Abstract
Background Children in low-income settings suffering from frequent diarrhoea episodes are also at a high risk of acute lower respiratory infections (ALRI). We explored whether this is due to common risk factors for both conditions or whether diarrhoea can increase the risk of ALRI directly.
Methods We used a dynamic time-to-event analysis of data from two large child studies in low-income settings in Ghana and Brazil, with the cumulative diarrhoea prevalence over 2 weeks as the exposure and severe ALRI as outcome. The analysis was adjusted for baseline risk of ALRI and diarrhoea, seasonality and age.
Results The child population from Ghana had a much higher risk of diarrhoea, malnutrition and death than the children in Brazil. In the data from Ghana, every additional day of diarrhoea within 2 weeks increased the risk of ALRI by a factor of 1.08 (95% CI 1.00–1.15). In addition, we found a roughly linear relationship between the number of diarrhoea days over the last 28 days and the risk of ALRI. In the Ghana data, 26% of ALRI episodes may be due to recent exposure to diarrhoea. The Brazilian data gave no evidence for an association between diarrhoea and ALRI.
Conclusion Diarrhoea may contribute substantially to the burden of ALRI in malnourished child populations.
Keywords: Diarrhoea, pneumonia, malnutrition, ALRI
Introduction
Diarrhoeal diseases and acute lower respiratory infections (ALRI) are regarded as the leading proximate causes of death among children in low-income settings.1 It has been shown that children who suffer from repeated or severe episodes of diarrhoea are also at a higher risk of ALRI.2 However, it is not clear whether these conditions are causally related, or whether their observed co-occurrence merely reflects the presence of common risk factors, for example a weak immune system and malnutrition.3
Diarrhoeal disease is an established risk factor for acute weight loss, malnutrition and stunting.4–8 Likewise, malnutrition and failure to thrive are known risk factors for ALRI.9,10 A causal link between diarrhoeal disease and subsequent risk of ALRI is therefore biologically plausible and would be of considerable public health importance because effective interventions targeting diarrhoeal diseases11 would then also contribute to a reduction of ALRI. Diarrhoea may also increase the risk of ALRI in the short term by causing acute micronutrient loss, stress on the immune system, dehydration or immobilization, thereby creating a vulnerable period of increased risk of infections. There is some evidence that ALRI is a common finding and cause of death of malnourished children hospitalized for severe diarrhoea.12–14 However, in hospital case series it can be difficult to distinguish between cause, effect and co-occurrence due to common risk factors.
Longitudinal population-based studies are more suitable to investigate the dependence of ALRI risk on recent diarrhoea, but we were unable to identify a study in the literature addressing this question. The aim of this study was to explore whether diarrhoea increases the risk of ALRI in the short term using a dynamic time-to-event analysis of data from two large child studies in low-income settings.
Materials and methods
Study populations
We used data from two large vitamin A trials in northern Ghana15 and north-eastern Brazil,16 both conducted in semi-arid low-income settings between 1990 and 1991. The Ghana study took place in a poor rural setting with extended families living in compounds and practicing subsistence farming. The study area was chosen due to a high prevalence of vitamin A deficiency. A full census of all residents was carried out prior to the trial. All compounds in the study area were included in the trial. All children aged 0–60 months living in these compounds were eligible for inclusion (10% loss to follow up). The Brazil trial was conducted in Serrinha, a town with 30 000 inhabitants 170 km north-west of Bahia. At the beginning of the study, children were identified by a census of the poorest neighbourhoods in the area. Eligible children were between 6 and 48 months of age, and had no active xerophthalmia, recent measles or recent vitamin A supplementation (10% loss to follow up). In both trials children were followed up for ∼1 year. Socio-economic status was poorer in Ghana, where a large majority of households relied on open wells, bore holes and rivers as water source. In Brazil, over 95% of households had piped-in water supply and over 90% had electricity.
Diarrhoea exposure
In Ghana, weekly field worker visits measured the prevalence of diarrhoeal disease symptoms since the last visit and respiratory signs; in Brazil field workers visited up to three times a week. In the Ghana dataset, diarrhoea was defined based on mothers’ definitions and local disease perception. In the Brazil data diarrhoea was defined as the occurrence of three or more loose stools over 24 h.
