Abstract
Acute recurrent pancreatitis is a clinical entity largely associated with pancreatic ductal obstruction. This latter includes congenital variants, of which pancreas divisum is the most frequent but also controversial, chronic pancreatitis, tumors of the pancreaticobiliary junction and sphincter of Oddi dysfunction. This review summarizes current knowledge about diagnostic work-up and therapy of these conditions.
Keywords: Pancreatitis, Congenital variants, Sphincter of Oddi dysfunction, Pancreatic tumors, Magnetic resonance cholangiopancreatography, Endoscopic retrograde cholangiopancreatography
INTRODUCTION
Acute recurrent pancreatitis (ARP) represents a clinical entity in which acute pancreatitis occurs more than once in the setting of a normal morpho-functional pancreas[1].
The diagnosis of acute pancreatitis is generally based on clinical and laboratory findings. Imaging studies are obtained to confirm clinical diagnosis, to rule out mechanical factors that may induce a transient obstruction of pancreatic juice flow, to assess the extension of the inflammatory process in and around the pancreas, and to detect possible complications.
In patients presenting with ARP and a normal gland visualized on transabdominal ultrasound and abdominal CT, a primary role for secretin-enhanced magnetic resonance cholangiopancreatography (S-MRCP) has been suggested[2].
Indeed, the exogenous administration of secretin stimulates the secretion of fluid and bicarbonate by the exocrine pancreas[3]. Consequently, the volume of fluid in the pancreatic ducts increases which permits a more accurate visualization of the pancreatic ductal system, a better detection of anatomical variants, an evaluation of the pancreatic flow dynamics and therefore an indirect assessment of the sphincter of Oddi and of the pancreatic exocrine reserve[4].
S-MRCP offers several advantages over endoscopic retrograde cholangiopancreatography (ERCP) such as its non invasiveness, the absence of procedure-related complications, the absence of contrast injection or radiation exposure and its performance in post-surgical patients. Secretin administration is safe, even in the setting of acute pancreatitis[5] but additional time and cost are required, therefore restricting the use of secretin in warranted indications i.e. assessment of ARP.
ERCP should be performed only in those patients in whom the etiology of pancreatitis cannot be achieved by S-MRCP (for inspection of the papilla, for brush cytology and biopsy sampling, for bile or pancreatic juice aspiration) or for therapeutic purposes.
An etiology can be found in 70%-80% of patients after an attack of acute pancreatitis, with alcohol abuse and gallstone disease most often implicated[6,7]. Any factor capable of causing an initial episode of acute pancreatitis has the potential to initiate recurrent episodes[2].
The purpose of this review focuses on mechanical factors that promote recurrent episodes of pancreatitis by inducing a persistent or transient obstruction to pancreatic juice flow into the duodenum with a subsequent rise in intraductal pancreatic pressure.
Potential causes of pancreatic ductal obstruction include: (1) anatomical congenital variations of the biliopancreatic ductal system; (2) acquired obstructive conditions at the level of major/minor papilla, the level of the main pancreatic duct (MPD), or the level of the duodenal wall; (3) pancreatic sphincter of Oddi dysfunction (SOD) (Table 1).
Table 1.
Potential causes of pancreatic ductal obstruction in acute recurrent pancreatitis
| Potential causes | Diagnostic evaluation patient selection | Potential treatment strategies |
| Congenital variants | ||
| -PD ± Santorinicele | S-MRCP | ERCP ± EPS at the minor papilla |
| ± transient dorsal duct stenting | ||
| -APBU | S-MRCP | ES |
| -Choledochocele | S-MRCP | EBS |
| -TypeI& IV choledochal cyst | S-MRCP | Surgical resection + HJ |
| -Duodenal duplication cyst | S-MRCP | Endoscopic snare resection |
| or surgical resection | ||
| -Annular pancreas | S-MRCP | ERCP + EPS |
| or surgical gastrojejunostomy | ||
| Acquired obstructive conditions | ||
| -Suspected neoplasm | ||
| ampullary tumor | EUS, S-MRCP | Curative/palliative endotherapy |
| MPD stricture | EUS, S-MRCP | or |
| IPMT | EUS, S-MRCP | surgery |
| -Suspected early CP | EUS, S-MRCP | ERCP + EPS |
| ± transient MPD stenting | ||
| -Groove pancreatitis (CDDW) | EUS, S-MRCP | ERCP + dual ES + MPD stenting |
| or surgery or somatostatin | ||
| -Pancreatic SOD typeI | ||
| Type II | S-MRCP | ERCP + dual ES + transient MPD stenting |
| Type III |
PD: Pancreas divisum; APBU: Anomalous pancreaticobiliary union; MPD: Main pancreatic duct; IPMT: Intraductal papillary mucinous tumor; CP: Chronic pancreatitis; CDDW: Cystic dystrophy of the duodenal wall; SOD: Sphincter of Oddi dysfunction; S-MRCP: Secretin-enhanced magnetic resonance cholangiopancreatography; EUS: Endoscopic ultrasonography; ERCP: Endoscopic retrograde cholangiopancreatography; EPS: Endoscopic pancreatic sphincterotomy; ES: Endoscopic sphincterotomy; EBS: Endoscopic biliary sphincterotomy; HJ: Hepaticojejunostomy.
A detailed discussion of pancreatic ductal system obstruction as specific causes of ARP follows with emphasis on recent developments.
