Abstract
The application of gene therapy to human disease is currently restricted by the relatively low efficiency and potential hazards of methods of oligonucleotide or gene delivery. Antisense or transcription factor decoy oligonucleotides have been shown to be effective at altering gene expression in cell culture expreriments, but their in vivo application is limited by the efficiency of cellular delivery, the intracellular stability of the compounds, and their duration of activity. We report herein the development of a highly efficient method for naked oligodeoxynucleotide (ODN) transfection into cardiovascular tissues by using controlled, nondistending pressure without the use of viral vectors, lipid formulations, or exposure to other adjunctive, potentially hazardous substances. In this study, we have documented the ability of ex vivo, pressure-mediated transfection to achieve nuclear localization of fluorescent (FITC)-labeled ODN in approximately 90% and 50% of cells in intact human saphenous vein and rat myocardium, respectively. We have further documented that pressure-mediated delivery of antisense ODN can functionally inhibit target gene expression in both of these tissues in a sequence-specific manner at the mRNA and protein levels. This oligonucleotide transfection system may represent a safe means of achieving the intraoperative genetic engineering of failure-resistant human bypass grafts and may provide an avenue for the genetic manipultation of cardiac allograft rejection, allograft vasculopathy, or other transplant diseases.
The manipulation of gene expression in vivo, via either the expression of a transduced gene or the blockade of endogenous gene expression, has been shown in recent years to influence experimental models of cardiovascular disease (1–6). However, effective in vivo delivery into intact tissues of either short-sequence nucleic acids or full-length gene constructs remains a major hurdle in the application of these strategies to human disease. In the cardiovascular system, a number of groups have reported successful adenoviral transduction of endothelial and myocardial cells in vivo, and viral gene delivery to vascular smooth muscle cells has been achieved in conjunction with mechanical disruption of the endothelium (3, 7, 8). Viral protein/liposomes complexes have been used to transfect blood vessels and the heart with either oligodeoxynucleotides (ODN) or plasmid DNA (4, 5, 9). Viral vectors, however, pose potential hazards of viral mutations and of immunologic and cytotoxic complications. Other nonviral methods for in vivo transfection, such as hydrogel balloon delivery (10) or cationic lipid preparations (11), have demonstrated only low in vivo transfection efficiencies of either ODN or plasmid DNA.
Using the hemagglutinating virus of Japan (HVJ)–liposome method for ODN delivery, we have recently transfected into rabbit vein grafts ODN that block expression of cell cycle regulatory genes (5, 12). Genetic inhibition of vascular cell-cycle progression prevented significant neointima formation, induced an adaptive medial hypertrophy, and resulted in a stabilization of graft endothelial function. Furthermore, when implanted in rabbits fed a high-cholesterol diet, ODN-treated grafts proved resistant to atherosclerosis. In models of cardiac transplantation, the manipulation of adhesion molecule expression via postoperative systemic treatment with antisense (AS) ODN targeted against intercellular adhesion molecule 1 (ICAM-1) has been reported to delay allograft rejection and, when combined with mAbs against the ligand for ICAM-1, to induce graft tolerance (13).
Human therapy based on ODN blockade of gene expression requires a delivery method that is safe and efficient. We report a method for efficient delivery of ODN to cells throughout intact cardiovascular tissues without the use either of live or inactivated viral particles or of lipid formulations. Our results demonstrate that under carefully controlled conditions of increased pressure, nuclear localization of fluorescent (FITC)-labeled ODN can be achieved in >90% of cells in human saphenous vein segments and ≈50% of cells in the rat myocardium. Delivery was pressure-dependent but did not rely on distension of the vessel wall or the heart. The functional capacity of this transfection system to alter gene expression in a sequence-specific manner was then tested in a human organ culture system and in a model of rat heterotopic heart transplantation.
MATERIALS AND METHODS
Oligonucleotide.
Phosphorothioate ODN were obtained from CLONTECH and Keystone Laboratories (Menlo Park, CA). A random sequence (5′-TCCAGCTTCGTAGC-3′) 14-mer was either FITC- or 32P-labeled (5′ labeling it, Amersham Pharmacia). A second random sequence (5′-ACGACTAAATCAGCAGAC-3′) 18-mer was also used to verify that ODN uptake was not a sequence-specific phenomenon. ODN with previously reported antisense sequences were: hIL-6 sequence A, 5′-GGAGTTCATAGCTGG-3′ (14); hIL-6 sequence B, 5′-TCCTGGGGGTACTGG-3′ (15), and rat ICAM-1 antisense sequence, 5′-TGCATCCCCAGGCCACTGT-3′ (based on a reported mouse antisense sequence) (13). Reverse antisense sequences were used as controls.
Pressure-Mediated Transfection of Human Saphenous Vein.
