Abstract
AIM: To study the origin of calcium necessary for agonist-induced contraction of the distal colon in rats.
METHODS: The change in intracellular calcium concentration ([Ca2+]i) evoked by elevating external Ca2+ was detected by fura 2/AM fluorescence. Contractile activity was measured with a force displacement transducer. Tension was continuously monitored and recorded using a Powerlab 4/25T data acquisition system with an ML110 bridge bioelectric physiographic amplifier.
RESULTS: Store depletion induced Ca2+ influx had an effect on [Ca2+]i. In nominally Ca2+-free medium, the sarco-endoplasmic reticulum Ca2+-ATPase inhibitor thapsigargin (1 μmol/L) increased [Ca2+]i from 68 to 241 nmol/L, and to 458 (P < 0.01) and 1006 nmol/L (P < 0.01), respectively, when 1.5 mmol/L and 3.0 mmol/L extracellular Ca2+ was reintroduced. Furthermore, the change in [Ca2+]i was observed with verapamil (5 μmol/L), La3+ (1 mmol/L) or KCl (40 mmol/L) in the bathing solution. These channels were sensitive to La3+ (P < 0.01), insensitive to verapamil, and voltage independent. In isolated distal colons we found that in normal Krebs solution, contraction induced by acetylcholine (ACh) was partially inhibited by verapamil, and the inhibitory rate was 41% (P < 0.05). On the other hand, in Ca2+-free Krebs solution, ACh induced transient contraction due to Ca2+ release from the intracellular stores. The transient contraction lasted until the Ca2+ store was depleted. Restoration of extracellular Ca2+ in the presence of atropine produced contraction, mainly due to Ca2+ influx. Such contraction was not inhibited by verapamil, but was decreased by La3+ (50 μmol/L) from 0.96 to 0.72 g (P < 0.01).
CONCLUSION: The predominant source of activator Ca2+ for the contractile response to agonist is extracellular Ca2+, and intracellular Ca2+ has little role to play in mediating excitation-contraction coupling by agonists in rat distal colon smooth muscle in vitro. The influx of extracellular Ca2+ is mainly mediated through voltage-, receptor- and store-operated Ca2+ channels, which can be used as an alternative to develop new drugs targeted on the dysfunction of digestive tract motility.
Keywords: Distal colon, Source of Ca2+, Store-operated Ca2+ channel, Voltage-operated Ca2+ channel, Acetylcholine
INTRODUCTION
Ca2+ is involved in many cellular transduction pathways, and regulation of different cellular phenotypes by [Ca2+]i has been observed in many cell systems, including smooth muscle cells (SMC). Various agonists induce contraction in a variety of smooth muscle preparations, with a concomitant increase in intracellular Ca2+ concentration ([Ca2+]i)[1,2]. The source for this ion is extracellular Ca2+ influx and Ca2+ release from intracellular stores. The relative contribution of intracellular and extracellular sources of Ca2+ depends on the tissue and mode of stimulation[3]. Moreover, agonist-induced increases in the cytosolic Ca2+ concentration produce smooth muscle contraction, characterized by a fast initial peak, the phasic component, followed by a decline to a lower maintained tension level, the tonic component[4,5].
In the former, we think two different Ca2+ channels participate in extracellular Ca2+ influx: voltage-operated Ca2+ channels (VOCCs) and receptor-operated Ca2+ channels (ROCCs)[6,7]. Participation of these channels in contraction varies among the types of smooth muscle present, the agonists used, and the experimental conditions planned.
In addition to these influx pathways, there is now significant data from different cell types that demonstrate controlled Ca2+ influx also occurs in response to depletion of intracellular Ca2+ stores. We call these store-operated Ca2+ channels (SOCCs) or capacitative Ca2+ entry (CCE) channels. Previous studies have suggested SOCCs are restricted in non-excitable cells[8,9], but they have only recently been investigated in excitable cells , such as smooth muscle cells and neurons, which are involved in smooth muscle tone and regulation of synaptic plasticity. It has been reported there is a relationship between CCE and the regulation of smooth muscle tone with respect to their involvement in pulmonary, stomach, bronchial, vascular, gallbladder, and basilar artery tissue[10–15]. There is thus far no data to show that CCE are necessary as a source of Ca2+ in the smooth muscle of the rat distal colon.
The neurotransmitter acetylcholine (ACh) induces smooth muscle contraction via activating muscarinic receptors. ACh has been shown to elicit contraction that consists of a rapid phasic phase, followed by a tonic phase, by activation of M3 muscarinic receptors in the rat and guinea pig gastric fundus and ileum, and the human ileum[16–19]. However, there have been some different results concerning the source of Ca2+ for muscarinic receptor-mediated contraction. It has been postulated that the sources of Ca2+ for phasic and tonic contraction induced by activation of muscarinic receptors differ among regions of the digestive tract and species of animals used.
It has been suggested that pharmacological manipulation of Ca2+ channels may be therapeutically useful in treating colonic motility disorders. Therefore, we tried to clarify the source of Ca2+ in the contraction induced by ACh. Sources of activator Ca2+ for muscarinic-mediated contraction of proximal colonic smooth muscle in rats have been well documented. With respect to the rat distal colon, the relative importance of the contribution of the different Ca2+ influx mechanisms, and the release of Ca2+ from the sarcoplasmic reticulum (SR), has not been assessed. The aim of this study was to evaluate the contribution of VOCCs, non-VOCCs and intracellular Ca2+ release to ACh-induced contraction in the smooth muscle of rat distal colon in vitro.