It has been recognized that the adverse effects of diarrhoea such as malnutrition and death (and potentially ALRI) depend not so much on the number of diarrhoea episodes, but on the duration of these episodes17 as well as the number of days with diarrhoea over a certain period of time, also known as the longitudinal prevalence.6–8 Restricting the analysis to (rare) prolonged episodes would have led to a loss of power. We therefore used as a measure of recent diarrhoea experience the number of days with diarrhoea in an individual during a fixed time window looking back from the date on which an episode of ALRI occurred, subsequently named ‘index day’. We decided to apply a 2-week window for the primary analysis, since episodes of this duration have been shown to be associated with adverse outcomes. In a sensitivity analysis we also tested 4-week windows. Days that were preceded by <14 days (or 28 days) of observation were excluded from the analysis.
ALRI measurement
We based the diagnosis of ALRI on clinical findings documented by trained field workers during regular visits. In both the Brazil and the Ghana data we defined ALRI as the presence of rapid breathing (more than 50 breaths per minute) plus one of the following ‘danger signs’ indicating severe ALRI: chest indrawing, nasal flaring and stridor.18 Thus, we used criteria slightly more stringent than the WHO definition for ‘severe ALRI’ (the WHO definition for severe ALRI does not require fast breathing), which should have a higher specificity for pneumonia than ALRI without danger signs.19 Due to the remaining uncertainty we maintain the term ALRI (rather than pneumonia) throughout this text. We defined a new episode of ALRI as one following a 2-week interval without ALRI.18
Data analysis
We used Cox regression for the time-to-event analysis. Since ALRI can occur as recurrent events in the same individual, we used an extension of the Cox model, the Prentice–Williams–Peterson model (PWP-CP), a dynamic time-to-event model with incident ALRI as the outcome variable.20 The model is suitable to fit complex multivariate time-to-event data and accounts for the changing diarrhoea exposure of individuals over time. We chose calendar time as the time scale in order to control fully for seasonal variation of diarrhoea and ALRI. To control for potential confounding due to common risk factors for both diarrhoea and ALRI we stratified the analysis by the individual ALRI rate in five categories (including the individual ALRI rate as a covariate led to over-fitting). In addition, we adjusted for the individual proportion-of-time-ill (longitudinal prevalence over the whole observation period), age and clustering at the child level by using robust variance estimation.21 Thus, by controlling for the overall risk of diarrhoea and ALRI, which should represent the effect of common risk factors for the two conditions (e.g. socio-economic, environmental, water, hygiene) the model allowed focussing on the short-term temporal association.
We fitted a separate model for each stratum defined by the ALRI rate, and then combined estimators of the stratum specific models to an overall estimate.20 In other words, each time an ALRI episode occurs in any individual (‘index day’), the model compares the diarrhoea exposure over the 14-day time window in that individual with the diarrhoea exposure at that calendar time in other individuals with a similar overall rate of ALRI and diarrhoea, adjusted for age.
We explored the impact of diarrhoea in the weeks prior to the index day by moving the time window to the past starting on the day before the index day. For example, exposure to diarrhoea during the time window closest to the index day was specified as the sum of diarrhoea days from Day −15 to Day −1 prior to the index day. This window was moved to the past in weekly intervals. For each time window at weekly intervals we fitted a separate model.
The population attributable fraction (PAF), i.e. the proportion of ALRI episodes due to diarrhoea was calculated using the formula PAF = p′ × (HR − 1)/HR, where p′ is the proportion of cases exposed and HR the adjusted hazard ratio.22
Results
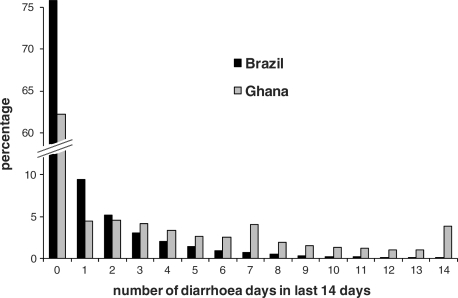
Basic epidemiological characteristics of the two study populations are shown in Table 1. The children in Ghana had a higher risk of diarrhoea and longer episodes. The mortality rate was more than 10 times higher in Ghana than in Brazil. Figure 1 shows the distribution of the number of days with diarrhoea over the past 14 days. In Brazil, 76% of all days observed were not preceded by any diarrhoea within the prior 14 days. In Ghana this figure was only 62%. Time periods with many diarrhoea days were much more common in Ghana. Nearly 4% of all days in the Ghana study were preceded by at least 14 days of diarrhoea. As expected, the longitudinal prevalence of diarrhoea and the incidence of ALRI in an individual child were correlated both in Ghana (r = 0.15, P < 0.001) and in Brazil (r = 0.18, P < 0.001).