ANATOMICAL VARIATIONS OF THE BILIOPANCREATIC DUCTAL SYSTEM
Pancreas divisum
Pancreas divisum (PD) is the most common congenital variant of the pancreas, affecting 5%-14% of the Caucasian population[6,8–10]. This variant occurs when the embryological ventral and dorsal ducts fail to fuse during the second month of life in utero. In this case, the ventral duct only drains the ventral pancreas through the major papilla, whereas the majority of the pancreas drains via the dorsal duct through the minor papilla. The diagnosis of PD can be done safely and accurately by S-MRCP showing the dorsal pancreatic duct crossing the common bile duct anteriorly and separated from a smaller ventral duct[11] (Figure 1). A relative obstruction at the small papillary orifice of the minor papilla overburdened by draining the larger dorsal pancreas has been proposed more than 25 years ago as the mechanism of pancreatitis associated with PD[12].
Figure 1.
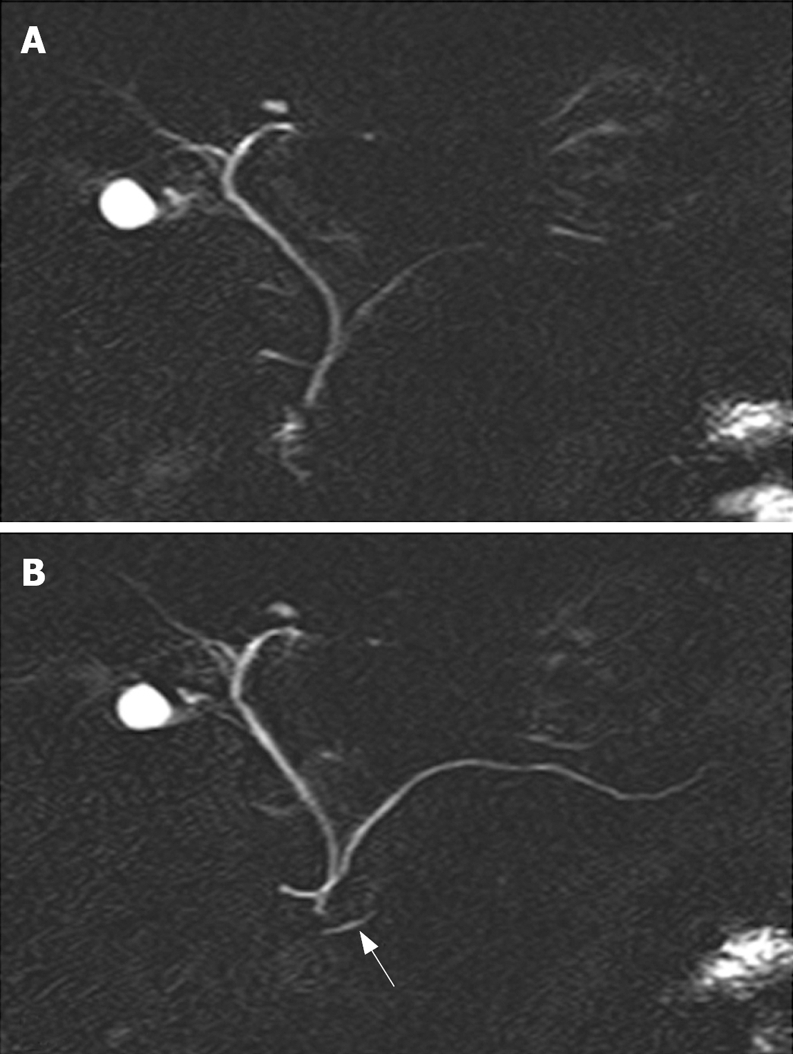
Normal pancreas divisum. Unenhanced (A) and secretin-enhanced magnetic resonance cholangiopancreatography (B). The ventral pancreatic duct (arrow in B) and the entire course of the main dorsal pancreatic duct are seen only after secretin administration.
However, the clinical significance of PD remains controversial. Indeed, only a minority of patients with PD (less than 5%) becomes symptomatic with ARP, chronic pancreatitis or chronic abdominal pain[10,13].
In a large ERCP series, we failed to find a significantly increased frequency of pancreatic disease among patients with PD compared with control patients[14].
Recruitment bias (greater frequency of PD diagnosis in patients referred after unsuccessful opacification of the pancreatic ductal system) may have resulted in an overestimation of the prevalence of PD in previous other ERCP studies investigating ARP and PD[15].
More recently, in an S-MRCP study (where the bias of referral to a tertiary center after failure of ERCP was avoided), the frequency of PD was similar in 54 control patients, in 68 patients with established chronic pancreatitis and in 157 patients in whom pancreatic disease was suspected on the basis of idiopathic acute pancreatitis (n = 67), increased serum levels of pancreatic enzymes (n = 42) or pancreatic-type pain (n = 48)[16].
As most patients with PD do not develop pancreatitis, a stenosis of the minor papilla or a Santorinicele (Figure 2) (defined as a cystic dilatation of the distal dorsal duct just proximal to the minor papilla and considered as resulting from a combination, either acquired or congenital, of relative obstruction and weakness of the distal ductal wall) could be the additional necessary factors to cause an acute pancreatitis in a few patients with PD[17].
