Abstract
AIM: To investigate the predictive value of Ki67 and p53 and their correlation with thymidylate synthase (TS) gene expression in a rectal cancer patient cohort treated according to a standardized recommended neoadjuvant treatment regimen.
METHODS: Formalin fixed, paraffin embedded pre-therapeutical tumor biopsies (n = 22) and post-therapeutical resection specimens (n = 40) from patients with rectal adenocarcinoma (clinical UICC stage II/III) receiving standardized neoadjuvant 5-fluorouracil (5-FU) based chemoradiotherapy were studied for Ki67 and p53 expression by immunohistochemistry and correlated with TS mRNA expression by quantitative TaqMan real-time PCR after laser microdissection. The results were compared with histopathological tumor regression according to a standardized semiquantitative score grading system.
RESULTS: Responders (patients with high tumor regression) showed a significantly lower Ki67 expression than non-responders in the pre-therapeutical tumor biopsies (81.2% vs 16.7%; P < 0.05) as well as in the post-therapeutical resection specimens (75.8% vs 14.3%; P < 0.01). High TS mRNA expression was significantly correlated with a high Ki67 index and low TS mRNA expression was significantly correlated with a low Ki67 index in the pre-therapeutical tumor biopsies (corr. coef. = 0.46; P < 0.01) as well as in the post-therapeutical resection specimens (corr. coef. = 0.40; P < 0.05). No significant association was found between p53 and TS mRNA expression or tumor regression.
CONCLUSION: Ki67 has, like TS, predictive value in rectal cancer patients after neoadjuvant 5-FU based chemoradiotherapy. The close correlation between Ki67 and TS indicates that TS is involved in active cell cycle processes.
Keywords: p53, Ki67, Neoadjuvant treatment, Rectal cancer, Thymidylate synthase
INTRODUCTION
According to the results of the German Rectal Cancer Study Group (CAO-/AIO-/ARO-94 trial) pre-operative (neoadjuvant) 5-fluorouracil (5-FU) based chemoradiotherapy (CT/RT) is now the recommended therapy regimen for rectal cancer UICC stage II/III[1].
The antimetabolite 5-FU plays a central role in the treatment of colorectal cancer and, additionally, was shown to enhance the effectiveness of radiation therapy in the treatment of rectal cancer[2,3]. One of the most important molecular targets of 5-FU is thymidylate synthase (TS), which is essential for the de novo DNA synthesis[4].
Several clinical studies have shown that high TS expression is a predictive marker for a low sensitivity to adjuvant 5-FU-based chemotherapy in colorectal cancer. Furthermore, high TS expression is also a prognostic marker for poor disease free and overall survival in colorectal cancer patients not treated with chemotherapy after surgery[5]. Recently, we demonstrated that high TS gene as well as protein expression predicts low therapy-induced tumor regression in neoadjuvantly treated rectal cancer, thus indicating poor treatment efficacy[6,7]. In addition, we showed that persistent positive lymph node status and high TS gene expression after pre-operative 5-FU-based CT/RT are predictive for the development of cancer recurrence and therefore for an unfavorable prognosis in patients with rectal cancer UICC stage II/III[8].
Since TS is mandatory for DNA synthesis, there is a close relationship between TS expression and proliferation rate: the faster the cell proliferation, the higher the TS expression and activity[9,10].
The Ki67-antigen recognizes the nuclei of proliferating cells throughout the cell cycle, with the exception during the G0 and early G1 phases. Several studies showed that a high S-phase fraction is associated with a greater risk for tumor recurrence and diminished survival in the adjuvant setting, although this has not been universally observed[11,12].
Mutations in p53 were found in 40% to 60% of patients with colorectal cancer[13]. Several clinical studies showed a variable, and particular controversial role of the p53 status with regard to therapy response and prognosis in the neoadjuvant and adjuvant setting, respectively, depending on the kind of treatment and the p53 detection technique used[14–17]. Generally, mutation or overexpression of p53 seems to be associated with an unfavorable prognosis for patients with locally advanced colon cancer[18–20]. However, there are also investigations with contrary results or without any associations[11,21–24]. Thus, the role of p53 as a predictive or prognostic marker is still controversial.
The aim of this study was to examine the predictive value of p53 and Ki67 alone or in combination with TS in patients with locally advanced rectal cancer neoadjuvantly treated with 5-FU based CT/RT. Furthermore, the association between both markers and TS was investigated.