MATERIALS AND METHODS
Tissue preparation
A segment of distal colon (about 2 cm) was removed from Sprague-Dawley rats (150-250 g, supplied by the animal center of Anhui Medical University, China) killed by cervical dislocation. Pieces of colon were cleaned in a Petri dish, by perfusing with Krebs solution (114.0 mmol/L NaCl, 4.7 mmol/L KCl, 1.2 mmol/L MgCl2, 2.5 mmol/L CaCl2, 1.8 mmol/L NaH2PO4, 11.5 mmol/L glucose, 18.0 mmol/L NaHCO3, pH 7.4). The adipose and connective tissues were carefully stripped off with fine forceps. The endothelium of the colon was removed by gently rubbing the inner lumen of the vessels with a rough wooden stick.
Tension measurement
Pieces of distal colon were mounted in 40-mL organ baths containing Krebs solution, maintained at 37°C and gassed with 95% O2 and 50% CO2, pH 7.4. One end of each piece was fixed to a hook on the bottom of the bath and the other attached to a force displacement transducer (Xinhang, Beijing, China). A resting preload of 1 g was applied to each colon, which was then allowed to equilibrate for 1 h. During this time, the Krebs solution was changed every 20 min. Tension was continuously monitored and recorded using a Powerlab 4/25T data acquisition system (Australia AD Instruments) with an ML110 bridge bioelectric physiographic amplifier (Australia AD Instruments). ACh and atropine were dissolved in the perfusate on the day of use.
Cell preparation
A segment of the distal colon was removed and placed in oxygenated HEPES/Ringer buffer solution (126 mmol/L NaCl, 6 mmol/L KCl, 6 mmol/L HEPES, 11 mmol/L MgCl2, 1.5 mmol/L CaCl2), pH 7.4 with 5 mol/L NaOH. The fecal contents were removed by repeated rinsing with HEPES/Ringer solution. After the segment was cut open longitudinally and pinned out in a dissection dish. Under a microscope, longitudinal and circular muscle layers were freed of the mesentery, serosa and mucosa, and cut into small pieces (2 mm2) and rinsed in HEPES/Ringer solution for 20 min (at 37°C). Dispersion of smooth muscle cells was accomplished by three incubations at 31°C with agitation in low-Ca2+ HEPES/Ringer solution containing collagenase type I (0.6 mg/mL), bovine serum albumin (2 mg/mL) and soybean trypsin inhibitor (0.6 mg/mL). They were then suspended in enzyme-free low-Ca2+ HEPES/Ringer solution and gently agitated through the tip of a wide-bore glass pipette to liberate single smooth muscle cells. Isolated cells were collected by low-speed centrifugation and resuspended in HEPES/Ringer solution. Trypan blue exclusion was used to verify cell viability (> 90% of all cells).
Measurement of [Ca2+]i in distal colon smooth muscle cells
Measurement of [Ca2+]i in colonic smooth muscle cells using fura-2 was performed using dual excitation wavelength fluorescence microscopy. Aliquots of cell suspension in HEPES/Ringer solution (106 cells/mL) were placed in Quartz cuvettes and incubated with 5 μmol/L fura-2/AM at 37°C for 45 min. After being loaded with fura-2/AM, the cells were rinsed with Ca2+-free solution, centrifuged (1500 r/min) and suspended in Ca2+-free solution for the experiments that were carried out in a 960 MC spectrofluorophotometer (Shanghai Precision & Scientific Instruments, Shanghai, China). Cells were alternately illuminated at 340 and 380 nm and the intensity of fluorescence emission was measured at 500 nm. The [Ca2+]i was calculated according to Grynkiewicz’s equation: [Ca2+]i = Kd [(F - Fmin)/(Fmax - F)]: where the dissociation constant Kd was 224 nmol/L at 37°C; F was fluorescence intensity under different experiment conditions; Fmax was the maximum fluorescence intensity obtained in the presence of 0.1% Triton ×-100; and the minimum value (Fmin) was obtained by using 5 mmol/L EGTA in the external medium. Before calculation, the autofluorescence measured from cells that unloaded fura-2/AM should be subtracted. All the measurements were finished in 40 min.
Drugs and solutions
Reagents were procured from the following vendors: thapsigargin, DMSO, ACh, atropine, lanthanum, fura-2/AM, Triton ×-100, EGTA, collagenase type I, bovine serum albumin (BSA) and verapamil were from Sigma (USA), and soybean trypsin inhibitor was from GIBCO (USA). The solutions of thapsigargin and fura-2/AM were prepared by dilution with DMSO. The concentration of DMSO in the final solution was < 0.1% and did not affect [Ca2+]i. In cell experiments, the Ca2+-free HEPES/Ringer solution was prepared without CaCl2 and with addition of EGTA (0.2 mmol/L). The low-Ca2+ solution had the same composition, except CaCl2 was 0.03 mmol/L.
Statistical analysis
Biostatistical analysis was performed using the SPSS 11.0 software package. All experiments were repeated at least three times. Results of multiple experiments are given as the mean ± SE. Unpaired Student’s t test was used for statistical evaluation in two-group comparisons. P < 0.05 was accepted as statistically significant.
RESULTS
Establishment of SOCCs
We first established whether SOCCs were present in rat distal colon smooth muscle cells. As shown in Table 1, for the fura-2/AM loaded cells, the incubation in Ca2+ free solution lasted 3 min, followed by the addition of two concentrations of Ca2+ (1.5, 3.0 mmol/L). It can be seen that cytosolic calcium rose from 68.32 in Ca2+-free solution to 104.81 and 194.44 nmol/L for the two concentrations of 1.5 mmol/L and 3.0 mmol/L, respectively. The incubation of cells in Ca2+-free solution in the presence of thapsigargin (1 μmol/L), followed by the addition of Ca2+, 1.5 and 3.0 mmol/L, increased [Ca2+]i that was significantly greater (455.77 and 1005.9 nmol/L) than that in the absence of thapsigargin.