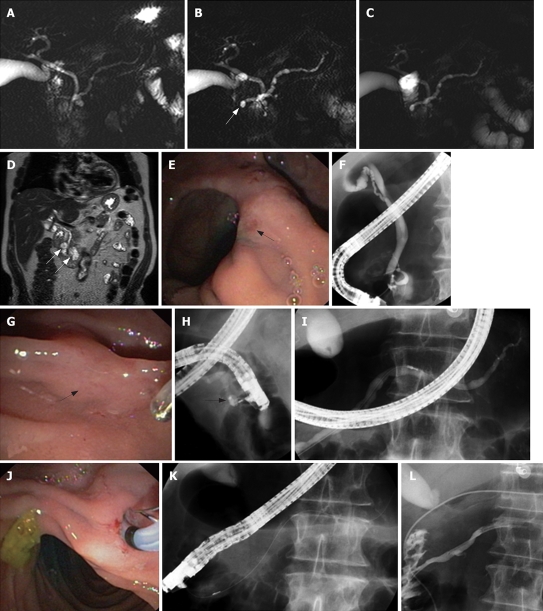
Figure 2.
An 80-year-old man presented with 3 attacks of acute pancreatitis over 5 yr. There was no history of alcohol abuse, smoking, nor medications. There were no gallbladder stones at transabdominal ultrasound and no common bile duct stones at endoscopic ultrasonography. Unenhanced (A) and secretin-enhanced magnetic resonance cholangiography (B) 3 min after secretin injection and C: 10 min after secretin injection). Ectasia of the minor papilla (Santorinicele) (arrow in B) is seen only after secretin administration. Dilatation of the main pancreatic duct persists 10 min after secretin stimulation (C), suggesting obstruction at the level of the minor papilla. Coronal view on T2-weighted magnetic resonance imaging (D) shows 2 paraduodenal diverticula (arrows in D). The major papilla is located on a fold between 2 diverticula (arrow in E), whereas the minor papilla is extremely difficult to locate even after secretin injection and methylene blue staining (arrow in G). During ERCP, cannulation of the major papilla allows opacification of a normal common bile duct and a short ventral pancreatic duct (F). Cannulation of the minor papilla using the needle, in the long scope position, allows opacification of a cystic dilatation of the distal part of the dorsal pancreatic duct (Santorinicele, arrow in H). The main dorsal pancreatic duct is dilated (maximal diameter: 8.3 mm after correction for magnification) (I). Endoscopic minor papillotomy is performed with a standard pull-type sphincterotome (J-K) and subsequently a 6 Fr nasopancreatic catheter is inserted for 24 h (L). After sphincterotomy, contrast medium flows freely alongside the nasopancreatic catheter into the duodenum.
Identification of a Santorinicele at S-MRCP (Figure 2B) or a minor papillary stenosis (suggested by an abnormal response to secretin, i.e. persistent dilatation of the MPD greater than 3 mm at 10 min after secretin injection in patients younger than 60 years) might be helpful in selecting a subset of patients with PD and ARP who might benefit from pancreatic ductal drainage[16–18].
Indeed, patients with PD and Santorinicele showed a larger MPD in the head of the gland compared with that in patients with only PD (compare Figures 1B and 2B), both at rest and after secretin stimulation[17]. Moreover, the mean interval between the injection of secretin and the onset of duodenal filling was significantly longer in patients with Santorinicele compared with that in patients without Santorinicele[17]. These observations suggest the presence of impeded pancreatic secretion outflow at the level of the minor papilla with subsequent increased intraductal pressure.
In a prospective study including 279 patients investigated by S-MRCP, an abnormal response of the MPD to secretin stimulation could be detected in 12% of the patients with ARP but the dynamics of pancreatic duct filling after secretin stimulation did not differ between patients with or without PD[16]. Whether the observation of an abnormal response of the dorsal pancreatic duct to secretin stimulation at S-MRCP is clinically relevant still remains to be demonstrated.
There is also no consensus regarding the appropriate treatment for ARP associated with PD but available data suggest that some patients may benefit from dorsal duct drainage[19]. For example, it was reported that patients with Santorinicele may benefit from endoscopic papillotomy of the minor papilla (Figure 2G-L) as evidenced by a remarkable reduction in size of the MPD and of the Santorinicele on follow-up S-MRCP images and by symptomatic improvement[17]. The same might be true for patients with a persistent ductal dilation after secretin administration visualized at MRCP or ultrasound.
Dorsal duct drainage could be achieved by surgical sphincteroplasty of the minor papilla[20] or endoscopic therapy including minor papilla sphincterotomy, transient dorsal duct stenting or combination[13,19,21]. The overall success rate of endoscopic therapy is similar to the results of surgical sphincteroplasty. Patients with well defined bouts of pancreatitis had a significantly higher response rate (70% to 80%) to dorsal duct drainage than those with chronic pancreatitis or chronic abdominal pain[13,22].
Endoscopic minor papillotomy may be performed with a standard pull-type sphincterotome (Figure 2J-K) and subsequent placement of a 5 Fr unflanged stent into the dorsal pancreatic duct to protect against early scarring and post-ERCP pancreatitis, or with a needle knife over a previously placed dorsal pancreatic duct stent. The stent should be removed within 2-4 wk if it has not migrated spontaneously into the duodenal lumen to avoid stent-induced damage to the pancreatic duct[21].
The majority of therapeutic trials are small, retrospective case series with only one randomized controlled trial on 19 patients presenting with ARP and PD, showing a clinical benefit after dorsal duct stenting at a mean follow-up of 12 mo after retrieval of the stent[23]. However, prolonged stenting of the dorsal MPD should be avoided because of the risk of inducing pancreatic damage mainly when the ductal morphology is normal initially[22]. Complication and papillary restenosis rates were reported in 9% and 16% respectively among 69 patients with ARP and PD treated by pancreatic sphincterotomy of the minor papilla[21].
