Abstract
Chemokine and opioid receptors are G-protein-coupled receptors that play important roles in both the central nervous system and the immune system. The long-term goal of our research is to establish whether opioids regulate the activity of the chemokine receptor CXCR4 (one of the major HIV co-receptors) in the brain. In this research, we studied the anatomical distribution of functional receptors in young and adult animals by using the [35S]GTPγS “binding” assay as an indication of G-protein activation by CXCL12 (the natural CXCR4 ligand) or by μ-opioid agonists. Brain slices or homogenates from Holtzmann rats of different ages (from 2 to 21 days old and adult animals) were treated with CXCL12 (0.001–100 nM), D-ala2,MePhe4,gly-ol5]enkephalin (DAMGO; 0.0003–10 μM) or morphine (0.0003–10 μM) and then processed for the assay. Our results show stimulation of both μ-OR and CXCR4 in several brain areas, including cortex and hippocampus (p<0.001); this effect is dose and age dependent, and the magnitude of response varies among different brain regions. Furthermore, AMD3100 (100 ng/ml), a specific CXCR4 antagonist, abolished CXCL12 stimulation in all the brain regions analyzed (p<0.001). Our findings suggest a similar pattern of expression for μ-OR and CXCR4 in the brain, supporting the possibility of an interaction between the two G-protein-coupled receptors in vivo. This might be relevant to the role of opiates in HIV neuropathogenesis.
Keywords: chemokine, DAMGO, morphine, CXCL12, GPCR, CNS
Introduction
Chemokines (chemotactic cytokines) and their seven transmembrane G-protein-coupled receptors (GPCRs) regulate important biological processes, such as cell migration, organogenesis, and establishment of functional microenvironment. Several studies have shown that both chemokines and chemokine receptors are expressed in the brain and are involved in intercellular communication under physiological and pathological conditions. For instance, chemokines regulate migration, proliferation, and differentiation of neural progenitor cells during development, as well as immune responses in neuroinflammatory disease states (Zou et al. 1998; Bacon and Harrison 2000; Belmadani et al. 2005). The chemokine stromal cell-derived factor 1 (SDF-1α), recently renamed CXCL12, is one of the best-characterized chemokines in the central nervous system (CNS); CXCL12 and its specific receptor CXCR4 have been increasingly studied in recent years because of their critical roles in neuronal patterning and survival (Lazarini et al. 2003; Tran and Miller 2003). Both CXCL12 and its receptor are expressed from early embryonic ages through adulthood in different CNS regions, such as cerebral cortex, hippocampus, thalamus, cerebellum, brainstem, and spinal cord; their expression within these structures has been reported in neurons, astrocytes, endothelial cells, microglia, and meninges (van der Meer et al. 2000; Stumm et al. 2002; Berger et al. 2007). In vivo studies using either CXCR4- or CXCL12-deficient animals indicate that lack of CXCR4 or CXCL12 leads to serious abnormalities in the cardiovascular, gastrointestinal, immune, and nervous systems (Ma et al. 1998; Zou et al. 1998; Stumm et al. 2003), and their role in these organs is still under intense investigation. Furthermore, CXCR4 also serves as one of the major co-receptor for HIV-1 (along with CCR5), and it is involved in HIV neuropathogenesis (Miller and Meucci 1999).
A substantial proportion of the HIV-1-infected individuals are intravenous drug users, many of which abuse opiates (Donahoe and Vlahov 1998). In many parts of the world, drug abuse and HIV-1 are interrelated epidemics, and AIDS is widely spread through injection drug use or drug-seeking behavior. It has been suggested that opiate abuse promotes HIV-1 infection and disease progression, exacerbating the pathogenesis and neurological complications of HIV-1 through direct actions in the CNS (Hauser et al. 2005), and it is quite well established that opioids can modulate the immune system at different levels (Donahoe and Vlahov 1998; Bell et al. 2002). HIV-1 induces a syndrome of cognitive and motor dysfunction that can affect about 30% of untreated patients and about 10–20% of patients treated with antiretroviral drugs. This syndrome, originally known as HIV-1-associated dementia (HAD) and more recently designated as minor cognitive motor disorder (MCMD), is caused by neuronal loss and dysfunction (i.e., loss of communication between neurons) in various regions of the brain, including the hippocampus, frontal cortex, and basal ganglia (Gonzalez-Scarano and Martin-Garcia 2005; Ellis et al. 2007).
