Abstract
Critical care physicians are increasingly facing patients receiving oral anticoagulation for either cessation of major haemorrhage or to reverse the effects of vitamin K antagonists ahead of emergency surgery. Rapid reversal of anticoagulation is particularly essential in cases of life-threatening bleeding. In these situations, guidelines recommend the concomitant administration of prothrombin complex concentrates (PCCs) and oral or intravenous vitamin K for the fastest normalisation of the international normalised ratio (INR). Despite their universal recommendation, PCCs remain underused by many physicians who prefer to opt for fresh frozen plasma despite its limitations in anticoagulant reversal, including time to reverse INR and high risk of transfusion-related acute lung injury. In contrast, the lower volume required to normalise INR with PCCs and the room temperature storage facilitate faster preparation and administration time, thus increasing the speed at which haemorrhages can be treated. PCCs therefore allow faster, more reliable and complete reversal of vitamin K anticoagulation, especially when administered immediately following confirmation of haemorrhage. In the emergency setting, probabilistic dosing may be considered.
Introduction
Since the introduction of oral anticoagulants over 50 years ago, there has been a dramatic increase in their use in the developed world due to their high success in preventing thromboembolic events. In fact, 0.8% to 2.0% of the population in these countries receives oral anticoagulation therapy with the vitamin K antagonists warfarin, acenocoumarol, fluinidone or phenprocoumon [1,2]. The most common indication for the use of vitamin K antagonists is atrial fibrillation, but they are also widely used to prevent a range of other thromboembolic complications, such as deep vein thrombosis, pulmonary embolisms and strokes from mechanical heart valves [3].
Oral anticoagulation therapy carries the inherent risk of haemorrhagic complications. Many patients receiving vitamin K antagonists have an international normalised ratio (INR) higher than the target of 2.0 to 3.0 for over 50% of the time [3,4], increasing their risk of bleeding; those with an INR within the therapeutic range may still be at risk. A rate of major haemorrhage of 7.2 per 100 person-years was reported in the United States, with most events occurring in patients aged over 80 years (Figure 1) [5]. Major bleeding can occur at a number of sites, with gastrointestinal and urinary tract bleeds the most frequently observed, affecting approximately 1% to 4% of patients being treated with vitamin K antagonists per year [6,7]. Intracranial haemorrhage (ICH) is less common, with reported annual risk ranging between 0.25% and 1% among patients receiving vitamin K antagonists [8-11]; however, it is the most life-threatening of bleeds and is associated with a high mortality rate [6,7]. This review highlights the clinical need for emergency reversal of anticoagulation in the critical care setting and outlines the available treatment options.
Figure 1.
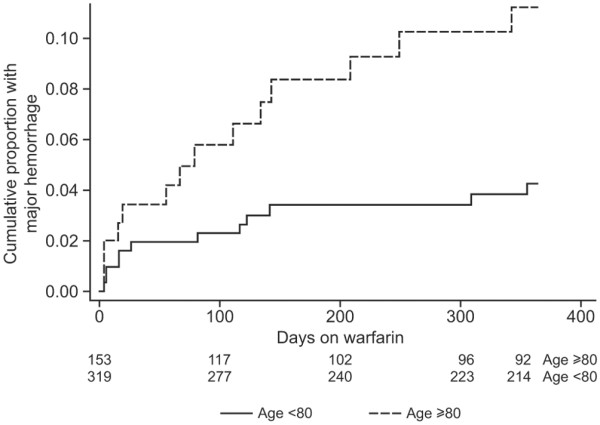
Cumulative bleeding of patients receiving warfarin [5]. Reproduced with permission from Lippincott Williams & Wilkins (http://www.lww.com/)
The need for anticoagulant reversal in a bleeding emergency
Emergency reversal of vitamin K antagonists is often necessary in the critical care setting and many guidelines recommend rapid reversal as soon as diagnosis of haemorrhage is confirmed in cases of life-threatening bleeding, major trauma or specific haematoma localisations (Table 1). Reversal should normalise coagulation as quickly as possible to reduce blood loss, and consequently improve prognosis in terms of both morbidity and mortality. Moreover, in patients without haemorrhage, rapid anticoagulant reversal may be required prior to immediate emergency surgery (Table 1) [12].
Table 1.
Reasons for emergency anticoagulant reversal
| Severity of haemorrhage |
| Shock |
| Need for red blood cell transfusion |
| Haemorrhage localisation |
| Brain |
| Gastrointestinal tract |
| Deep muscles |
| Retro-ocular bleeds |
| Joints (functional prognosis) |
| Need for urgent surgery |
| Ischaemic surgical events |
| Septic shock |
| Treatment of open fractures |
Severe haemorrhage may be diagnosed either by the level of vital signs (for example, shock) or by the localisation of the bleed – for example, intracranial haemorrhage is defined as a bleeding emergency (Table 1). Anticoagulant-induced ICHs are larger than non-anticoagulant-induced events, carry a higher risk of mortality (44% to 68% at 1 to 6 months), and occur more frequently [13]. The progression of events in patients with anticoagulant-induced ICH generally takes around 24 hours, with increasing neurological deterioration observed in the first 24 to 48 hours [13-15]. The increased mortality in patients receiving warfarin appears related to increased in-hospital haematoma expansion and not to the initial volume of haematoma at the time of admission [14]. Rapid normalisation of INR (<2 hours) limits growth of the haematoma [15]. These results highlight the importance of rapid anticoagulant reversal upon admission.
Treatment options for anticoagulant reversal
In theory, there are a number of potential treatment options for anticoagulant reversal, including administration of vitamin K (oral or intravenous), human plasma products (for example, fresh frozen plasma (FFP)), prothrombin complex concentrates (PCCs; concentrates that contain coagulation factors II, VII, IX and X), or single coagulation factors such as activated recombinant factor VII (rFVIIa).
Vitamin K
Normalisation with vitamin K alone is slow to take effect because of the time required for hepatic de novo synthesis of vitamin K-dependent coagulation factors. After intravenous vitamin K administration, the INR falls within 4 hours, but this may be misleading as it is almost entirely due to a rise in factor VII [16]. The more important rise in factor II takes approximately 24 hours [16], and correction of coagulation factor levels takes longer following oral vitamin K administration. The delayed effect of vitamin K on endogenous coagulation factors complements the immediate effect of PCC therapy, providing a rationale for co-administration of the two products. Most studies of vitamin K alone examine the restoration of INR to levels within the therapeutic zone in non-haemorrhagic, over-anticoagulated patients [17]. In this situation, where there is no need for complete reversal, administration of vitamin K (1 to 2.5 mg) is recommended [17-19]. When complete reversal of anticoagulation is needed – that is, prior to surgery or when INR is very high – a vitamin K dose of up to 5 mg is recommended [18,19]. In patients with serious bleeding and elevated INR, the recommended dose of vitamin K is 10 mg [18]. However, vitamin K therapy alone is inadequate during an emergency situation in which rapid cessation of bleeding or correction of INR is required [1,2,20]. More rapid reversal can only be achieved by the supplementation of vitamin K-dependent clotting factors through the use of human plasma products, PCCs or rFVIIa.
