Abstract
AIM: To investigate whether KAI1, as a metastasis suppressor gene, is associated with invasive and metastatic ability of pancreatic cancer cells.
METHODS: KAI1 gene was transfected into pancreatic cancer cell line MiaPaCa II by liposomes selected with G418. Expression of transfected cells was measured by Western blotting, immunofluorescence and immunocytochemistry. Tumor cell invasion and metastatic ability were detected through gelatinase activity and reconstituted basement membrane (Matrigel) assay. pCMV-KAI1 was directly injected into the heterotopic human pancreatic adenocarcinoma successfully established in the groin of BALB/C nude mice, by subcutaneous injection of MiaPaCa II pancreatic cancer cells. The statistical analysis between groups was determined by Student’s two tailed t test.
RESULTS: By Western blotting, MiaPaCa II cells transfected by KAI1 gene indicated KAI1 expression at approximately 29.1 kDa. Cytoplasm staining was positive and uniformly spread in transfected cancer cells, using immunohistochemistry and immunofluorescence. The most obvious difference was present after 30 h (MiaPaca II 43.6 ± 9.42, pCMV-MiaPaca II 44.8 ± 8.56, pCMV-KAI1-MiaPaca II 22.0 ± 4.69, P < 0.05). Gelatinolysis revealed a wider and clearer band of gelatinolytic activity in non-transfected than in transfected cells (MiaPaCa II cells 30.8 ± 0.57, transfected cells 28.1 ± 0.65, P < 0.05). In vivo tumor growth rates of KAI1 transfectants with KAI1-Lipofectamine 1.22 ± 0.31 in A group were lower than control 4.61 ± 1.98 and pCMV-KAI 11.67 ± 0.81. Analyses of metastases with and without KAI1 transfection in mice were different in liver and lung between controls 1.62 ± 0.39, 0.45 ± 0.09, pCMV-KAI 1.01 ± 0.27, 0.33 ± 0.09 and KAI1-Lipofectamine 0.99 ± 0.21, 0.30 ± 0.09 respectively (P < 0.05).
CONCLUSION: High expression of KAI1 gene was found in transfected MiaPaCa II human pancreatic cancer cells with lower metastatic ability. KAI1 gene plays an important role in inhibiting metastasis of pancreatic cancer after direct injection into pancreatic adenocarcinoma. These results show that the suppressed invasion and motor function of pancreatic cancer cells may be a key reason why the KAI1 gene controls pancreatic cancer cell metastasis.
Keywords: KAI1, Pancreatic cancer cell line, Transfection, Immunocytochemistry, Western blotting, Immunofluorescence, Gelatinolysis
INTRODUCTION
Invasion and metastasis are remarkable characteristics in pancreatic cancer. During invasion and metastasis, primary pancreatic cancer cells have to move out, invade adjacent tissue, and circulate to distant organs where they may form new colonies[1]. Tumor invasion encompasses the process of tumor cell penetration or infiltration into adjacent tissue; therefore, metastasis depends on host immunological ability, local and systemic expansion of the tumor mass, and angiogenesis[2]. Once metastatic cells settle in a distant organ, nutrients and spaces are no longer limited because newly formed metastases arise as islets of cancer cells surrounded by normal host cells. Invasion and metastasis are complex processes that involve molecular mechanisms, which lead to multiple gene alterations in pancreatic carcinoma, including alterations to KAI1, K-ras, DPC4 and P53[3–6]. KAI1 was originally identified as a metastasis suppressor gene.
KAI1 has been mapped to human chromosome 11p11.2. The expression level of KAI1 mRNA in human cell lines derived from metastatic prostate cancers is undetectable or much lower than that in normal prostate tissue[7]. Moreover, the gene transfer of KAI1 into rat AT6.1 prostate cancer cells results in significant suppression of pulmonary metastases. KAI1, which is identical to CD82, belongs to a structurally distinct family of membrane glycoproteins named transmembrane 4 (TM4). KAI1 is expressed ubiquitously, not only in prostate tissue, but also in a wide variety of other tissues, which suggests that it may play a role in suppressing metastases of other tissue-derived cancers, such as pancreatic, non-small cell lung, bladder, gastric and breast cancer[8–12]. These findings, taken together with the fact that KAI1 protein has a nearly ubiquitous tissue distribution, suggest that KAI1 may function as a metastasis suppressor gene in other types of cancers.
