Abstract
Background Some studies suggest that weight gain in childhood may increase the risk of chronic diseases in adulthood, and recent studies have noticed that the timing of weight gain may be related to its long-term consequence. However, weight gain in childhood has clear short-term benefits, and the literature on the pro and cons of weight gain in childhood is limited.
Methods In 1982, all 5914 hospital births (over 99% of all deliveries) occurring in Pelotas, Southern Brazil, were identified and studied prospectively on several occasions. In 2004–05, we attempted to trace the whole cohort and information on offspring birthweight was collected. Conditional growth modelling was used to assess the association between offspring birthweight and weight gain from birth to 20 months, and from 20 to 42 months.
Results In 2004–05, we interviewed 4297 subjects, with a follow-up rate of 77.4%. This manuscript includes data from 848 women who had already delivered a child and 525 men who were fathers at the mean age of 23 years. Maternal birthweight, weight and length for age Z-score at 20 months of age were positively associated with next-generation birthweight, whereas paternal variables were not related to the outcome. Conditional growth modelling analyses showed that women whose weight gain in the first 20 months of life was faster than predicted had heavier babies, whereas paternal weight gain was not associated. The association was strongest for mothers whose birthweight for gestational age was in the lowest tertile.
Conclusion Maternal, but not paternal birthweight and weight gain in early childhood are positively associated with next-generation birthweight.
Keywords: Weight gain, intergenerational effect, birthweight
Introduction
Birthweight is an important determinant of child survival.1,2 It also has long-term consequences: adults born with a low birthweight have lower human capital3 and are at increased risk of some chronic diseases.4,5 The literature suggests that every 100 g increase in maternal birthweight leads to a 10–20 g gain in offspring birthweight.6–14 Paternal birthweight has also been associated with offspring birthweight, but this association is not as strong as for maternal birthweight.9,10,15–18
Fewer studies are available on the effect of parental post-natal growth on birthweight. Two reports from the UK have assessed the effects of leg length, a proxy for childhood nutritional status. Martin et al.14 observed that maternal height and leg length in childhood were positively associated with offspring birthweight, whereas Lawlor et al.19 reported that maternal leg length in adulthood was positively associated with offspring birthweight regardless of maternal birthweight. Hyppönen et al.10 used data from three generations of the 1958 British National Cohort and reported that maternal height at age 7 years, but not body mass index, was associated with offspring birthweight. A joint analysis of four cohorts from low-and middle-income countries, including our own, showed that maternal weight and height for age at around 2 years of age are positively associated with offspring birthweight.6 To our knowledge, no previous studies have assessed the associations of parental size or weight gain at different ages on offspring birthweight.
In developed countries, most small for gestational age (SGA) infants catch up in growth during the first years of life,20 whereas in developing countries this is not as common.1,21 Catch up has clear short-term benefits, including lower mortality and fewer hospital admissions.22 Higher systolic blood pressure has been reported among subjects who were lighter at birth and showed rapid weight gain after 4 years of age.23 Insulin resistance24 and endothelial dysfunction25 have also been reported to be related to rapid growth. This conflict between a positive effect of catch-up on child health and a detrimental one on adult health was described as ‘the catch-up dilemma’.26 More recent evidence suggests that the period when rapid weight gain occurs is important, because weight gain in the first 1–2 years of life may have beneficial effects whereas later weight gain is associated with higher risk of obesity and chronic diseases.6,27–29
The present study was aimed at assessing the association between rapid weight gain in different age ranges in early childhood and offspring birthweight. We also investigate possible interactions between parental weight gain and intrauterine growth restriction (IUGR) or stunting.
Methods
All 6011 hospital births occurring during the calendar year 1982 were identified in Pelotas, Brazil (current population 320 000) by the research team. The 5914 liveborns were examined and their mothers interviewed. Birthweight was recorded by the maternity hospital staff using calibrated scales; low birthweight was defined as <2500 g. Gestational age was calculated according to the recalled date of the mother's last menstrual period, and preterm birth was defined as gestational age <37 weeks. Children whose birthweight was below the 10th centile for gestational age and sex, according to the reference developed by Williams et al.30 were classified as having IUGR.
In 1984 (mean age 20 months) and 1986 (mean age 42 months) all households in the city were visited in search of cohort children; 87 and 84% of the original cohort were located, respectively. Standardized interviews were carried out in each round and children were weighed using a portable spring scale with an accuracy of ±100 g and had their length (1984) and height (1986) measured with a portable stadiometer.