Human saphenous vein (patients age 48–82 yr) was obtained from the operating room and divided into 1- to 2-cm segments. The veins were cannulated distally and secured with 3–0 silk ties, flushed with DMEM/F12 medium (GIBCO) and then with ODN in balanced salt solution (137 mM NaCl/5.4 mM KCl/10 mM Tris⋅HCl, pH7.6). The veins were then clamped proximally, and ODN solution was infused via the cannula to pressures ranging from 0 to 760 mmHg (1 mmHg = 133 Pa) by using a standard angioplasty insufflator. On release of pressure, the veins were washed in DMEM/F12 and placed in culture condition consisting of DMEM/F12 with 30% fetal bovine serum and incubated at 37°C with 5% CO2.
In some specimens, pressure-mediated transfection also was carried out by using ODN complexed with the cationic lipid formulation, Lipofectamine (GIBCO). ODN and lipid were mixed in 1:0.5, 1:1, 1:5, and 1:10 ratios (wt/wt) and incubated at room temperature in Opti-Mem medium (GIBCO) for 20 minutes before transfection.
Finally, we developed a device for the transfection of vein segments that eliminated pressure-induced distension. Vessel segments were secured to a cannula as described above and then surrounded by a plastic sheath mounted proximally on the cannula. This sheath was sealed distal to the open end of the vein, and transfection solution was introduced under pressure through the lumen of the vein as well as into the sheath cavity. Thus, the vein segment was surrounded by pressurized ODN solution both lumenally and ablumenally. Preliminary experiments indicated that pressurization did not effect vascular cell viability, measured either via exclusion of propidium iodide or via LDH release; neither was vessel histology altered by the pressurization.
Pressure-Mediated Transfection of Rat Myocardium.
Hearts were harvested from adult male Sprague–Dawley or PVG rats (≈300 g) after induction of anesthesia with intraperitoneal ketamine (35 mg/kg)/xylazine (7 mg/kg) as described (16). The aortic root was cannulated just before excision, and the coronary circulation was perfused with either Stanford cardioplegia solution or heparinized saline solution (1 unit/ml). After removal of the hearts and ligation of the pulmonary veins and vena cavae, this cannula was used to deliver either ODN or physiologic saline solution (1 ml) to the coronary circulation. The hearts were then placed in a small bath of ODN solution at 4°C, and the entire bath was placed in a custom-designed chamber that was subsequently pressurized to 0.5, 1, or 2 atm (1 atm = 101.3 kPa) above ambient pressure. The hearts were then transplanted heterotopically into the abdominal aorta and vena cava of Sprague–Dawley (isograft) or ACI (allograft) recipients as described (16). The recipients were monitored postoperatively, and beating of the donor heart was confirmed by manual palpation until the time of harvest. Preliminary studies confirmed that nondistending myocardial pressurization did not influence posttransplant survival and did not have any detrimental effect either on neutrophil infiltration, cardiac edema, or histologic injury characterized by contraction band necrosis.
Measurement of Transfection Efficiency and DNA Delivery.
Transfection efficiency was measured at 24 hours after treatment. Four equally spaced 5-μm cryosections of each FITC–ODN-transfected vein and three of each heart were examined in a blinded manner, and FITC–ODN-labeled nuclei were recorded as a percent of total Hoechst 33342-labeled nuclei in four high-powered fields of each section. Nuclear labeling did not vary among specimens that were either unfixed or fixed with 10% formalin before sectioning.
Veins transfected with 32P-labeled ODN were washed in acidic glycine solution (0.2 M, pH 2.8) after removal from organ culture at 2 hours. Total ODN delivery was then calculated based on the specific activity of the 32P-labeled ODN as counted on a scintillation counter.
IL-6 Protein Measurement.
Human IL-6 concentration was determined in cultured medium from vein organ culture with an ELISA kit (Endogen, Cambridge, MA). No difference was observed between normalization for protein content and wet weight. IL-6 protein inhibition was calculated as IL-6 level in ODN-transfected vein segments as a percent of IL-6 level in adjacent untransfected segments.
ICAM-1 Immunohistochemistry.
ICAM-1 protein was detected in 5-μm cryosections of native and heterotopically transplanted rat hearts by using a mAb (R & D Systems) and an immunoperoxidase technique. ICAM-1 was detected in the endothelium, but not myocytes, of native hearts. Up-regulation of ICAM-1 was measured as the percentage of area with myocyte ICAM-1 staining as determined by computerized image analysis. Percentages were obtained for six sections of each heart, and average values were calculated.
Quantitative Reverse Transcription–PCR (RT-PCR) Measurement of IL-6 and ICAM-1 mRNA.
Competitive RT-PCR with RNA mimics was performed as described (17). Briefly, RNA mimics for human IL-6 and rat ICAM-1 were generated to yield RT-PCR amplification products of 519 and 279 bp using primers that yielded cDNA of 628 and 470 bp from native hIL-6 and rICAM-1 mRNA, respectively. Various known amounts of mimic RNA were mixed with 250 ng of total sample RNA for quantitation. Reverse transcription was carried out at 42°C for 1 hour before 35 cycles of PCR. Electrophoresis allowed comparison of mimic and sample cDNA products in each reaction tube; the reaction mixture that yielded bands of equivalent visual density represented the point at which sample mRNA approximately equaled the known concentration of mimic RNA. Water controls and DNase and RNase treatments were used to eliminate the possibility of false positives caused by genomic DNA or cDNA contamination.
