blythe L, Moore K, Gonzalez JM, Jagust W. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc. 2006;54:1046–54. doi: 10.1111/j.1532-5415.2006.00796.x. [DOI] [PubMed] [Google Scholar]
- 20.Dowd JB, Haan MN, Blythe L, Moore K, Aiello AE. Socioeconomic gradients in immune response to latent infection. Am J Epidemiol. 2007;167:112–120. doi: 10.1093/aje/kwm247. [DOI] [PubMed] [Google Scholar]
- 21.Hsich E, Zhou YF, Paigen B, Johnson TM, Burnett MS, Epstein SE. Cytomegalovirus infection increases development of atherosclerosis in Apolipoprotein-E knockout mice. Atherosclerosis. 2001;156:23–28. doi: 10.1016/s0021-9150(00)00608-0. [DOI] [PubMed] [Google Scholar]
- 22.Alber DG, Powell KL, Vallance P, Goodwin DA, Grahame-Clarke C. Herpesvirus infection accelerates atherosclerosis in the apolipoprotein E-deficient mouse. Circulation. 2000;102:779–85. doi: 10.1161/01.cir.102.7.779. [DOI] [PubMed] [Google Scholar]
- 23.Berencsi K, Endresz V, Klurfeld D, Kari L, Kritchevsky D, Gonczol E. Early atherosclerotic plaques in the aorta following cytomegalovirus infection of mice. Cell Adhes Commun. 1998;5:39–47. doi: 10.3109/15419069809005597. [DOI] [PubMed] [Google Scholar]
- 24.Fabricant CG, Fabricant J, Minick CR, Litrenta MM. Herpesvirus-induced atherosclerosis in chickens. Fed Proc. 1983;42:2476–79. [PubMed] [Google Scholar]
- 25.Stassen FR, Vega-Cordova X, Vliegen I, Bruggeman CA. Immune activation following cytomegalovirus infection: more important than direct viral effects in cardiovascular disease? J Clin Virol. 2006;35:349–53. doi: 10.1016/j.jcv.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Fong IW. Infections and their role in atherosclerotic vascular disease. J Am Dent Assoc. 2002;133(Suppl):7S–13S. doi: 10.14219/jada.archive.2002.0376. [DOI] [PubMed] [Google Scholar]
- 27.Leinonen M, Saikku P. Evidence for infectious agents in cardiovascular disease and atherosclerosis. Lancet Infect Dis. 2002;2:11–17. doi: 10.1016/s1473-3099(01)00168-2. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson AC, Hajjar DP. Herpesvirus in atherosclerosis and thrombosis: etiologic agents or ubiquitous bystanders? Arterioscler Thromb Vasc Biol. 1998;18:339–48. doi: 10.1161/01.atv.18.3.339. [DOI] [PubMed] [Google Scholar]
- 29.Epstein SE. The multiple mechanisms by which infection may contribute to atherosclerosis development and course. Circ Res. 2002;90:2–4. [PubMed] [Google Scholar]
- 30.Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: Is there a link? Lancet. 1997;350:430–36. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- 31.Epstein SE, Zhou YF, Zhu J. Infection and atherosclerosis: emerging mechanistic paradigms. Circulation. 1999;100:e20–28. doi: 10.1161/01.cir.100.4.e20. [DOI] [PubMed] [Google Scholar]
- 32.Zhu J, Nieto FJ, Horne BD, Anderson JL, Muhlestein JB, Epstein SE. Prospective study of pathogen burden and risk of myocardial infarction or death. Circulation. 2001;103:45–51. doi: 10.1161/01.cir.103.1.45. [DOI] [PubMed] [Google Scholar]
- 33.Zhu J, Quyyumi AA, Norman JE, et al. Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Am J Cardiol. 2000;85:140–46. doi: 10.1016/s0002-9149(99)00653-0. [DOI] [PubMed] [Google Scholar]
- 34.Smieja M, Gnarpe J, Lonn E, et al. Multiple infections and subsequent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation. 2003;107:251–57. doi: 10.1161/01.cir.0000044940.65226.1f. [DOI] [PubMed] [Google Scholar]
- 35.Vahdat K, Jafari SM, Pazoki R, Nabipour I. Concurrent increased high sensitivity C-reactive protein and chronic infections are associated with coronary artery disease: a population-based study. Indian J Med Sci. 2007;61:135–43. [PubMed] [Google Scholar]