From October 2004 to August 2005, we visited all households located in urban area of the city. For those who had not been located and were not known to have died, we used the last known address and existing databases (including universities, secondary schools and telephone directories) for another attempt. Subjects answered a questionnaire on socio-demographic, health and behavioural variables, as well as on whether they had had any children and birthweight of all children. In the present study, we used information from the first live-born child. Further details on the methodology of the study are available elsewhere.31
Because the ages of children seen at a given follow-up visit varied slightly, we used Z-scores of weight for age and sex, using the 2006 WHO growth standards.32 Stunting (low height for age) and underweight (low weight for age) were defined by using the –2 Z-score cut-off. Birthweight for gestational age Z-scores were also calculated. As Williams et al.30 did not provide the mean birthweight and standard deviation for each gestational age and sex group, the 50th centile was used as the mean birthweight, and the standard deviation was estimated by subtracting the 10th from the 50th centile and dividing by 1.28.
Mean offspring birthweight for different groups was compared using analysis of variance. Analysis of covariance (ANCOVA) was used to adjust for possible confounding variables, testing for heterogeneity and linear trend. The multivariable analysis was based on a conceptual model with three levels of determination: (i) socio-economic and demographic variables (family income at delivery, maternal schooling and maternal skin colour); (ii) birth condition (birthweight, gestational age and intrauterine growth); and (iii) nutritional status in childhood (height and weight/height Z-score). Because family income and maternal schooling are highly collinear, the latter was not included in the model that assessed the adjusted effect of family income. However, when assessing the effect of variables located in the other levels, all variables from the first level (maternal schooling, family income and maternal skin colour) were included in the model.
The analyses took into account the correlation between weight gain in subsequent age ranges, as well as regression to the mean, by using conditional growth modelling.33,34 First, birthweight for gestational Z-score was used to predict weight for age Z-score at 20 months; the residual or difference between actual and predicted weight Z-score, for each child, was calculated. The regression equation that assessed the effect of weight gain in the first 20 months on offspring birthweight included parental birthweight and this residual. Next, weight for age Z-score at 42 months was predicted from both birthweight and weight for age Z-score at 20 months; the equation for offspring birthweight included parental birthweight, the weight residual at 20 months and the weight residual at 42 months.
The following variables were also included in the analyses as potential confounders:
family income at delivery: total income earned by family members during the month before the interview;
parental schooling at delivery: years of schooling completed with success;
maternal smoking during pregnancy (non-smokers, 0–14 or 15 cigarettes or more per day);
breastfeeding duration: the age at which breastfeeding stopped completely.
Separate analyses were carried out for women and for men who had fathered a child by the 2004–05 visit. One does not expect the post-natal growth in males to affect offspring birthweight; their inclusion in the analyses is aimed at testing the specificity of findings relative to the growth of young women, thus contributing to rule out confounding effects.
The confidentiality of all information was ensured and informed consent was obtained in all phases of the study (verbal consent in the 1980s and written consent in 2000). The Medical Ethics Committee of the University of Pelotas, affiliated with the Brazilian Medical Research Council, approved the study protocol.
Results
In the 2004–05 follow-up visit, 4297 subjects were interviewed. Added to the 282 known to have died, they represented a follow-up rate of 77.4%. Table 1 shows that follow-up rates were independent of birthweight, sex and maternal skin colour. On the other hand, children born at either the upper or lower ends of family income distribution and those whose mother had ≥12 years of schooling were less likely to be traced in adulthood. At the 2004–05 visit, 848 women had already delivered a child, and 525 men were fathers. All analyses are restricted to these subgroups, who were considerably poorer than the cohort as a whole because early parenthood is associated with low socio-economic position (data not shown).
Table 1.
Percentage of cohort located in 2004–05 according to socio-economic and demographic variables
| Variable | Original cohort (number)a | Percent locatedb 2004—05 (%) |
|---|---|---|
| Sex | ||
| Boys | 3037 | 78 |
| Girls | 2876 | 77 |
| Birthweight (g) | ||
| <2500 | 534 | 77 |
| ≥2500 | 5375 | 78 |
| Monthly family income (US$) | ||
| ≤50 | 1288 | 75 |
| 51–150 | 2789 | 81 |
| 151–300 | 1091 | 76 |
| 301–500 | 382 | 68 |
| >500 | 335 | 74 |
| Maternal schooling (years) | ||
| 0–4 | 1960 | 78 |
| 5–8 | 2454 | 79 |
| 9–11 | 654 | 76 |
| ≥12 | 839 | 71 |
| Maternal skin colour | ||
| White | 4851 | 77 |
| Black | 1060 | 80 |
| Total | 5914a | 77.4 |
aUp to 29 subjects had missing information in baseline variables.
bIncludes subjects interviewed as well as those who are known to have died.