Fresh frozen plasma
After a decrease in FFP consumption in the 1990s, probably due to HIV epidemics, the use of FFP has increased in recent years. In patients with haemorrhagic trauma, recent data suggest that, contrary to traditional beliefs and guidelines, early use of FFP may be associated with better patient outcome [21]. Nevertheless, several audits have shown that the rate of inappropriate FFP transfusion remains high considering the fact that FFP is the most frequently implicated blood product in transfusion-related acute lung injury (TRALI) [22]. Moreover, FFP continues to be used in patients who may be better treated with other strategies [21]. FFP contains all vitamin K-dependent factors but there are no in vitro studies about its effectiveness in the reversal of vitamin K antagonists; all clinical studies are observational and all underline the longer time required by FFP to reverse INR [15,23-25].
Prothrombin complex concentrates
Historically, PCCs were approved for the treatment of bleeding associated with haemophilia B, due to their inclusion of factor IX. Although this remains the case in some European countries, most now have a specific factor IX product for treatment of bleeding in haemophilia B. PCCs are now formulated and approved specifically for vitamin K deficiency. They generally contain a balance of coagulation factors II, VII, IX and X, with ratios designed to limit accumulation of factors with a long half-life, particularly factor II [26]. PCCs often also include anticoagulants, such as Proteins C, S and Z and antithrombin III, to balance the opposing needs of haemorrhage control and thrombosis avoidance (Table 2) [27]. PCCs approved for use in anticoagulant reversal include Beriplex® P/N (CSL Behring, Marburg, Germany) and Octaplex® (Octapharma, Vienna, Austria), which are widely available in Europe, and other country-specific formulations are available. The effectiveness of PCCs for reversal of anticoagulation has been reported in a number of clinical studies in patients with haemorrhage and those requiring emergency intervention [12,28-36]. The rapid onset of action of PCCs makes them ideal for anticoagulant reversal in an emergency situation. However, there are no clinical data comparing the efficacy of different PCCs in the treatment of acute haemorrhage and only one in vitro study has attempted to describe possible differences between different PCCs, analysing reversal efficiency [37].
Table 2.
Constituents of commercially available PCCs in Europe (based on product labeling*)
| Factor content | Antithrombotic content | ||||||||||||
| II | VII | IX | X | Protein C, S, Z (label IU/ml) | |||||||||
| Product (manufacturer); international availability | Label IU/ml | Ratio | Label IU/ml | Ratio | Label IU/ml | Ratio | Label IU/ml | Ratio | C | S | Z | ATIII (label IU/ml) | Heparin (label IU/ml) |
| Beriplex P/N (CSL Behring); major western European countries | 20–48 | 133% | 10–25 | 69% | 20–31 | 100% | 22–60 | 161% | 15–45 | 13–26 | Not in label | 0.2–1.5 | 0.4–2.0 |
| Octaplex (Octapharma); major western European countries | 11–38 | 98% | 9–24 | 66% | 25 | 100% | 18–30 | 96% | 7–31 | 7–32 | Not in label | Not in label | Not in label |
| Prothromplex Total/S-TIM 4 Immuno (Baxter); Sweden, Germany, Austria | 30 | 100% | 25 | 83% | 30 | 100% | 30 | 100% | >20 | Not in label | Not in label | 0.75–1.5 | <15 |
| Prothromplex TIM 3 (Baxter); Italy, Austria | 25 | 100% | Not in label | - | 25 | 100% | 25 | 100% | Not in label | Not in label | Not in label | Not in label | 3.75 |
| Cofact/PPSB SD (Sanquin/CAF); Netherlands, Belgium, Austria, Germany | ≥ 15 | 75% | ≥ 5 | 25% | ≥ 20 | 100% | ≥ 15 | 75% | Not in label | Not in label | Not in label | Present, not quantified | Not in label |
| Kaskadil (LFB); France | 40 | 160% | 25 | 100% | 25 | 100% | 40 | 160% | Not in label | Not in label | Not in label | Not in label | Present, not quantified |
| Uman Complex DI (Kedrion); Italy | 25 | 100% | Not in label | 0% | 25 | 100% | 20 | 80% | Not in label | Not in label | Not in label | Present, not quantified | Present, not quantified |
| PPSB-human SD/Nano (Octapharma); Germany | 25–55 | 130% | 7.5–20 | 45% | 24–37.5 | 100% | 25–55 | 130% | 20–50 | 5–25 | Not in label | 0.5–3 | 0.5–6 |
Factor content ratios are based on the content of factor IX [27]. *In Europe, ranges are usually given on the product label, in accordance with the European Pharmacopoeia; single values are generally from older, national registrations. ATIII, antithrombin III; PCC, prothrombin complex concentrate. Reproduced from Levy et al. Perioperative hemostatic management of patients treated with vitamin K antagonists [27]. Reproduced with permission from Lippincott Williams & Wilkins (http://www.lww.com/)
Activated recombinant factor VII
rFVIIa was originally used for the treatment of bleeding in patients with trauma [38] and haemophilia; it is currently licensed for the treatment of haemophilia and Glanzmann's thrombasthenia. For the treatment of haemophilia, an alternative to rFVIIa is FEIBA (factor VIII inhibitor bypassing activity). This is essentially a PCC with activated coagulation factors, but it is not recommended for reversal of vitamin K antagonists. The use of rFVIIa for treating patients on vitamin K antagonist therapy who have life-threatening haemorrhage has been examined in a number of studies [39-43], but there is less clinical experience with rFVIIa than with PCCs in this setting. Comparative investigation of rFVIIa and PCCs is required to further elucidate differences in treatments and their relative contribution to improved prognosis. However, the high number of thrombotic events reported to the US Food and Drug Administration [44] compared with the low incidence of case reports associated with PCC use for anticoagulation reversal, and the shorter half-life of rFVIIa (approximately 2 hours), which complicates the transition to the effect of vitamin K, suggest that there is not a strong enough argument to favour rFVIIa for reversal of oral anticoagulation [18,19].
Prothrombin complex concentrates or fresh frozen plasma?
Guideline recommendations
Current guidelines for anticoagulant reversal are based on the severity of the situation [18,20]. In cases of non-life-threatening bleeding or where emergency surgery is not necessary, the use of PCCs or FFP is not required. In cases of severe haemorrhage, however, rapid reversal of INR is required to prevent further bleeding. In a review by Dentali and colleagues [20], rapid reversal of anticoagulation with PCCs or human plasma, always administered with vitamin K, is recommended for life-threatening bleeding with any increase in INR, and should also be considered in major but non-life-threatening bleeds. US recommendations also suggest the use of PCCs in any serious or life-threatening bleed (Table 3) [18]. Similarly, European and Australian guidelines advocate the use of PCCs in a bleeding emergency, largely due to the rapid response time with these products [45-47]. In clinical practice, however, the choice between different treatment options often leads to confusion.