MATERIALS AND METHODS
Patients and treatment
Surgical specimens from 40 patients (male: n = 33; female: n = 7; median age = 62 years) with rectal adenocarcinoma (cUICC stage II/III), histopathologically proven by rectal biopsy between 1998 and 2001 at the Department of General Surgery, University Medical Center, Göttingen, Germany were analyzed[6]. In all patients, locally curative (R0) tumor resection was achieved after standardized pre-operative CT/RT. During radiotherapy with a total dose of 50.4 Gy (single dose 1.8 Gy delivered in 28 fractions) 5-FU was administered as a 120-h continuous intravenous infusion of 1000 mg/m2 per day during the first and fifth weeks. Standardized surgery including total mesorectal excision was scheduled 5 wk after completion of pre-operative treatment and clinical restaging. All patients of this study received the same therapy protocol according to the CAO-/AIO-/ARO-94 trial of the German Rectal Cancer Study Group[1]. Treatment was completed by post-operative application of 4 cycles of bolus 5-FU chemotherapy. The trial was approved by the medical ethics committee of the University of Göttingen (No 20-9-95).
Assessment of tumor regression
Histopathological tumor regression was graded according to the semiquantitative 5-point tumor regression grading (TRG) system proposed by Dworak et al: TRG 0 = no regression; TRG 1 = dominant tumor mass with obvious fibrosis or mucin; TRG 2 = dominantly fibrotic or mucinous changes with few tumor cells or groups; TRG 3 = very few tumor cells in fibrotic or mucinous tissue; TRG 4 = no tumor cells, only fibrotic or mucinous mass (total regression or response)[25]. As previously described, samples with dominant fibrous tissue (TRG 2 to 4) were defined as responders, those with dominant tumor mass (TRG 0 and 1) were defined as non-responders[6].
Microdissection, RNA extraction, and quantitation of TS gene expression
Microdissection of the tumor was performed by laser microbeam technique using the P.A.L.M. Robot-Microbeam (P.A.L.M. Bernried, Germany). Tumors in form of macroclusters (> 3 mm) or tumor areas with little fibrosis were manually microdissected using sterile needles. Extraction of total RNA from formalin-fixed, paraffin-embedded tissue was done as previously described[6]. RNA was transcribed into cDNA using M-MLV reverse transcriptase (Invitrogen, Karlsruhe, Germany) and random hexamers (Amersham Pharmacia, Freiburg, Germany) according to the manufacturer’s instructions. TS-cDNA was quantified by TaqMan real-time PCR in relation to beta-actin cDNA using the standard curve method. cDNA obtained from HT29 cell line was used as a standard. Primer sequences were based on the GenBank accession numbers AB004047 (gene sequence of beta-actin) and X02308 (gene sequence of TS). PCR conditions used have been described[26].
Immunohistochemistry
Protein expression was assessed on 2-μm sections of paraffin-embedded tissue samples. Deparaffinization and heat-induced antigen retrieval were done in PT ModuleTM (PTM) buffer (Lab Vision Corporation, Fremont, CA, USA) in a pressure steam-cooker at 98°C for 45 min. The slides were cooled down at room temperature for 20 min and then loaded onto a Lab Vision Microm 2D automated cell stainer (Microm, Walldorf, Germany). Endogenous peroxidase activity was inhibited by incubating the slides in 3% H2O2 for 10 min. The tissues were incubated at room temperature with the primary MIB1 antibody (DAKO, Hamburg, Germany) at a 1:400 dilution for 30 min and the primary p53 D0-1 antibody (Calbiochem, Frankfurt, Germany) at a 1:50 dilution for 30 min, respectively. The slides were then incubated with a biotinylated secondary antibody for 15 min, and after this incubated with avidin-biotinylated peroxidase complex (ABC) for 15 min. The chromogen 3, 3-diaminobenzidine (DAB; 0.6 mg/mL) was applied for 2 min × 4 min. The tissues were counter-stained with Mayer’s hematoxylin. In each experiment positive and negative controls were included; isotype controls were done and proved negative.
Evaluation of immunohistochemistry
The immunostained sections were independently reviewed by two investigators (CJ and DEA). Conflicts in scores were resolved by consensus. For the tissue evaluation of Ki-67, each slide was scored based on the percentage of positively stained malignant nuclei. The following ranges were used: 0% to 20%, > 20% to 40%, > 40% to 60%, > 60% to 80%, and > 80% to 100%. According to the recommended classification in previous studies[21,27], samples with Ki67 nuclear staining equal or above 40% were considered having a high proliferative index, whereas nuclear positivity below 40% was considered a low proliferative index. P53 was considered overexpressed when ≥ 10% of the malignant nuclei were positive. If fewer than 10% of the nuclei were stained, the slide was scored as having normal p53 expression.