Further studies using S-MRCP should evaluate the outcome of minor papilla sphincterotomy in patients with PD and ARP in order to provide selection criteria that indicate which patients would be most amenable for therapy.
Other congenital variants
Various congenital abnormalities of the pancreaticobiliary system are associated with ARP[24]. In 1.5% to 3% of individuals there is an anomalous union of the pancreatic and bile ducts outside the duodenal wall, which results in an unusually long common channel measuring more than 15 mm proximal to the duodenum[25]. This common channel facilitates free reflux of bile and pancreatic juice into the alternative duct. Bile entering the pancreas may potentially induce acute pancreatitis by increasing intraductal pressure, and reflux of pancreatic enzymes into the bile duct predisposes to the development of the choledochal cyst[26].
Pathogenesis of ARP in cases of anomalous pancreaticobiliary union (APBU) has also been associated with temporary occlusion of pancreatic secretion by stone, protein plugs, or sphincter of Oddi dysfunction, all leading to a rise in pancreaticobiliary intraductal pressure[25]. Endoscopic ultrasonography and S-MRCP show a good overall accuracy for detecting ductal variations[27] and demonstrating associated reflux[28].
Acute pancreatitis was reported to occur in 3% to 31% of APBU patients[24]. It was usually mild and resolved in a few days with conservative treatment. Sphincter ablation by endoscopic sphincterotomy may decrease the risk of ARP[9].
Choledochocele is a rare congenital or acquired condition, depicted as a dilatation of the intraduodenal segment of the common bile duct. It was initially included in the Todani classification of the choledochal cysts as type III[29], but recently type II (diverticulum of the common bile duct related to a form of gallbladder duplication), type III (choledochocele) and type V (Caroli disease associated with congenital hepatic fibrosis) were considered as unrelated to choledochal cysts[30].
Pancreatitis develops when the cystic dilatation or its content (sludge or stones) obstruct pancreatic duct outflow. Recommended treatment consists of uprooting the choledochocele by endoscopic sphincterotomy[31].
TypesIand IV choledochal cysts (congenital dilatation of the extrahepatic bile ducts with a variable amount of intrahepatic involvement) account for more than 90% of the patients with choledochal cysts and among them, 16% presented with pancreatitis[30]. In such patients, the entire extrahepatic biliary tree and gallbladder should be removed with reconstruction by a Roux-en-Y hepaticojejunostomy to prevent the risk of further cholangiocarcinoma.
Duodenal duplication is a cyst-like structure bulging into the duodenal lumen just distal to the papillary orifice. The duodenal duplication cysts contain Brunner’s glands within their wall. The association of pancreatitis with duodenal duplication is infrequent[32]. Pancreatitis may result from occlusion of the pancreatic ductal system by the distended duodenal cyst filled with secretions or stones. Endoscopic snare resection of the top of the cyst has been described[33,34].
Annular pancreas refers to a part of pancreatic tissue partially or completely encircling the duodenum usually at the level of or just proximal to the major papilla. This anomaly occurs when the ventral bud fails to rotate with the duodenum during embryological development. This rare variant is often associated with duodenal or biliary obstructive symptoms and pancreatitis may affect the annulus or the remaining pancreas. Recommended treatment consists in gastrojejunostomy[35] in case of duodenal occlusion.
Congenital variants of the biliopancreatic ductal system provide interesting challenges when discovered during the diagnostic workup of ARP. However, most of these variants are clinically irrelevant. Therefore methods to select patients who are likely to benefit from therapy would be of the utmost importance.
ACQUIRED OBSTRUCTIVE CONDITIONS
Tumors of the pancreaticobiliary junction
It is estimated that 5% to 7% of patients with pancreaticobiliary tumors, benign or malignant, present with idiopathic ARP[9]. Mechanical blockage of the papillary orifice by ampullary tumors, may lead to painless jaundice, anemia, but also idiopathic ARP. Therefore careful papilla inspection for size and shape should be included in the diagnostic workup of ARP of unknown etiology and papilla biopsies should be taken when indicated.
Adenomas are the most common ampullary tumors and represent premalignant conditions which need endoscopic snare resection or surgical resection according to their size and histological grade[36]. Surveillance remains an option, especially for those occurring in the setting of familial adenomatous polyposis which are not symptomatic[37].
Similarly, unexplained ARP in a patient 45 years or older requires that an underlying pancreatic carcinoma be excluded[38]. The incidence of malignant pancreatic neoplasm as the underlying cause of acute pancreatitis is low (only 3%) in patients younger than 40 years of age compared to patients aged 40 to 60 years (21%) and to patients aged older than 60 years (25%)[39].
Endoscopic ultrasonography and S-MRCP are helpful procedures in assessing strictures of the MPD and associated parenchymal and ductal changes.
Cytologic and histologic intraductal sampling of pancreatic ductal strictures, tumoral molecular markers searched on pure pancreatic juice or brushing samples may help to unravel the suspicion of malignancy.
Chronic pancreatitis
ARP may also be the clinical presentation of early chronic pancreatitis (CP). Indeed, according to the recently proposed Sentinel Acute Pancreatitis Event (SAPE) hypothesis, repeated attacks of acute pancreatitis may evolve to a clinically chronic disease[40].
Early stages of chronic pancreatitis can be specifically detected by endoscopic ultrasonography (3 to 5 positive criteria among echogenic duct walls, irregular duct contour, dilated side branches, parenchymal echogenic foci or strands, lobularity of the gland,..)[41] and by S-MRCP (abnormal ductal response to secretin, progressive enhancement of the pancreatic parenchyma related to a loss of ductal or parenchymal compliance, characteristic side-branch involvement, changes in gadolinium uptake,…)[4,42].