Table 3.
Example recommendations (United States) for managing oral anticoagulation patients who need their INR lowered because of actual or potential bleeding [73]
| Condition | Description |
| INR above therapeutic range but <5.0; no significant bleeding | Lower dose or omit dose, monitor more frequently and resume at lower dose when INR is therapeutic; if only minimally above the therapeutic range, no dose reduction may be required (Grade 1C) |
| INR ≥ 5.0 but <9.0; no significant bleeding | Omit next one or two doses, monitor more frequently and resume at an appropriately adjusted dose when INR is in the therapeutic range. Alternatively, omit dose and give vitamin K (1 to 2 mg orally), particularly if at increased risk of bleeding. If more rapid reversal is required because the patient requires urgent surgery, vitamin K (≤ 5 mg orally) can be given with the expectation that a reduction of the INR will occur in 24 h. If the INR is still high, additional vitamin K (1 to 2 mg orally) can be given (Grade 2C) |
| INR ≥ 9.0; no significant bleeding | Hold warfarin therapy and give higher dose of vitamin K (2.5 to 5 mg orally) with the expectation that the INR will be reduced substantially in 24 to 48 h (Grade 1B). Monitor more frequently and use additional vitamin K if necessary. Resume therapy at an appropriately adjusted dose when the INR is therapeutic |
| Serious bleeding at any elevation of INR | Hold warfarin therapy and give vitamin K (10 mg by slow intravenous infusion), supplemented with FFP, PCC or rFVIIa, depending on the urgency of the situation; vitamin K can be repeated every 12 hours (Grade 1C) |
| Life-threatening bleeding | Hold warfarin therapy and give FFP, PCC or rFVIIa supplemented with vitamin K (10 mg by slow intravenous infusion); repeat if necessary, depending on INR (Grade 1C) |
In cases of life-threatening bleeding, one probabilistic dose of vitamin K (10 mg) is proposed; there are no specified doses for prothrombin complex concentrate (PCC) or recombinant activated coagulation factor VII (rFVIIa). Note: if continuing warfarin therapy is indicated after high doses of vitamin K, then heparin or low molecular weight heparin can be given until the effects of vitamin K have been reversed and the patient becomes responsive to warfarin therapy. It should be noted that international normalised ratio (INR) values > 4.5 are less reliable than values in or near the therapeutic range. Thus, these guidelines represent an approximate guide for high INRs. FFP, fresh frozen plasma. Reproduced with permission from American College of Chest Physicians.
Why are prothrombin complex concentrates under-used?
While all current guidelines recommend the use of PCCs in conjunction with vitamin K for the treatment of severe haemorrhage [18,20,45-47], they remain underused in many surgical units [13,48]. The reasons for this are multifactorial. First, there is a lack of knowledge of both the guidelines and the options available for rapid reversal of anticoagulation amongst many critical care clinicians. In some countries, PCCs have only recently been licensed for anticoagulation reversal. Second, there is a fear of thrombosis, reinforced by historical reports on the use of PCCs where they were associated with a risk of thromboembolic complications [26,49] and many clinicians are not aware of the recent improvements to these products and evidence indicating that PCC use per se does not cause thromboembolic complications. There may be an element of fatalism, especially regarding ICH in patients on oral anticoagulant therapy; such self-fulfilling prophecies are well known in neurological events and especially in ICH [50]. Many clinicians continue to use FFP due to its apparently lower cost [48]. However, although the unit cost is lower, larger volumes of FFP are required for anticoagulant reversal; therefore, the overall cost of FFP administration is often similar to that with PCCs, while there are significant benefits with PCCs in terms of time, volume and safety.
Rapid and effective correction of INR
The main priority for a patient with life-threatening haemorrhage is to achieve rapid cessation of bleeding. A number of comparative studies and clinical reports have demonstrated better correction of INR in anticoagulant reversal in patients receiving PCCs than in those receiving FFP, reflecting current recommendations [15,23,25,51-53]. In a study of 41 patients requiring anticoagulant reversal for either bleeding or emergency intervention, all patients receiving PCCs (25 to 50 IU/kg; n = 29) demonstrated complete correction of INR to a mean post-treatment level of 1.3 within 15 minutes following infusion [25]. Conversely, the mean post-treatment INR in 12 patients receiving 4 units of FFP was 2.3, the lowest INR in these patients being 1.6, higher than the cut-off required for treatment to be considered successful. Moreover, nearly all individual measured concentrations of vitamin K-dependent factors were above 50% in the PCC group, while the respective concentrations remained consistently under 40% in the FFP group, clearly demonstrating the superiority of PCC in normalising the levels of the deficient factors [25]. PCCs are also reported to result in more effective reversal of INR in patients with ICH [15,23,51]. In a retrospective study of patients experiencing ICH in German critical care units by Huttner and colleagues [15], the incidence and progression of haematoma related to ICH was significantly lower in patients receiving PCCs (19% (incidence) and 44% (progression)) compared with FFP (33% (incidence) and 54% (progression)).
One of the major advantages of PCCs over FFP is the speed at which correction of INR is achieved. In the study by Fredriksson and colleagues [23], a mean post-treatment INR of 1.22 was reached within 4.8 hours in patients receiving PCCs (mean baseline INR 2.83) for treatment of ICH compared with a reduction to INR 1.74 within 7.3 hours in those receiving FFP (mean baseline INR 2.97). In a study of eight patients requiring emergency reversal of phenprocoumon, the mean baseline INR of 3.4 decreased to less than 1.3 within 10 minutes of administration of PCC in the majority of patients [30]. Faster normalisation of INR with PCCs is also due to faster preparation (no thawing required) and faster infusion of the product.
Impact of perfusion
Another factor influencing the speed of correction is related to the volume of PCC required for a therapeutic effect. The volume of PCC required to reverse INR is 25-fold less than the volume of FFP required; that is, 60 mL of PCC is equivalent to 2,000 mL of FFP [25,54]. The lower volume of PCCs required for anticoagulant reversal decreases the risk of fluid overload compared to FFP.
Perfusion of human plasma products, especially FFP, is also known to increase the risk of TRALI [22,55], which is a major cause of death associated with transfusion [55-57]. In contrast, no reports of transfusion-related acute lung injury following administration of PCCs have been documented.
Viral safety
The safety of PCCs compared with human plasma products has been the subject of much debate in recent years. There is a risk of pathogen transmission associated with the use of any human products. PCCs, whilst plasma-derived, undergo a number of viral inactivation steps to minimise the risk of pathogen transmission; in fact, some commercially available PCCs are subject to two stages of manufacture [1,2,36,58-60]. Such two-step inactivation processes are highly effective in preventing transmission of a wide number of pathogens, including HIV, hepatitis virus, herpes simplex virus 1 and influenza viruses. The purification processes involved in the manufacture are also likely to remove prion proteins [61]. The different forms of human plasma products are subject to differing degrees of viral inactivation; while solvent/detergent-inactivated and methylene blue-inactivated plasma are treated to improve their safety, single-donor plasma is often not treated for viral inactivation. Furthermore, it is common in Europe to quarantine FFP for 4 to 6 months to minimise the risk of viral contamination [62,63].