In our previous studies, we have demonstrated that KAI1 expression, at both mRNA and protein levels, is inversely correlated with the metastatic potential of some established human pancreatic cancer cell lines. In addition, we have also assessed KAI1 protein expression in human pancreatic cancer specimens from patients with known clinical outcome. We have found that more malignant tumor types express significantly lower levels of KAI1 protein, and down regulation of KAI1 at the RNA level is correlated with lymph node and distant metastases in patients with advanced pancreatic cancer[12,13]. The function of KAI1 with anti-metastasis has been unclear up till now. The aim of this study was to examine the expression of KAI1 protein in pancreatic cancer cells transfected by the gene, and to determine whether KAI1 transfected into pancreatic cancer cells can suppress invasive and metastatic ability of the cancer cells.
MATERIALS AND METHODS
Cells and cell culture
The human pancreatic cancer cell MiaPaCa II was kindly provided by Dr. H. Friess. The cells was cultivated in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal calf serum (FCS), 2 mmol/L L-glutamine, 100 IU/mL penicillin G and 100 μg/mL streptomycin, and passaged with 0.25% trypsin.
Transfection
Expression plasmids encoding KAI1 (pCMV-KAI1 DNA) were obtained from Dr. J. Dong (University of Virginia Health System). The day before transfection, cells were trypsinized and counted, and plated at 1 × 105 cells per well, so that they were 80%-90% confluent on the day of transfection. Cells were plated in 0.5 mL normal growth medium. For each well of cells to be transfected, we diluted 1.0 μg pCMV-KAI1 DNA into 50 μL medium without serum (this can be prepared in bulk for multiple wells). For each well of cells, we diluted 1 μL LF2000 Reagent (GibcoBRL, Maryland, USA) into 50 μL medium without serum and incubated for 5 min at room temperature. The diluted DNA was combined with the diluted LF2000 Reagent, and incubated at room temperature for 20 min. We added 0.5 mL medium without serum, then 100 μL DNA-LF2000 Reagent complexes was added directly to each well and mixed gently by rocking the plate back and forth. The cells were incubated at 37°C in a CO2 incubator for 24 h, and passaged at a 1:10 into fresh growth medium. After 24 h, we added 400 μg/mL G418 selection medium for the cells transfected by KAI1. At 4 wk after transfection, stable transfected cells were obtained. MiaPaCa II cells transfected with pCMV served as a negative control.
Western blotting analysis
1 × 107 cells for Western blotting were prepared in 0.5 mL Lysis Buffer (10 mmol/L Tris/HCl, 5 mmol/L EDTA, 50 mmol/L NaCl, 30 mmol/L Na3PO4, 50 mmol/L NaF, 0.1 mmol/L Na3VO4, 1 mmol/L PMSF, 5 mg/mL aprotinin, 0.1% Triton X-100, pH 7.6). Homogenates were centrifuged for 10 min at 10 000 rpm at 4°C. The supernatant was collected and maintained at -20°C. After denaturing at 100°C for 5 min, the supernatant was subjected to SDS-PAGE on 5% concentrated gels and 7.5% segregated gels. Separated proteins were electrophoretically transferred to PVDF membranes for 1.5 h at 4°C. These membrane transfers were dried and incubated over night at 4°C with 5% defatted milk powder/TBS/0.1% Tween-20. After washing with TBS, the membrane transfers were incubated with purified rabbit polyclonal antibody against KAI1 (CD82) (BD Biosciences, New Jersey, USA) 1:200 in buffer (10 mmol/L Tris/HCl, 100 mmol/L NaCl, 5% BSA, and 0.1% Tween-20, pH 7.4). Following three washes of 20 min in TBS/Tween-20, membrane transfers were incubated with goat anti-rabbit IgG/horseradish peroxidase conjugate for 1 h. Each was removed by three 20 min washes in TBS/Tween-20. Immunoreactive proteins in peroxidase-treated transfers were visualized using an ECL Western blotting detection system (Amersham Life Sciences, Little Chalfont, United Kingdom) with exposure to X-ray film for 30-120 s. Prestained SDS-PAGE standard proteins (Bio-Rad, Richmond, CA, USA) were employed as molecular size markers.
Immunofluorescence
The pancreatic cells before and after transfection were allowed to attach overnight to chambered glass slides. After incubation, cells were rinsed in PBS, fixed in ice-cold methanol, washed, and blocked in a PBS solution containing globulin-free BSA (1%), Triton X-100 (0.3%) and goat serum (3%). The cells were incubated with primary antibodies (CD82, 1:80 dilution) in a humidified chamber for 1 h at room temperature, washed in PBS, and incubated again for 1 h in the dark with a secondary antibody (1:80 dilution) coupled to the fluorophore. After washing, the cell lines were covered with DAPI solution (Roche, New Jersey USA). Slides were mounted with coverslips and examined by fluorescence microscopy.