It was shown that a substantial number of patients with ARP indeed have evidence of CP (42%-47%) during follow-up evaluation[41,43] and that CP was twice as frequent in patients with ARP compared with those with a single episode of pancreatitis[41].
Strictures of the MPD, generally due to inflammation or fibrosis around the MPD and obstructing pancreatic stones may contribute to abdominal pain or ARP in patients with CP. In these advanced stages of CP (which are not called ARP anymore), S-MRCP demonstrates the ductal anatomy, the degree and level of pancreatic duct obstruction, the associated complications (pseudocysts, common bile duct stricture) and the possible duodenal filling impairment suggesting pancreatic exocrine insufficiency. This imaging procedure is mainly useful before planning therapy and also for follow-up after relief of ductal obstruction[2].
Groove pancreatitis or cystic dystrophy of the duodenal wall is an uncommon type of CP affecting the "groove" between the head of the pancreas and the duodenum. The most characteristic finding is a sheet-like mass between the head of the pancreas and the thickened duodenal wall associated with cystic changes[44]. The cysts in the duodenal wall develop from foci of heterotrophic pancreas lying close to the pancreas. In a series of CP patients, the frequency of cystic dystrophy of the duodenal wall is to the order of 25% with a strong male predominance, a mean age of 45 years and frequent weight loss and vomiting associated to the ARP clinical presentation[45].
Endoscopic ultrasonography is the exploration of choice to search for cystic dystrophy of the duodenal wall. MR imaging can also visualize cystic formations and a band of low-intensity signals on T1 and T2 sequences, lying between the second portion of the duodenum and the pancreas, and corresponding to heterotopic pancreatic tissue.
Pancreaticoduodenectomy remains the most effective treatment since the diseased tissue is removed but endoscopic cystic and pancreatic ductal drainage and somatostatin analogs are also alternatively proposed therapies[44].
Pancreatic sphincter of Oddi dysfunction
Sphincter of Oddi dysfunction (SOD) is defined as a benign, non-calculous partial obstruction (organic or functional) of the biliary and/or the pancreatic segment of the sphincter of Oddi, giving rise to episodic upper abdominal pain or pancreatitis[46].
SOD is reported to be present in about 30% of patients with ARP of unknown etiology[7,9]. Organic or functional obstruction of the sphincter of Oddi may promote reflux of bile into the MPD or impairment of pancreatic duct outflow, causing pancreatitis.
Patients with pancreatic SOD may be classified similarly to those with biliary SOD[6]. Pancreatic typeISOD corresponds to a papillary stenosis and is thought when pancreatic-type pain or documented ARP are associated with elevated serum amylase/lipase and a dilated MPD (> 5 mm in the body of the pancreas). In pancreatic type II SOD, pancreatic-type pain or ARP are combined with one additional factor defining typeI, and pancreatic type III SOD includes patients having recurrent pancreatic type pain alone with none of the above findings[1,6,9].
TypeIis considered to be due to a chronic inflammatory process which becomes a fibrosis with subsequent stenosis of a part or the entire sphincter. Pancreatic type II SOD patients are thought to suffer from sphincter of Oddi dyskinesia that means a functional alteration of the physiological motility of the sphincter which causes some delay in the passage of pancreatic juice into the duodenum[47].
In suspected pancreatic type III SOD, an objective diagnosis of dysfunction should rely on sphincter of Oddi manometry (basal pancreatic sphincter pressure > 40 mmHg) or less invasively on MRCP evaluation of changes in the size of the MPD after secretin stimulation (1). However, data regarding pancreatic SOD are sparse, making the management of this disorder even more controversial than its biliary counterpart[13].
Nowadays, sphincter of Oddi manometry tends to be replaced by the non-invasive S-MRCP[48,49] providing information regarding the sphincter of Oddi function by measuring changes in MPD diameter over time, after secretin injection. At S-MRCP, the diagnosis of pancreatic SOD may be suggested when MPD diameter remains increased by greater than 1 mm compared with baseline, throughout the 15 min testing interval. Based on MPD kinetics at S-MRCP, Mariani et al found concordant results between S-MRCP and sphincter of Oddi manometry in 13 of 15 patients with idiopathic ARP[48]. Moreover, a recent study suggests that S-MRCP may be useful in selecting those patients with suspected SOD who might benefit from endotherapy[49]. Interestingly, S-MRCP could replace sphincter of Oddi manometry more effectively in type II SOD than in type III, this latter group being the most controversial for potential therapy[49].
Available data suggest that, in suspected pancreatic SOD (on the basis of either a basal dilatation of the MPD, or an abnormal dynamic response of the pancreatic duct at S-MRCP), a dual endoscopic biliary and pancreatic sphincterotomy, whether at single or separate sessions, yields significantly better response than endoscopic biliary sphincterotomy alone[50]. However, patients with SOD have a complication rate from endoscopic sphincterotomy 5 times higher than that of patients with bile duct stones[51]. Placement of a transient pancreatic stent reduces this risk especially when the accessory duct is not patent[52]. This stent usually migrates spontaneously but in case migration does not occur, it should be removed 10-14 d following dual endoscopic sphincterotomy.