Statistical analysis
The correlation between expression levels in tumor biopsies and resection specimens was evaluated with linear regression analysis. The correlation between TS and Ki67 or p53, respectively, was evaluated with the students-t-test. For TS gene expression, the maximal χ2 method was adapted to determine which cut-off value best separates tumors into low and high expression subgroups[28,29]. The correlation between TS, Ki67, and p53 expression and tumor regression was assessed with the chi-square test. The combination of the marker expressions was tested regarding a better discrimination between responders and non-responders with a multivariate discriminant analysis. P values < 0.05 were considered statistically significant.
RESULTS
Pre- and post-therapeutical tumor stage and histopathological tumor regression
The results of the histopathological tumor regression after neoadjuvant CT/RT with respect to downsizing and downstaging have been reported previously[6]. The data are summarized in Table 1. According to our criteria, eight tumors were non-responders and 32 were responders. Representative examples of tumors with different regression grades are shown in Figure 1.
Table 1.
Tumor regression after neoadjuvant chemoradiotherapy[6]
|
Histopathological tumor regression |
|||||||||||
|
Primary tumor (downsizing) |
Tumor stage (downstaging) |
||||||||||
| ypT | TRG0 | TRG1 | TRG2 | TRG3 | TRG4 | ypUICC | TRG0 | TRG1 | TRG2 | TRG3 | TRG4 |
| 0 | - | - | - | - | 3 | 0 | - | - | - | - | 2 |
| 1 | - | - | - | 1 | - | I | - | 2 | 3 | 4 | - |
| 2 | - | 2 | 4 | 6 | - | II | 1 | 2 | 4 | 3 | - |
| 3 | 1 | 3 | 10 | 8 | - | III | - | 3 | 7 | 7 | 1 |
| 4 | - | 2 | - | - | - | IV | - | - | - | 1 | - |
| Total | 1 | 7 | 14 | 15 | 3 | Total | 1 | 7 | 14 | 15 | 3 |
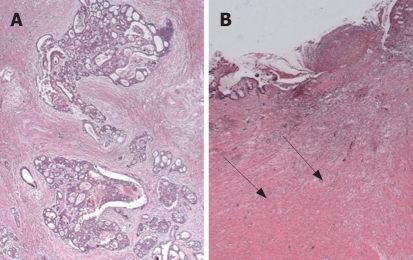
Figure 1.
Rectal adenocarcinomas with different tumor regression grade (TRG) after preoperative chemoradiotherapy: dominant tumor with obvious fibrosis in a non-responder, TRG1 (A); dominant fibrosis with very few tumor cell groups in a responder, TRG3 (B).
TS gene, p53 and Ki67 protein expression
The median of TS gene expression in pre-therapeutical tumor biopsies and post-therapeutical resection specimens was 1.07 and 0.70, respectively.
Tumor cells showed a nuclear staining pattern for Ki67 or p53. Cytoplasmic (without nuclear) immunoreactivity was not encountered. Epithelial cells located in the generative zone and the basal layers of normal rectal mucosa were consistently immunoreactive for Ki67. No apparent p53 expression was detected in the non-neoplastic cells. Representative immunohistochemical staining examples are shown in Figures 2 and 3.
Figure 2.
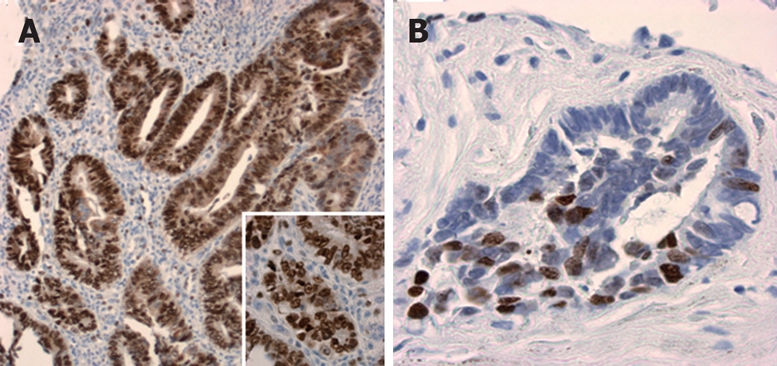
Ki67 expression in post-therapeutical resection specimens. High proliferative index with more than 90% positive nuclei (magnification × 10; insert: × 40) (A), and low proliferative index with less than 40% positive nuclei (magnification × 20) (B).
Figure 3.