Thrombogenicity
As previously mentioned, historically, the main safety concern with PCCs has been their association with thrombogenic events. There is a common misconception that PCCs are likely to cause thrombogenic events in patients requiring reversal of oral anticoagulation. A review of studies with a total of 460 patients revealed seven thrombotic complications, but the extent to which these complications were caused by PCCs cannot be determined [64]. The low incidence (2%) must be considered in light of the fact that all patients receiving warfarin therapy do so for underlying tendency to thrombosis [65]. Therefore, the real risk of thrombosis is more likely related to the underlying disease. In patients with atrial fibrillation or mechanical valves, the risk of thrombosis is calculated as 4% per patient per year without oral anticoagulation therapy; this falls to 1% when undergoing oral anticoagulation therapy, but returns to 4% when coagulation is normalised. This is equivalent to a risk of 0.02% per day [66]. Therefore, in cases of life-threatening haemorrhage, reversal of anticoagulation should be the priority. In most cases, anticoagulant therapy should be reintroduced after ensuring that haemorrhage has been stopped. Current PCC formulations have been improved relative to the older formulations; they now ordinarily contain anticoagulants such as Proteins C, S and Z and antithrombin III, and have lower levels of factor II relative to other factors to reduce the risk of thrombogenic events. A recent pharmacokinetic study of a PCC examined the post-treatment levels of the thrombogenicity marker D-dimer in addition to the coagulation factors [59]. Whilst increases in factors II, VII, IX, X, and Proteins C and S were observed within 5 minutes of treatment, there was no increase in D-dimer and no clinical evidence of thrombosis. Cases of thrombogenic complications have also been reported in patients receiving other human plasma products such as solvent/detergent-inactivated and methylene blue-inactivated plasma [67,68].
Practical use of prothrombin complex concentrates
Speed to correction of INR
In emergency situations, critical care clinicians are likely to have to deal with patients requiring urgent anticoagulant reversal, with little time available to consult haematologists. Whatever the site of a haemorrhage, a rapid therapeutic effect is essential [31,69].
Due to the lower therapeutic volume of PCCs, they can be infused faster than human plasma. The fastest infusion rate of PCCs to date was reported in patients requiring anticoagulant reversal in a prospective cohort study; in this study, a median dose of 3,600 IU of PCCs was delivered over a median of 6 minutes, an infusion rate of 17 mL/minute [30].
However, the fastest approved infusion speed of any PCC is approximately 8 mL/minute. Along with the faster preparation time for PCCs, the lack of cross-matching and thawing required, this leads to a significant reduction in the time from presentation of bleeding or diagnosis to INR correction [1,52,59], with PCCs providing INR correction within about 15 minutes [1], often achieved through the administration of a single-dose bolus [33].
Co-administration of prothrombin complex concentrates with vitamin K
To maximise their therapeutic effect, PCCs should be administered simultaneously with vitamin K. In a recent pharmacokinetic study by Ostermann and colleagues [59], the median terminal half-lives of factors II, VII, IX and X were 59.7, 4.2, 16.7 and 30.7 hours, respectively. The clinical effect will last around 5 hours, in accordance with the half-life of factor VII [70]. Notably, re-injecting PCCs after this time can cause accumulation of factors with a long half-life (for example, factor II), which can exaggerate the thrombotic risk. Vitamin K raises levels of endogenous factor VII 5 to 6 hours following administration [1]. Simultaneous administration of vitamin K with PCCs therefore allows a sustained recovery, often without the need for further administration of PCCs.
Dosing of prothrombin complex concentrates and general management of reversal
Recommended dosing of PCCs for anticoagulant reversal has historically been based on the dose used in treatment of haemophilia, which reflects the factor IX content administered per bodyweight [1,32]. While fixed doses are used commonly and are effective in reversal of anticoagulation [34], other studies advocate the use of individualised dosing based on the INR at the time of presentation (Figure 2). This approach seems to be associated with low variability in the actual INR after treatment [12,31,32]. However, it requires physicians to wait for the INR at arrival to be determined before reversal can be initiated. In the absence of a device for bedside testing, this time delay can be very deleterious in cases of life-threatening haemorrhage.
Figure 2.
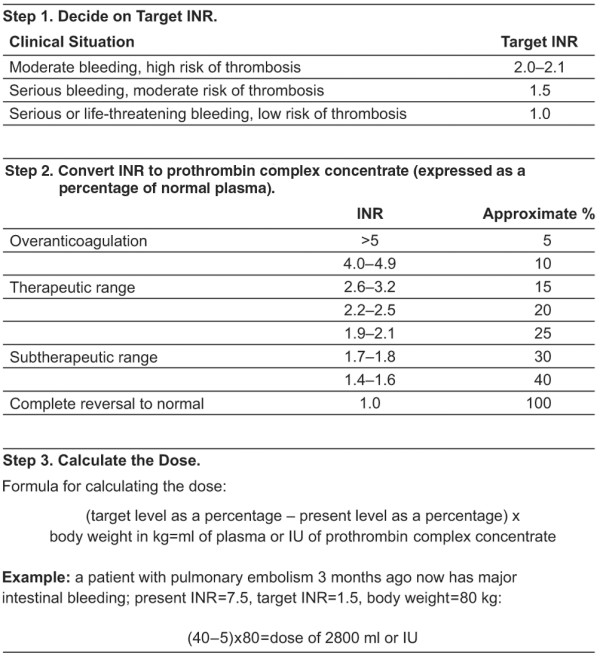
Calculation of prothrombin complex concentrate dose for anticoagulant reversal in bleeding patients [2]. The proposed 'calculation of dose' method is difficult to manage in an emergency situation when immediate normalisation of the international normalised ratio (INR) is required to stop life-threatening bleeding. Reproduced with permission from Massachusetts Medical Society.
The dose-response between coagulation time and PCC dose is not proven and studies remain ambivalent about this. For example, an in vitro study measuring changes in prothrombin time following administration of different doses of PCCs at a range of different INRs (2.1, 3.6, 4.7, 5.1 and 6.7) failed to demonstrate a dose-response curve, with better reversal observed at an initial INR of 6.7 compared to that with an initial INR of 5.1 with the same dose of PCC [71]. The balance between coagulant and anticoagulant factors in a patient after perfusion of PCC with pro- and anti-coagulant factors (for example, Proteins C and S) may provide some answers to this issue. Factor II is likely to have been mainly responsible for previous thrombogenic events associated with PCCs [26] and, due to its long half-life, would accumulate after repeated administration. Therefore, avoidance of repeated administration is recommended to minimise the risk of thrombogenic events.
It is strongly recommended that PCCs should be rapidly available to critical care physicians. Many PCCs have a short preparation time since they are stored at room temperature in the emergency department. Several clinical studies have demonstrated reversal within 3 to 10 minutes [12,33].