Immunocytochemical detection of KAI1 protein
Briefly, cells were washed in PBS, fixed in ice-cold acetone for 20 min at 4°C, soaked in 0.3% H2O2 for 30 min, and blocked in a PBS solution containing NP40 and goat serum (10%). The cells were incubated with specific KAI1 antibody, diluted 1:20 in PBS containing 10% goat serum and NP40, at 4°C overnight. After washing, we added a secondary antibody (1:100 dilution), coupled to biotin for 1 h at room temperature, and the samples were stained with DAB.
Cell invasion assay
In Transwell cell culture chambers (Costar 3422, Cambridge, MA, USA), polycarbonate membranes with 8.0-μm pore size were spread with 40 μL (10 μg) of Matrigel (BD Biosciences) on the upper surface at 37°C. After 1 h, each insert was placed in 500 μL DMEM containing 0.1% BSA, for hydration at 37°C for 30 min. The membranes were precoated with 40 μL (2 μg) fibronectin (Invitrogen) on the lower surface of the filters. The filters were dried at room temperature for 45 min. MiaPaCa II and KAI1-MiaPaCa II cells (1 × 105) suspended in 100 μL DMEM containing 0.1% BSA were added to the upper compartment and incubated at 37°C for 10-60 h. Every 10 h, the filters were fixed with 95% ethanol for 15 min and stained with hematoxylin and eosin. After gently rinsing with water, the cells on the upper surface of the filters were removed by wiping with a cotton swab. The filter containing the stained cells that invaded to the lower surface was manually counted under a microscope at × 400 magnification. Each assay was performed in triplicate.
Gelatin zymography
MiaPaCa II and KAI1-MiaPaCa II cells (1 × 105) were cultured overnight using ordinary serum-containing DMEM, washed with serum-free DMEM, and further cultured using serum-free DMEM for 24 h. The supernatant was collected, and the cellular fragment was removed by low-speed centrifugation and concentrated 30-fold with a membrane dialysis concentrator. The conditioned medium was used for the detection of gelatinase activity. Gelatin zymograms were obtained according to the method reported by Heussen and Dowdle[18], with some modifications. In brief, the conditioned medium was resolved by SDS-PAGE using 8% separating gel containing 0.1% (w/v) gelatin and 5% stacking gel. The gels were washed twice in 2.5% (w/v) Triton X-100 for 30 min at room temperature to remove SDS, then incubated in reaction buffer containing 50 mmol/L Tris/HCl, pH 7.6, 10 mmol/L CaCl2 and 1 μmol/L ZnCl2 for 12-16 h at 37°C. The gelatin gels were stained with 0.1% (w/v) Coomassie brilliant blue R-250 in 45% (v/v) methanol and 10% (v/v) acetic acid for 4 h. After destaining with 45% methanol and 10% acetic acid, the gelatinolytic activity on the gel was detected as clear bands on a blue background of undigested gelatin, and quantitated using a Master Scan gel analysis system (Scanalytics, Billerica, MA, USA).
In vivo growth and experimental metastasis assay
To investigate the effect of KAI1 on in vivo metastasis, 1 ×106 cells in 0.1 mL 0.9% NaCl were injected subcutaneously into the flank region of 36 nu/nu mice (20 g body weight; China Medical University Breeding Laboratories, Shenyang, China). Mice were randomly divided into two groups: group A (n = 18) was injected with the KAI1 gene 10 d after injection of pancreatic cancer cells, and group B (n = 18) was treated 21 d after injection as our report[14]. The mice in group A were randomly divided into three (six mice per group), including pCMV-KAI1, pCMV-KAI1-Lipofectamine and control. Group B was divided the same as group A. One hundred micrograms of recombinant plasmid pCMV-KAI1, 100 μg pCMV-KAI1-Lipofectamine (1:1) and 100 μL 0.9% NaCl were directly injected into pancreatic adenocarcinoma every 7 d, and repeated three times. Tumor size was monitored 1 wk after inoculation of tumor cells. Tumor volume was determined using the formula V = A × B × 0.4, where A and B are the larger and smaller diameters of the tumor, respectively. After 50 d, the mice were necropsied, and the lungs were removed. Visible lung metastases were counted in Bouin’s fixed tissues, with the aid of a dissecting microscope.
Statistical analysis
The statistical significance of differences between the groups was determined by applying Student’s two-tailed t test. P < 0.05 was considered statistically significant. Values are indicated as the mean ± SD.