Table 1.
Measurement of [Ca2+]i in freshly dispersed suspension of smooth muscle cells from rat distal colon
| Group |
[Ca2+]i (nmol/L) |
||
| Ca2+-free | 1.5 mmol/L CaCl2 | 3.0 mmol/L CaCl2 | |
| medium | medium | medium | |
| Control | 68.32 ± 3.43 | 104.81 ± 6.37 | 194.44 ± 3.91 |
| Thapsigargin | 240.85 ± 12.65 | 457.55 ± 19.80b | 1005.93 ± 54.62b |
P < 0.01 vs control.
Sensitivity to verapamil
To distinguish SOCC-mediated Ca2+ influx from that via L-type Ca2+ channels, we conducted experiments in a separate set of cells using 5 μmol/L verapamil. In these cells pretreated with verapamil, the [Ca2+]i response to rapid reintroduction of [Ca2+]o in the presence of thapsigargin (n = 7) was determined as above. We found the presence of verapamil during rapid reintroduction of [Ca2+]o (1.5 mmol/L) did not significantly influence Ca2+ influx induced by thapsigargin. Previous studies in other tissues have found that SOCC-mediated Ca2+ influx is inhibited by La3+[20,21]. We investigated the sensitivity of multivalent cations to such Ca2+ influx. In a separate group of cells, pretreatment with La3+ and Ca2+ influx to rapid reintroduction in the presence of thapsigargin was greatly changed (Figure 1). Taken together, these results suggest the observed influx of extracellular Ca2+ in both instances was not mediated via L-type Ca2+ channels, but capacitative Ca2+ entry through SOCCs.
Figure 1.
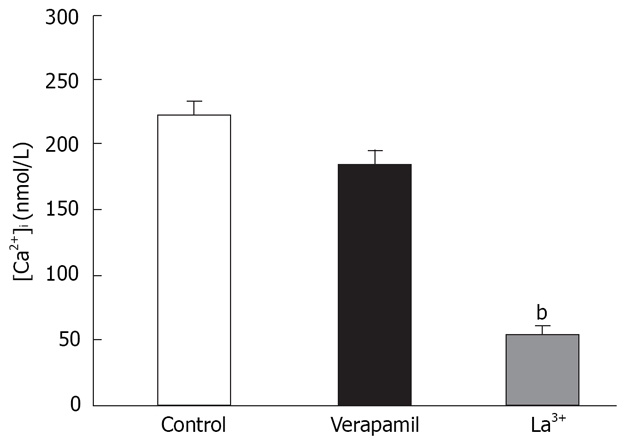
Ca2+ influx through SOCCs in enzymatically dissociated smooth muscle cells of rats distal colon. After depletion of the SR by thapsigargin in the absence of extracellular Ca2+, subsequent rapid reintroduction of extracellular Ca2+ resulted in activation of CCE via SOCCs. CCE were insensitive to verapamil, but blocked by La3+. bP < 0.01 vs control.
Role of membrane potential
A potential confounding factor in SOCC-mediated Ca2+ influx is the membrane potential, which alters the driving forces for Ca2+ entry. Accordingly, separate experiments were performed to determine the role of membrane potential. In the presence of verapamil (5 μmol/L), and pretreatment with high KCl solution (final concentrations of 40, 60 mmol/L), cells were re-exposed to thapsigargin, and [Ca2+]o was then rapidly reintroduced (final concentration, 1.5 mmol/L). Figure 2 shows the presence of 40 mmol/L KCl did not significantly influence Ca2+ influx induced by thapsigargin. However, in the presence of 60 mmol/L KCl, Ca2+ influx was decreased (P < 0.05).
Figure 2.
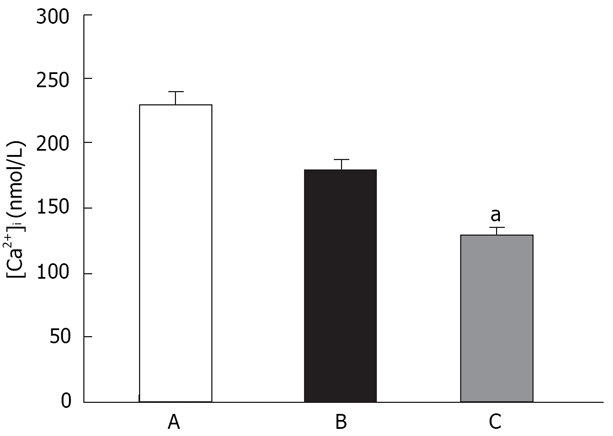
CCE are not mediated via L-type Ca2+ channels. (A) Controls; (B) 40 mmol/L K+; (C) 60 mmol/L K+. Only 60 mmol/L K+ significantly decreased the Ca2+ influx mediated by CCE. aP < 0.05 vs control.