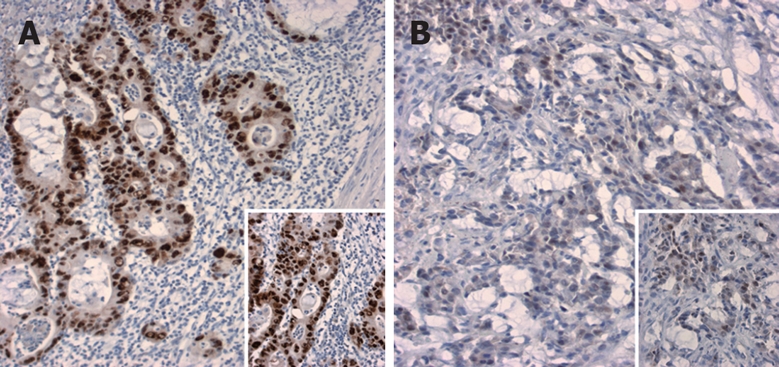
P53 expression in post-therapeutical resection specimens. Overexpression of p53 with more than 75% (A) and about 25% positive nuclei (B) (magnification × 10; inserts × 20).
Correlation between TS, Ki67, and p53 expression and tumor regression in pre-therapeutical rectal biopsies and post-therapeutical resection specimens
Low TS gene expression and low Ki67 expression, respectively, were significantly associated with high tumor regression (response) in the pre-therapeutical tumor biopsies as well as in the post-therapeutical resection specimens. No significant association was found between TS gene and p53 protein expression. The results are summarized in Tables 2 and 3.
Table 2.
Correlations in pre-therapy tumor biopsies
| Marker expression | Responders | Non-responders | P |
| TS < 1.201 | 10 | 1 | |
| TS ≥ 1.20 | 0 | 3 | < 0.05 |
| Ki67 < 40% | 13 | 1 | |
| Ki67 ≥ 40% | 3 | 5 | < 0.05 |
| p53 < 10% | 6 | 3 | |
| p53 ≥ 10% | 10 | 3 | 0.60 |
cut-off value.
Table 3.
Correlations in post-therapy resection specimens
| Marker expression | Responders | Non-responders | P |
| TS < 0.881 | 26 | 2 | < 0.01 |
| TS ≥ 0.88 | 4 | 4 | |
| Ki67 < 40% | 22 | 1 | < 0.01 |
| Ki67 ≥ 40% | 7 | 6 | |
| p53 < 10% | 10 | 3 | 0.98 |
| p53 ≥ 10% | 19 | 4 |
cut-off value.
Comparison of expression levels between pre-therapeutical biopsies and post-therapeutical resection specimens
A significant correlation between pre-therapeutical tumor biopsies and post-therapeutical resection specimens was observed for the TS gene expression (P < 0.05) and p53 expression (P < 0.05). No significant correlation was found for Ki67.
Correlation between TS gene expression and Ki67 or p53 expression
High TS mRNA expression was significantly correlated with a high Ki67 index and low TS mRNA expression was significantly correlated with a low Ki67 index in the pre-therapeutical tumor biopsies (P < 0.01) as well as in the post-therapeutical resection specimens (P < 0.05). No significant correlation was found between TS and p53.
Multivariate analysis
Although both, TS gene and Ki67 expression were significantly correlated with histopathological tumor regression, a combination of the markers did not significantly improve the discrimination between responders and non-responders.
DISCUSSION
There is very little and conflicting data about the predictive value of Ki67 and p53 in rectal cancer after neoadjuvant CT/RT[14–17] and almost no data on the correlation with TS expression. The aim of this study therefore was, to define the predictive value of Ki67 and p53 and their correlation with TS gene expression in a patient cohort treated according to the standardized recommended neoadjuvant treatment regimen[1]. We used histopathologically assessed tumor regression as a surrogate endpoint for early determination of the treatment efficacy[6].
TS is mandatory for DNA replication, and thus a critical target for fluoropyrimidines. Furthermore, TS is a cell cycle enzyme and is present in proliferating cells[9,10]. Previous observations in cell lines showed that the TS expression and activity in asynchronously growing cancer cells were significantly related to the cell doubling time: the faster the cell proliferation, the greater the expression and activity of TS. The expression and activity of TS were strongly related to the doubling time, and not to the number of cycling cells or to the number of cells in S phase[10]. Rapidly proliferating cells have to synthesise a greater amount of DNA- and consequently need a greater amount of TS- than slowly proliferating cells. Thus, TS expression is closely correlated with the cell proliferation rate. The positive association between TS and Ki67 expression in our study supports this thesis. Additionally, on the basis of our results, this relationship seems not to be influenced by (neoadjuvant) therapy because we found it in the pre-therapeutical biopsies as well as in the post-therapeutical resection specimens. As a consequence of the close relationship of Ki67 and TS, we could show that non-responders had a significantly higher Ki67 expression than responders. The combination of both markers (Ki67 and TS expression) did not significantly improve the response prediction.
In the light of the known relationship between tumor cell kinetic and efficacy of chemotherapy our results are somewhat surprising, since one would expect that rapidly growing tumors with high TS and/or high Ki67 levels would benefit more from chemotherapy. Though Ki67 reflects active cell cycling, it does not necessarily quantitate the speed of the cell cycling process that may be the critical factor in sensitivity to chemotherapy[30]. Thus, it is conceivable that cancers with relatively high Ki67 levels may still have relatively slow doubling times.