- 36.Gnann J, Jr, Whitley R. Herpes Zoster. N Engl J Med. 2002;347:340–46. doi: 10.1056/NEJMcp013211. [DOI] [PubMed] [Google Scholar]
- 37.Schillinger JA, Xu F, Sternberg MR, et al. National seroprevalence and trends in herpes simplex virus type 1 in the United States, 1976–1994. Sex Transm Dis. 2004;31:753–60. doi: 10.1097/01.olq.0000145852.43262.c3. [DOI] [PubMed] [Google Scholar]
- 38.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43:1143–51. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 39.Davey Smith G, Hart C. Life-course socioeconomic and behavioral influences on cardiovascular disease mortality: the collaborative study. Am J Public Health. 2002;92:1295–98. doi: 10.2105/ajph.92.8.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aboderin I, Kalache A, Ben-Shlomo Y, et al. Life Course Perspectives on Coronary Heart Disease. Stroke and Diabetes: Key Issues and Implications for Policy and Research, 2002. Geneva, World Health Organization. [Google Scholar]
- 41.Centers for Disease Control and Prevention (CDC) Prevalence of heart disease—United States, 2005. Morb Mortal Wkly Rep. 2007;56:113–18. [PubMed] [Google Scholar]
- 42.Stoops N. Washington, DC: U.S. Census Bureau; Educational Attainment in the United States: 2003 (P20-550). 2004. [Google Scholar]
- 43.Hennekens CH. Increasing burden of cardiovascular disease: current knowledge and future directions for research on risk factors. Circulation. 1998;97:1095–102. doi: 10.1161/01.cir.97.11.1095. [DOI] [PubMed] [Google Scholar]
- 44.Glaser R, Gotlieb-Stematsky T, editors. Human Herpesvirus Infections: Clinical Aspects. New York: Marcel Dekker, Inc.; 1982. [Google Scholar]
- 45.Schmader K. Herpes zoster in older adults. Clin Infect Dis. 2001;32:1481–86. doi: 10.1086/320169. [DOI] [PubMed] [Google Scholar]
- 46.Dowd JB, Aiello AE, Alley DE. Socioeconomic gradients in cytomegalovirus seropositivity in the U.S. population: NHANES III. Epidemiol Infect. 2008;16:1–8. doi: 10.1017/S0950268808000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wikby A, Nilsson BO, Forsey R, et al. The immune risk phenotype is associated with IL-6 in the terminal decline stage: findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech Ageing Dev. 2006;127:695–704. doi: 10.1016/j.mad.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev. 2005;205:257–68. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 49.Almanzar G, Schwaiger S, Jenewein B, et al. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79:3675–83. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koch S, Solana R, Dela Rosa O, Pawelec G. Human cytomegalovirus infection and T cell immunosenescence: a mini review. Mech Ageing Dev. 2006;127:538–43. doi: 10.1016/j.mad.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Pawelec G, Koch S, Franceschi C, Wikby A. Human immunosenescence: does it have an infectious component? Ann NY Acad Sci. 2006;1067:56–65. doi: 10.1196/annals.1354.009. [DOI] [PubMed] [Google Scholar]
- 52.Akbar AN, Fletcher JM. Memory T cell homeostasis and senescence during aging. Curr Opin Immunol. 2005;17:480–85. doi: 10.1016/j.coi.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 53.Aiello AE, Haan MN, Pierce CM, Simanek AM, Liang J. Persistent infection, inflammation, and functional impairment in older latinos. J Gerontol A Biol Sci Med Sci. 2008;63:610–18. doi: 10.1093/gerona/63.6.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Itzhaki RF, Wozniak MA. Herpes simplex virus type 1, apolipoprotein E, and cholesterol: a dangerous liaison in Alzheimer's disease and other disorders. Prog Lipid Res. 2006;45:73–90. doi: 10.1016/j.plipres.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Sorlie PD, Nieto FJ, Adam E, Folsom AR, Shahar E, Massing M. A prospective study of cytomegalovirus, herpes simplex virus 1, and coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Arch Intern Med. 2000;160:2027–32. doi: 10.1001/archinte.160.13.2027. [DOI] [PubMed] [Google Scholar]