When considering major haemorrhage, it is important to fix a goal for reversal. Different goals have been set for normalisa-tion of INR, often lowering it to 1 to <2 [2,12,31,72,73]. INR recommendations for coagulation control in case of traumatic haemorrhagic shock, where the coagulation capacity is considered efficient, is equal to or less than 1.5 [74]. For the moment, an INR of 1.5 or less seems to be the optimum goal for oral anticoagulant reversal [18] but future studies are required to confirm this.
Since normalisation of coagulation should be as rapid as possible, treatment with a 'probabilistic dose' of PCC may be considered as soon as the diagnosis of haemorrhage is confirmed (Figure 3) [19]. This avoids time delays in determining the dose, which arise through waiting for test results such as INR or complicated calculations recommended in some reports [2]. To ensure the best chance of ultra-rapid coagulation, we propose a probabilistic dose of 25 IU/kg (1 mL/kg) of factor IX with an INR performed post-perfusion to control the reversal [33]. Probabilistic dosing may be controversial because most PCC dosing recommendations are calculated using additional parameters such as INR; it is therefore possible that INR might not be completely normalised using this approach. However, this has never been reported, and an immediate probabilistic dose minimises haemorrhagic risk.
Figure 3.
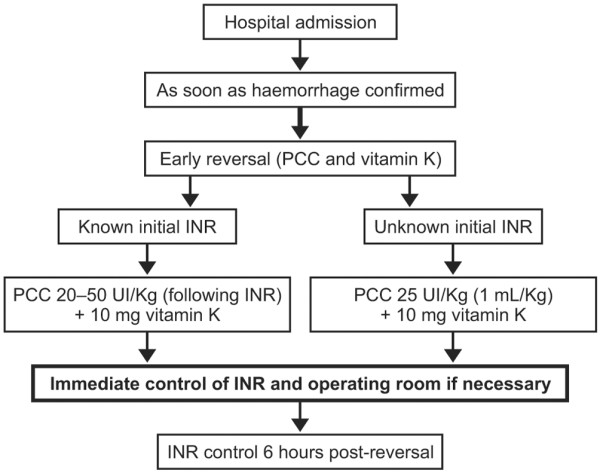
Algorithm for anticoagulant reversal with prothrombin complex concentrates. INR, international normalised ratio; PCC, prothrombin complex concentrate.
Monitoring international normalised ratio
After anticoagulant reversal with PCCs, other haemostatic treatments (surgery or artery embolism) should not be delayed by waiting for normalisation of INR. If following the initial PCC administration the INR is not less than 1.5, further doses can be administered based on the post-treatment INR. Information on the INR at arrival may not be essential for reversal, given the option of probabilistic dosing. However, PCC dosing may often be determined by INR and it can also provide interesting information on the reasons for the haemorrhage, which may influence future treatments. In general, therefore, it is preferable to take a blood sample before attempting to reverse anticoagulation.
The most interesting INR is that taken after treatment to assess the success of the reversal. The time for distribution of factors in the extracellular space is not well-known but pharmacokinetic studies in healthy volunteers are in favour of an ultra-rapid dispersion with a steady-state observed at the first point sampled, 5 minutes after perfusion [59]. Therefore, it can be assumed that control of INR is achieved within 5 to 30 minutes after injection.
An INR measurement taken 6 hours after reversal demonstrates control of the capacity of endogenous hepatic production of factors. At this point there may be an added thrombotic risk from further administration of PCC at higher INR ranges because of the long half-life of factor II and its possible accumulation. Conversely, if the initial dose of PCC was low (25 IU/kg), it is possible to administer a second dose of 12.5 to 25 IU/kg (0.5 to 1.0 mL/kg) if the INR at 6 hours indicates a high risk of bleeding (INR >1.5). This approach provides a further 5 hours of control before vitamin K increases production of endogenous coagulation factors. A final assessment at 24 hours can be useful to discuss the treatment after the haemorrhagic event and examine the thrombotic and haemorrhagic risk for the patient.
The development of new point-of-care devices for INR testing could change attitudes towards INR testing as it is useful to determine whether doses related to INR are related to clinical reality. For the moment, INR testing at point-of-care and the bedside has been investigated in patients receiving oral anticoagulants, with conflicting reports of the accuracy of this method [75-77]. Further improvements in bedside INR monitoring will improve diagnosis, and achieve more rapid and accurate tailored treatment.
Conclusion
Severe vitamin K antagonist-related haemorrhages are being encountered more frequently by critical care clinicians and, in line with the increasing use of oral anticoagulant therapy, the need for rapid reversal of anticoagulation before emergency surgery is more common. These situations must be treated rapidly to stop bleeding and improve patient outcomes. Supplementation of coagulation factors via the administration of PCCs provides effective correction of coagulation more rapidly than the alternative treatment options. Moreover, PCCs are associated with a reduced risk of pathogen transmission and volume overload compared with human plasma. Although PCCs are widely recommended in current guidelines, their use remains limited due to a lack of understanding of their clinical benefits. PCCs are an effective treatment for the cessation of severe bleeding and for rapid normalisation of INR in patients receiving vitamin K antagonists, and their use should be considered immediately at the time of presentation or diagnosis in emergency situations.
Abbreviations
FFP: fresh frozen plasma; ICH: intracranial haemorrhage; INR: international normalised ratio; PCC: prothrombin complex concentrate; rFVIIa: recombinant activated coagulation factor VII; TRALI: transfusion-related acute lung injury.
Competing interests
During the past 5 years Dr Vigué has received fees from CSL Behring, Octapharma and LFB (Laboratoires Français des Biotechnologies). Financial support for this manuscript was provided by CSL Behring.