Requirement of extracellular Ca2+ for distal colon contraction
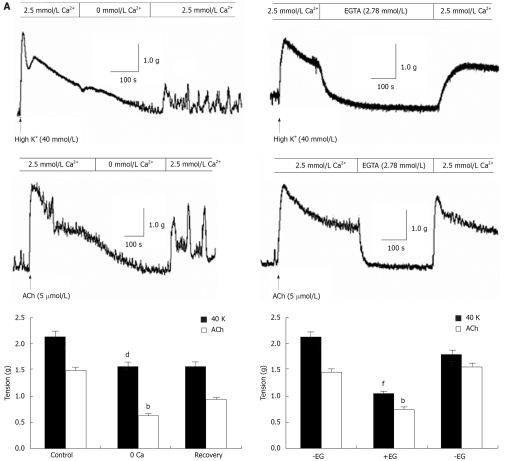
In isolated rat distal colon, removal of extracellular Ca2+ almost abolished the contraction induced by 40 mmol/L KCl or 5 μmol/L ACh, a muscarinic receptor agonist (n = 11, Figure 3A). Chelation of extracellular Ca2+ with 2.78 mmol/L EGTA, which decreases free Ca2+ concentration to 0.5 μmol/L in Krebs solution containing 2.5 mmol/L CaCl2, had the same inhibitory effect on contraction induced by 40 mmol/L K+ or 5 μmol/L ACh (n = 7, Figure 3B). Furthermore, extracellular application of the L-type VOCC blocker verapamil (5 μmol/L) almost abolished the 40 mmol/L K+-induced tension. The tension decreased from 2.1 to 0.52 g and the inhibitory rate was 74% (n = 7, P < 0.001). The tension induced by 5 μmol/L ACh was also inhibited, but the inhibitory rate was only 41%. The tension decreased from 1.44 to 0.85 g (n = 7, P < 0.05) (Figure 4). These observations indicated ACh induced rat distal colon contraction by activating multiple Ca2+ entry pathways, whereas 40 mmol/L K+-induced rat distal colon contraction was solely dependent on the membrane-depolarization-mediated opening of VOCCs.
Figure 3.
Role of Ca2+ in rat distal colon contraction. A: Representative results showing that removal of extracellular Ca2+ abolished contraction induced by high K+ and 5 μmol/L ACh. Summarized data showing that tension induced by high K+ and 5 μmol/L ACh before, during and after application of Ca2+-free Krebs solution. bP < 0.01, dP < 0.001 vs Control; B: Representative results showing that chelation of extracellular Ca2+ with 2.78 mmol/L EGTA abolished contraction induced by high K+ and 5 μmol/L ACh. Summarized data showing that tension induced by high K+ and 5 μmol/L ACh in the presence of EGTA bP < 0.01, fP < 0.001 vs-EG (before).
Figure 4.
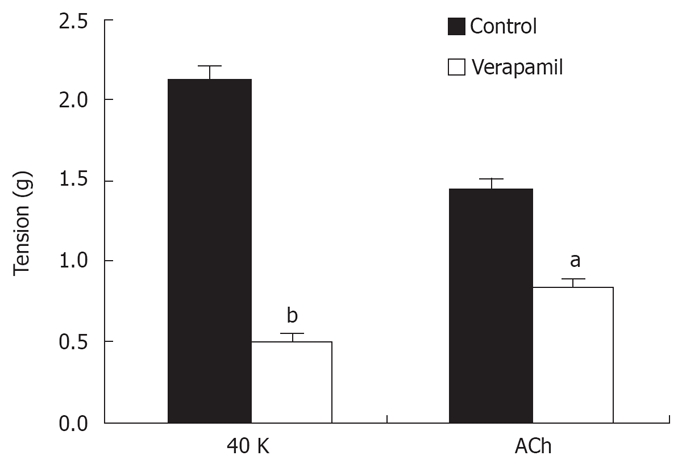
Effects of the VOCC blocker verapamil on distal colon contraction induced by high K+ and ACh. aP < 0.05, bP < 0.001 vs control.
CCE-mediated distal colon contraction
In the absence of extracellular Ca2+, ACh induced a transient contraction that was apparently due to Ca2+ release from the SR. The muscarinic receptor blocker atropine (10 μmol/L) was applied to the distal colon when the ACh-induced contraction returned to baseline. In the presence of atropine, restoration of extracellular Ca2+ caused tonic contraction (Figure 5Aa). We further showed tonic contraction was unaffected by verapamil (n = 5, Figure 5Ab). The CCE-mediated contraction in the presence of atropine was about 0.46-fold greater than that induced by SR Ca2+ release and the total peak contraction induced by ACh in the presence of extracellular Ca2+ (n = 9, Figure 5B). Taken together, these results showed that Ca2+ release from the SR and CCE via SOCCs both contribute to agonist-mediated distal colon contraction.
Figure 5.