In 1982, the prevalence of low birthweight, preterm delivery and SGA among parous women were 8.0, 4.1 and 16.7%, respectively. About 16% of the women were stunted at 20 and 42 months of age. Offspring birthweight was positively associated with family income and maternal birthweight. After controlling for socio-economic status, the offspring of IUGR women were 252 g lighter than those whose birthweight for gestational age was above the Williams mean curve. Maternal stunting at 20 months was also associated with a lower birthweight in the next generation, even after controlling for confounding by socio-economic status and maternal birth conditions. On the other hand, stunting at 42 months and underweight in the childhood were not associated with offspring birthweight (Table 2).
Table 2.
Offspring birthweight according to maternal socio-economic status, birth condition and nutritional status in childhood
| Offspring birthweight Mean (95% CI) |
|||
|---|---|---|---|
| Maternal variables in childhood | n (%) | Crude | Adjusted |
| Monthly family income in tertiles | P = 0.002* | P = 0.007*,@ | |
| 1st tertile | 375 (44.2) | 3025 (2968–3082) | 3029 (2970–3087) |
| 2nd tertile | 309 (36.4) | 3118 (3056–3180) | 3114 (3051–3177) |
| 3rd tertile | 164 (19.3) | 3175 (3092–3259) | 3171 (3085–3257) |
| Birthweight (g) | P < 0.001* | P < 0.001*,§ | |
| <2500 | 68 (8.0) | 2958 (2817–3098) | 2962 (2817–3106) |
| 2500–2999 | 263 (31.1) | 2971 (2907–3034) | 2966 (2880–3053) |
| 3000–3499 | 307 (36.2) | 3116 (3051–3180) | 3110 (3029–3192) |
| ≥3500 | 209 (24.7) | 3238 (3166–3310) | 3225 (3133–3318) |
| Gestational age (weeks) | P = 0.95# | P = 0.89 #,§ | |
| <37 | 26 (4.1) | 3126 (2878–3374) | 3130 (2907–3353) |
| ≥37 | 605 (95.9) | 3119 (3075–3163) | 3115 (3040–3189) |
| Birthweight for gestational age Z-score | P < 0.001* | P < 0.001*,§ | |
| <−1.28 | 105 (16.7) | 2949 (2857–3042) | 2952 (2831–3072) |
| −1.28 to 0 | 300 (47.8) | 3109 (3045–3173) | 3107 (3020–3194) |
| >0 | 224 (35.6) | 3215 (3141–3288) | 3204 (3111–3297) |
| Length for age Z-score at mean age of 20 months | P < 0.001* | P = 0.02#,ŧ | |
| ≤−2 | 129 (16.6) | 2882 (2791–2973) | 2940 (2779–3100) |
| −1.99 to −1 | 223 (28.7) | 3102 (3033–3171) | 3137 (2979–3296) |
| >−1 | 424 (54.6) | 3148 (3093–3203) | 3129 (2980–3278) |
| Weight for age Z-score at mean age of 20 months | P < 0.001* | P = 0.06*,ŧ | |
| ≤−2 | 33 (4.3) | 2826 (2618–3033) | 2891 (2609–3172) |
| −1.99 to −1 | 92 (11.9) | 2956 (2841–3072) | 3001 (2821–3181) |
| >−1 | 649 (83.9) | 3122 (3079–3165) | 3101 (2960–3243) |
| Height for age Z-score at mean age of 42 months | P = 0.001* | P = 0.13*,ŧ | |
| ≤−2 | 113 (15.1) | 2953 (2853–3052) | 3017 (2823–3211) |
| −1.99 to −1 | 252 (33.6) | 3050 (2984–3116) | 3061 (2889–3232) |
| >−1 | 385 (51.3) | 3149 (3090–3208) | 3125 (2950–3299) |
| Weight for age Z-score at mean age of 42 months | P = 0.02* | P = 0.16*,ŧ | |
| ≤−2 | 30 (4.0) | 2927 (2699–3154) | 3064 (2802–3325) |
| −1.99 to −1 | 118 (15.7) | 2958 (2860–3056) | 2992 (2819–3166) |
| >−1 | 602 (80.3) | 3119 (3074–3165) | 3121 (2980–3261) |
| Total | 848 | ||
*Test for linear trend.
#Test for heterogeneity.
@Adjusted for maternal skin colour.
§Adjusted for maternal skin colour, maternal schooling and family income at delivery.