Recent data have shown a direct interaction of opioids and their receptors with the HIV co-receptors in immune cells, which is distinct from the general immunosuppressive actions of opioids (Hu et al. 2000; Szabo et al. 2003). Furthermore, previous studies from our group indicate that crosstalk between chemokines and opioids also occur in the CNS (Patel et al. 2006). Specifically, we found that long-term treatment of primary cortical cultures with μ-opioid agonists, such as D-ala2,MePhe4,gly-ol5]enkephalin (DAMGO), morphine, or the endogenous peptide endomorphin-1, inhibits the activation of neuronal survival pathways (i.e., ERK1/2 and AKT phosphorylation) by CXCL12—an effect that is not mediated by changes in CXCR4 levels on neuronal surface. Our studies also show that μ-OR and CXCR4 are co-expressed in cortical neurons, suggesting a possible interaction/crosstalk between the two receptors.
The present report represents an initial step toward the characterization of the interactions between these two receptors in vivo. Our next goal is to establish whether in vivo morphine treatment alters CXCR4 coupling to G proteins. This would validate our previous studies in cultured neurons (Patel et al. 2006), and test the clinical relevance of a potential interaction between CXCR4 and opioid ligands in HIV-positive drug users. To this end, in this study, we have used the [35S]GTPγS assay to measure levels of G-protein activation after stimulation of CXCR4 and opioid receptors; the advantage of this assay is that it measures a functional consequence of receptor occupancy at one of the earliest receptor-mediated events. Combining the [35S]GTPγS incorporation assay with autoradiography allows G protein activation by specific receptors to be anatomically defined and quantified (Sim et al. 1995). Using this technique, we were able to study the functional localization of opioid receptors and CXCR4 in different rat brain areas. We have been focusing on the cerebral cortex and hippocampus formation, where the presence of μ-OR and CXCR4 has been previously reported (Mansour et al. 1987; Sharif and Hughes 1989; Banisadr et al. 2002, 2003), as these areas are affected in HIV neuropathology. Other studies from different groups previously investigated the brain regional specificity of opioid activation of G proteins using [35S]GTPγS autoradiography and showed that the μ-selective peptide DAMGO acts as a full agonist, whereas morphine is a high-efficacy partial agonist (Sim et al. 1995, 1996; Maher et al. 2000; Talbot et al. 2005). We examined the brain regional distribution of G protein activation by CXCL12 and compared it to the action of DAMGO or morphine. AMD3100 (100 ng/ml), a specific and well-characterized CXCR4 antagonist, was used to determine the role of CXCR4 in these experiments. Both [35S]GTPγS autoradiography (brain slices) and incorporation (brain homogenates) were used to test the effect of these compounds in rats of different ages to identify potential age-related differences.
Materials and methods
Animals
All animals were treated according to protocols approved by the Drexel University Animal Research Committee. Tissues were obtained from Holtzmann rats of the indicated ages, i.e., postnatal day 2 (P2) to 21 (P21) and adult female animals (2 or more months old); immediately following sacrifice, the brain was rapidly removed and quickly frozen using liquid nitrogen and stored at −80°C. For the studies on homogenized tissue, brain was removed, and cortex/hippocampus was dissected on ice, frozen on dry ice, and stored at −80°C until used to prepare membrane fractions.
[35S]GTPγS binding in brain homogenates
The effect of μ-opioid receptors or CXCR4 activation in cortex or hippocampus was determined using the μ-agonists DAMGO (0.0003–10 μM) or morphine (0.0003–10 μM) and using CXCL12 (0.001–100 nM). Briefly, animals were killed, the brain removed, and the cortex and/or hippocampus immediately dissected and frozen at −80°C. The following day, the tissue was weighed and homogenized with a Brinkmann Polytron for 10 s in 10 volumes (w/v) of ice-cold 50-mM Tris–HCl buffer (pH 7.4). The suspension was centrifuged (40,000×g at 4°C for 20 min), and the pellet was resuspended in 50 volumes (w/v) of ice-cold 50-mM Tris–HCl buffer (pH 7.4). The suspension was recentrifuged (40,000×g at 4°C for 20 min), and the resulting pellet was resuspended in 50 mM Tris–HCl, pH 7.4 at room temperature, to give a final concentration of 0.5–1 mg original tissue per milliliter. The [35S]GTPγS binding was determined by incubating washed membranes for 45–60 min at 30°C in a total volume of 1 ml of 50 mM Tris buffer, containing 100 mM NaCl, 0.2 mM ethylene glycol bis(2-aminoethyl ether)-N,N,N′N′-tetraacetic acid (EGTA), 3 mM MgSO4, 30 μM guanosine diphosphate (GDP), with or without the indicated drugs. Non-specific binding was defined by the addition of excess unlabeled GTPγS(10 μM, final concentration). Assays were performed in triplicate or quadruplicate. The reaction was terminated by rapid filtration through Whatman GF/B filters, using a Brandel Cell Harvester (Brandel, Gaithersburg, MD); the filters were washed (3×5 ml) with ice-cold 20-mM Tris–HCl buffer (pH 7.4) and radioactivity retained on the filters counted by liquid scintillation spectrophotometry.