Table 1.
Epidemiological characteristics of the two study populations
| Ghana | Brazil | |
|---|---|---|
| Number of children | 1877 | 1209 |
| Person-years | 1455 | 1104 |
| Diarrhoea | ||
| Incidence rate/PYa | 9.0 | 7.0 |
| Longitudinal prevalence (%) | 17 | 5 |
| Mean duration of episodes (days) | 6.1 | 2.7 |
| ALRIb | ||
| Number of episodes | 162 | 128 |
| Incidence rate/PY | 0.11 | 0.12 |
| Malnutrition | ||
| Weight-for-age z-score < −2 (%) | 30 | 13 |
| Mortality | ||
| Deaths (n) | 77 | 4 |
| Mortality rate/1000 PY | 52.9 | 3.6 |
aPY = person-years of observation.
bDefined as increased breathing rate (more than 50 breaths per minute) plus presence of any danger sign (see text).
Figure 1.
Exposure to diarrhoea. Shown is the distribution of the number of diarrhoea days in the 14 days for all individuals and days under observation
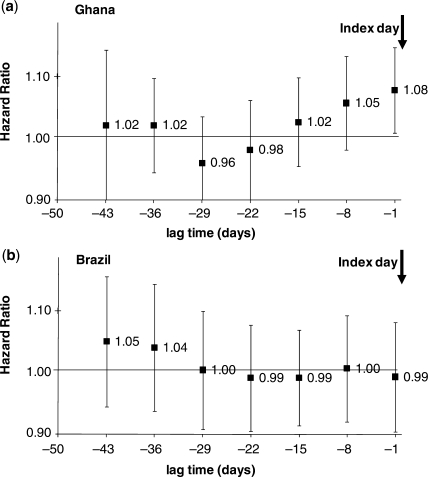
The results of the Cox regression analysis are shown in Figure 2. The graphs show the increase in the ALRI hazard rate with every additional day of diarrhoea over 14 days during time windows at increasing distance from the index day. In Ghana, every additional day of diarrhoea during the 14 days preceding the index day increased the hazard rate of ALRI by a factor of 1.08 (95% CI 1.00–1.15). There was no indication that the effect differed between different categories of ALRI risk. The effect also did not depend on whether children without any ALRI episode were excluded (hazard ratio = 1.07, 95% CI 0.99–1.14). We also found that additional adjustment for weight-for-age z-score (which can be regarded as a marker for nutritional status but also for socio-economic and environmental health conditions) hardly changed this association (hazard ratio = 1.07, 95% CI 1.0–1.15).
Figure 2.
The risk of ALRI depending on diarrhoea over a 14-day window at different lag times for (a) Ghana and (b) Brazil. The index day 0 is the day on which ALRI presence or absence was ascertained. The first estimate on the right (1.08 for Ghana) denotes the increase in the risk (hazard) of ALRI with every additional day with diarrhoea during the 14 days preceding the index day starting from Day −1. The next estimate (1.05 for Ghana) denotes the increase in the risk of ALRI with every additional diarrhoea day during the 14 days from Day −8 to Day −21, and so on
Moving the 14-day time window to the past by 1 week still revealed a trend towards an increased hazard rate of ALRI (HR = 1.05, 95% 0.98–1.13). Time windows further into the past did not show an obvious association between diarrhoea and ALRI. Due to decreasing power of the analysis for the time windows further away from the index day, these estimates have very wide confidence intervals.
In striking contrast to Ghana, the Brazilian data gave no evidence for an association between diarrhoea over the last 14 days and ALRI for any of the time windows (Figure 2b). The hazard ratios were very close to unity for the time windows close to the index day. There was some indication of an association for the lag times further into the past, but—as the wide confidence intervals suggest—this may easily be due to chance.