ŧAdjusted for maternal skin colour, maternal schooling and family income at delivery, birthweight and gestational age.
The association between maternal family income—measured at birth—and offspring birthweight was largely mediated through intrauterine growth retardation and stunting in childhood. After controlling for maternal intrauterine growth and length for age Z-score at 20 months, the mean difference between the third and the first tertile of family income was reduced from 142 to 34 g [95% confidence interval (CI): –97 to 165).
Unlike what was observed among women, paternal variables were not related to birthweight in the next generation (Table 3).
Table 3.
Offspring birthweight according to paternal socio-economic status, birth condition and nutritional status in childhood
|
Offspring birthweight Mean (95% CI) |
|||
|---|---|---|---|
| Paternal variables in childhood | n (%) | Crude | Adjusted |
| Monthly family income in tertiles | P = 0.40# | P = 0.36*,@ | |
| 1st tertile | 226 (43.0) | 3144 (3050–3238) | 3116 (3024–3209) |
| 2nd tertile | 211 (40.2) | 3208 (3116–3300) | 3130 (3020–3241) |
| 3rd tertile | 88 (16.8) | 3101 (2971–3231) | 3018 (2862–3174) |
| Birthweight (g) | P = 0.36# | P = 0.33#,§ | |
| <2500 | 32 (6.1) | 3279 (2988–3570) | 3230 (2983–3477) |
| 2500–2999 | 115 (21.9) | 3122 (2994–3250) | 3046 (2887–3206) |
| 3000–3499 | 206 (39.2) | 3121 (3030–3211) | 3071 (2942–3200) |
| ≥3500 | 172 (32.8) | 3215 (3114–3316) | 3155 (3014–3295) |
| Gestational age (weeks) | P = 0.25# | P = 0.29#,§ | |
| <37 | 18 (4.5) | 2985 (2582–3388) | 2916 (2593–3239) |
| ≥37 | 378 (95.5) | 3168 (3099–3237) | 3083 (2953–3214) |
| Weight for gestational age Z-score | P = 0.17* | P = 0.10*,§ | |
| <−1.28 | 75 (18.9) | 3122 (2960–3284) | 3021 (2818–3224) |
| −1.28 to 0 | 178 (44.9) | 3111 (3012–3211) | 3031 (2886–3176) |
| >0 | 143 (36.1) | 3233 (3117–3348) | 3160 (3001–3319) |
| Length for age Z-score at mean age of 20 months | P = 0.02# | P = 0.21#,ŧ | |
| ≤−2 | 89(18.6) | 3176(3035–3316) | 3006 (2756–3256) |
| −1.99 to −1 | 147 (30.8) | 3045 (2923–3167) | 2891 (2664–3119) |
| >−1 | 242 (50.6) | 3238 (3158–3318) | 3040 (2826–3254) |
| Weight for age Z-score at mean age of 20 months | P = 0.84# | P = 0.67*,ŧ | |
| ≤−2 | 19 (4.0) | 3097 (2704–3490) | 3128 (2683–3574) |
| −1.99 to −1 | 51 (10.7) | 3206 (2947–3464) | 2960 (2654–3265) |
| >−1 | 408 (85.4) | 3165 (3101–3228) | 2978 (2773–3182) |
| Height for age Z-score at mean age of 42 months | P = 0.03* | P = 0.05#,ŧ | |
| ≤−2 | 61 (13.0) | 3084 (2901–3268) | 2890 (2590–3190) |
| −1.99 to −1 | 135 (28.8) | 3068 (2939–3197) | 2800 (2600–3041) |
| >−1 | 273 (58.2) | 3231 (3153–3309) | 3015 (2835–3233) |
| Weight for age Z-score at mean age of 42 months | P = 0.64* | P = 0.52*,ŧ | |
| ≤−2 | 11 (2.4) | 3088 (2708–3469) | 3065 (2448–3683) |
| −1.99 to −1 | 63 (13.5) | 3138 (2931–3346) | 2980 (2706–3254) |
| >−1 | 394 (84.2) | 3170 (3102–3238) | 2923 (2704–3142) |
| Total | 525 | ||
*Test for linear trend.
#Test for heterogeneity.
@Adjusted for maternal skin colour.
§Adjusted for maternal skin colour, maternal schooling and family income at delivery.
ŧAdjusted for maternal skin colour, maternal schooling and family income at delivery, birthweight and gestational age.