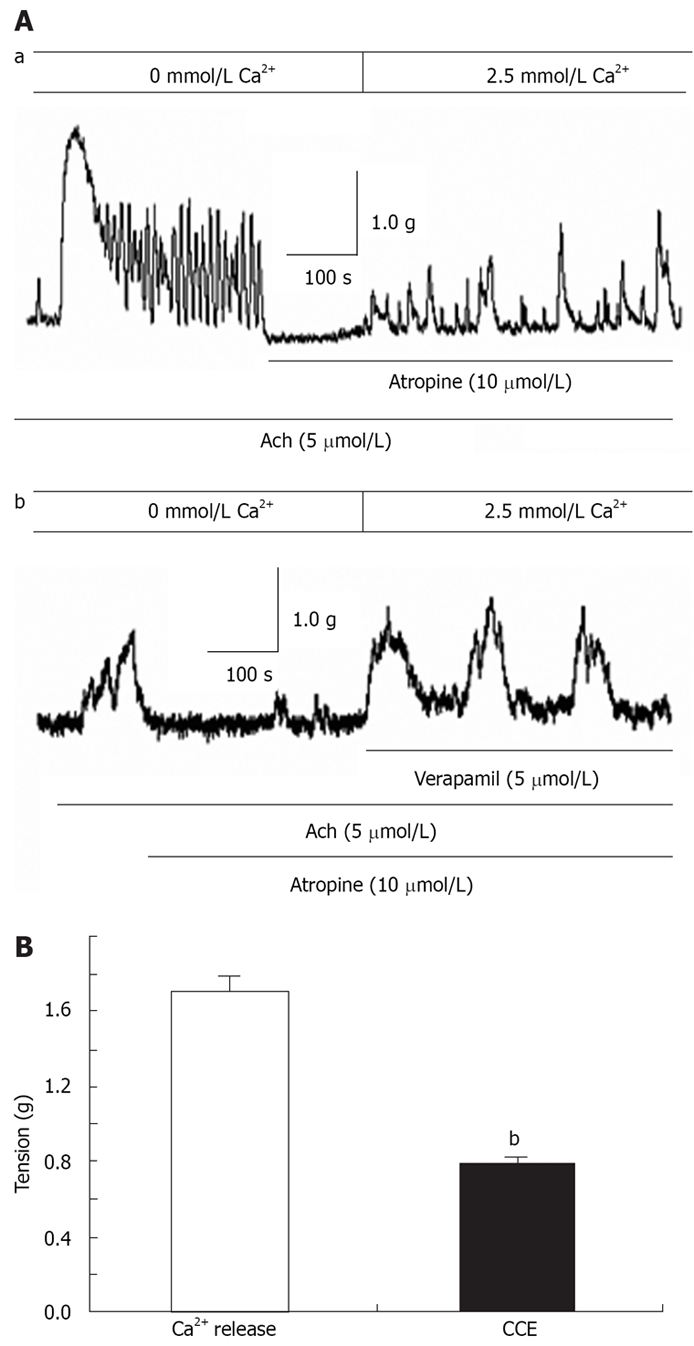
Contribution of Ca2+ release from SR stores and Ca2+ influx via CCE in ACh-induced distal colon contraction. Aa: In the presence of atropine, restoration of extracellular Ca2+ induced contraction, most likely due to Ca2+ influx via CCE; Ab: VOCC blocker verapamil (5 μmol/L) negligibly affected CCE-mediated contraction in the presence of atropine; B: Summarized data showing that CCE-mediated contraction is about 0.46-fold greater than the contraction induced by SR Ca2+ release in ACh-induced rat distal colon contraction. bP < 0.001 vs Ca2+ release.
Inhibitory effects of La3+ on CCE-mediated distal colon contraction
La3+ has been shown to block SOCCs in many cell types[11,31]. La3+ (50 μmol/L) was applied to the distal colon when CCE-induced contraction was maximized, and the CCE-mediated contraction was significantly attenuated (n = 7, Figure 6A and B). These results indicated the properties of SOCCs in the distal colon are similar to those found in other tissues.
Figure 6.
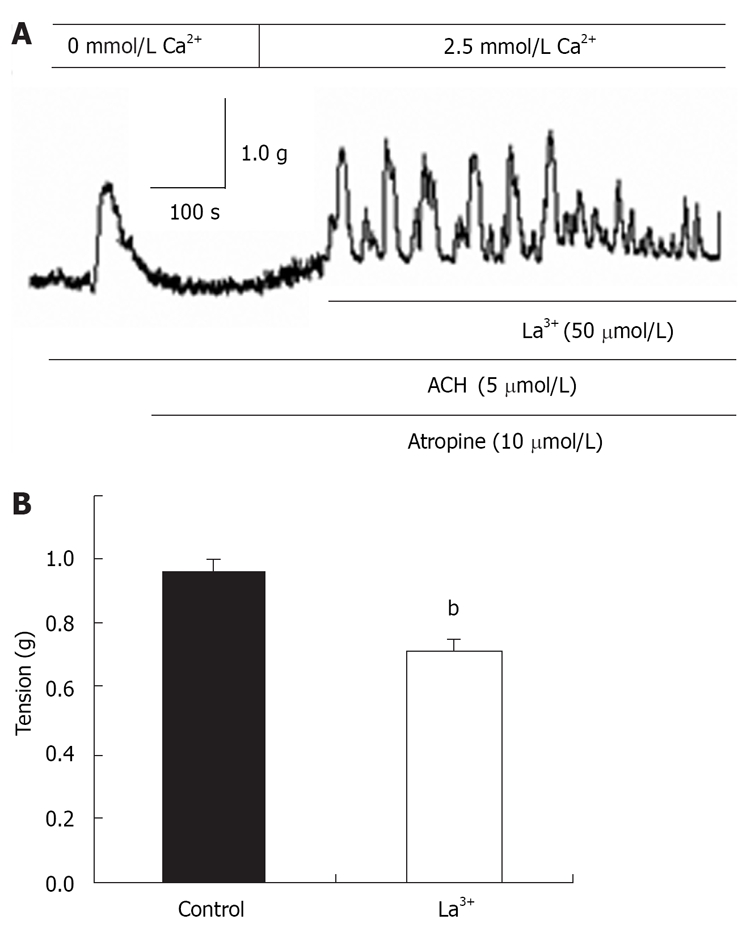
Inhibitory effects of La3+ on CCE-mediated distal colon contraction. bP < 0.01 vs control. Control: CCE-mediated contraction.
DISCUSSION
The results of this study reveal smooth muscle cells of rat distal colon express functional SOCCs, which play a vital role in agonist-induced contraction.