References
- Makris M, Watson HG. The management of coumarin-induced overanticoagulation annotation. Br J Haematol. 2001;114:271–280. doi: 10.1046/j.1365-2141.2001.02908.x. [DOI] [PubMed] [Google Scholar]
- Schulman S. Clinical practice. Care of patients receiving long-term anticoagulant therapy. N Engl J Med. 2003;349:675–683. doi: 10.1056/NEJMcp025373. [DOI] [PubMed] [Google Scholar]
- Wittkowsky AK, Devine EB. Frequency and causes of overanticoagulation and underanticoagulation in patients treated with warfarin. Pharmacotherapy. 2004;24:1311–1316. doi: 10.1592/phco.24.14.1311.43144. [DOI] [PubMed] [Google Scholar]
- Samsa GP, Matchar DB, Goldstein LB, Bonito AJ, Lux LJ, Witter DM, Bian J. Quality of anticoagulation management among patients with atrial fibrillation: results of a review of medical records from 2 communities. Arch Intern Med. 2000;160:967–973. doi: 10.1001/archinte.160.7.967. [DOI] [PubMed] [Google Scholar]
- Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115:2689–2696. doi: 10.1161/CIRCULATIONAHA.106.653048. [DOI] [PubMed] [Google Scholar]
- McMahan DA, Smith DM, Carey MA, Zhou XH. Risk of major hemorrhage for outpatients treated with warfarin. J Gen Intern Med. 1998;13:311–316. doi: 10.1046/j.1525-1497.1998.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang MC, Go AS, Chang Y, Hylek EM, Henault LE, Jensvold NG, Singer DE. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med. 2007;120:700–705. doi: 10.1016/j.amjmed.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fihn SD, McDonell M, Martin D, Henikoff J, Vermes D, Kent D, White RH. Risk factors for complications of chronic anticoagulation. A multicenter study. Warfarin Optimized Outpatient Follow-up Study Group. Ann Intern Med. 1993;118:511–520. doi: 10.7326/0003-4819-118-7-199304010-00005. [DOI] [PubMed] [Google Scholar]
- Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med. 1993;95:315–328. doi: 10.1016/0002-9343(93)90285-W. [DOI] [PubMed] [Google Scholar]
- Leissinger CA, Blatt PM, Hoots WK, Ewenstein B. Role of prothrombin complex concentrates in reversing warfarin anticoagulation: A review of the literature. Am J Hematol. 2008;83:137–143. doi: 10.1002/ajh.21046. [DOI] [PubMed] [Google Scholar]
- Palareti G, Leali N, Coccheri S, Poggi M, Manotti C, D'Angelo A, Pengo V, Erba N, Moia M, Ciavarella N, Devoto G, Berrettini M, Musolesi S. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet. 1996;348:423–428. doi: 10.1016/S0140-6736(96)01109-9. [DOI] [PubMed] [Google Scholar]
- Pabinger I, Brenner B, Kalina U, Knaub S, Nagy A, Ostermann H. Prothrombin complex concentrate (Beriplex P/N) for emergency anticoagulation reversal: a prospective anticoagulation reversal: a prospective multinational clinical trial. J Thromb Haemost. 2008;6:622–631. doi: 10.1111/j.1538-7836.2008.02904.x. [DOI] [PubMed] [Google Scholar]
- Sjoblom L, Hardemark HG, Lindgren A, Norrving B, Fahlen M, Samuelsson M, Stigendal L, Stockelberg D, Taghavi A, Wallrup L, Wallvik J. Management and prognostic features of intracerebral hemorrhage during anticoagulant therapy: a Swedish multicenter study. Stroke. 2001;32:2567–2574. doi: 10.1161/hs1101.098523. [DOI] [PubMed] [Google Scholar]
- Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63:1059–1064. doi: 10.1212/01.wnl.0000138428.40673.83. [DOI] [PubMed] [Google Scholar]
- Huttner HB, Schellinger PD, Hartmann M, Kohrmann M, Juettler E, Wikner J, Mueller S, Meyding-Lamade U, Strobl R, Mansmann U, Schwab S, Steiner T. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke. 2006;37:1465–1470. doi: 10.1161/01.STR.0000221786.81354.d6. [DOI] [PubMed] [Google Scholar]
- Watson HG, Baglin T, Laidlaw SL, Makris M, Preston FE. A comparison of the efficacy and rate of response to oral and intravenous Vitamin K in reversal of overanticoagulation with warfarin. Br J Haematol. 2001;115:145–149. doi: 10.1046/j.1365-2141.2001.03070.x. [DOI] [PubMed] [Google Scholar]
- Dezee KJ, Shimeall WT, Douglas KM, Shumway NM, O'Malley PG. Treatment of excessive anticoagulation with phytonadione (vitamin K): a meta-analysis. Arch Intern Med. 2006;166:391–397. doi: 10.1001/.391. [DOI] [PubMed] [Google Scholar]
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Chest. 8. Vol. 133. 2008. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines; pp. 160S–198S. [DOI] [PubMed] [Google Scholar]
- Haute Autorité de Santé Prise en charge des surdosages en antivitamines K, des situations à risque hémorragiques et des accidents hémorragiques chez les patients traités par antivitamine K en ville et en milieu hospitalier http://www.has-sante.fr/portail/jcms/c_682188/prise-en-charge-des-surdosages-des-situations-a-risque-hemorragique-et-des-accidents-hemorrag-iques-chez-les-patients-traites-par-antivitamines-k-en-ville-et-en-milieu-hospitalier [DOI] [PubMed]
- Dentali F, Ageno W, Crowther M. Treatment of coumarin-associated coagulopathy: a systematic review and proposed treatment algorithms. J Thromb Haemost. 2006;4:1853–1863. doi: 10.1111/j.1538-7836.2006.01986.x. [DOI] [PubMed] [Google Scholar]
- Benhamou D. The use of fresh frozen plasma (FFP) in 2007 in France. Transfus Clin Biol. 2007;14:557–559. doi: 10.1016/j.tracli.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Holness L, Knippen MA, Simmons L, Lachenbruch PA. Fatalities caused by TRALI. Transfus Med Rev. 2004;18:184–188. doi: 10.1016/j.tmrv.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Fredriksson K, Norrving B, Stromblad LG. Emergency reversal of anticoagulation after intracerebral hemorrhage. Stroke. 1992;23:972–977. doi: 10.1161/01.str.23.7.972. [DOI] [PubMed] [Google Scholar]
- Goldstein JN, Thomas SH, Frontiero V, Joseph A, Engel C, Snider R, Smith EE, Greenberg SM, Rosand J. Timing of fresh frozen plasma administration and rapid correction of coagulopathy in warfarin-related intracerebral hemorrhage. Stroke. 2006;37:151–155. doi: 10.1161/01.STR.0000195047.21562.23. [DOI] [PubMed] [Google Scholar]
- Makris M, Greaves M, Phillips WS, Kitchen S, Rosendaal FR, Preston EF. Emergency oral anticoagulant reversal: the relative efficacy of infusions of fresh frozen plasma and clotting factor concentrate on correction of the coagulopathy. Thromb Haemost. 1997;77:477–480. [PubMed] [Google Scholar]
- Dusel CH, Grundmann C, Eich S, Seitz R, Konig H. Identification of prothrombin as a major thrombogenic agent in prothrombin complex concentrates. Blood Coagul Fibrinolysis. 2004;15:405–411. doi: 10.1097/01.mbc.0000114437.81125.2b. [DOI] [PubMed] [Google Scholar]