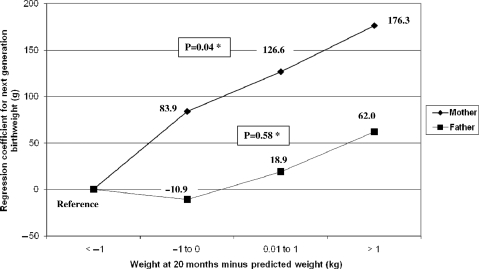
Table 4 shows the results of conditional growth modelling. Weight gain is expressed in Z-scores of the difference between actual weight and that predicted from earlier weights. Women whose weight gain in the first 20 months of life was faster than predicted had heavier babies, whereas paternal growth showed no such effect. No associations were observed between weight gain from 20 to 42 months for either mothers or fathers. In Figure 1, the same results are expressed in terms of kilograms, i.e. the equation predicted the weight in kilograms instead of weight for age Z-score. Women whose attained weight was >1 kg below what would be predicted delivered babies who were on average 176 g lighter than those whose attained weight was >1 kg above the prediction.
Table 4.
Conditional growth analyses of next-generation birthweight according to parental weight gain in childhood
|
Regression coefficient for next-generation birthweight (g) and 95% CI |
||||
|---|---|---|---|---|
| Mother |
Father |
|||
| Crude | Adjusted# | Crude | Adjusted# | |
| Weight at 20 months minus | P = 0.01& | P = 0.02& | P = 0.37& | P = 0.33& |
| predicted weight (Z-score) | 63.2 (15.0 to 111.4) | 61.8 (11.2–112.4) | 34.4 (−41.5 to 110.3) | 36.8 (−39.7 to 113.3) |
| Weight at 42 months minus | P = 0.93$ | P = 0.86$ | P = 0.64$ | P = 0.62$ |
| predicted weight (Z-score) | 2.2 (−45.8 to 50.2) | −4.4 (−52.7 to 43.9) | 17.6 (−57.3 to 92.5) | 19.0 (−56.1 to 94.1) |
#Adjusted for family income, maternal schooling, maternal smoking during pregnancy and breastfeeding duration.
&Also adjusted for birthweight.
$Also adjusted for birthweight and weight residual at 20 months.
Figure 1.
Next-generation birthweight according to parental weight gain from birth to 20 months. Adjusted for family income, maternal schooling, maternal smoking during pregnancy, breastfeeding duration and birthweight *Indicates test for linear trend
Table 5 shows that the effect of maternal weight gain in the first 20 months of life was strongest among women whose birthweight for gestational age Z-score was in the lower tertile (regression coefficient 126.9; 95% CI: 52.1–201.8), that is, those who showed catch-up growth. No associations were found for weight gain between 20 and 42 months.
Table 5.
Conditional growth analyses of next-generation birthweight according to maternal weight gain in childhood, by maternal birthweight for gestational age Z-score
|
Regression coefficient for next-generation birthweight (g) and 95% CI |
|||
|---|---|---|---|
| Maternal birthweight for gestational age Z-score |
|||
| 1st tertile | 2nd tertile | 3rd tertile | |
| Weight gain from birth to | P = 0.001& | P = 0.52& | P = 0.06& |
| mean age of 20 months (Z-score) | 126.9 (52.1 to 201.8) | −35.8 (−144.9 to 73.4) | 81.6 (−1.7 to 164.8) |
| Weight gain from mean age of 20 to | P = 0.36$ | P = 0.85$ | P = 0.42$ |
| 43 months (Z-score) | 33.8 (−39.3 to 106.8) | 10.0 (−96.6 to 116.6) | −32.2 (−110.7 to 46.3) |
#Adjusted for birthweight, family income, maternal schooling, maternal smoking during pregnancy and breastfeeding duration.
&Also adjusted for birthweight.
$Also adjusted for birthweight and weight residual at 20 months.
No associations were found for weight gain between 20 and 42 months (data not shown).
Independent of the length-for-age Z-score at 20 months of age, maternal weight gain from 20 to 42 months was not associated with offspring birthweight. Similar results were observed when the analysis was stratified according to weight/length Z-score at 20 months (data not shown).
Discussion
The prospective nature of the study, its population basis and the use of standardized methods for anthropometric evaluation in infancy reduced the likelihood of selection and information bias. Possible confounding factors were also measured in childhood. On the other hand, we relied on parental recall of the offspring birthweight, but previous studies in the city showed that recall was reasonably accurate, with about 80% of the mothers recalling the birthweight of their child within 100 g of the real value.35 This study also reported that recall was not related to family socio-economic status. Information bias would only affect our results if there was differential recall of birthweight according to parental birthweight or catch-up growth in early childhood. Furthermore, as previously described, our sample was poorer than the whole cohort. Because the effect of weight gain in childhood was similar among socio-economic categories, we do not believe that our results were biased because fertility was higher among low-income cohort members.