RESULTS
Confirmation of KAI1 gene transfection
The pCMV-KAI1 DNA was transformed into CM109 using the Maxi preparation. KAI1 DNA was identified in the correct position. The correct orientation was confirmed by PCR and sequencing analysis. MiaPaCa II cells transfected with pCMV-KAI1 DNA retained G418 resistance for 10 generations. The transfection of MiaPaCa II cells indicated KAI1 expression.
Expression of KAI1 protein
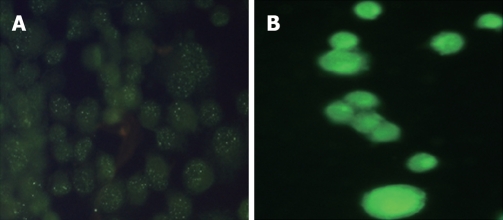
The stably transfected cells were analyzed by Western blotting to evaluate the intracellular amounts of KAI1 protein. The major specific KAI1 protein was clearly visible at about 29.1 kDa in MiaPaCa II pancreatic cells transfected with pCMV-KAI1 DNA, but almost undetectable in cells transfected with pCMV. The most positively staining was found in the cytoplasm in transfected cancer cells. Negative staining intensity in non-transfected cells was shown to be comparable with that in transfected cells. Strong green immunofluorescence (Figure 1) was seen in the transfected MiaPaCa II pancreatic cancer cells, whereas very weak immunofluorescence was found in cells transfected with pCMV.
Figure 1.
A: Very weak immunofluorescence in cells with empty plasmids without KAI1; B: Strong green immunofluorescence in cells transfected with KAI1.
Invading ability of cells transfected with KAI1
The transfection of KAI1 cells on tumor invasion of Matrigel was evaluated using a Transwell chamber assay. The data in Table 1 shows that the stained cells invading the lower surface of the filters of pCMV-KAI1-MiaPaca II were less than pCMV-MiaPaca II and MiaPaca II cells without transfection. There was no distinct difference in the number of invasive cells before and 10 and 20 h after transfection, while there was an obvious difference after 30 h (P < 0.05). There was no difference in the number of invasive cells observed between cells transfected by pCMV vector control and MiaPaca II (P > 0.05). The results show that transfection of the KAI1 gene significantly reduced the invasive ability of MiaPaCa II cells.
Table 1.
Invasive ability of pancreatic cancer cells (mean ± SD)
| Cell type |
Number of cells at different times |
|||||
| 10 h | 20 h | 30 h | 40 h | 50 h | 60 h | |
| MiaPaca II | 20.6 ± 6.43 | 23.6 ± 7.50 | 43.6 ± 9.42 | 59.8 ± 6.06 | 86.8 ± 4.32 | 117.4 ± 4.88 |
| pCMV-MiaPaca II | 21.0 ± 7.21 | 24.8 ± 6.46 | 44.8 ± 8.56 | 62.6 ± 3.11 | 87.4 ± 1.82 | 115.4 ± 3.58 |
| pCMV-KAI1-MiaPaca II | 14.8 ± 6.69 | 16.4 ± 4.51 | 22.0 ± 4.69 | 25.6 ± 5.55 | 29.4 ± 4.93 | 33.6 ± 6.11 |
| P > 0.05 | P > 0.05 | P < 0.05 | P < 0.05 | P < 0.05 | P < 0.05 | |
P < 0.05 between KAI1-transfected and non-transfected cells.
Gelatinolysis in MiaPaCa II pancreatic cells
We investigated the effect of transfection on the gelatinolytic activity of MMP-2 by gelatin zymography. Zymograms from MiaPaCa II cells revealed a wider and clearer band of gelatinolytic activity than that from transfected cells. Values ofIA were 30.8 ± 0.57 of MiaPaCa II cells, moreover 28.1 ± 0.65 of transfected cells (P < 0.05).
Tumor growth
Tumor volume was assessed at 7 d after injection of tumor cells and continuously every 3 d until 50 d. Time curves showing the percentage increase in tumor size in saline-treated and KAI1-treated mice are shown in Figure 2. At d 21, the KAI1 transfectants had significantly lower tumor volume compared with the controls (P < 0.05). As a result of KAI1 administration, tumor growth was dramatically reduced in pCMV KAI1 in group A.
Figure 2.