Means and standard errors were calculated for all values, and statistical significance was based on Student’s t test. When comparison was made between two sample measurements and a common control value, a P value of 0.025 was considered statistically significant after Bonferroni correction.
RESULTS
Transfection Efficiency.
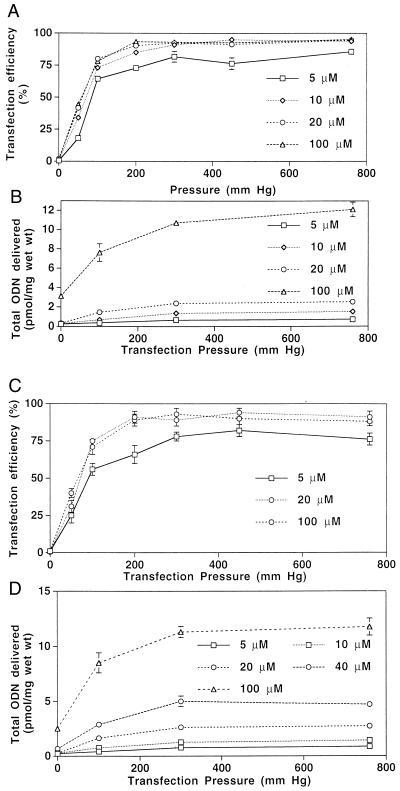
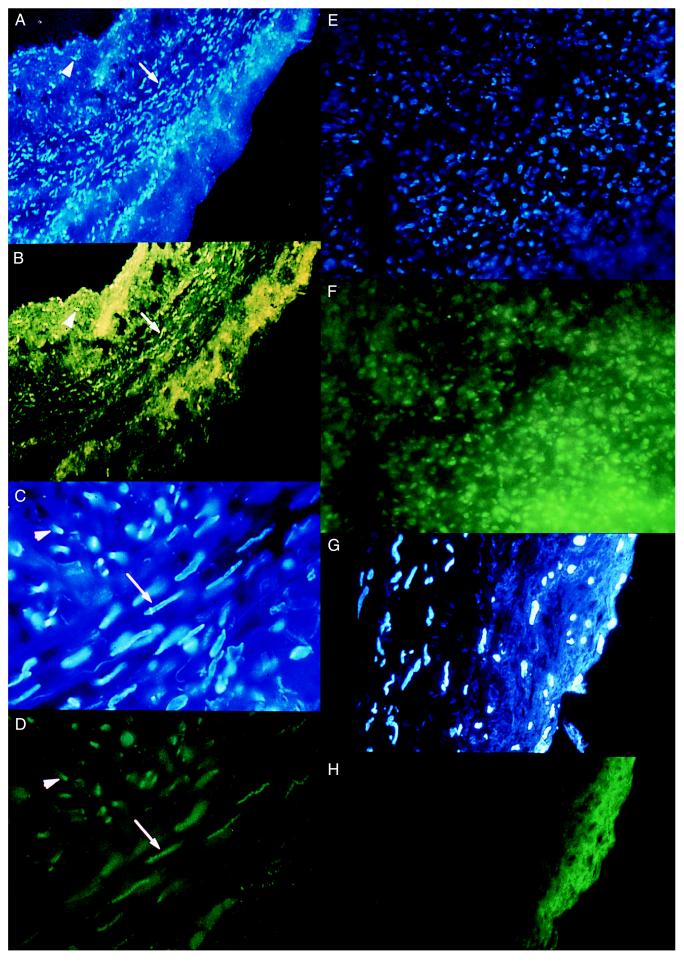
Human saphenous vein. Pressure-mediated ex vivo transfection was studied in vein segments from 60 different patients. As shown in Fig. 1A for 10-minute incubations, transfection increased as incubation pressure increased from 0 to 300 mmHg, plateauing at an efficiency of ≈90% of intimal and medial cells. Peak efficiency was slightly reduced at incubation durations of 1 and 3 min. Increasing ODN concentration from 5 to 20 μM yielded greater transfection efficiencies at each pressure, but these efficiencies were not further increased at 100 μM. A successfully transfected cell was defined as one in which the nuclear FITC signal was clearly stronger than that of the cell cytoplasm, thereby documenting nuclear localization of ODN (Fig. 2 A–D). Evidence for transfection of adventitial cells also was observed in many specimens. The transfection efficiencies measured under various transfection conditions were not affected by the degrees of intimal thickening of the specimens or the ages of the patients. No nuclear localization was observed when nonconjugated FITC was substituted for FITC–ODN, indicating that the fluorescent nuclear signal observed after transfection was not caused by free unconjugated FITC, but rather to intact FITC–ODN (Fig. 2 G and H). Furthermore, similar transfection efficiencies (88 ± 5% after 10 minutes incubation with 10 μM ODN at 300 mm Hg) were achieved by using FITC-labeled ODN bearing a nonrelated random sequence.
Figure 1.