- Levy JH, Kenichi KA, Dietrich W. Perioperative hemostatic management of patients treated with vitamin K antagonists. Anesthesiology. 2008;109:918–926. doi: 10.1097/ALN.0b013e3181895bd8. [DOI] [PubMed] [Google Scholar]
- Evans G, Luddington R, Baglin T. Beriplex P/N reverses severe warfarin-induced overanticoagulation immediately and completely in patients presenting with major bleeding. Br J Haematol. 2001;115:998–1001. doi: 10.1046/j.1365-2141.2001.03214.x. [DOI] [PubMed] [Google Scholar]
- Lankiewicz MW, Hays J, Friedman KD, Tinkoff G, Blatt PM. Urgent reversal of warfarin with prothrombin complex concentrate. J Thromb Haemost. 2006;4:967–970. doi: 10.1111/j.1538-7836.2006.01815.x. [DOI] [PubMed] [Google Scholar]
- Lorenz R, Kienast J, Otto U, Kiehl M, Schreiter D, Haertel S, Barthels M. Successful emergency reversal of phenprocoumon anticoagulation with prothrombin complex concentrate: a prospective clinical study. Blood Coagul Fibrinolysis. 2007;18:565–570. doi: 10.1097/MBC.0b013e3282010d7a. [DOI] [PubMed] [Google Scholar]
- Preston FE, Laidlaw ST, Sampson B, Kitchen S. Rapid reversal of oral anticoagulation with warfarin by a prothrombin complex concentrate (Beriplex): efficacy and safety in 42 patients. Br J Haematol. 2002;116:619–624. doi: 10.1046/j.0007-1048.2001.03295.x. [DOI] [PubMed] [Google Scholar]
- van Aart L, Eijkhout HW, Kamphuis JS, Dam M, Schattenkerk ME, Schouten TJ, Ploeger B, Strengers PF. Individualized dosing regimen for prothrombin complex concentrate more effective than standard treatment in the reversal of oral anticoagulant therapy: an open, prospective randomized controlled trial. Thromb Res. 2006;118:313–320. doi: 10.1016/j.thromres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Vigué B, Ract C, Tremey B, Engrand N, Leblanc PE, Decaux A, Martin L, Benhamou D. Ultra-rapid management of oral anticoagulant therapy-related surgical intracranial hemorrhage. Intensive Care Med. 2007;33:721–725. doi: 10.1007/s00134-007-0528-z. [DOI] [PubMed] [Google Scholar]
- Yasaka M, Sakata T, Naritomi H, Minematsu K. Optimal dose of prothrombin complex concentrate for acute reversal of oral anticoagulation. Thromb Res. 2005;115:455–459. doi: 10.1016/j.thromres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Riess HB, Meier-Hellmann A, Motsch J, Elias M, Kursten FW, Dempfle CE. Prothrombin complex concentrate (Octaplex®) in patients requiring immediate reversal of oral anticoagulation. Thromb Res. 2007;121:9–16. doi: 10.1016/j.thromres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Lubetsky A, Hoffman R, Zimlichman R, Eldor A, Zvi J, Kostenko V, Brenner B. Efficacy and safety of a prothrombin complex concentrate (Octaplex) for rapid reversal of oral anticoagulation. Thromb Res. 2004;113:371–378. doi: 10.1016/j.thromres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Dargaud Y, Desmurs-Clavel H, Marin S, Bordet JC, Poplavsky JL, Negrier C. Comparison of the capacities of two prothrombin complex concentrates to restore thrombin generation in plasma from orally anticoagulated patients: an in vitro study. J Thromb Haemost. 2008;6:962–968. doi: 10.1111/j.1538-7836.2008.02964.x. [DOI] [PubMed] [Google Scholar]
- Boffard KD, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, Axelsen M, Kluger Y. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. J Trauma. 2005;59:8–15. doi: 10.1097/01.TA.0000171453.37949.B7. [DOI] [PubMed] [Google Scholar]
- Deveras RA, Kessler CM. Reversal of warfarin-induced excessive anticoagulation with recombinant human factor VIIa concentrate. Ann Intern Med. 2002;137:884–888. doi: 10.7326/0003-4819-137-11-200212030-00009. [DOI] [PubMed] [Google Scholar]
- Brody DL, Aiyagari V, Shackleford AM, Diringer MN. Use of recombinant factor VIIa in patients with warfarin-associated intracranial hemorrhage. Neurocrit Care. 2005;2:263–267. doi: 10.1385/NCC:2:3:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton RP, McCunn M, Hyder M, D'Angelo M, O'Connor J, Hess JR, Scalea TM. Factor VIIa for correction of traumatic coagulopathy. J Trauma. 2004;57:709–718. doi: 10.1097/01.TA.0000140646.66852.AB. [DOI] [PubMed] [Google Scholar]
- Freeman WD, Brott TG, Barrett KM, Castillo PR, Deen HG, Jr, Czervionke LF, Meschia JF. Recombinant factor VIIa for rapid reversal of warfarin anticoagulation in acute intracranial hemorrhage. Mayo Clin Proc. 2004;79:1495–1500. doi: 10.4065/79.12.1495. [DOI] [PubMed] [Google Scholar]
- Lin J, Hanigan WC, Tarantino M, Wang J. The use of recombinant activated factor VII to reverse warfarin-induced anticoagulation in patients with hemorrhages in the central nervous system: preliminary findings. J Neurosurg. 2003;98:737–740. doi: 10.3171/jns.2003.98.4.0737. [DOI] [PubMed] [Google Scholar]
- O'Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA. 2006;295:293–298. doi: 10.1001/jama.295.3.293. [DOI] [PubMed] [Google Scholar]
- Baker RI, Coughlin PB, Gallus AS, Harper PL, Salem HH, Wood EM. Warfarin reversal: consensus guidelines, on behalf of the Australasian Society of Thrombosis and Haemostasis. Med J Aust. 2004;181:492–497. doi: 10.5694/j.1326-5377.2004.tb06407.x. [DOI] [PubMed] [Google Scholar]
- Spahn DR, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Gordini G, Stahel PF, Hunt BJ, Komadina R, Neugebauer E, Ozier Y, Riddez L, Schultz A, Vincent JL, Rossaint R. Management of bleeding following major trauma: a European guideline. Crit Care. 2007;11:R17. doi: 10.1186/cc5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglin TP, Keeling DM, Watson HG. Guidelines on oral anticoagulation (warfarin): third edition – 2005 update. Br J Haematol. 2006;132:277–285. doi: 10.1111/j.1365-2141.2005.05856.x. [DOI] [PubMed] [Google Scholar]
- Appelboam R, Thomas EO. The headache over warfarin in British neurosurgical intensive care units: a national survey of current practice. Intensive Care Med. 2007;33:1946–1953. doi: 10.1007/s00134-007-0765-1. [DOI] [PubMed] [Google Scholar]
- Kohler M, Hellstern P, Lechler E, Uberfuhr P, Muller-Berghaus G. Thromboembolic complications associated with the use of prothrombin complex and factor IX concentrates. Thromb Haemost. 1998;80:399–402. [PubMed] [Google Scholar]
- Becker KJ, Baxter AB, Cohen WA, Bybee HM, Tirschwell DL, Newell DW, Winn HR, Longstreth WT., Jr Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology. 2001;56:766–772. doi: 10.1212/wnl.56.6.766. [DOI] [PubMed] [Google Scholar]