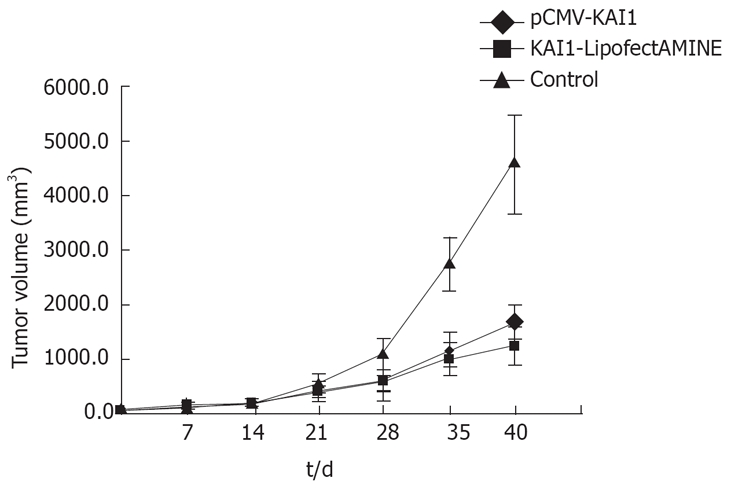
Effect of KAI1 expressions on in vivo tumor growth in group A.
In group A (Table 2), the tumor volume inhibitory rate of the KAI1 and KAI1-Lipofectamine groups was 63.79% and 73.51%, and the lung metastatic nodule number was 0.5 ± 0.83 and 2.33 ± 1.63, respectively. The tumor volume, lung weight, liver weight and lung metastatic nodule number of the KAI1-treated group were significantly lower than those in the control group (P < 0.05; Table 3). These data showed no significant difference between the KAI1 and KAI1-Lipofectamine groups. In group B, there was no significant difference between the gene therapy and saline control groups. There was a significant difference in tumor volume, lung weight, liver weight and lung metastatic nodule number between group A and B (P < 0.05).
Table 2.
Effect of KAI1 expression on in vivo tumor growth (mean ± SD)
| Group | n | Tumor volume (cm3) | t | P | |
| Control | 6 | 4.61 ± 1.98 | - | - | |
| A | KAI1-LipofectAMINE | 6 | 1.22 ± 0.31 | 2.860 | < 0.05 |
| pCMV-KAI1 | 6 | 1.67 ± 0.81 | 2.414 | < 0.05 | |
| Control | 6 | 4.61 ± 1.98 | - | - | |
| B | KAI1-LipofectAMINE | 6 | 4.87 ± 1.58 | 0.199 | > 0.05 |
| pCMV-KAI1 | 6 | 4.16 ± 2.81 | 0.286 | > 0.05 |
P < 0.05 between KAI1 transfection and controls.
Table 3.
Analyses of liver and lung metastases with and without KAI1 transfection in mice in vivo (mean ± SD)
| Group | n | Liver weight (g) | t | P | Lung weight (g) | t | P | |
| Control | 6 | 1.62 ± 0.39 | 0.45 ± 0.09 | |||||
| A | KAI1-LipofectAMINE | 6 | 0.99 ± 0.21 | 2.661 | < 0.05 | 0.30 ± 0.09 | 3.181 | < 0.05 |
| pCMV-KAI1 | 6 | 1.01 ± 0.27 | 2.295 | < 0.05 | 0.33 ± 0.09 | 2.291 | < 0.05 | |
| Control | 6 | 1.62 ± 0.39 | 0.45 ± 0.09 | |||||
| B | KAI1-LipofectAMINE | 6 | 1.69 ± 0.17 | 0.312 | > 0.05 | 0.38 ± 0.03 | 1.348 | > 0.05 |
| pCMV-KAI1 | 6 | 1.46 ± 0.28 | 1.286 | > 0.05 | 0.36 ± 0.04 | 1.956 | > 0.05 |
P < 0.05 between KAI1 transfection and controls.
DISCUSSION
The KAI1 gene as a metastasis suppressor gene may be a useful marker for the metastatic/invasive potential of several human tumor types[9–11,15]. In the case of pancreatic cancer, KAI1 appears to be crucial for the development of distant organ metastasis, since loss of KAI1 expression is found in lymph node and distant metastases[12,13,16], therefore, down-regulation of the KAI1 gene during cancer development may be associated with methylation of the promoter or p53 regulation, but does not commonly interfere either mutation or allelic loss of the KAI1 gene[17]. Although the mechanisms of invasion and metastasis of the KAI1 gene have not been elucidated in pancreatic and other cancers, a better understanding of these mechanisms that lead to cancer remains a major goal because it may help develop strategies for earlier diagnosis and better treatment.