(A) Efficiency of pressure-mediated transfection of FITC–ODN into human saphenous vein. FITC-labeled nuclei were counted after a transfection period of 10 min and are reported as a percentage of total vein wall nuclei. n = 4–6 at each point. (B) Total 32P-labeled ODN delivery to human saphenous vein after pressure-mediated transfection for 10 min. n = 4 at each point. Transfection efficiency (C) and total ODN delivery (D) in human saphenous vein 2 hours after 10-minute, nondistending pressure-mediated transfection. n = 6 at each point.
Figure 2.
Nuclear localization of FITC–ODN in human vein and rat heart. Fluorescent photomicrographs indicate concentrated green FITC–ODN signals (A, C, and E) that correspond to nuclei labeled with the blue intercalating chromatin dye, Hoechst 33342 (B, D, and F). FITC–ODN localization is seen in nuclei of the medial (arrow) and intimal (arrowhead) layers in human saphenous vein at ×100 (A and B) and ×400 (C and D) magnification. (E and F) Pressure-mediated FITC–ODN transfection of cells in rat myocardium (magnification ×400). (G and H) Control human saphenous vein pressure-treated with free, non-ODN-conjugated FITC. Note the absence of nuclear localization of FITC signal despite background green fluorescence (magnification ×400).
Total ODN delivered also increased with increasing pressures, again plateauing at 300 mm Hg (Fig. 1B). Longer durations of pressure up to 10 minutes again yielded greater total ODN uptake. An increase from 10 to 100 μM yielded an 8-fold increase in total 32P-labeled ODN delivered despite similar histologic distributions of FITC–ODN at the two concentrations. Addition of the cationic lipid Lipofectamine at varying ODN/lipid ratios did not improve either transfection efficiency or total ODN delivery under any set of conditions (data not shown).
We then tested a device that allowed pressure-mediated transfection of human vein without distension of the vessel structure. This device allowed application of pressurized ODN solution simultaneously to the luminal and ablumenal surfaces of the vessel, and thereby enhanced ODN delivery to the adventitia, as well. In this system, pressure alone, without vessel wall stretch or distension, proved effective at achieving high transfection efficiencies (Fig. 1 C and D).
Rat myocardium.
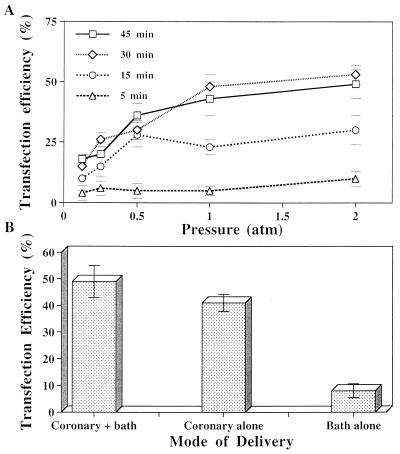
Pressure-mediated transfection efficiently delivered ODN to the more complex, muscular tissue of the rat myocardium, as well. The highest efficiency, 53 ± 5.4%, was achieved when ODN solution was applied both via the coronary circulation and the epicardial and endocardial surfaces at an incubation pressure of 2 atm for 30 min (Figs. 2 E and F and 3). Although areas near the epicardial and endocardial surfaces still contained regions of locally high transfection when hearts were exposed to pressurized ODN solution without coronary ODN perfusion, only 8 ± 2.6% of total myocardial cells were transfected. Omission of ODN from the solution surrounding the heart tissue also reduced maximal transfection efficiency at 2 atm for 45 min to 41 ± 3.2% (vs. 50 ± 2.2% with surrounding ODN solution and coronary perfusion, P < 0.0), suggesting that delivery of ODN to the cell surface occurred both via the coronary bed and directly through the myocardial wall. Only rare (3.5 ± 2.4%), diffusely scattered myocardial cells were transfected when hearts were incubated for 45 min at ambient pressure.
Figure 3.
Ex vivo pressure-mediated transfection of rat myocardium. (A) Transfection efficiency increased with increasing pressure and with prolonged incubation in hearts both perfused with and surrounded on epicardial and endocardial surfaces by FITC–ODN solution (80 μM). (B) Optimum transfection was achieved with both coronary perfusion and submersion in a bath of FITC–ODN solution, the former contributing more significantly to total delivery. *, n = 4.
Functional Gene Blockade.
Human saphenous vein. An organ culture system was used to document the biological efficacy of ODN delivery to human vein. Consistent with previously published observations (18), stimulation of vein segments with 30% FBS resulted in up-regulated expression of the cytokine IL-6. IL-6 protein levels were undetectable in cultured media during the first 6 hours of organ culture, rose dramatically between hours 9 and 15, and peaked at hour 24. RT-PCR up to 42 cycles was unable to detect IL-6 mRNA in veins during the first few hours of organ culture; IL-6 mRNA levels then peaked between hours 12 and 18. Specimens for quantitative RT-PCR were subsequently taken from this 12–18 hour time period.