CONCLUSION
ARP should be evaluated in referral centers as diagnosis is time-consuming, usually expensive and may expose the patient to a substantial morbidity. Initial modalities of choice involve CT scan and S-MRCP to detect alterations in the pancreatic parenchyma including calcifications and tumors and in order to obtain a high resolution imaging of the pancreatic ducts.
Endoscopic ultrasonography and ERCP are the next logical steps for the detection of early CP, unappreciated malignancy and for a trial of transpapillary ductal drainage.
Future prospective trials should define which patients, with idiopathic ARP, whether or not it is associated with anatomical variants, are most likely to benefit from pancreatic ductal drainage.
These studies should allow appropriate patient selection and the development of novel, effective, preventive and therapeutic strategies to improve the clinical condition of these patients.
Peer reviewer: Giuseppe Brisinda, MD, Department of Surgery, Catholic School of Medicine “Agostino Gemelli”, Largo Agostino Gemelli 8-00168 Rome, Italy
S- Editor Liu Y L- Editor Alpini GD E- Editor Wang HF
References
- 1.Testoni PA, Caporuscio S, Bagnolo F, Lella F. Idiopathic recurrent pancreatitis: long-term results after ERCP, endoscopic sphincterotomy, or ursodeoxycholic acid treatment. Am J Gastroenterol. 2000;95:1702–1707. doi: 10.1111/j.1572-0241.2000.02292.x. [DOI] [PubMed] [Google Scholar]
- 2.Matos C, Bali MA, Delhaye M, Deviere J. Magnetic resonance imaging in the detection of pancreatitis and pancreatic neoplasms. Best Pract Res Clin Gastroenterol. 2006;20:157–178. doi: 10.1016/j.bpg.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Matos C, Metens T, Devière J, Nicaise N, Braude P, Van Yperen G, Cremer M, Struyven J. Pancreatic duct: morphologic and functional evaluation with dynamic MR pancreatography after secretin stimulation. Radiology. 1997;203:435–441. doi: 10.1148/radiology.203.2.9114101. [DOI] [PubMed] [Google Scholar]
- 4.Manfredi R, Costamagna G, Brizi MG, Maresca G, Vecchioli A, Colagrande C, Marano P. Severe chronic pancreatitis versus suspected pancreatic disease: dynamic MR cholangiopancreatography after secretin stimulation. Radiology. 2000;214:849–855. doi: 10.1148/radiology.214.3.r00mr24849. [DOI] [PubMed] [Google Scholar]
- 5.Arvanitakis M, Delhaye M, De Maertelaere V, Bali M, Winant C, Coppens E, Jeanmart J, Zalcman M, VanGansbeke D, Devière J, et al. Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology. 2004;126:715–723. doi: 10.1053/j.gastro.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Somogyi L, Martin SP, Venkatesan T, Ulrich CD 2nd. Recurrent acute pancreatitis: an algorithmic approach to identification and elimination of inciting factors. Gastroenterology. 2001;120:708–717. doi: 10.1053/gast.2001.22333. [DOI] [PubMed] [Google Scholar]
- 7.Coyle WJ, Pineau BC, Tarnasky PR, Knapple WL, Aabakken L, Hoffman BJ, Cunningham JT, Hawes RH, Cotton PB. Evaluation of unexplained acute and acute recurrent pancreatitis using endoscopic retrograde cholangiopancreatography, sphincter of Oddi manometry and endoscopic ultrasound. Endoscopy. 2002;34:617–623. doi: 10.1055/s-2002-33245. [DOI] [PubMed] [Google Scholar]
- 8.Testoni PA. Aetiologies of recurrent acute pancreatitis: acute or chronic relapsing disease? JOP. 2001;2:357–367. [PubMed] [Google Scholar]
- 9.Levy MJ, Geenen JE. Idiopathic acute recurrent pancreatitis. Am J Gastroenterol. 2001;96:2540–2555. doi: 10.1111/j.1572-0241.2001.04098.x. [DOI] [PubMed] [Google Scholar]
- 10.Klein SD, Affronti JP. Pancreas divisum, an evidence-based review: part I, pathophysiology. Gastrointest Endosc. 2004;60:419–425. doi: 10.1016/s0016-5107(04)01815-2. [DOI] [PubMed] [Google Scholar]
- 11.Bret PM, Reinhold C, Taourel P, Guibaud L, Atri M, Barkun AN. Pancreas divisum: evaluation with MR cholangiopancreatography. Radiology. 1996;199:99–103. doi: 10.1148/radiology.199.1.8633179. [DOI] [PubMed] [Google Scholar]
- 12.Cotton PB. Congenital anomaly of pancreas divisum as cause of obstructive pain and pancreatitis. Gut. 1980;21:105–114. doi: 10.1136/gut.21.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khalid A, Slivka A. Approach to idiopathic recurrent pancreatitis. Gastrointest Endosc Clin N Am. 2003;13:695–716, x. doi: 10.1016/s1052-5157(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 14.Delhaye M, Engelholm L, Cremer M. Pancreas divisum: congenital anatomic variant or anomaly? Contribution of endoscopic retrograde dorsal pancreatography. Gastroenterology. 1985;89:951–958. doi: 10.1016/0016-5085(85)90193-3. [DOI] [PubMed] [Google Scholar]