In contrast to the expectation that tumors with high proliferation and therefore high TS expression would benefit most from chemotherapy, high TS expression was associated with poor response to 5-FU and poor survival in most patients with colorectal cancer in the majority of the published studies[5]. Therefore, other-obviously TS-independent - events seem to be involved in 5-FU resistance. Inherent or acquired resistance to 5-FU based chemotherapy is obviously multifactorial, since 5-FU sensitivity is for instance influenced by the expression of dihydropyrimidine dehydrogenase[31], the genetic status of p53[32,33], nuclear factor (NF)-kappa B[34], DNA mismatch-repair genes[35], and cell cycle disturbance[36].
The relationship between TS and p53 was investigated in several clinical studies, mostly in the adjuvant setting. Generally, it has been suggested that mutation of the p53 gene or p53 overexpression (as a surrogate marker for p53 mutation) is significantly and independently associated with resistance to 5-FU therapy and with poor prognosis in colorectal cancer[18–20]. Some studies showed that p53 expression may be used to improve the predictive value of TS for response to 5-FU therapy[21,23,37]. However, some investigations failed to demonstrate such an association[38], and others have shown an improved clinical outcome and overexpression of p53[39].
There are only very rare data of p53 and TS in the neoadjuvant setting. Kamoshida et al. demonstrated in gastric cancer neoadjuvantly treated with S-1/cisplatin chemotherapy that high expression of TS and/or p53 in the pre-treatment biopsies predicted chemoresistance[40]. Other investigators[41] were not able to find any correlation between p53 and either TS or tumor regression. Our data do not suggest a predictive value of p53 immunohistochemistry in the neoadjuvant therapy of rectal cancer, either. Like us, most investigators have used immunohistochemistry to detect mutant p53, with the assumption that overexpression of p53 is often associated with a mutation, while the lack of expression is usually associated with a wild-type p53 genotype. This assumption has been validated in approximately 60% to 80% of the cases in which mutational analysis and p53 detection by immunohistochemistry were compared[42,43]. But there may be discrepancies between p53 protein expression and p53 mutation status which may at least partly explain the missing correlation between p53, TS and tumor regression.
In conclusion, we demonstrated that Ki67 has, like TS, predictive value in rectal cancer patients after neoadjuvant 5-FU based CT/RT as responders had a significantly lower Ki67 expression than non-responders. The positive association between Ki67 and TS indicates that TS is involved in active cell cycle processes.
COMMENTS
Background
In locally advanced rectal cancer, neoadjuvant 5-fluorouracil (5-FU) based chemoradiotherapy is now the recommended therapy regimen. One of the most important molecular targets of 5-FU is thymidylate synthase (TS), essential for de novo DNA synthesis. A number of clinical studies have shown that TS expression is a predictive marker in colorectal cancer. Since TS is mandatory for DNA synthesis there seems to be a close relationship between TS expression and other cell cycle related markers like p53 and Ki67, which could serve as predictive markers for response to neoadjuvant 5-FU based therapy in rectal cancer.
Research frontiers
Despite the advances in cancer therapy, there are still a number of patients who do not benefit from treatment. Hence, there is a need to identify novel molecular markers discriminating between patients who will benefit from treatment and those who will not.
Innovations and breakthroughs
To date, there is very little and conflicting data about the predictive value of Ki67 and p53 in rectal cancer after neoadjuvant CT/RT. The results of our study suggest that Ki67, like TS, may be used as a predictive marker in rectal cancer patients treated with neoadjuvant 5-FU based chemoradiotherapy.
Applications
The integration of predictive markers like TS and Ki67 into the therapeutical planning will support the advance of individualized treatment, maximizing therapeutic effect and minimizing exposure toxicity.
Terminology
Prognostic markers distinguish between patients with more or less favourable outcome. Predictive markers determine the likelihood of response to therapy and also the potential toxicity; they are always treatment specific.
Peer review
This paper is valuable particularly to colorectal surgeons, oncologists and gastroenterologists interested in rectal cancer biology and biomarkers. It is overall well designed, well written, with interesting results.