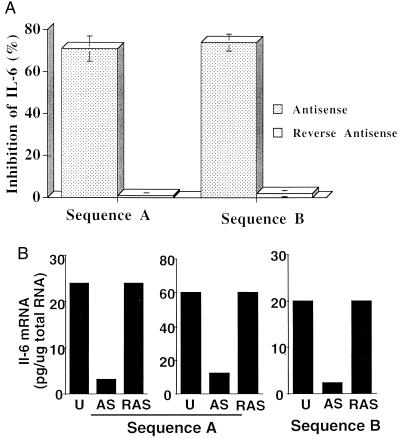
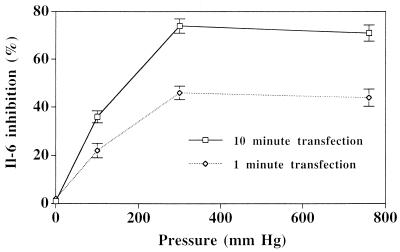
Pressure-mediated transfection with either IL-6 AS ODN sequence A or sequence B was effective at inhibiting IL-6 expression (Fig. 4), whereas neither scrambled ODN nor reverse antisense (RAS) ODN sequences had any significant effect on levels of IL-6 protein or mRNA. Transfection of either antisense sequence at a concentration of 10 μM with a 10-minute incubation at 300 mmHg resulted in a 70–75% mean inhibition of IL-6 protein expression (n = 6, P < 0.001). Quantitative RT-PCR analysis of two sets of vein segments treated with AS and RAS sequence A ODN and one set treated with sequence B indicated that AS ODN transfection reduced mRNA levels. AS ODN transfection achieved a 70–80% inhibition of IL-6 protein in all of these samples, and a similar sequence-specific inhibition of IL-6 mRNA was observed. Inhibition of IL-6 expression was found to be pressure- and dose-dependent, paralleling observations of transfection efficiency (Fig. 5).
Figure 4.
(A) Sequence-specific inhibition of IL-6 protein production determined by ELISA after pressure-mediated transfection of cultured human saphenous vein at 300 mmHg for 10 min with either sequence A or sequence B ODN (n = 4). (B) IL-6 mRNA measured by quantitative RT-PCR. One vein segment from each of three patients was either left untreated (U) or was treated with antisense (AS) or reverse antisense (RAS) ODN of IL-6 sequence A or sequence B (see text for details).
Figure 5.
Relationship between transfection pressure and IL-6 protein inhibition by antisense ODN (10 μM) in human saphenous vein. n = 3 at each point.
Rat myocardium.
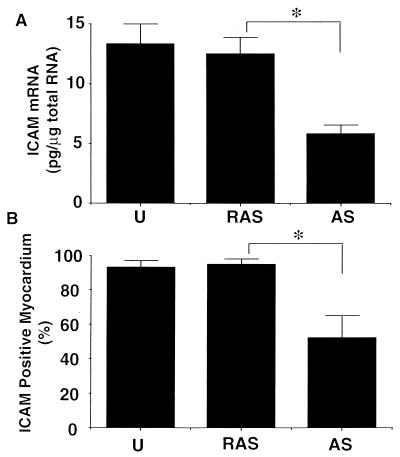
Up-regulated expression of ICAM-1 has been documented in cardiac myocytes after allotransplantation (16), and this adhesion molecule may play an important role in mediating host immune cell interactions with the donor tissue (19, 20). We therefore tested the ability of our ODN delivery system to modulate expression of ICAM-1 in rat hearts during the posttransplantation period. Myocyte expression of this adhesion molecule, absent in normal hearts, was induced in nearly 100% of examined areas of both untreated and ICAM-1 RAS ODN-transfected myocardium harvested 3 days after heterotopic transplantation from PVG donor rats into ACI recipients. In contrast, AS ODN directed against rat ICAM-1 succeeded in reducing ICAM-1-positive areas to 53 ± 14%. Quantitative RT-PCR analysis again indicated that this sequence-specific influence on myocardial target protein expression was mediated by a reduction of mRNA levels (Fig. 6).
Figure 6.
Quantitative analysis of ICAM-1 inhibition by pressure-mediated transfection of allotransplanted rat hearts. (A) ICAM-1 mRNA levels measured by quantitative RT-PCR are similarly reduced in a sequence-specific manner after antisense ODN transfection. (B) Percentage of total myocardium stained positive with ICAM-1 immunohistochemistry is reduced by antisense (AS) but not reverse antisense (RAS) ODN transfection. ∗, P < 0.02; n = 3–4.