- 15.Delhaye M, Engelholm L, Cremer M. Pancreas divisum: controversial clinical significance. Dig Dis. 1988;6:30–39. doi: 10.1159/000171182. [DOI] [PubMed] [Google Scholar]
- 16.Matos C, Metens T, Devière J, Delhaye M, Le Moine O, Cremer M. Pancreas divisum: evaluation with secretin-enhanced magnetic resonance cholangiopancreatography. Gastrointest Endosc. 2001;53:728–733. doi: 10.1067/mge.2001.114784. [DOI] [PubMed] [Google Scholar]
- 17.Manfredi R, Costamagna G, Brizi MG, Spina S, Maresca G, Vecchioli A, Mutignani M, Marano P. Pancreas divisum and “santorinicele”: diagnosis with dynamic MR cholangiopancreatography with secretin stimulation. Radiology. 2000;217:403–408. doi: 10.1148/radiology.217.2.r00nv29403. [DOI] [PubMed] [Google Scholar]
- 18.Costamagna G, Ingrosso M, Tringali A, Mutignani M, Manfredi R. Santorinicele and recurrent acute pancreatitis in pancreas divisum: diagnosis with dynamic secretin-stimulated magnetic resonance pancreatography and endoscopic treatment. Gastrointest Endosc. 2000;52:262–267. doi: 10.1067/mge.2000.107711. [DOI] [PubMed] [Google Scholar]
- 19.Klein SD, Affronti JP. Pancreas divisum, an evidence-based review: part II, patient selection and treatment. Gastrointest Endosc. 2004;60:585–589. doi: 10.1016/s0016-5107(04)01896-6. [DOI] [PubMed] [Google Scholar]
- 20.Bradley EL 3rd, Stephan RN. Accessory duct sphincteroplasty is preferred for long-term prevention of recurrent acute pancreatitis in patients with pancreas divisum. J Am Coll Surg. 1996;183:65–70. [PubMed] [Google Scholar]
- 21.Attwell A, Borak G, Hawes R, Cotton P, Romagnuolo J. Endoscopic pancreatic sphincterotomy for pancreas divisum by using a needle-knife or standard pull-type technique: safety and reintervention rates. Gastrointest Endosc. 2006;64:705–711. doi: 10.1016/j.gie.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 22.Gerke H, Byrne MF, Stiffler HL, Obando JV, Mitchell RM, Jowell PS, Branch MS, Baillie J. Outcome of endoscopic minor papillotomy in patients with symptomatic pancreas divisum. JOP. 2004;5:122–131. [PubMed] [Google Scholar]
- 23.Lans JI, Geenen JE, Johanson JF, Hogan WJ. Endoscopic therapy in patients with pancreas divisum and acute pancreatitis: a prospective, randomized, controlled clinical trial. Gastrointest Endosc. 1992;38:430–434. doi: 10.1016/s0016-5107(92)70471-4. [DOI] [PubMed] [Google Scholar]
- 24.Kamisawa T, Egawa N, Tsuruta K, Okamoto A, Mtsukawa M. Pancreatitis associated with congenital abnormalities of the pancreaticobiliary system. Hepatogastroenterology. 2005;52:223–229. [PubMed] [Google Scholar]
- 25.Sugiyama M, Atomi Y, Kuroda A. Pancreatic disorders associated with anomalous pancreaticobiliary junction. Surgery. 1999;126:492–497. [PubMed] [Google Scholar]
- 26.Misra SP, Dwivedi M. Pancreaticobiliary ductal union. Gut. 1990;31:1144–1149. doi: 10.1136/gut.31.10.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yusuf TE, Bhutani MS. Role of endoscopic ultrasonography in diseases of the extrahepatic biliary system. J Gastroenterol Hepatol. 2004;19:243–250. doi: 10.1111/j.1440-1746.2003.03142.x. [DOI] [PubMed] [Google Scholar]
- 28.Park DH, Kim MH, Lee SK, Lee SS, Choi JS, Lee YS, Seo DW, Won HJ, Kim MY. Can MRCP replace the diagnostic role of ERCP for patients with choledochal cysts? Gastrointest Endosc. 2005;62:360–366. doi: 10.1016/j.gie.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 29.Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts: Classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977;134:263–269. doi: 10.1016/0002-9610(77)90359-2. [DOI] [PubMed] [Google Scholar]
- 30.Visser BC, Suh I, Way LW, Kang SM. Congenital choledochal cysts in adults. Arch Surg. 2004;139:855–860; discussion 860-862. doi: 10.1001/archsurg.139.8.855. [DOI] [PubMed] [Google Scholar]
- 31.Ladas SD, Katsogridakis I, Tassios P, Tastemiroglou T, Vrachliotis T, Raptis SA. Choledochocele, an overlooked diagnosis: report of 15 cases and review of 56 published reports from 1984 to 1992. Endoscopy. 1995;27:233–239. doi: 10.1055/s-2007-1005677. [DOI] [PubMed] [Google Scholar]
- 32.Procacci C, Portuese A, Fugazzola C, Pederzoli P, Caudana R, Gallo E, Bergamo Andreis IA, Spiller M, Zonta L, Graziani R. Duodenal duplication in the adult: its relationship with pancreatitis. Gastrointest Radiol. 1988;13:315–322. doi: 10.1007/BF01889089. [DOI] [PubMed] [Google Scholar]
- 33.Dave P, Romeu J, Clary S, Rybak B, Messer J. Endoscopic removal of an obstructing duodenal duplication cyst. Endoscopy. 1984;16:75–76. doi: 10.1055/s-2007-1018537. [DOI] [PubMed] [Google Scholar]
- 34.Delhay M, Matos C, Deviere J. Acute relapsing pancreatitis. Congenital variants: diagnosis, treatment, outcome. JOP. 2001;2:373–381. [PubMed] [Google Scholar]