Peer reviewer: Daniel L Worthley, Dr, Department of Gastroenterology and Hepatology, Flinders Medical Centre, Room 3D230, Bedford Park, SA 5042, Australia
S- Editor Zhu LH L- Editor Negro F E- Editor Wang HF
References
- 1.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 2.Minsky BD, Cohen AM, Kemeny N, Enker WE, Kelsen DP, Reichman B, Saltz L, Sigurdson ER, Frankel J. Enhancement of radiation-induced downstaging of rectal cancer by fluorouracil and high-dose leucovorin chemotherapy. J Clin Oncol. 1992;10:79–84. doi: 10.1200/JCO.1992.10.1.79. [DOI] [PubMed] [Google Scholar]
- 3.Moertel CG. Chemotherapy for colorectal cancer. N Engl J Med. 1994;330:1136–1142. doi: 10.1056/NEJM199404213301608. [DOI] [PubMed] [Google Scholar]
- 4.Heidelberger C, Chaudhari N, Danenberg PV. Fluorinate pyrimidines: a new class of tumor-inhibitory compounds. Nature. 1957;179:663–666. doi: 10.1038/179663a0. [DOI] [PubMed] [Google Scholar]
- 5.Popat S, Matakidou A, Houlston RS. Thymidylate synthase expression and prognosis in colorectal cancer: a systematic review and meta-analysis. J Clin Oncol. 2004;22:529–536. doi: 10.1200/JCO.2004.05.064. [DOI] [PubMed] [Google Scholar]
- 6.Jakob C, Aust DE, Meyer W, Baretton GB, Schwabe W, Hausler P, Becker H, Liersch T. Thymidylate synthase, thymidine phosphorylase, dihydropyrimidine dehydrogenase expression, and histological tumour regression after 5-FU-based neo-adjuvant chemoradiotherapy in rectal cancer. J Pathol. 2004;204:562–568. doi: 10.1002/path.1663. [DOI] [PubMed] [Google Scholar]
- 7.Jakob C, Liersch T, Meyer W, Baretton GB, Hausler P, Schwabe W, Becker H, Aust DE. Immunohistochemical analysis of thymidylate synthase, thymidine phosphorylase, and dihydropyrimidine dehydrogenase in rectal cancer (cUICC II/III): correlation with histopathologic tumor regression after 5-fluorouracil-based long-term neoadjuvant chemoradiotherapy. Am J Surg Pathol. 2005;29:1304–1309. doi: 10.1097/01.pas.0000170346.55304.88. [DOI] [PubMed] [Google Scholar]
- 8.Jakob C, Liersch T, Meyer W, Baretton GB, Schwabe W, Hausler P, Kulle B, Becker H, Aust DE. Prognostic value of histologic tumor regression, thymidylate synthase, thymidine phosphorylase, and dihydropyrimidine dehydrogenase in rectal cancer UICC Stage II/III after neoadjuvant chemoradiotherapy. Am J Surg Pathol. 2006;30:1169–1174. doi: 10.1097/01.pas.0000213302.13435.6e. [DOI] [PubMed] [Google Scholar]
- 9.McGinn CJ, Pestalozzi BC, Drake JC, Glennon MC, Kunugi K, Otterson G, Allegra CJ, Johnston PG, Kinsella TJ. Cell cycle regulation of the G0/G1 transition in 5-fluorouracil-sensitive and -resistant human colon cancer cell lines. Cancer J. 2000;6:234–242. [PubMed] [Google Scholar]
- 10.Pestalozzi BC, McGinn CJ, Kinsella TJ, Drake JC, Glennon MC, Allegra CJ, Johnston PG. Increased thymidylate synthase protein levels are principally associated with proliferation but not cell cycle phase in asynchronous human cancer cells. Br J Cancer. 1995;71:1151–1157. doi: 10.1038/bjc.1995.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahnen DJ, Feigl P, Quan G, Fenoglio-Preiser C, Lovato LC, Bunn PA Jr, Stemmerman G, Wells JD, Macdonald JS, Meyskens FL Jr. Ki-ras mutation and p53 overexpression predict the clinical behavior of colorectal cancer: a Southwest Oncology Group study. Cancer Res. 1998;58:1149–1158. [PubMed] [Google Scholar]
- 12.Witzig TE, Loprinzi CL, Gonchoroff NJ, Reiman HM, Cha SS, Wieand HS, Katzmann JA, Paulsen JK, Moertel CG. DNA ploidy and cell kinetic measurements as predictors of recurrence and survival in stages B2 and C colorectal adenocarcinoma. Cancer. 1991;68:879–888. doi: 10.1002/1097-0142(19910815)68:4<879::aid-cncr2820680434>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Crapez E, Bibeau F, Thezenas S, Ychou M, Simony-Lafontaine J, Thirion A, Azria D, Grenier J, Senesse P. p53 status and response to radiotherapy in rectal cancer: a prospective multilevel analysis. Br J Cancer. 2005;92:2114–2121. doi: 10.1038/sj.bjc.6602622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rebischung C, Gerard JP, Gayet J, Thomas G, Hamelin R, Laurent-Puig P. Prognostic value of P53 mutations in rectal carcinoma. Int J Cancer. 2002;100:131–135. doi: 10.1002/ijc.10480. [DOI] [PubMed] [Google Scholar]