- 56.Lee FK, Pereira L, Griffin C, Reid E, Nahmias A. A novel glycoprotein for detection of herpes simplex virus type 1-specific antibodies. J Virol Meth. 1986;14:111–18. doi: 10.1016/0166-0934(86)90041-8. [DOI] [PubMed] [Google Scholar]
- 57.Galobardes B, Lynch J, Smith GD. Measuring socioeconomic position in health research. Br Med Bull. 2007;81–82:21–37. doi: 10.1093/bmb/ldm001. [DOI] [PubMed] [Google Scholar]
- 58.Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc. 2007;99:1013–23. [PMC free article] [PubMed] [Google Scholar]
- 59.Green MS, Cohen D, Slepon R, Robin G, Wiener M. Ethnic and gender differences in the prevalence of anti-cytomegalovirus antibodies among young adults in Israel. Int J Epidemiol. 1993;22:720–23. doi: 10.1093/ije/22.4.720. [DOI] [PubMed] [Google Scholar]
- 60.Van der Horst Graat JM, Terpstra JS, Kok FJ, Schouten EG. Alcohol, smoking, and physical activity related to respiratory infections in elderly people. J Nutr Health Aging. 2007;11:80–85. [PubMed] [Google Scholar]
- 61.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164:2206–16. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 62.Bertoni AG, Saydah S, Brancati FL. Diabetes and the risk of infection-related mortality in the U.S. Diabetes Care. 2001;24:1044–49. doi: 10.2337/diacare.24.6.1044. [DOI] [PubMed] [Google Scholar]
- 63.Braunwald E. Shattuck lecture—cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–69. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 64.Baron R, Kenny D. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Personality Social Psychol. 1968;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 65.MacKinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Eval Rev. 1993;17:144–58. [Google Scholar]
- 66.Rothman KJ, Greenland S, editors. Modern Epidemiology. 2nd. Boston: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 67.Prigerson HG, Maciejewski PK, Rosenheck RA. Population attributable fractions of psychiatric disorders and behavioral outcomes associated with combat exposure among US men. Am J Public Health. 2002;92:59–63. doi: 10.2105/ajph.92.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–91. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 69.Hsieh FY, Bloch DA, Larsen MD. A simple method of sample size calculation for linear and logistic regression. Stat Med. 1998;17:1623–34. doi: 10.1002/(sici)1097-0258(19980730)17:14<1623::aid-sim871>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 70.Itzhaki RF, Dobson CB, Shipley SJ, Wozniak MA. The role of viruses and of APOE in dementia. Ann N Y Acad Sci. 2004;1019:15–18. doi: 10.1196/annals.1297.003. [DOI] [PubMed] [Google Scholar]
- 71.Chiu B, Viira E, Tucker W, Fong IW. Chlamydia pneumoniae, cytomegalovirus, and herpes simplex virus in atherosclerosis of the carotid artery. Circulation. 1997;96:2144–48. doi: 10.1161/01.cir.96.7.2144. [DOI] [PubMed] [Google Scholar]
- 72.Espinola-Klein C, Rupprecht HJ, Blankenberg S, et al. Are morphological or functional changes in the carotid artery wall associated with Chlamydia pneumoniae, Helicobacter pylori, cytomegalovirus, or herpes simplex virus infection? Stroke. 2000;31:2127–33. doi: 10.1161/01.str.31.9.2127. [DOI] [PubMed] [Google Scholar]