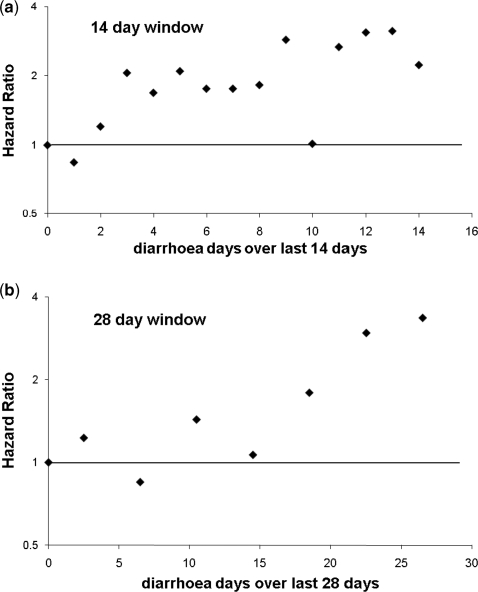
To further examine the association between the number of days with diarrhoea and ALRI found in the Ghana data, we treated the number of diarrhoea days as a categorical variable and compared the ALRI risk of each category with the risk in time windows without any diarrhoea. Figure 3a shows the association between the number of days with diarrhoea and ALRI risk compared with 0 days of diarrhoea during the last 14 days. There appears to be a roughly linear association between the two conditions, at least from≥3 days with diarrhoea over 14 days (as expected, the Brazil data revealed no such trend—not shown). To explore whether this association extends further into the past, we constructed a 28-day window, also looking back from the first day after the index day (Figure 3b). The graph suggests that the association is also present for the longer time window.
Figure 3.
The risk of ALRI in Ghana depending on the number of diarrhoea days during (a) the last 14 and (b) the last 28 days prior to the index day (time window closest to the index day). To avoid convergence problems, the number of diarrhoea days during the 28-day window was collapsed into groups of four
Population attributable fraction
For Ghana, we estimated the population attributable fraction, i.e. the proportion of ALRI in the population due to short-term exposure to diarrhoea, by assuming that diarrhoea affects ALRI risk predominantly within 2 weeks. The study population was exposed to any diarrhoea within 2 weeks during 38% of the person-time of observation (Figure 1). The proportion of ALRI episodes exposed (i.e. with any diarrhoea in the preceding 2 weeks) was 68%. Any exposure to diarrhoea within 2 weeks was associated with an adjusted hazard ratio of HR = 1.61 (95% CI: 0.89, 2.95). The estimated PAF is 0.68 × (1.61 − 1)/1.61 = 26%, suggesting that 26% of ALRI cases may be due to diarrhoea in the previous 2 weeks.
Discussion
Our analysis suggests that diarrhoea contributed substantially to the risk of ALRI within a few weeks of its occurrence in a child population in Ghana, but not in a child population in Brazil. In Ghana, the relationship was roughly linear: every additional day of diarrhoea over 2–4 weeks appeared to increase the risk of ALRI.
The striking socio-demographic and epidemiological differences between the two study populations may be a plausible explanation for the contrasting findings. The Ghana study included children under 6 months who were excluded in Brazil. In Ghana, the mortality rate was more than 10 times higher and diarrhoea was more common, especially in long episodes (Table 1). Malnutrition was more prevalent in the Ghana study site, as was clinical Vitamin A deficiency,15 which was mostly sub-clinical in Brazil.16 ALRI incidence was similar, but this is likely to be due to the three times more frequent household visits in Brazil, which increased the chance of a child presenting with all respiratory signs necessary to meet the case definition. Socio-economic status and water access were much more favourable in the Brazilian households compared with those included in the Ghana study.
The higher risk profile in the Ghana study may suggest that diarrhoea increases the risk of ALRI predominantly in malnourished children with a severe diarrhoea burden, who are at high risk of death. In line with our findings, hospital studies have shown that ALRI is a common comorbidity and cause of death among malnourished children admitted with severe diarrhoea.12–14 Hospital case series have many limitations. The strength of our study is that longitudinal data allow a much better exploration and quantification of the temporal link, which is difficult to establish in hospital studies. Our population-based study also allowed calculation of the population attributable fraction, which in the case of Ghana suggests a substantial contribution of diarrhoea to the burden of ALRI.