- 16.Rodel C, Grabenbauer GG, Papadopoulos T, Bigalke M, Gunther K, Schick C, Peters A, Sauer R, Rodel F. Apoptosis as a cellular predictor for histopathologic response to neoadjuvant radiochemotherapy in patients with rectal cancer. Int J Radiat Oncol Biol Phys. 2002;52:294–303. doi: 10.1016/s0360-3016(01)02643-8. [DOI] [PubMed] [Google Scholar]
- 17.Smith FM, Reynolds JV, Miller N, Stephens RB, Kennedy MJ. Pathological and molecular predictors of the response of rectal cancer to neoadjuvant radiochemotherapy. Eur J Surg Oncol. 2006;32:55–64. doi: 10.1016/j.ejso.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Bosari S, Viale G, Bossi P, Maggioni M, Coggi G, Murray JJ, Lee AK. Cytoplasmic accumulation of p53 protein: an independent prognostic indicator in colorectal adenocarcinomas. J Natl Cancer Inst. 1994;86:681–687. doi: 10.1093/jnci/86.9.681. [DOI] [PubMed] [Google Scholar]
- 19.Gallego MG, Acenero MJ, Ortega S, Delgado AA, Cantero JL. Prognostic influence of p53 nuclear overexpression in colorectal carcinoma. Dis Colon Rectum. 2000;43:971–975. doi: 10.1007/BF02237362. [DOI] [PubMed] [Google Scholar]
- 20.Zeng ZS, Sarkis AS, Zhang ZF, Klimstra DS, Charytonowicz E, Guillem JG, Cordon-Cardo C, Cohen AM. p53 nuclear overexpression: an independent predictor of survival in lymph node--positive colorectal cancer patients. J Clin Oncol. 1994;12:2043–2050. doi: 10.1200/JCO.1994.12.10.2043. [DOI] [PubMed] [Google Scholar]
- 21.Allegra CJ, Paik S, Colangelo LH, Parr AL, Kirsch I, Kim G, Klein P, Johnston PG, Wolmark N, Wieand HS. Prognostic value of thymidylate synthase, Ki-67, and p53 in patients with Dukes' B and C colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project collaborative study. J Clin Oncol. 2003;21:241–250. doi: 10.1200/JCO.2003.05.044. [DOI] [PubMed] [Google Scholar]
- 22.Etienne MC, Chazal M, Laurent-Puig P, Magne N, Rosty C, Formento JL, Francoual M, Formento P, Renee N, Chamorey E, et al. Prognostic value of tumoral thymidylate synthase and p53 in metastatic colorectal cancer patients receiving fluorouracil-based chemotherapy: phenotypic and genotypic analyses. J Clin Oncol. 2002;20:2832–2843. doi: 10.1200/JCO.2002.09.091. [DOI] [PubMed] [Google Scholar]
- 23.Paradiso A, Simone G, Petroni S, Leone B, Vallejo C, Lacava J, Romero A, Machiavelli M, De Lena M, Allegra CJ, et al. Thymidilate synthase and p53 primary tumour expression as predictive factors for advanced colorectal cancer patients. Br J Cancer. 2000;82:560–567. doi: 10.1054/bjoc.1999.0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang R, Wang JY, Fan CW, Tsao KC, Chen HH, Wu CM, Chen JS, Changchien CR, Hsieh LL. p53 is an independent pre-treatment markers for long-term survival in stage II and III colorectal cancers: an analysis of interaction between genetic markers and fluorouracil-based adjuvant therapy. Cancer Lett. 2004;210:101–109. doi: 10.1016/j.canlet.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23. doi: 10.1007/s003840050072. [DOI] [PubMed] [Google Scholar]
- 26.Kornmann M, Danenberg KD, Arber N, Beger HG, Danenberg PV, Korc M. Inhibition of cyclin D1 expression in human pancreatic cancer cells is associated with increased chemosensitivity and decreased expression of multiple chemoresistance genes. Cancer Res. 1999;59:3505–3511. [PubMed] [Google Scholar]
- 27.van Triest B, Pinedo HM, Blaauwgeers JL, van Diest PJ, Schoenmakers PS, Voorn DA, Smid K, Hoekman K, Hoitsma HF, Peters GJ. Prognostic role of thymidylate synthase, thymidine phosphorylase/platelet-derived endothelial cell growth factor, and proliferation markers in colorectal cancer. Clin Cancer Res. 2000;6:1063–1072. [PubMed] [Google Scholar]