Concerning the intergenerational effect of maternal birthweight, the consistency with previous studies6–14 and the dose–response effect support the hypothesis of a causal association. This is reinforced by the specificity of the association, as paternal birthweight did not have an effect on the offspring.
The literature is scarce in relation to associations between parental nutritional status and offspring birthweight.6 To our knowledge, no studies investigated whether nutritional status or weight gain in different ages in childhood had similar effects on offspring birthweight. This is the main contribution of the present analyses. Our findings were specific for mothers, and an effect was detected at 20 but not at 42 months. Being heavier or taller at the age of 20 months, or gaining weight rapidly from birth to this age, was associated with higher birthweight in the next generation. The use of conditional growth models took into account the association between weight gain in subsequent age ranges, a problem that affects many studies of the long-term consequences of childhood growth. This finding reinforces the other beneficial effects of weight gain in the first 2 years of life in low- and middle-income countries.6
The classic use of the term ‘catch-up growth’ implies that the infant or child presents accelerated rates of growth following a period of growth failure.36 Nevertheless, this term has been frequently defined in reviews and research reports as weight or length gain above a certain parameter, independent from nutritional status at the beginning of the period.5 Most studies, therefore, have failed to separate the effects of catch-up growth from those of rapid growth per se. We were able to assess catch-up by stratifying the analysis according to birthweight-for-gestational age Z-score; and observed that the most marked effect of early weight gain occurred for mothers in the lowest tertile of birthweight for gestational age. On the other hand, there was no difference in the effect of weight gain from 20 to 42 months on offspring birthweight according to maternal stunting at 20 months.
Because the association between maternal weight gain in childhood and offspring birthweight, among those mothers whose birthweight was in the lowest tertile, persisted after controlling for maternal height, body mass index and parity (regression coefficient 102.0; 95% CI: 21.4–182.7), our results suggest that the effect of catch-up growth is not mediated by maternal size in adulthood or parity. There is some evidence that growth in early childhood is positively associated with vascular function.37,38 Therefore, weight gain in the first 2 years of life would influence vascularization of the mother's placenta, increasing offspring birthweight. A more plausible explanation concerns the size of the reproductive tract. Ibanez39 reported that uterine size is reduced in girls who are born small; hence, they may exert greater maternal constraint when they in turn become pregnant.
Previous analyses of our cohort data show that rapid weight gain in the first 2 years of life has short-term benefits on morbidity and mortality.22 A recent set of analyses from five low- and middle-income country cohorts, including our own,6 confirmed the associations between early nutritional status and human capital outcomes, and the importance of the critical growth window from conception to 2 years of life. This review also suggested that weight gain in later childhood may have more harmful than beneficial effects.6 Our results confirm these findings, showing for the first time that weight gain in the first 2 years, but not from 2 to 4 years, is associated with birthweight of the offspring. The prevention of early undernutrition is a valuable investment that will influence future generations as well as the present one.
Funding
World Health Organization (Department of Child and Adolescent Health); Wellcome Trust initiative entitled Major Awards for Latin America on Health Consequences of Population Change. 1982 cohort study was funded by the International Development Research Center (Canada); the World Health Organization (Department of Child and Adolescent Health and Development, and Human Reproduction Programme); the Overseas Development Administration (UK); United Nations Development Fund for Women; the National Program for Centers of Excellence (Brazil); the National Research Council (Brazil); Ministry of Health (Brazil).
Conflict of interest: None declared.
KEY MESSAGES.
Maternal birthweight and undernutrition in childhood are associated with next generation birthweight.
Early maternal weight gain (birth to 20 months), but not later weight gain (20–42 months), is associated with offspring birthweight.
Paternal weight gain showed no association with offspring birthweight.