- 73.Muhlestein JB, Anderson JL. Chronic infection and coronary artery disease. Cardiol Clin. 2003;21:333–62. doi: 10.1016/s0733-8651(03)00054-7. [DOI] [PubMed] [Google Scholar]
- 74.De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005;579:2035–39. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 75.Pawelec G, Gouttefangeas C. T-cell dysregulation caused by chronic antigenic stress: the role of CMV in immunosenescence? Aging Clin Exp Res. 2006;18:171–73. doi: 10.1007/BF03327436. [DOI] [PubMed] [Google Scholar]
- 76.Vasto S, Colonna-Romano G, Larbi A, Wikby A, Caruso C, Pawelec G. Role of persistent CMV infection in configuring T cell immunity in the elderly. Immun Ageing. 2007;4:2. doi: 10.1186/1742-4933-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scrimshaw N, SanGiovanni J. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997;66:464S–77S. doi: 10.1093/ajcn/66.2.464S. [DOI] [PubMed] [Google Scholar]
- 78.Glaser R. Stress-associated immune dysregulation and its importance for human health: a personal history of psychoneuroimmunology. Brain Behav Immun. 2005;19:3–11. doi: 10.1016/j.bbi.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 79.Aiello AE, Simanek AM, Galea S. Population levels of psychological stress, herpesvirus reactivation and HIV. AIDS Behav. 2008 doi: 10.1007/s10461-008-9358-4. . Epub ahead of print (Feb. 9, 2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steptoe A, Shamaei-Tousi A, Gylfe A, Henderson B, Bergstrom S, Marmot M. Socioeconomic status, pathogen burden, and cardiovascular disease risk. Heart. 2007;93:1567–70. doi: 10.1136/hrt.2006.113993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhong J, Khanna R. Vaccine strategies against human cytomegalovirus infection. Expert Rev Anti Infect Ther. 2007;5:449–59. doi: 10.1586/14787210.5.3.449. [DOI] [PubMed] [Google Scholar]
- 83.Schleiss MR. Cytomegalovirus vaccine development. Curr Top Microbiol Immunol. 2008;325:361–82. doi: 10.1007/978-3-540-77349-8_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pass RF, Burke RL. Development of cytomegalovirus vaccines: prospects for prevention of congenital CMV infection. Semin Pediatr Infect Dis. 2002;13:196–204. doi: 10.1053/spid.2002.125863. [DOI] [PubMed] [Google Scholar]
- 85.Colugnati FA, Staras SA, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis. 2007;7:71. doi: 10.1186/1471-2334-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wacholder S, Benichou J, Heineman EF, Hartge P, Hoover RN. Attributable risk: advantages of a broad definition of exposure. Am J Epidemiol. 1994;140:303–09. doi: 10.1093/oxfordjournals.aje.a117252. [DOI] [PubMed] [Google Scholar]
- 87.Greenland S, Robins JM. Conceptual problems in the definition and interpretation of attributable fractions. Am J Epidemiol. 1988;128:1185–97. doi: 10.1093/oxfordjournals.aje.a115073. [DOI] [PubMed] [Google Scholar]
- 88.Bodeus M, Feyder S, Goubau P. Avidity of IgG antibodies distinguishes primary from non-primary cytomegalovirus infection in pregnant women. Clin Diagn Virol. 1998;9:9–16. doi: 10.1016/s0928-0197(97)10016-2. [DOI] [PubMed] [Google Scholar]
- 89.Petersson U, Ostgren CJ, Brudin L, Ovhed I, Nilsson PM. Predictors of successful, self-reported lifestyle changes in a defined middle-aged population: the Soderakra Cardiovascular Risk Factor Study, Sweden. Scand J Public Health. 2008;36:389–96. doi: 10.1177/1403494808089561. [DOI] [PubMed] [Google Scholar]