Among the methodological limitations, recall bias and misclassification may be the most important. In Ghana, diarrhoea was defined based on local disease concepts identified during extensive formative research. The Brazil study applied the WHO standard definition. Both approaches have been used extensively in epidemiological studies, but do not fully avoid misclassification and biased disease estimates. Recall bias could have increased the estimated hazard ratio of ALRI for the diarrhoea time window immediately prior to the index day. Mothers caring for a child with ALRI may have been more inclined to report diarrhoea during the time since the last visit than those with a healthy child. The risk for recall bias may be more pronounced in Ghana, where field workers only visited once per week rather than two to three times per week as in Brazil. Recall bias may therefore explain some of the association between diarrhoea during the 7 days before a field worker visit, but is unlikely to have affected the estimates including diarrhoea days reported at the previous visit or earlier where no ALRI was present. In Ghana, we found a fairly linear dose–response relationship between the number of diarrhoea days for up to 28 days of diarrhoea (Figure 3) which is well beyond 7 diarrhoea days and cannot be explained by recall bias alone.
We used an outcome definition similar to the definitions recommended by WHO for the presumptive treatment of ALRI. There are inherent methodological limitations in defining diarrhoea and ALRI in large field studies.18,23 Thorough clinical examination and imaging by medical staff are often not possible in the field, where presumptive treatment of signs likely to be due to ALRI predominates. Due to the low specificity and sensitivity of most surveillance methods, misclassification of disease status is unavoidable, even when using (as in our analysis) stringent criteria for severe ALRI resulting in comparatively low figures for overall risk (Table 1).19 Non-differential misclassification of diarrhoea may have decreased the size of a potential association between the two conditions; differential misclassification may have biased the estimate in either direction.24 Misclassification of ALRI may have lowered the precision of the estimate. In some cases of severe diarrhoea, hypovolaemic or septic shock may cause metabolic acidosis with rapid breathing misclassified as ALRI, which could have inflated the effect sizes seen.3 However, we think it is unlikely that this could account for a substantial proportion of the estimate, since rapid breathing due to metabolic acidosis represents a relatively rare, as well as short-lived and terminal sign of diarrhoea, unlikely to be regularly picked up by weekly field worker visits. Also, metabolic acidosis is unlikely to cause danger signs of ALRI, on which ALRI definition was also dependent.
By stratifying the analysis by the individual ALRI risk, and adjusting for diarrhoea longitudinal prevalence, we believe we have minimized the potential for confounding by common underlying risk factors for diarrhoea and ALRI. Residual confounding should have affected the estimate at all time lags, not exclusively those close to the index day. To confirm the temporal association between diarrhoea and subsequent ALRI we reversed the time axis in the Ghana data, by simply moving the time window forward in time from the index day. There was no evidence that diarrhoea in the 14 days after the index day was associated with ALRI (HR = 0.99, 95% CI 0.93–1.05), which lends supports to a causative role of diarrhoea.
On the whole, we believe that methodological limitations of the analysis are a possible, but not the most likely, reason for the pronounced differences in the relationship between diarrhoea and ALRI identified between the two child populations from Ghana and Brazil. Differences in the demographic, nutritional and socio-economic profile are in our view a more plausible explanation. While we acknowledge that methodological limitations potentially inflated the effect size, the population attributable fraction resulting from the Ghana data may on the other hand underestimate the true effect of diarrhoea on ALRI. Our analysis only allowed us to explore the short-term association between diarrhoea and ALRI. The longer-term association between diarrhoea, malnutrition and subsequent risk of ALRI is difficult to study and would require close follow up of a large number of children over several years and careful consideration of a wide range of potential confounders, data that are usually not available in settings where the problem is greatest. Despite high biological plausibility, it remains unknown whether the long-term effect of frequent and severe diarrhoea episodes on the nutritional status of children25 also increases the risk of ALRI. There is a need for further large-scale longitudinal studies to confirm our findings and explore long-term effects.
Bearing in mind that ALRI and diarrhoea probably represent the two most common proximate causes of child mortality worldwide, an association between the two conditions as suggested by our analysis would have considerable public health implications. Our analysis reinforces the idea that ALRI is largely the endpoint of a long chain of causal factors,26 including poverty, nutrition and environmental health (water, sanitation, indoor air pollution). According to our results, diarrhoea may be an important mediator in this causal chain in very poor settings. Global figures on causes of child mortality, which often place ALRI or pneumonia at the top of the list,27 need to be seen in this light. While specific measures to reduce ALRI incidence and case fatality such as vaccination28,29 and case treatment30 proved effective and worthwhile, this analysis underscores that the underlying causes may be the ultimate problem.