In many cells, depletion of intracellular Ca2+ stores is coupled to activation of CCE. The CCE model has gained support from experiments that used inhibitors of sarcoplasmic endoplasmic reticulum Ca2+-ATPase (SERCA), such as thapsigargin[22,23]. These drugs cause passive store depletion of Ca2+ by blocking the reuptake of Ca2+ into the SR storage pools[24,25]. Studies in a variety of preparations have demonstrated that SERCA inhibitors increase Ca2+ influx and/or smooth muscle tone[26,27]. To investigate the role of CCE in the contractile response in rat distal colon smooth muscle, freshly dissociated cells were incubated with thapsigargin in Ca2+-free HEPES/Ringer solution, followed by reintroduction of extracellular Ca2+, which greatly elevated [Ca2+]i. The lack of effect of thapsigargin resulted in only a slightly elevated [Ca2+]i. Moreover, the properties of the pathway were consistent with the CCE through SOCC found in several types of non-excitable cells. They are insensitive to verapamil (L-type VOCC inhibitor), but sensitive to La3+ (SOCC inhibitor). Unlike VOCCs, SOCCs do not appear to be greatly affected by changes in membrane potential. If thapsigargin-induced elevation of [Ca2+]i in the presence of verapamil is mediated via VOCCs, most positive membrane potentials should increase Ca2+ influx. However, in this study, such Ca2+ influx was not significantly altered by 40 mmol/L K+. Nevertheless, we do not rule out the role of VOCCs in distal colon smooth muscle cells, because 60 mmol/L K+ greatly altered elevation of [Ca2+]i. The relative contribution of VOCCs and SOCCs depends on whether a specific agonist causes membrane potential depolarization and/or only intracellular Ca2+ release.
In the rat distal colon, ACh and high K+ induced the contraction of smooth muscle in a phasic and tonic phase. It has been suggested the phasic contraction is induced by Ca2+ released from the SR, whereas the tonic phase is induced by Ca2+ that enters from outside the cell[28]. Both pathways for inducing contraction were found in the rat distal colon in the present study. The tonic phase was abolished or significantly inhibited by removal of extracellular Ca2+ or chelation of extracellular Ca2+ with 2.78 mmol/L EGTA. The present results suggest extracellular Ca2+ is necessary for the contraction induced by high K+ and ACh. ACh studied in the rat distal colon activates Ca2+ influx, which may be mediated by different mechanisms, from outside of the smooth muscle cells.
Having established in the present study that the contractile response to ACh in rat distal colon smooth muscle is dependent on extracellular Ca2+ influx, we then investigated the contributions of VOCCs and non-VOCCs to this response. Tissue pretreatment with verapamil essentially abolished the 40 mmol/L K+-induced contraction, but only partially attenuated the response to 5 μmol/L ACh, and the inhibitory rate was 41%. These results indicate the contraction induced by high K+ is mainly dependent on Ca2+ release from SR stores and Ca2+ influx mediated by VOCCs. Ca2+ influx mediated via VOCCs and non-VOCCs is most important for the contraction of rat distal colon smooth muscle induced by ACh.
The muscarinic receptor plays a key role in the parasympathetic nervous control of various peripheral tissues including the gastrointestinal tract[29]. Pharma-cological characterization of the contractile response to ACh has suggested the M3/Gq/11/PLCβ pathway, which acts through the production and release of inositol 1,4,5-trisphosphate (IP3) to produce mobilization of stored Ca2+, is largely responsible for the contraction[28]. Rather, it is believed the depletion of intracellular Ca2+ stores by IP3 generates an as-yet-uncharacterized signal that activates CCE[30,31]. Because in the present study, ACh induced transient contraction that was apparently due to Ca2+ release from the SR in the absence of extracellular Ca2+, the question arises, does CCE has any role in distal colon motility? We found that after the ACh-mediated transient contraction in the absence of extracellular Ca2+ and the return to baseline, the subsequent restoration of extracellular Ca2+ induced a sustained contraction in the presence of the muscarinic-receptor blocker atropine. Such sustained contraction was not sensitive to verapamil, and was decreased by 50 μmol/L La3+. This contraction was independent of: (1) activation of the muscarinic receptor, (2) intact endothelium, and (3) opening of VOCCs. The result indicated that in the absence of extracellular Ca2+, ACh actively depleted Ca2+ from the SR by increasing IP3 production; therefore, activation of SOCCs and induction of CCE may be the trigger for this contraction. Taken together, the results shows that Ca2+ release from the SR is responsible for the phasic contraction, and that CCE via SOCC is necessary for the sustained contraction of ACh-induced rat distal colon contraction.
In summary, this study demonstrated the predominant source of activator Ca2+ for the contractile response to ACh is extracellular Ca2+, and that intracellular Ca2+ has little role to play in mediating excitation-contraction coupling by ACh in rat distal colon smooth muscle in vitro. Influx of extracellular Ca2+ is mainly mediated by VOCCs, ROCCs and SOCCs. The study of Takeuchi et al[32] has shown that the source of Ca2+ in smooth muscle cells in the guinea pig proximal colon is mainly dependent on VOCCs. It is postulated that the source of activator Ca2+ for the contractile response varies among different regions of the gastrointestinal tract and the animals used. Further studies will hopefully be helpful in developing new drugs with unique properties, such as those that inhibit CCE mediated Ca2+ influx.
COMMENTS
Background
Various agonists induce contraction in a variety of smooth muscle preparations, with a concomitant increase in [Ca2+]i. It is suggested the sources of Ca2+ for phasic and tonic contraction induced by activation of muscarinic receptors differ among regions of the digestive tract and species of animals used.
Research frontiers
Intracellular Ca2+ is a critical second messenger responsible for linking external stimuli to contraction, proliferation and gene expression. It has been suggested some of the motor changes associated with intestinal infection, stress and inflammation are associated with alterations in [Ca2+]. Further studies of pharmacological manipulation of Ca2+ channels may be therapeutically useful in treating colonic motility disorders.