References
- 1.Barros FC, Huttly SR, Victora CG, Kirkwood BR, Vaughan JP. Comparison of the causes and consequences of prematurity and intrauterine growth retardation: a longitudinal study in southern Brazil. Pediatrics. 1992;90:238–44. [PubMed] [Google Scholar]
- 2.Kramer MS, Victoria CG. Low birth weight and neonatal mortality. In: Semba RD, Bloem MW, editors. Nutrition and Health in Developing Countries. Totowa, NJ: Humana Press; 2008. pp. 63–86. [Google Scholar]
- 3.Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–57. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drake AJ, Walker BR. The intergenerational effects of fetal programming: non-genomic mechanisms for the inheritance of low birth weight and cardiovascular risk. J Endocrinol. 2004;180:1–16. doi: 10.1677/joe.0.1800001. [DOI] [PubMed] [Google Scholar]
- 5.Adair L, Dahly D. Developmental determinants of blood pressure in adults. Annu Rev Nutr. 2005;25:407–34. doi: 10.1146/annurev.nutr.25.050304.092538. [DOI] [PubMed] [Google Scholar]
- 6.Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–57. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klebanoff MA, Graubard BI, Kessel SS, Berendes HW. Low birth weight across generations. JAMA. 1984;252:2423–27. [PubMed] [Google Scholar]
- 8.Little RE. Mother's and father's birthweight as predictors of infant birthweight. Paediatr Perinat Epidemiol. 1987;1:19–31. doi: 10.1111/j.1365-3016.1987.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 9.Veena SR, Kumaran K, Swarnagowri MN, et al. Intergenerational effects on size at birth in South India. Paediatr Perinat Epidemiol. 2004;18:361–70. doi: 10.1111/j.1365-3016.2004.00579.x. [DOI] [PubMed] [Google Scholar]
- 10.Hypponen E, Power C, Smith GD. Parental growth at different life stages and offspring birthweight: an intergenerational cohort study. Paediatr Perinat Epidemiol. 2004;18:168–77. doi: 10.1111/j.1365-3016.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- 11.Emanuel I, Kimpo C, Moceri V. The association of maternal growth and socio-economic measures with infant birthweight in four ethnic groups. Int J Epidemiol. 2004;33:1236–42. doi: 10.1093/ije/dyh269. [DOI] [PubMed] [Google Scholar]
- 12.Godfrey KM, Barker DJ, Robinson S, Osmond C. Maternal birthweight and diet in pregnancy in relation to the infant's thinness at birth. Br J Obstet Gynaecol. 1997;104:663–67. doi: 10.1111/j.1471-0528.1997.tb11975.x. [DOI] [PubMed] [Google Scholar]
- 13.Ramakrishnan U, Martorell R, Schroeder DG, Flores R. Role of intergenerational effects on linear growth. J Nutr. 1999;129:544S–4S. doi: 10.1093/jn/129.2.544S. [DOI] [PubMed] [Google Scholar]
- 14.Martin RM, Smith GD, Frankel S, Gunnell D. Parents’ growth in childhood and the birth weight of their offspring. Epidemiology. 2004;15:308–16. doi: 10.1097/01.ede.0000120042.16363.e3. [DOI] [PubMed] [Google Scholar]
- 15.Emanuel I, Filakti H, Alberman E, Evans SJ. Intergenerational studies of human birthweight from the 1958 birth cohort. 1. Evidence for a multigenerational effect. Br J Obstet Gynaecol. 1992;99:67–74. doi: 10.1111/j.1471-0528.1992.tb14396.x. [DOI] [PubMed] [Google Scholar]
- 16.Klebanoff MA, Mednick BR, Schulsinger C, Secher NJ, Shiono PH. Father's effect on infant birth weight. Am J Obstet Gynecol. 1998;178:1022–26. doi: 10.1016/s0002-9378(98)70542-3. [DOI] [PubMed] [Google Scholar]
- 17.Coutinho R, David RJ, Collins JW., Jr Relation of parental birth weights to infant birth weight among African Americans and whites in Illinois: a trans-generational study. Am J Epidemiol. 1997;146:804–9. doi: 10.1093/oxfordjournals.aje.a009197. [DOI] [PubMed] [Google Scholar]
- 18.Magnus P, Gjessing HK, Skrondal A, Skjaerven R. Paternal contribution to birth weight. J Epidemiol Community Health. 2001;55:873–77. doi: 10.1136/jech.55.12.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawlor DA, Davey Smith G, Ebrahim S. Association between leg length and offspring birthweight: partial explanation for the trans-generational association between birthweight and cardiovascular disease: findings from the British Women's Heart and Health Study. Paediatr Perinat Epidemiol. 2003;17:148–55. doi: 10.1046/j.1365-3016.2003.00479.x. [DOI] [PubMed] [Google Scholar]