In the present study, KAI1 expression was examined using immunohistochemistry. Positive staining for the protein in cells transfected with the KAI1 gene should be observed mainly at the cell membrane, because KAI1 is a member of the TM4 superfamily, however protein localization in the transfected cells was mainly found in the cytoplasm, similar to that in previous studies of human pancreatic cancer[18]. We used a rabbit polyclonal antibody and found that KAI1 was expressed abundantly in transfected cells, but there was negative staining in the non-transfected cells. Our data suggests that the protein upregulation in KAI1 transfected cells is the same as the expression in human pancreatic cancer samples. This may suggest that KAI1 inhibits metastasis in pancreatic cancer cells associated with the human samples[12].
Cancer mortality is due largely to distant metastases and subsequent organ failure. Metastasis involves the degradation of the basement membrane and stromal extracellular matrix (ECM) and migration into adjoining blood vessels, which results in tumor growth in distant organs[19,20]. Degradation of the basement membrane and ECM involve the secretion of several proteases, such as one or more members of the MMP family[21–23]. Among more than 20 MMPs that have been identified[24], MMP-2 has been described as a negative prognostic marker of metastasis and disease-free interval[25–27]. We transfected KAI1 cDNA into highly metastatic pancreatic cancer cells, MiaPaCa II, and investigated whether KAI1 gene reduced invasive ability of transfected cells and decreased gelatinase activity. In other words, the gelatinolytic activity by gelatin zymography was shown to increase in cells that were not transfected with the KAI1 gene. Our results showed that after KAI1 gene transfection, the activity of gelatinase decreased markedly, which suggests that KAI1 may suppress expression of gelatinase. Through a reconstituted basement membrane (Matrigel) in vitro, we found that stained cells invading the lower surface of the filters of pCMV-KAI1-MiaPaca II were less than pCMV-MiaPaca II and MiaPaca II cells without transfection. The number of invasive cells observed did not obviously differ between cells transfected with the pCMV vector and non-transfected cells. This indicates that, as with any malignancy, the propensity for local/regional invasion and distant metastasis of pancreatic cancer may be based on its ability to invade the basement membrane.
The time of gene therapy selected was at 10 d (group A) and 21 d (group B) after injecting pancreatic cells, according to our previous research[14]. In agreement with studies of prostate cancer, colon cancer and melanoma cells[7,28,29], our data also indicated that KAI1 expression suppressed metastasis in vivo when transfected into pancreatic cancer cells, and revealed a decrease in tumor volume of KAI1 transfectants 3 wk after inoculation, similar to breast cancer cells[11]. These results showed that KAI1 cDNA may be directly injected into the body of pancreatic adenocarcinoma to inhibit its metastasis and growth, without being mediating by liposomes, but the inhibition distinctly correlated with the time at which KAI1 gene was injected. The present data, along with our previous results[30], showed that the KAI1 gene may have play a key role in inhibiting metastasis of pancreatic cancer, by controlling cancer cell growth at secondary metastatic loci.
KAI1, as a metastasis suppressor gene, has drawn special attention as a new therapeutic target, in the hope that re-activation of the gene reduces the metastatic ability of malignant cells. We found that down-regulation of KAI1 mRNA level was correlated with lymph node and distant metastases in patients with advanced pancreatic cancer[11], whereas the results suggested that the activity of gelatinase, ability to invade the basement membrane, and metastasis of pancreatic cancer in mice, were decreased in the cells transfected with the KAI1 gene. In general, the results demonstrated that a decreased level of KAI1 mRNA expression in advanced pancreatic cancer involves both invasion and metastasis based on principles of cellular and molecular pathology.
COMMENTS
Background
Metastasis is an important feature in pancreatic cancer. However, recently no new breakthroughs have been found. Therefore it is necessary to use new techniques to detect early-stage metastasis in pancreatic cancer.
Research frontiers
This is a frontier of the molecular biology of tumor genetics interfering with up-regulation of the KAI1 gene to inhibit growth and metastasis of primary pancreatic cancer in mice. The propensity for local and regional invasion and distant metastasis of pancreatic cancer may be associated with its ability to invade the basement membrane.
Innovations and breakthroughs
A leading phenomenon on controlling metastasis of pancreatic cancer was found in KAI1 gene regulation. It may suggest mechanisms for inhibiting metastases of other tumors in the human body.
Applications
The findings help to explain some of the mechanisms behind metastasis of cancer. This research may contribute to providing new gene treatment options for pancreatic cancer patients.
Peer review
This is a good study. The authors have shown that there was high expression of the KAI1 gene in transfected MiaPaCa II human pancreatic cancer cells with lower metastatic ability. They conclude that the KAI1 gene plays an important role in inhibiting the metastasis of pancreatic cancer.