DISCUSSION
In vivo transfection of AS and transcription factor decoy ODN has been shown to have significant biological effects in different animal models (1, 5, 9, 21, 22). Therapeutically significant results in human patients, however, are likely to require high in vivo transfection efficiency, because the activity of AS or decoy ODN takes place only within transfected cells. Whereas simple exposure of cells in vitro to naked ODN solution can result in ODN uptake, high transfection efficiencies in vivo have previously been difficult to achieve. This difficulty may be due in part to problems with delivery of ODN to the surface of cells in intact tissues. Pressure-mediated transfection may enhance the hydrostatic movement of ODN through interstitial spaces, thereby delivering ODN to the cell surface. In fact, pressure gradients across vascular tissues have been shown in the past to allow convective forces to enhance delivery of solutes through extracellular matrices (23). However, the pressure-mediated DNA delivery system described here does not depend on the establishment of a pressure gradient across the target tissue, and DNA movement is therefore expected to occur via simple diffusion rather than convection. Enhanced delivery may instead be explained by an influence of absolute pressure on the microstructure of the tissue interstitium or may simply result from an altered interaction of the cell membrane with the DNA that is present at the cell surface. In fact, pressure has been found to alter both the fluid phase of biological membranes and the organization of membrane proteins and cytoskeletal elements, and these effects may be particularly important in protein-rich areas of cell membranes involved in channel formation or transmembrane transport (24, 25). Hydrostatic pressure has also been documented to influence both sodium and potassium transport as well as Mg2+- and Na+-K+-ATPase activity in human erythrocytes (26).
In vitro, simple ODN uptake is believed to proceed via receptor-mediated endocytosis that is associated consequently with lysosomal degradation of most of the ODN (27–29). Incorporation of ODN into cationic lipid or HVJ–liposome complexes has been shown to enhance nuclear localization of ODN in vitro and may improve antisense effect (30, 31). Although the cellular pathway has not yet been delineated, pressure-mediated transfection not only yields cellular uptake of ODN but appears to trigger a mechanism for delivery of the ODN to the cell nucleus, bypassing lysosomal degredation without the need for potentially toxic lipid formulations or viral particles. Studies have suggested that entry into the cell cytpolasm without passage through endosomes or lysosomes results in rapid accumulation of ODN in the cell nucleus (32). In any event, this pattern of intracellular trafficking likely increases the bioavailability of the ODN, provides a high concentration of ODN at the site of mRNA synthesis, and, by protection from intracellular degradation pathways, is likely to increase its stability and duration of activity.
In this study, we observed that optimized pressure-mediated transfection can achieve ODN delivery with nuclear localization in over 90% of cells in intact human vascular tissue, and approximately 50% of cells in intact rat myocardium. Although both types of cardiovascular tissues examined in this study possess luminal spaces that allowed dual-surface incubation, our results indicated that the majority of uptake in the myocardium was achieved as a result of ODN delivery via the coronary arteries rather than via movement of ODN from the organ surface. These observations suggest that a similar pressure-mediated transfection mediated by intravascular delivery to other nonluminal solid organs may be feasible, as well. Although the methods of this study did not allow measurement of intracellular or intranuclear ODN concentration, it might be reasonable to extrapolate higher ODN concentrations in these locations from higher total ODN delivery. Of interest, once a maximal effect was reached, even an 8-fold further increase in ODN delivery did not affect AS inhibitory activity in human veins, suggesting a saturation effect.
We next documented the function of pressure-delivered ODN. The cytokine IL-6 is expressed by vascular cells after injury and after stimulation with factors such as IL-1, tumor necrosis factor, and lipopolysaccharide (18), and its secretion may play a role in disease processes such as atherosclerosis and transplant vasculopathy. IL-6 was chosen as a target gene in this study, however, primarily because of its very high level of expression and ease of detection in organ culture. Pressure-mediated delivery of AS ODN inhibited hIL-6 expression in a sequence-specific manner. Although sequence B contains five adjacent guanosine residues, for which a nonspecific “aptameric” effect on cellular proliferation has been reported (33), the five adjacent guanosine residues in the RAS control did not influence IL-6 expression. Quantitative RT-PCR results also indicated that the mechanism of inhibition with both sequences involved reduction in levels of IL-6 mRNA.
In the rat myocardium, pressure-mediated transfection of AS ODN achieved a widely distributed, sequence-specific inhibition of ICAM-1 mRNA and protein levels after allotransplantation. Although myocardial ICAM-1 expression was not completely abrogated, the 50% decrease observed correlated with the measured efficiency of ODN uptake. Furthermore, previous studies that used systemic treatment with ICAM-1 AS ODN have not documented the success or degree of inhibition of target protein expression, and it is therefore unknown what level of inhibition is necessary to achieve a therapeutic effect. In fact, preliminary studies in our laboratories have suggested that pressure-mediated transfection of PVG donor hearts with ICAM-1 AS ODN before transplantation into ACI recipients can influence the induction of tolerance achieved by short-term treatment with mAbs against the ICAM-1 ligand, leukocyte function-associated antigen-1 (34). This type of organ-specific immunomodulation may reduce the need for highly toxic systemic immunosuppression.
To standardize the transfection method and to avoid injury to the vessel wall, we developed a device that allows a simultaneous application of pressurized ODN solution to the luminal and ablumenal surfaces of vein segments without causing any distension. Distension of venous tissue results in acute injury to and prolonged dysfunction of endothelial as well as medial cells, and prevention of this distension during exposure to pressure has been found to eliminate this injury (35–39). Our system proved effective not only at delivering ODN to vein wall cells with nuclear localization, but also at achieving AS ODN inhibition of target gene expression. These observations imply that pressure-mediated transfection does not rely on the disruption of cell–cell or cell–matrix interactions and that high efficiency transfection can be accomplished in human tissue in a safe and effective manner. A similar, nondirectional application of pressure succeeded in achieving nuclear localization of FITC–ODN in 50% of the cells in the intact rat myocardium. One avenue for ODN movement to the cell surface appears to involve movement directly through the myocardial wall, although perfusion of the hearts via the coronary bed before pressurization yielded a much higher uniformity and overall efficiency of transfection.