Funding
Wellcome Trust, UK (WT082569AIA).
Acknowledgements
The authors are grateful to Saul Morris and David Ross for providing data and comments; to Lucy Smith, Simon Cousens and Kim Mulholland for comments on the draft.
Conflict of interest: None declared.
Key Messages.
Children in poor settings with frequent diarrhoea episodes are often found to be also at high risk of ALRI, but it is unclear whether this is due to common risk factors or whether diarrhoea can increase the risk of ALRI directly.
In this analysis, we found that diarrhoea may increase the risk of ALRI over a vulnerable period of 2–4 weeks in malnourished child populations. The results suggest that prevention of diarrhoea may contribute to a reduction in ALRI, the leading immediate cause of death in children.
References
- 1.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003;361:2226–34. doi: 10.1016/S0140-6736(03)13779-8. [DOI] [PubMed] [Google Scholar]
- 2.Fenn B, Morris SS, Black RE. Comorbidity in childhood in northern Ghana: magnitude, associated factors, and impact on mortality. Int J Epidemiol. 2005;34:368–75. doi: 10.1093/ije/dyh335. [DOI] [PubMed] [Google Scholar]
- 3.Mulholland K. Commentary: comorbidity as a factor in child health and child survival in developing countries. Int J Epidemiol. 2005;34:375–77. doi: 10.1093/ije/dyi028. [DOI] [PubMed] [Google Scholar]
- 4.Guerrant RL, Schorling JB, Mcauliffe JF, de Souza MA. Diarrhea as a cause and an effect of malnutrition: diarrhea prevents catch-up growth and malnutrition increases diarrhea frequency and duration. Am J Trop Med Hyg. 1992;47:28–35. doi: 10.4269/ajtmh.1992.47.28. [DOI] [PubMed] [Google Scholar]
- 5.Lutter CK, Mora JO, Habicht JP, et al. Nutritional supplementation: effects on child stunting because of diarrhea. Am J Clin Nutr. 1989;50:1–8. doi: 10.1093/ajcn/50.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Morris SS, Cousens SN, Kirkwood BR, Arthur P, Ross DA. Is prevalence of diarrhea a better predictor of subsequent mortality and weight gain than diarrhea incidence? Am J Epidemiol. 1996;144:582–88. doi: 10.1093/oxfordjournals.aje.a008968. [DOI] [PubMed] [Google Scholar]
- 7.Rowland MG, Rowland SG, Cole TJ. Impact of infection on the growth of children from 0 to 2 years in an urban West African community. Am J Clin Nutr. 1988;47:134–38. doi: 10.1093/ajcn/47.1.134. [DOI] [PubMed] [Google Scholar]
- 8.Torres AM, Peterson KE, de Souza AC, Orav EJ, Hughes M, Chen LC. Association of diarrhoea and upper respiratory infections with weight and height gains in Bangladeshi children aged 5 to 11 years. Bull World Health Organ. 2000;78:1316–23. [PMC free article] [PubMed] [Google Scholar]
- 9.Savitha MR, Nandeeshwara SB, Pradeep Kumar MJ, ul-Haque F, Raju CK. Modifiable risk factors for acute lower respiratory tract infections. Indian J Pediatr. 2007;74:477–82. doi: 10.1007/s12098-007-0081-3. [DOI] [PubMed] [Google Scholar]
- 10.Caulfield LE, de Onis M, Blossner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. 2004;80:193–98. doi: 10.1093/ajcn/80.1.193. [DOI] [PubMed] [Google Scholar]
- 11.Laxminarayan R, Chow J, Shahid-Salles SA. Intervention cost-effectiveness: overview of main messages. In: Jamison DT, Breman JG, Measham AR, editors. Disease Control Priorities in Developing Countries. New York: Oxford University Press and The World Bank; 2006. pp. 35–58. [PubMed] [Google Scholar]
- 12.Mitra AK, Khan MR, Alam AN. Complications and outcome of disease in patients admitted to the intensive care unit of a diarrhoeal diseases hospital in Bangladesh. Trans R Soc Trop Med Hyg. 1991;85:685–87. doi: 10.1016/0035-9203(91)90396-g. [DOI] [PubMed] [Google Scholar]