Acknowledgments
We thank Dr. TJ Dong and Dr. H Friess for their generous gifts of the KAI1 plasmid and MinPaCa II pancreatic cancer cells, respectively.
Supported by Grant-in-aid No. 39970344 and No. 30470798 (to Dr. Xiao-Zhong Guo) from the National Nature Science Foundation, China in 1999 and 2004
Peer reviewer: Yoshiharu Motoo, MD, PhD, FACP, FACG, Professor and Chairman, Department of Medical Oncology, Kanazawa Medical University,1-1 Daigaku, Uchinada, Ishikawa 920-0293, Japan
S- Editor Zhu LH L- Editor Kerr C E- Editor Wang HF
References
- 1.DiMagno EP, Reber HA, Tempero MA. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. American Gastroenterological Association. Gastroenterology. 1999;117:1464–1484. doi: 10.1016/s0016-5085(99)70298-2. [DOI] [PubMed] [Google Scholar]
- 2.Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808–812. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- 3.Tascilar M, Skinner HG, Rosty C, Sohn T, Wilentz RE, Offerhaus GJ, Adsay V, Abrams RA, Cameron JL, Kern SE, et al. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2001;7:4115–4121. [PubMed] [Google Scholar]
- 4.Shi X, Friess H, Kleeff J, Ozawa F, Buchler MW. Pancreatic cancer: factors regulating tumor development, maintenance and metastasis. Pancreatology. 2001;1:517–524. doi: 10.1159/000055854. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Yamaguchi Y, Watanabe H, Ohtsubo K, Motoo Y, Sawabu N. Detection of p53 gene mutations in the supernatant of pancreatic juice and plasma from patients with pancreatic carcinomas. Pancreas. 2004;28:13–19. doi: 10.1097/00006676-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Sun C, Yamato T, Furukawa T, Ohnishi Y, Kijima H, Horii A. Characterization of the mutations of the K-ras, p53, p16, and SMAD4 genes in 15 human pancreatic cancer cell lines. Oncol Rep. 2001;8:89–92. doi: 10.3892/or.8.1.89. [DOI] [PubMed] [Google Scholar]
- 7.Dong JT, Lamb PW, Rinker-Schaeffer CW, Vukanovic J, Ichikawa T, Isaacs JT, Barrett JC. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science. 1995;268:884–886. doi: 10.1126/science.7754374. [DOI] [PubMed] [Google Scholar]
- 8.Guo XZ, Friess H, Maurer C, Berberat P, Tang WH, Zimmermann A, Naef M, Graber HU, Korc M, Buchler MW. KAI1 is unchanged in metastatic and nonmetastatic esophageal and gastric cancers. Cancer Res. 1998;58:753–758. [PubMed] [Google Scholar]
- 9.Adachi M, Taki T, Ieki Y, Huang CL, Higashiyama M, Miyake M. Correlation of KAI1/CD82 gene expression with good prognosis in patients with non-small cell lung cancer. Cancer Res. 1996;56:1751–1755. [PubMed] [Google Scholar]
- 10.Jackson P, Grimm MO, Kingsley EA, Brosius U, Antalis T, Yardley G, Russell PJ. Relationship between expression of KAI1 metastasis suppressor gene, mRNA levels and p53 in human bladder and prostate cancer cell lines. Urol Oncol. 2002;7:99–104. doi: 10.1016/s1078-1439(01)00175-2. [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Wei LL, Tang C, Slack R, Mueller S, Lippman ME. Overexpression of KAI1 suppresses in vitro invasiveness and in vivo metastasis in breast cancer cells. Cancer Res. 2001;61:5284–5288. [PubMed] [Google Scholar]
- 12.Guo X, Friess H, Graber HU, Kashiwagi M, Zimmermann A, Korc M, Buchler MW. KAI1 expression is up-regulated in early pancreatic cancer and decreased in the presence of metastases. Cancer Res. 1996;56:4876–4880. [PubMed] [Google Scholar]
- 13.Friess H, Guo XZ, Berberat P, Graber HU, Zimmermann A, Korc M, Buchler MW. Reduced KAI1 expression in pancreatic cancer is associated with lymph node and distant metastases. Int J Cancer. 1998 Aug 21;79:349–355. doi: 10.1002/(sici)1097-0215(19980821)79:4<349::aid-ijc7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Ren LN, Xu JH, Guo XZ, Liu MP. Establishment and significance of mice model in pancreatic cancer. Yixianbingxue Zazhi. 2004;4:109–110. [Google Scholar]