We have previously used ODN inhibition of cell cycle regulatory genes in the successful development of a rabbit vein graft resistant to atherogenesis. The current study demonstrates that pressure-mediated transfection can be used to achieve an effective delivery of ODN in human vein tissue ex vivo, and may therefore provide an avenue for the safe genetic engineering of human graft conduits that will similarly resist atherosclerotic occlusion and improve the long-term treatment of human vascular disease. We have also demonstrated that delivery of ODN to nonvascular cells in an intact organ can be significantly enhanced via exposure to the DNA in a pressurized environment. This transfection method may also prove useful in other ODN-based gene therapy strategies, such as antigene, decoy, or ribozyme approaches, and may be applicable to many tissues in a variety of other clinical settings.
Acknowledgments
The authors thank Dr. Bob Hu and Dr. Elazar Edelman for their review and input. This study was supported in part by the Fred and Edna Mandel Fund. V.J.D. is the recipient of an National Heart, Lung, and Blood Institute Merit Award. M.J.M. is supported by the William Randolph Hearst Endowment for Young Investigators.
ABBREVIATIONS
- ODN
oligodeoxynucleotide
- AS
antisense
- RAS
reverse AS
- ICAM-1
intercellular adhesion molecule 1
- RT-PCR
reverse transcription–PCR
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Simons M, Edelman E R, DeKeyser J L, Langer R, Rosenberg R D. Nature (London) 1992;359:67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- 2.Ohno T, Gordon D, San H, Pompili V J, Imperiale M J, Nabel G J, Nabel E G. Science. 1994;265:781–784. doi: 10.1126/science.8047883. [DOI] [PubMed] [Google Scholar]
- 3.Chang M W, Barr E, Seltzer J, Jiang Y Q, Nabel G J, Nabel E G, Parmacek M S, Leiden J M. Science. 1995;26:518–522. doi: 10.1126/science.7824950. [DOI] [PubMed] [Google Scholar]
- 4.von der Leyen H E, Gibbons G H, Morishita R, Lewis N P, Zhang L, Nakajima M, Kaneda Y, Cooke J P, Dzau V J. Proc Natl Acad Sci USA. 1995;92:1137–1141. doi: 10.1073/pnas.92.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mann M J, Gibbons G H, Kernoff R S, Diet F P, Tsao P, Cooke J P, Kaneda Y, Dzau V J. Proc Natl Acad Sci USA. 1995;92:4502–4506. doi: 10.1073/pnas.92.10.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giordano F J, Ping P, McKirnan M D, Nozaki S, DeMaria A N, Dillmann W H, Mathieu-Costello O, Hammond HK. Nat Med. 1996;2:534–539. doi: 10.1038/nm0596-534. [DOI] [PubMed] [Google Scholar]
- 7.Schulick A H, Dong G, Newman K D, Virmani R, Dichek D A. Circ Res. 1995;77:475–485. doi: 10.1161/01.res.77.3.475. [DOI] [PubMed] [Google Scholar]
- 8.Guzman R J, Lemarchand P, Crystal R G, Epstein S E, Finkel T. Circ Res. 1993;73:1202–1207. doi: 10.1161/01.res.73.6.1202. [DOI] [PubMed] [Google Scholar]
- 9.Morishita R, Sugimoto T, Aoki M, Kida I, Tomita N, Moriguchi A, Maeda K, Sawa Y, Kaneda Y, Higaki J, Ogihara T. Nat Med. 1997;3:894–899. doi: 10.1038/nm0897-894. [DOI] [PubMed] [Google Scholar]
- 10.Riessen R, Rahimizadeh H, Blessing E, Takeshita S, Barry J J, Isner J M. Hum Gene Ther. 1993;4:749–758. doi: 10.1089/hum.1993.4.6-749. [DOI] [PubMed] [Google Scholar]
- 11.Nabel E G, Plautz G, Nabel G J. Science. 1990;249:1285–1288. doi: 10.1126/science.2119055. [DOI] [PubMed] [Google Scholar]
- 12.Mann M J, Gibbons G H, Tsao P S, von der Leyen H E, Buitrago R, Kernoff R, Cooke J P, Dzau V J. J Clin Invest. 1997;99:1295–1301. doi: 10.1172/JCI119288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stepkowski S M, Tu Y, Condon T P, Bennett C F. J Immunol. 1994;153:5336–5346. [PubMed] [Google Scholar]
- 14.Reddy S V, Takahashi S, Dallas M, Williams R E, Neckers L, Roodman G D. J Bone Miner Res. 1994;9:753–757. doi: 10.1002/jbmr.5650090522. [DOI] [PubMed] [Google Scholar]