- 28.Halpern J. Maximally selected chi-square statistics for small samples. Biometrics. 1982;38:1017–1023. [Google Scholar]
- 29.Miller R, Siegmund D. Maximally selected chi-square statistics. Biometrics. 1982;38:1011–1016. [Google Scholar]
- 30.Grem JL, Danenberg KD, Behan K, Parr A, Young L, Danenberg PV, Nguyen D, Drake J, Monks A, Allegra CJ. Thymidine kinase, thymidylate synthase, and dihydropyrimidine dehydrogenase profiles of cell lines of the National Cancer Institute's Anticancer Drug Screen. Clin Cancer Res. 2001;7:999–1009. [PubMed] [Google Scholar]
- 31.Salonga D, Danenberg KD, Johnson M, Metzger R, Groshen S, Tsao-Wei DD, Lenz HJ, Leichman CG, Leichman L, Diasio RB, et al. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res. 2000;6:1322–1327. [PubMed] [Google Scholar]
- 32.Boyer J, McLean EG, Aroori S, Wilson P, McCulla A, Carey PD, Longley DB, Johnston PG. Characterization of p53 wild-type and null isogenic colorectal cancer cell lines resistant to 5-fluorouracil, oxaliplatin, and irinotecan. Clin Cancer Res. 2004;10:2158–2167. doi: 10.1158/1078-0432.ccr-03-0362. [DOI] [PubMed] [Google Scholar]
- 33.Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L, Williams J, Lengauer C, Kinzler KW, Vogelstein B. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104:263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W, McLeod HL, Cassidy J. Disulfiram-mediated inhibition of NF-kappaB activity enhances cytotoxicity of 5-fluorouracil in human colorectal cancer cell lines. Int J Cancer. 2003;104:504–511. doi: 10.1002/ijc.10972. [DOI] [PubMed] [Google Scholar]
- 35.Meyers M, Wagner MW, Hwang HS, Kinsella TJ, Boothman DA. Role of the hMLH1 DNA mismatch repair protein in fluoropyrimidine-mediated cell death and cell cycle responses. Cancer Res. 2001;61:5193–5201. [PubMed] [Google Scholar]
- 36.Mirjolet JF, Didelot C, Barberi-Heyob M, Merlin JL. G(1)/S but not G(0)/G(1)cell fraction is related to 5-fluorouracil cytotoxicity. Cytometry. 2002;48:6–13. doi: 10.1002/cyto.10087. [DOI] [PubMed] [Google Scholar]
- 37.Lenz HJ, Danenberg KD, Leichman CG, Florentine B, Johnston PG, Groshen S, Zhou L, Xiong YP, Danenberg PV, Leichman LP. p53 and thymidylate synthase expression in untreated stage II colon cancer: associations with recurrence, survival, and site. Clin Cancer Res. 1998;4:1227–1234. [PubMed] [Google Scholar]
- 38.Watanabe T, Wu TT, Catalano PJ, Ueki T, Satriano R, Haller DG, Benson AB 3rd, Hamilton SR. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001;344:1196–1206. doi: 10.1056/NEJM200104193441603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soong R, Grieu F, Robbins P, Dix B, Chen D, Parsons R, House A, Iacopetta B. p53 alterations are associated with improved prognosis in distal colonic carcinomas. Clin Cancer Res. 1997;3:1405–1411. [PubMed] [Google Scholar]
- 40.Kamoshida S, Suzuki M, Shimomura R, Sakurai Y, Komori Y, Uyama I, Tsutsumi Y. Immunostaining of thymidylate synthase and p53 for predicting chemoresistance to S-1/cisplatin in gastric cancer. Br J Cancer. 2007;96:277–283. doi: 10.1038/sj.bjc.6603546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong NA, Brett L, Stewart M, Leitch A, Longley DB, Dunlop MG, Johnston PG, Lessells AM, Jodrell DI. Nuclear thymidylate synthase expression, p53 expression and 5FU response in colorectal carcinoma. Br J Cancer. 2001;85:1937–1943. doi: 10.1054/bjoc.2001.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hashimoto T, Tokuchi Y, Hayashi M, Kobayashi Y, Nishida K, Hayashi S, Ishikawa Y, Tsuchiya S, Nakagawa K, Hayashi J, et al. p53 null mutations undetected by immunohistochemical staining predict a poor outcome with early-stage non-small cell lung carcinomas. Cancer Res. 1999;59:5572–5577. [PubMed] [Google Scholar]
- 43.Sjogren S, Inganas M, Norberg T, Lindgren A, Nordgren H, Holmberg L, Bergh J. The p53 gene in breast cancer: prognostic value of complementary DNA sequencing versus immunohistochemistry. J Natl Cancer Inst. 1996;88:173–182. doi: 10.1093/jnci/88.3-4.173. [DOI] [PubMed] [Google Scholar]