- 20.Karlberg J, Albertsson-Wikland K. Growth in full-term small-for-gestational-age infants: from birth to final height. Pediatr Res. 1995;38:733–39. doi: 10.1203/00006450-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Kebede A, Larson C. The health consequences of intrauterine growth retardation in southwestern Ethiopia. Trop Doct. 1994;24:64–69. doi: 10.1177/004947559402400207. [DOI] [PubMed] [Google Scholar]
- 22.Victora CG, Barros FC, Horta BL, Martorell R. Short-term benefits of catch-up growth for small-for-gestational-age infants. Int J Epidemiol. 2001;30:1325–30. doi: 10.1093/ije/30.6.1325. [DOI] [PubMed] [Google Scholar]
- 23.Horta BL, Barros FC, Victora CG, Cole TJ. Early and late growth and blood pressure in adolescence. J Epidemiol Community Health. 2003;57:226–30. doi: 10.1136/jech.57.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fewtrell MS, Doherty C, Cole TJ, Stafford M, Hales CN, Lucas A. Effects of size at birth, gestational age and early growth in preterm infants on glucose and insulin concentrations at 9–12 years. Diabetologia. 2000;43:714–17. doi: 10.1007/s001250051368. [DOI] [PubMed] [Google Scholar]
- 25.Singhal A, Cole TJ, Fewtrell M, Deanfield J, Lucas A. Is slower early growth beneficial for long-term cardiovascular health? Circulation. 2004;109:1108–13. doi: 10.1161/01.CIR.0000118500.23649.DF. [DOI] [PubMed] [Google Scholar]
- 26.Victora CG, Barros FC. Commentary: the catch-up dilemma—relevance of Leitch's low-high' pig to child growth in developing countries. Int J Epidemiol. 2001;30:217–20. doi: 10.1093/ije/30.2.217. [DOI] [PubMed] [Google Scholar]
- 27.Fisher D, Baird J, Payne L, et al. Are infant size and growth related to burden of disease in adulthood? A systematic review of literature. Int J Epidemiol. 2006;35:1196–210. doi: 10.1093/ije/dyl130. [DOI] [PubMed] [Google Scholar]
- 28.Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–9. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 29.Horta BL, Sibbritt DW, Lima RC, Victora CG. Weight catch-up and achieved schooling at 18 years of age in Brazilian males. Eur J Clin Nutr. 2009;63:369–74. doi: 10.1038/sj.ejcn.1602934. [DOI] [PubMed] [Google Scholar]
- 30.Williams RL, Creasy RK, Cunningham GC, Hawes WE, Norris FD, Tashiro M. Fetal growth and perinatal viability in California. Obstet Gynecol. 1982;59:624–32. [PubMed] [Google Scholar]
- 31.Victora CG, Barros FC. Cohort profile: the 1982 Pelotas (Brazil) birth cohort study. Int J Epidemiol. 2006;35:237–42. doi: 10.1093/ije/dyi290. [DOI] [PubMed] [Google Scholar]
- 32.de Onis M, Onyango AW. WHO child growth standards. Lancet. 2008;371:204. doi: 10.1016/S0140-6736(08)60131-2. [DOI] [PubMed] [Google Scholar]
- 33.Osmond C, Kajantie E, Forsen TJ, Eriksson JG, Barker DJ. Infant growth and stroke in adult life: the Helsinki birth cohort study. Stroke. 2007;38:264–70. doi: 10.1161/01.STR.0000254471.72186.03. [DOI] [PubMed] [Google Scholar]
- 34.Keijzer-Veen MG, Euser AM, van Montfoort N, Dekker FW, Vandenbroucke JP, Van Houwelingen HC. A regression model with unexplained residuals was preferred in the analysis of the fetal origins of adult diseases hypothesis. J Clin Epidemiol. 2005;58:1320–24. doi: 10.1016/j.jclinepi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Victora CG, Barros FC, Martines JC, Beria JU, Vaughan JP. As mães lembram o peso ao nascer de seus filhos ? Rev Saude Publica. 1985;19:195–200. doi: 10.1590/s0034-89101985000300001. [DOI] [PubMed] [Google Scholar]
- 36.Tanner JM. Catch-up growth in man. Br Med Bull. 1981;37:233–38. doi: 10.1093/oxfordjournals.bmb.a071708. [DOI] [PubMed] [Google Scholar]
- 37.Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Early growth and coronary heart disease in later life: longitudinal study. BMJ. 2001;322:949–53. doi: 10.1136/bmj.322.7292.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kajantie E, Barker DJ, Osmond C, Forsen T, Eriksson JG. Growth before 2 years of age and serum lipids 60 years later: the Helsinki Birth Cohort study. Int J Epidemiol. 2008;37:280–89. doi: 10.1093/ije/dyn012. [DOI] [PubMed] [Google Scholar]
- 39.Ibanez L, Potau N, Enriquez G, de Zegher F. Reduced uterine and ovarian size in adolescent girls born small for gestational age. Pediatr Res. 2000;47:575–77. doi: 10.1203/00006450-200005000-00003. [DOI] [PubMed] [Google Scholar]