Innovations and breakthroughs
In this study, we investigated the importance of Ca2+ influx in smooth muscle contraction. We also evaluated the contribution of the different Ca2+ influx mechanisms and the release of Ca2+ from the sarcoplasmic reticulum (SR) to contraction induced by activation of muscarinic receptors in rat distal colon in vitro. The results showed that Ca2+ necessary for contractile response in the distal colon of rat was supplied from outside the smooth muscle cells via voltage-operated Ca2+ channels (VOCCs), receptor-operated Ca2+ channels (ROCCs) and store-operated Ca2+ channels (SOCCs), and the SR had a minor role in the contractile response.
Applications
The results provide further evidence for the contraction of the rat distal colon, which hopefully will be of importance in developing new drugs with unique properties, such as those that inhibit capacitative Ca2+ entry (CCE)-mediated Ca2+ influx.
Terminology
Thapsigargin is a potent skin irritating sesquiterpene lactone isolated from the roots of Thapsia garganica L. It also acts as a non-phorbol-ester-type tumor promoter, which discharges intracellular Ca2+ stores by specific inhibition of sarcoplasmic endoplasmic reticulum Ca2+-ATPase (SERCA).
Peer review
This paper examined the relative contributions of internal calcium release and calcium influx to phasic and tonic contraction in the distal colon, and measured calcium concentration and calcium influx in isolated colon smooth muscle cells. The work addressed an old topic but the current discovery of SOCCs in excitable cells such as smooth muscle cell (SMC) is interesting.
Supported by Grants from the fund of Wannan Medical College Scientific Research, No. WK 200729
Peer reviewer: Zong-Jie Cui, PhD, Professor, Institute of Cell Biology, Beijing Normal University, 19 Xinjiekou Waidajie, Beijing 100875, China
S- Editor Zhu LH L- Editor Kerr C E- Editor Yin DH
References
- 1.Shuba MF, Melenevs’ka NV, Filippov IB, Miroshnychenko MS. Molecular mechanisms of receptor-dependent signaling in the cells of the longitudinal and circular intestinal smooth muscles. Ukr Biokhim Zh. 2006;78:15–21. [PubMed] [Google Scholar]
- 2.Luo DL, Gao J, Fan LL, Tang Y, Zhang YY, Han QD. Receptor subtype involved in alpha 1-adrenergic receptor-mediated Ca2+ signaling in cardiomyocytes. Acta Pharmacol Sin. 2007;28:968–974. doi: 10.1111/j.1745-7254.2007.00605.x. [DOI] [PubMed] [Google Scholar]
- 3.Kapur N, Banach K. Inositol-1,4,5-trisphosphate-mediated spontaneous activity in mouse embryonic stem cell-derived cardiomyocytes. J Physiol. 2007;581:1113–1127. doi: 10.1113/jphysiol.2006.125955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarron JG, Craig JW, Bradley KN, Muir TC. Agonist-induced phasic and tonic responses in smooth muscle are mediated by InsP(3) J Cell Sci. 2002;115:2207–2218. doi: 10.1242/jcs.115.10.2207. [DOI] [PubMed] [Google Scholar]
- 5.MacMillan D, Chalmers S, Muir TC, McCarron JG. IP3-mediated Ca2+ increases do not involve the ryanodine receptor, but ryanodine receptor antagonists reduce IP3-mediated Ca2+ increases in guinea-pig colonic smooth muscle cells. J Physiol. 2005;569:533–544. doi: 10.1113/jphysiol.2005.096529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogalis F, Lang RJ, Bywater RA, Taylor GS. Voltage-gated ionic currents in smooth muscle cells of guinea pig proximal colon. Am J Physiol. 1993;264:C527–C536. doi: 10.1152/ajpcell.1993.264.3.C527. [DOI] [PubMed] [Google Scholar]
- 7.Ward SM, Dixon RE, de Faoite A, Sanders KM. Voltage-dependent calcium entry underlies propagation of slow waves in canine gastric antrum. J Physiol. 2004;561:793–810. doi: 10.1113/jphysiol.2004.076067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao J, Li Q, Chen J, Muallem S. Subpopulation of store-operated Ca2+ channels regulate Ca2+-induced Ca2+ release in non-excitable cells. J Biol Chem. 2004;279:21511–21519. doi: 10.1074/jbc.M314028200. [DOI] [PubMed] [Google Scholar]
- 9.Launay P, Cheng H, Srivatsan S, Penner R, Fleig A, Kinet JP. TRPM4 regulates calcium oscillations after T cell activation. Science. 2004;306:1374–1377. doi: 10.1126/science.1098845. [DOI] [PubMed] [Google Scholar]
- 10.Niger C, Malassine A, Cronier L. Calcium channels activated by endothelin-1 in human trophoblast. J Physiol. 2004;561:449–458. doi: 10.1113/jphysiol.2004.073023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia Y, Wang X, Varty L, Rizzo CA, Yang R, Correll CC, Phelps PT, Egan RW, Hey JA. Functional TRPV4 channels are expressed in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L272–L278. doi: 10.1152/ajplung.00393.2003. [DOI] [PubMed] [Google Scholar]