- Cartmill M, Dolan G, Byrne JL, Byrne PO. Prothrombin complex concentrate for oral anticoagulant reversal in neurosurgical emergencies. Br J Neurosurg. 2000;14:458–461. doi: 10.1080/02688690050175265. [DOI] [PubMed] [Google Scholar]
- Hanley JP. Warfarin reversal. J Clin Pathol. 2004;57:1132–1139. doi: 10.1136/jcp.2003.008904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitu IC, Perry DJ, Lee CA. Clinical experience with the use of clotting factor concentrates in oral anticoagulation reversal. Clin Lab Haematol. 1998;20:363–367. doi: 10.1046/j.1365-2257.1998.00161.x. [DOI] [PubMed] [Google Scholar]
- Schulman S, Bijsterveld NR. Anticoagulants and their reversal. Transfus Med Rev. 2007;21:37–48. doi: 10.1016/j.tmrv.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Khan H, Belsher J, Yilmaz M, Afessa B, Winters JL, Moore SB, Hubmayr RD, Gajic O. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest. 2007;131:1308–1314. doi: 10.1378/chest.06-3048. [DOI] [PubMed] [Google Scholar]
- Bux J. Transfusion-related acute lung injury (TRALI): a serious adverse event of blood transfusion. Vox Sang. 2005;89:1–10. doi: 10.1111/j.1423-0410.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- Kleinman S, Caulfield T, Chan P, Davenport R, McFarland J, McPhedran S, Meade M, Morrison D, Pinsent T, Robillard P, Slinger P. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44:1774–1789. doi: 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- Lorenz R, Kienast J, Otto U, Egger K, Kiehl M, Schreiter D, Kwasny H, Haertel S, Barthels M. Efficacy and safety of a prothrombin complex concentrate with two virus-inactivation steps in patients with severe liver damage. Eur J Gastroenterol Hepatol. 2003;15:15–20. doi: 10.1097/00042737-200301000-00004. [DOI] [PubMed] [Google Scholar]
- Ostermann H, Haertel S, Knaub S, Kalina U, Jung K, Pabinger I. Pharmacokinetics of Beriplex P/N prothrombin complex concentrate in healthy volunteers. Thromb Haemost. 2007;98:790–797. [PubMed] [Google Scholar]
- Kessler CM. Urgent reversal of warfarin with prothrombin complex concentrate: where are the evidence-based data? J Thromb Haemost. 2006;4:963–966. doi: 10.1111/j.1538-7836.2006.01944.x. [DOI] [PubMed] [Google Scholar]
- Nowak T, Schafer W, Groener A. Effective pathogen reduction for a plasma-derived prothrombin complex concentrate through multiple dedicated measures. J Thromb Haemost. 2007;5(Suppl 2) P-M-100. [Google Scholar]
- Algora M, Barbolla L. Uses of plasma in Spain. Transfus Clin Biol. 2007;14:564–567. doi: 10.1016/j.tracli.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Humpe A, Legler TJ, Nubling CM, Riggert J, Unger G, Wolf C, Heermann KH, Kohler M. Hepatitis C virus transmission through quarantine fresh-frozen plasma. Thromb Haemost. 2000;84:784–788. [PubMed] [Google Scholar]
- Leissinger CA, Blatt PM, Hoots WK, Ewenstein B. Role of prothrombin complex concentrates in reversing warfarin anticoagulation: A review of the literature. Am J Hematol. 2008;83:137–143. doi: 10.1002/ajh.21046. [DOI] [PubMed] [Google Scholar]
- Torn M, Rosendaal FR. Oral anticoagulation in surgical procedures: risks and recommendations. Br J Haematol. 2003;123:676–682. doi: 10.1046/j.1365-2141.2003.04652.x. [DOI] [PubMed] [Google Scholar]
- Crawley F, Bevan D, Wren D. Management of intracranial bleeding associated with anticoagulation: balancing the risk of further bleeding against thromboembolism from prosthetic heart valves. J Neurol Neurosurg Psychiatry. 2000;69:396–398. doi: 10.1136/jnnp.69.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Noblejas A, Osorio S, Duran AI, Cordoba R, Nistal S, Aguado B, Loscertales J, Gomez N. Pulmonary embolism in a patient with severe congenital deficiency for factor V during treatment with fresh frozen plasma. Haemophilia. 2005;11:276–279. doi: 10.1111/j.1365-2516.2005.01091.x. [DOI] [PubMed] [Google Scholar]
- Stanworth SJ, Brunskill SJ, Hyde CJ, McClelland DB, Murphy MF. Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. Br J Haematol. 2004;126:139–152. doi: 10.1111/j.1365-2141.2004.04973.x. [DOI] [PubMed] [Google Scholar]
- Yasaka M, Sakata T, Minematsu K, Naritomi H. Correction of INR by prothrombin complex concentrate and vitamin K in patients with warfarin related hemorrhagic complication. Thromb Res. 2002;108:25–30. doi: 10.1016/S0049-3848(02)00402-4. [DOI] [PubMed] [Google Scholar]
- Martin DJ, Lucas CE, Ledgerwood AM, Hoschner J, McGonigal MD, Grabow D. Fresh frozen plasma supplement to massive red blood cell transfusion. Ann Surg. 1985;202:505–511. doi: 10.1097/00000658-198510000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka KA, Szlam F, Dickneite G, Levy JH. Effects of prothrombin complex concentrate and recombinant activated factor VII on vitamin K antagonist induced anticoagulation. Thromb Res. 2008;122:117–123. doi: 10.1016/j.thromres.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Cannegieter SC, Rosendaal FR, Briet E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89:635–641. doi: 10.1161/01.cir.89.2.635. [DOI] [PubMed] [Google Scholar]
- Ansell JE. 9th National Conference on Anticoagulant Therapy. Preface. J Thromb Thrombolysis. 2008;25:1. doi: 10.1007/s11239-007-0096-7. [DOI] [PubMed] [Google Scholar]
- Hardy JF, De Moerloose P, Samama M. Massive transfusion and coagulopathy: pathophysiology and implications for clinical management. Can J Anaesth. 2004;51:293–310. doi: 10.1007/BF03018233. [DOI] [PubMed] [Google Scholar]
- Yano Y, Kambayashi J, Murata K, Shiba E, Sakon M, Kawasaki T, Mori T. Bedside monitoring of warfarin therapy by a whole blood capillary coagulation monitor. Thromb Res. 1992;66:583–590. doi: 10.1016/0049-3848(92)90312-X. [DOI] [PubMed] [Google Scholar]
- Kemme MJ, Faaij RA, Schoemaker RC, Kluft C, Meijer P, Cohen AF, Burggraaf J. Disagreement between bedside and laboratory activated partial thromboplastin time and international normalized ratio for various novel anticoagulants. Blood Coagul Fibrinolysis. 2001;12:583–591. doi: 10.1097/00001721-200110000-00012. [DOI] [PubMed] [Google Scholar]
- Lavenne-Pardonge E, Itegwa MA, Kalaai M, Klinkenberg G, Loncke JL, Pelgrims K, Strengers PF. Emergency reversal of oral anticoagulation through PPSB-SD: the fastest procedure in Belgium. Acta Anaesthesiol Belg. 2006;57:121–125. [PubMed] [Google Scholar]