- 15.Schwab G, Siegall C B, Aarden L A, Neckers L M, Nordan R P. Blood. 1991;77:587–593. [PubMed] [Google Scholar]
- 16.Poston R S, Jr, Billingham M E, Pollard J, Hoyt E G, Robbins R C. Ann Thorac Surg. 1997;64:1004–1012. doi: 10.1016/s0003-4975(97)00816-3. [DOI] [PubMed] [Google Scholar]
- 17.Haywood G A, Gullestad L, Katsuya T, Hutchinson H G, Pratt R E, Horiuchi M, Fowler M B. Circulation. 1997;95:1201–1206. doi: 10.1161/01.cir.95.5.1201. [DOI] [PubMed] [Google Scholar]
- 18.Loppnow H, Libby P. J Clin Invest. 1990;85:731–738. doi: 10.1172/JCI114498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Springer T. Nature (London) 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 20.Isobe M, Yagita H, Okumura K, Ihara A. Science. 1992;255:1125–1127. doi: 10.1126/science.1347662. [DOI] [PubMed] [Google Scholar]
- 21.Morishita R, Gibbons G H, Horiuchi M. Proc Natl Acad Sci USA. 1995;92:5855–5859. doi: 10.1073/pnas.92.13.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Y, Fard A, Galeo A, Hutchinson H G, Vermani P, Dodge G R, Hall D J, Shaheen F, Zalewski A. Circulation. 1994;90:944–951. doi: 10.1161/01.cir.90.2.944. [DOI] [PubMed] [Google Scholar]
- 23.Kim W S, Tarbell J M. J Biomech Eng. 1994;116:156–163. doi: 10.1115/1.2895714. [DOI] [PubMed] [Google Scholar]
- 24.Barshtein G, Bergelson L, Dagan A, Gratton E, Yedgar S. Am J Phys. 1997;272:H538–H543. doi: 10.1152/ajpheart.1997.272.1.H538. [DOI] [PubMed] [Google Scholar]
- 25.Haskin C, Cameron I. Biochem Cell Biol. 1993;71:27–35. doi: 10.1139/o93-005. [DOI] [PubMed] [Google Scholar]
- 26.Goldinger J M, Kang B S, Choo Y E, Paganelli C V, Hong S K. J Appl Physiol. 1980;49:224–231. doi: 10.1152/jappl.1980.49.2.224. [DOI] [PubMed] [Google Scholar]
- 27.Loke S L, Stein C A, Zhang X H, Mori K, Nakanishi M, Subasinghe C, Cohen J S, Neckers L M. Proc Natl Acad Sci USA. 1989;86:3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yakubov L A, Deeva E A, Zarytova V F, Ivanova E M, Ryte A S, Yurchenko L V, Vlassov V V. Proc Natl Acad Sci USA. 1989;86:6454–6458. doi: 10.1073/pnas.86.17.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein C A, Mori K, Loke S L, Subasinghe K, Cohen J S, Neckers L M. Gene. 1988;72:333. doi: 10.1016/0378-1119(88)90160-6. [DOI] [PubMed] [Google Scholar]
- 30.Bennett C F, Chiang M Y, Chan H, Shoemaker J E, Mirabelli C K. Mol Pharmacol. 1992;41:1023–1033. [PubMed] [Google Scholar]
- 31.Mann M J, Morishita R, Gibbons G H, von der Leyen H E, Dzau V J. Mol Cell Biochem. 1997;172:3–12. [PubMed] [Google Scholar]
- 32.Leonetti J P, Mechti N, Degols G, Gagnor C, Lebleu B. Proc Natl Acad Sci USA. 1991;88:2702–2706. doi: 10.1073/pnas.88.7.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burgess T L, Fisher E F, Ross S L, Bready J V, Qian Y X, Bayewitch L A, Cohen A M, Herrera C J, Hu S S, Kramer T B, et al. Proc Natl Acad Sci USA. 1995;92:4051–4055. doi: 10.1073/pnas.92.9.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poston R, Mann M J, Hoyt E G, Dzau V J, Robbins R C. Transplantation. 1997;16:41. doi: 10.1097/00007890-199909270-00015. [DOI] [PubMed] [Google Scholar]
- 35.Ramos J R, Berger K, Mansfield P B, Sauvage L R. Ann Surg. 1976;183:205–228. doi: 10.1097/00000658-197603000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boerboom L E, Olinger G N, Bonchek L I, Gunay I I, Kissebah A H, Rodriguez E R, Ferrans V J. J Thorac Cardiovasc Surg. 1985;90:756–764. [PubMed] [Google Scholar]
- 37.Angelini G D, Bryan A J, Williams H M J, Morgan R, Newby A C. J Thorac Cardiovasc Surg. 1990;99:433–439. [PubMed] [Google Scholar]
- 38.Sayers R D, Watt P A, Muller S, Bell P R, Thurston H. Br J Surg. 1991;78:1256–1258. doi: 10.1002/bjs.1800781035. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz L B, O’Donohoe M K, Mikat E M, McCann R L, Hagen P O. Surgery. 1991;110:146–153. [PubMed] [Google Scholar]