- 90.Mallaghan M, Pemberton J. Some behavioural changes in 493 patients after an acute myocardial infarction. Br J Prev Soc Med. 1977;31:86–90. doi: 10.1136/jech.31.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lopez-Jimenez F, Wu CO, Tian X, et al. Weight change after myocardial infarction–the Enhancing Recovery in Coronary Heart Disease patients (ENRICHD) experience. Am Heart J. 2008;155:478–84. doi: 10.1016/j.ahj.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Flegal KM, Brownie C, Haas JD. The effects of exposure misclassification on estimates of relative risk. Am J Epidemiol. 1986;123:736–51. doi: 10.1093/oxfordjournals.aje.a114294. [DOI] [PubMed] [Google Scholar]
- 93.Casagrande SS, Gary TL, LaVeist TA, Gaskin DJ, Cooper LA. Perceived discrimination and adherence to medical care in a racially integrated community. J Gen Intern Med. 2007;22:389–95. doi: 10.1007/s11606-006-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van Houtven CH, Voils CI, Oddone EZ, et al. Perceived discrimination and reported delay of pharmacy prescriptions and medical tests. J Gen Intern Med. 2005;20:578–83. doi: 10.1111/j.1525-1497.2005.0123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Britt W. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr Top Microbiol Immunol. 2008;325:417–70. doi: 10.1007/978-3-540-77349-8_23. [DOI] [PubMed] [Google Scholar]
- 96.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 97.Rosenthal SL, Stanberry LR, Biro FM, et al. Seroprevalence of herpes simplex virus types 1 and 2 and cytomegalovirus in adolescents. Clin Infect Dis. 1997;24:135–39. doi: 10.1093/clinids/24.2.135. [DOI] [PubMed] [Google Scholar]
- 98.Finch CE, editor. Cells and Surveys: Should Biological Measures be Included in Social Science Research?. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 99.Mehta SK, Stowe RP, Feiveson AH, Tyring SK, Pierson DL. Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J Infect Dis. 2000;182:1761–64. doi: 10.1086/317624. [DOI] [PubMed] [Google Scholar]
- 100.Glaser R, Friedman SB, Smyth J, et al. The differential impact of training stress and final examination stress on herpesvirus latency at the United States Military Academy at West Point. Brain Behavior Immunity. 1999;13:240–51. doi: 10.1006/brbi.1999.0566. [DOI] [PubMed] [Google Scholar]
- 101.Glaser R, Kiecolt-Glaser JK. Chronic stress modulates the virus-specific immune response to latent herpes simplex virus type 1. Ann Behav Med. 1997;19:78–82. doi: 10.1007/BF02883323. [DOI] [PubMed] [Google Scholar]
- 102.Herbert TB, Cohen S. Stress and immunity in humans: a meta-analytic review. Psychosom Med. 1993;55:364–79. doi: 10.1097/00006842-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 103.Glaser R, Pearson GR, Jones JF, et al. Stress-related activation of Epstein-Barr virus. Brain Behavior Immunity. 1991;5:219–32. doi: 10.1016/0889-1591(91)90018-6. [DOI] [PubMed] [Google Scholar]
- 104.Stowe RP, Mehta SK, Ferrando AA, Feeback DL, Pierson DL. Immune responses and latent herpesvirus reactivation in spaceflight. Aviat Space Environ Med. 2001;72:884–91. [PubMed] [Google Scholar]
- 105.Compton T, Kurt-Jones EA, Boehme KW, et al. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003;77:4588–96. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grahame-Clarke C. Human cytomegalovirus, endothelial function and atherosclerosis. Herpes. 2005;12:42–45. [PubMed] [Google Scholar]
- 107.Zhou YF, Leon MB, Waclawiw MA, et al. Association between prior cytomegalovirus infection and the risk of restenosis after coronary atherectomy. N Engl J Med. 1996;335:624–30. doi: 10.1056/NEJM199608293350903. [DOI] [PubMed] [Google Scholar]