- 15.Brown PD, Bloxidge RE, Stuart NS, Gatter KC, Carmichael J. Association between expression of activated 72-kilodalton gelatinase and tumor spread in non-small-cell lung carcinoma. J Natl Cancer Inst. 1993;85:574–578. doi: 10.1093/jnci/85.7.574. [DOI] [PubMed] [Google Scholar]
- 16.Guo XZ, Xu JH, Liu MP, Ren LN, Wang D, Li HY, Wu CY. The mechanism of KAI1 gene in inhibition of metastasis of primary pancreatic cancer. Zhonghua Neike Zazhi. 2004;43:360–362. [PubMed] [Google Scholar]
- 17.Dong JT, Suzuki H, Pin SS, Bova GS, Schalken JA, Isaacs WB, Barrett JC, Isaacs JT. Down-regulation of the KAI1 metastasis suppressor gene during the progression of human prostatic cancer infrequently involves gene mutation or allelic loss. Cancer Res. 1996;56:4387–4390. [PubMed] [Google Scholar]
- 18.Friess H, Guo XZ, Tempia-Caliera AA, Fukuda A, Martignoni ME, Zimmermann A, Korc M, Buchler MW. Differential expression of metastasis-associated genes in papilla of vater and pancreatic cancer correlates with disease stage. J Clin Oncol. 2001;19:2422–2432. doi: 10.1200/JCO.2001.19.9.2422. [DOI] [PubMed] [Google Scholar]
- 19.Engbring JA, Kleinman HK. The basement membrane matrix in malignancy. J Pathol. 2003;200:465–470. doi: 10.1002/path.1396. [DOI] [PubMed] [Google Scholar]
- 20.Itoh Y, Nagase H. Matrix metalloproteinases in cancer. Essays Biochem. 2002;38:21–36. doi: 10.1042/bse0380021. [DOI] [PubMed] [Google Scholar]
- 21.Hornebeck W, Maquart FX. Proteolyzed matrix as a template for the regulation of tumor progression. Biomed Pharmacother. 2003;57:223–230. doi: 10.1016/s0753-3322(03)00049-0. [DOI] [PubMed] [Google Scholar]
- 22.Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. Faseb J. 1999;13:781–792. [PubMed] [Google Scholar]
- 23.Johansson N, Ahonen M, Kahari VM. Matrix metallo-proteinases in tumor invasion. Cell Mol Life Sci. 2000;57:5–15. doi: 10.1007/s000180050495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 25.Leppä S, Saarto T, Vehmanen L, Blomqvist C, Elomaa I. A high serum matrix metalloproteinase-2 level is associated with an adverse prognosis in node-positive breast carcinoma. Clin Cancer Res. 2004;10:1057–1063. doi: 10.1158/1078-0432.ccr-03-0047. [DOI] [PubMed] [Google Scholar]
- 26.Sheen-Chen SM, Chen HS, Eng HL, Sheen CC, Chen WJ. Serum levels of matrix metalloproteinase 2 in patients with breast cancer. Cancer Lett. 2001;173:79–82. doi: 10.1016/s0304-3835(01)00657-7. [DOI] [PubMed] [Google Scholar]
- 27.Uchima Y, Sawada T, Nishihara T, Umekawa T, Ohira M, Ishikawa T, Nishino H, Hirakawa K. Identification of a trypsinogen activity stimulating factor produced by pancreatic cancer cells: its role in tumor invasion and metastasis. Int J Mol Med. 2003;12:871–878. [PubMed] [Google Scholar]
- 28.Takaoka A, Hinoda Y, Sato S, Itoh F, Adachi M, Hareyama M, Imai K. Reduced invasive and metastatic potentials of KAI1-transfected melanoma cells. Jpn J Cancer Res. 1998;89:397–404. doi: 10.1111/j.1349-7006.1998.tb00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takaoka A, Hinoda Y, Satoh S, Adachi Y, Itoh F, Adachi M, Imai K. Suppression of invasive properties of colon cancer cells by a metastasis suppressor KAI1 gene. Oncogene. 1998;16:1443–1453. doi: 10.1038/sj.onc.1201648. [DOI] [PubMed] [Google Scholar]
- 30.Guo XZ, Xu JH, Liu MP, Kleeff J, Ho CK, Ren LN, Li HY, Köninger J, Cui ZM, Wang D, et al. KAI1 inhibits anchorage-dependent and -independent pancreatic cancer cell growth. Oncol Rep. 2005 Jul;14:59–63. [PubMed] [Google Scholar]