- 12.Geppetti P, Trevisani M. Activation and sensitisation of the vanilloid receptor: role in gastrointestinal inflammation and function. Br J Pharmacol. 2004;141:1313–1320. doi: 10.1038/sj.bjp.0705768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alcon S, Morales S, Camello PJ, Hemming JM, Jennings L, Mawe GM, Pozo MJ. A redox-based mechanism for the contractile and relaxing effects of NO in the guinea-pig gall bladder. J Physiol. 2001;532:793–810. doi: 10.1111/j.1469-7793.2001.0793e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villalba N, Stankevicius E, Garcia-Sacristan A, Simonsen U, Prieto D. Contribution of both Ca2+ entry and Ca2+ sensitization to the alpha1-adrenergic vasoconstriction of rat penile small arteries. Am J Physiol Heart Circ Physiol. 2007;292:H1157–H1169. doi: 10.1152/ajpheart.01034.2006. [DOI] [PubMed] [Google Scholar]
- 15.Kawanabe Y, Hashimoto N, Masaki T. Characterization of Ca2+ channels involved in endothelin-1-induced contraction of rabbit basilar artery. J Cardiovasc Pharmacol. 2002;40:438–447. doi: 10.1097/00005344-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Ghayur MN, Khan AH, Gilani AH. Ginger facilitates cholinergic activity possibly due to blockade of muscarinic autoreceptors in rat stomach fundus. Pak J Pharm Sci. 2007;20:231–235. [PubMed] [Google Scholar]
- 17.Elorriaga M, Anselmi E, Hernandez JM, D'Ocon P, Ivorra D. The sources of Ca2+ for muscarinic receptor-induced contraction in the rat ileum. J Pharm Pharmacol. 1996;48:817–819. doi: 10.1111/j.2042-7158.1996.tb03980.x. [DOI] [PubMed] [Google Scholar]
- 18.Parekh AB, Brading AF. The sources of calcium for carbachol-induced contraction in the circular smooth muscle of guinea-pig stomach. Br J Pharmacol. 1991;104:412–418. doi: 10.1111/j.1476-5381.1991.tb12444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitsui M, Karaki H. Dual effects of carbachol on cytosolic Ca2+ and contraction in intestinal smooth muscle. Am J Physiol. 1990;258:C787–C793. doi: 10.1152/ajpcell.1990.258.5.C787. [DOI] [PubMed] [Google Scholar]
- 20.Vassilopoulos S, Brocard J, Garcia L, Marty I, Bouron A. Retrograde regulation of store-operated calcium channels by the ryanodine receptor-associated protein triadin 95 in rat skeletal myotubes. Cell Calcium. 2007;41:179–185. doi: 10.1016/j.ceca.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Rao GK, Kaminski NE. Induction of intracellular calcium elevation by Delta9-tetrahydrocannabinol in T cells involves TRPC1 channels. J Leukoc Biol. 2006;79:202–213. doi: 10.1189/jlb.0505274. [DOI] [PubMed] [Google Scholar]
- 22.Lemonnier L, Trebak M, Lievremont JP, Bird GS, Putney JW Jr. Protection of TRPC7 cation channels from calcium inhibition by closely associated SERCA pumps. FASEB J. 2006;20:503–505. doi: 10.1096/fj.05-4714fje. [DOI] [PubMed] [Google Scholar]
- 23.Su Z, Guo X, Barker DS, Shoemaker RL, Marchase RB, Blalock JE. A store-operated nonselective cation channel in human lymphocytes. Cell Mol Neurobiol. 2005;25:625–647. doi: 10.1007/s10571-005-4005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elliott AC. Recent developments in non-excitable cell calcium entry. Cell Calcium. 2001;30:73–93. doi: 10.1054/ceca.2001.0215. [DOI] [PubMed] [Google Scholar]
- 25.Skutella M, Riiegg UT. Increase of empty pool-activated Ca2+ influx using an intracellular Ca2+ chelating agent. Biochem Biophys Res Commun. 1996;218:837–841. doi: 10.1006/bbrc.1996.0149. [DOI] [PubMed] [Google Scholar]
- 26.Quinn T, Feighery R, Baird AW. Role of Rho-kinase in guinea-pig gallbladder smooth muscle contraction. Eur J Pharmacol. 2006;534:210–217. doi: 10.1016/j.ejphar.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Grasa L, Rebollar E, Arruebo MP, Plaza MA, Murillo MD. The role of Ca2+ in the contractility of rabbit small intestine in vitro. J Physiol Pharmacol. 2004;55:639–650. [PubMed] [Google Scholar]
- 28.Unno T, Matsuyama H, Okamoto H, Sakamoto T, Yamamoto M, Tanahashi Y, Yan HD, Komori S. Muscarinic cationic current in gastrointestinal smooth muscles: signal transduction and role in contraction. Auton Autacoid Pharmacol. 2006;26:203–217. doi: 10.1111/j.1474-8673.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- 29.Moreau B, Nelson C, Parekh AB. Biphasic regulation of mitochondrial Ca2+ uptake by cytosolic Ca2+ concentration. Curr Biol. 2006;16:1672–1677. doi: 10.1016/j.cub.2006.06.059. [DOI] [PubMed] [Google Scholar]
- 30.Putney JW Jr. Type 3 inositol 1,4,5-trisphosphate receptor and capacitative calcium entry. Cell Calcium. 1997;21:257–261. doi: 10.1016/s0143-4160(97)90050-6. [DOI] [PubMed] [Google Scholar]
- 31.Putney JW Jr. TRP, inositol 1,4,5-trisphosphate receptors, and capacitative calcium entry. Proc Natl Acad Sci USA. 1999;96:14669–14671. doi: 10.1073/pnas.96.26.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeuchi T, Sumiyoshi M, Kitayama M, Hirayama N, Fujita A, Hata F. Origin of Ca2+ necessary for carbachol-induced contraction in longitudinal muscle of the proximal colon of rats. Jpn J Pharmacol. 2001;87:309–317. doi: 10.1254/jjp.87.309. [DOI] [PubMed] [Google Scholar]