- 13.Islam SS, Khan MU. Risk factors for diarrhoeal deaths: a case–control study at a diarrhoeal disease hospital in Bangladesh. Int J Epidemiol. 1986;15:116–21. doi: 10.1093/ije/15.1.116. [DOI] [PubMed] [Google Scholar]
- 14.Sibal A, Patwari AK, Anand VK, Chhabra AK, Chandra D. Associated infections in persistent diarrhoea—another perspective. J Trop Pediatr. 1996;42:64–67. doi: 10.1093/tropej/42.2.64. [DOI] [PubMed] [Google Scholar]
- 15.Ghana VAST Study Team. Vitamin A supplementation in northern Ghana: effects on clinic attendances, hospital admissions, and child mortality. Lancet. 1993;342:7–12. [PubMed] [Google Scholar]
- 16.Barreto ML, Santos LM, Assis AM, et al. Effect of vitamin A supplementation on diarrhoea and acute lower-respiratory-tract infections in young children in Brazil. Lancet. 1994;344:228–31. doi: 10.1016/s0140-6736(94)92998-x. [DOI] [PubMed] [Google Scholar]
- 17.Baqui AH, Black RE, Sack RB, Yunus MD, Siddique AK, Chowdhury HR. Epidemiological and clinical characteristics of acute and persistent diarrhoea in rural Bangladeshi children. Acta Paediatr Suppl. 1992;381:15–21. doi: 10.1111/j.1651-2227.1992.tb12366.x. [DOI] [PubMed] [Google Scholar]
- 18.Lanata CF, Rudan I, Boschi-Pinto C, et al. Methodological and quality issues in epidemiological studies of acute lower respiratory infections in children in developing countries. Int J Epidemiol. 2004;33:1362–72. doi: 10.1093/ije/dyh229. [DOI] [PubMed] [Google Scholar]
- 19.Rudan I, Tomaskovic L, Boschi-Pinto C, Campbell H. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Health Organ. 2004;82:895–903. [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly PJ, Lim LL. Survival analysis for recurrent event data: an application to childhood infectious diseases. Stat Med. 2000;19:13–33. doi: 10.1002/(sici)1097-0258(20000115)19:1<13::aid-sim279>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Lin DY, Wei LJ. The robust inference for the Cox Proportional Hazard model. J Am Stat Assoc. 1989;84:1074–78. [Google Scholar]
- 22.Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C. Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol. 1985;122:904–14. doi: 10.1093/oxfordjournals.aje.a114174. [DOI] [PubMed] [Google Scholar]
- 23.Morris SS, Cousens SN, Lanata CF, Kirkwood BR. Diarrhoea—defining the episode. Int J Epidemiol. 1994;23:617–23. doi: 10.1093/ije/23.3.617. [DOI] [PubMed] [Google Scholar]
- 24.Copeland KT, Checkoway H, McMichael AJ, Holbrook RH. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol. 1977;105:488–95. doi: 10.1093/oxfordjournals.aje.a112408. [DOI] [PubMed] [Google Scholar]
- 25.Checkley W, Buckley G, Gilman RH, et al. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol. 2009;37:816–30. doi: 10.1093/ije/dyn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulholland K. Childhood pneumonia mortality—a permanent global emergency. Lancet. 2007;370:285–89. doi: 10.1016/S0140-6736(07)61130-1. [DOI] [PubMed] [Google Scholar]
- 27.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:408–16. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulholland K, Smith PG, Broome CV, Gaye A, Whittle H, Greenwood BM. A randomised trial of a Haemophilus influenzae type b conjugate vaccine in a developing country for the prevention of pneumonia—ethical considerations. Int J Tuberc Lung Dis. 1999;3:749–55. [PubMed] [Google Scholar]
- 29.Levine OS, O'Brien KL, Knoll M, et al. Pneumococcal vaccination in developing countries. Lancet. 2006;367:1880–82. doi: 10.1016/S0140-6736(06)68703-5. [DOI] [PubMed] [Google Scholar]
- 30.Marsh DR, Gilroy KE, Van de WR, Wansi E, Qazi S. Community case management of pneumonia: at a tipping point? Bull World Health Organ. 2008;86:381–89. doi: 10.2471/BLT.07.048462. [DOI] [PMC free article] [PubMed] [Google Scholar]