- 35.Urayama S, Kozarek R, Ball T, Brandabur J, Traverso L, Ryan J, Wechter D. Presentation and treatment of annular pancreas in an adult population. Am J Gastroenterol. 1995;90:995–999. [PubMed] [Google Scholar]
- 36.Catalano MF, Linder JD, Chak A, Sivak MV Jr, Raijman I, Geenen JE, Howell DA. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004;59:225–232. doi: 10.1016/s0016-5107(03)02366-6. [DOI] [PubMed] [Google Scholar]
- 37.Burke CA, Beck GJ, Church JM, van Stolk RU. The natural history of untreated duodenal and ampullary adenomas in patients with familial adenomatous polyposis followed in an endoscopic surveillance program. Gastrointest Endosc. 1999;49:358–364. doi: 10.1016/s0016-5107(99)70013-1. [DOI] [PubMed] [Google Scholar]
- 38.Bank S, Indaram A. Causes of acute and recurrent pancreatitis. Clinical considerations and clues to diagnosis. Gastroenterol Clin North Am. 1999;28:571–589. doi: 10.1016/s0889-8553(05)70074-1. [DOI] [PubMed] [Google Scholar]
- 39.Choudari CP, Fogel EL, Sherman S, Lehman GA. Idiopathic pancreatitis: yield of ERCP correlated with patient age. Am J Gastroenterol. 1998;93:1654A. [Google Scholar]
- 40.Whitcomb DC. Value of genetic testing in the management of pancreatitis. Gut. 2004;53:1710–1717. doi: 10.1136/gut.2003.015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yusoff IF, Raymond G, Sahai AV. A prospective comparison of the yield of EUS in primary vs. recurrent idiopathic acute pancreatitis. Gastrointest Endosc. 2004;60:673–678. doi: 10.1016/s0016-5107(04)02018-8. [DOI] [PubMed] [Google Scholar]
- 42.Matos C, Devière J, Cremer M, Nicaise N, Struyven J, Metens T. Acinar filling during secretin-stimulated MR pancreatography. AJR. 1998;171:165–169. doi: 10.2214/ajr.171.1.9648781. [DOI] [PubMed] [Google Scholar]
- 43.Garg PK, Tandon RK, Madan K. Is biliary microlithiasis a significant cause of idiopathic recurrent acute pancreatitis? A long-term follow-up study. Clin Gastroenterol Hepatol. 2007;5:75–79. doi: 10.1016/j.cgh.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 44.Jouannaud V, Coutarel P, Tossou H, Butel J, Vitte RL, Skinazi F, Blazquez M, Hadège H, Bories C, Rocher P, et al. Cystic dystrophy of the duodenal wall associated with chronic alcoholic pancreatitis. Clinical features, diagnostic procedures and therapeutic management in a retrospective multicenter series of 23 patients. Gastroenterol Clin Biol. 2006;30:580–586. doi: 10.1016/s0399-8320(06)73231-6. [DOI] [PubMed] [Google Scholar]
- 45.Vullierme MP, Vilgrain V, Flejou JF, Zins M, O’Toole D, Ruszniewski P, Belghiti J, Menu Y. Cystic dystrophy of the duodenal wall in the heterotopic pancreas: radiopathological correlations. J Comput Assist Tomogr. 2000;24:635–643. doi: 10.1097/00004728-200007000-00023. [DOI] [PubMed] [Google Scholar]
- 46.Chen JW, Saccone GT, Toouli J. Sphincter of Oddi dysfunction and acute pancreatitis. Gut. 1998;43:305–308. doi: 10.1136/gut.43.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petersen BT. Sphincter of Oddi dysfunction, part 2: Evidence-based review of the presentations, with "objective" pancreatic findings (types I and II) and of presumptive type III. Gastrointest Endosc. 2004;59:670–687. doi: 10.1016/s0016-5107(04)00297-4. [DOI] [PubMed] [Google Scholar]
- 48.Mariani A, Curioni S, Zanello A, Passaretti S, Masci E, Rossi M, Del Maschio A, Testoni PA. Secretin MRCP and endoscopic pancreatic manometry in the evaluation of sphincter of Oddi function: a comparative pilot study in patients with idiopathic recurrent pancreatitis. Gastrointest Endosc. 2003;58:847–852. doi: 10.1016/s0016-5107(03)02303-4. [DOI] [PubMed] [Google Scholar]
- 49.Pereira SP, Gillams A, Sgouros SN, Webster GJ, Hatfield AR. Prospective comparison of secretin-stimulated magnetic resonance cholangiopancreatography with manometry in the diagnosis of sphincter of Oddi dysfunction types II and III. Gut. 2007;56:809–813. doi: 10.1136/gut.2006.099267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park SH, Watkins JL, Fogel EL, Sherman S, Lazzell L, Bucksot L, Lehman GA. Long-term outcome of endoscopic dual pancreatobiliary sphincterotomy in patients with manometry-documented sphincter of Oddi dysfunction and normal pancreatogram. Gastrointest Endosc. 2003;57:483–491. doi: 10.1067/mge.2003.138. [DOI] [PubMed] [Google Scholar]
- 51.Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909–918. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 52.Tarnasky PR, Palesch YY, Cunningham JT, Mauldin PD, Cotton PB, Hawes RH. Pancreatic stenting prevents pancreatitis after biliary sphincterotomy in patients with sphincter of Oddi dysfunction. Gastroenterology. 1998;115:1518–1524. doi: 10.1016/s0016-5085(98)70031-9. [DOI] [PubMed] [Google Scholar]