In situ [35S]GTPγS autoradiography
The procedure for [35S]GTPγS autoradiography was based on established methods (e.g., Sim et al. 1996). Twenty-micron, frozen coronal sections were cut throughout the hippocampus using a Leica cryostat (model CM3050, Deerfield, IL, USA) and thaw-mounted onto slides subbed with chrome alum. Slides were preincubated for 40 min in assay buffer (in millimolar: Tris–HCl, 50; MgCl2, 4; EGTA, 0.3; NaCl, 100; pH 7.4) at 25°C followed by a 20-min incubation in 2-mM GDP in assay buffer for 20 min at 25°C. Sections were then incubated in slide mailers for 2 h at 25°C in assay buffer containing both [35S]GTPγS (0.04 nM, 1,250 Ci/mmol; Perkin Elmer, Boston, MA, USA) and 2 mM GDP (Sigma-Aldrich, St. Louis, MO, USA). Each mailer contained either vehicle (basal condition) or 10 μM DAMGO (Bachem Bioscience, King of Prussia, PA, USA), 10 μM morphine sulfate (Sigma-Aldrich, St. Louis, MO, USA), 50 nM CXCL12 (R&S System, Minneapolis, MN), 100 ng/ml AMD3100 (Sigma-Aldrich, St. Louis, MO, USA), or 100 ng/ml AMD3100 (pre-treatment of 15 min) plus 50 nM CXCL12. Unlabeled GTPγS (10 μM, MP ICN Biomedicals, Irvine, CA, USA) was used to determine non-specific binding. Furthermore, 1 μM DPCPX (Tocris Bioscience, Ellisville, MO, USA), which blocks the activation of adenosine A1 receptors, was added to each experimental group to reduce basal binding and improve signal-to-noise ratio, as previously reported (Happe et al. 2001).
After incubation, slides were rinsed twice for 2 min each in cold (4°C) 50-mM Tris–HCl buffer, pH 7.4, rinsed briefly in cold deionized water, dried immediately with a cool stream of air, and desiccated overnight. Slides were apposed to Kodak Biomax MS film (Eastman Kodak Company, Rochester, NY, USA) for 24–48 h. Images from the developed films were scanned and quantified using Image Pro-Plus® version 4.5 software (MediaCybernetics; Newburyport, MA, USA). We determined the densities of [35S]GTPγS incorporated into three regions of the hippocampus (CA1, CA2, and CA3) and two of the cortex area (medial and lateral cortex) in each 20-μm section from each rat. For each region, both the right and left sides of the brain were analyzed. Measures of binding, therefore, represent a duplicate determination for each brain region (expressed as averaged values); the n value in each figure refers to the number of animals analyzed. Optical density (OD) values (including non-specific binding) were corrected for background of the film, but not for the different path lengths of isotopic emissions from 35S (tissue sections) and 14C (standards). Each anatomical set of sections per rat contained alternating sections for the non-specific, basal, DAMGO-stimulated, CXCL12-stimulated, morphine-stimulated, AMD3100-stimulated, and AMD3100/CXCL12-stimulated conditions through the brain. Non-specific binding was subtracted from each condition.
Statistical analysis
Actual values and conversion to percent stimulation of basal incorporation of [35S]GTPγS were analyzed by analysis of variance (ANOVA). The Student's Newman–Keuls as well as the Dunnet's test were used for post hoc comparisons of means. An alpha level of p<0.05 was the threshold for statistical significance for all tests (GraphPad PRISM 4.03).
Results
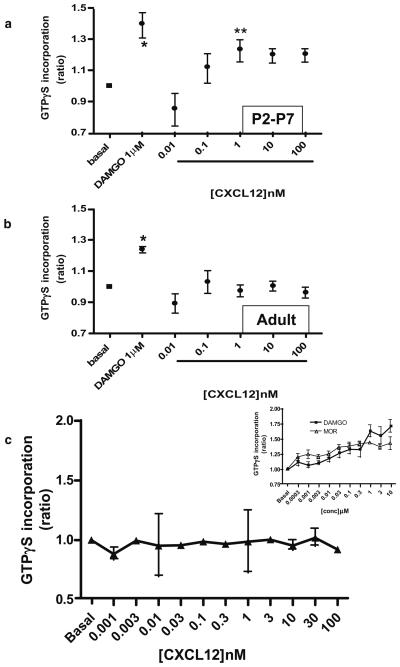
We first determined whether CXCL12 stimulates [35S]GTPγS binding in the cortex of newborn (P2–P7) and adult (2- to 3-month-old) rats and compared the chemokine-induced responses to DAMGO-induced stimulation. Brain tissue homogenates were used for these studies and incubated with 1 μM DAMGO or CXCL12 (0.01–100 nM). As expected, DAMGO increased GTPγS incorporation by approximately 30–40% in both adult- and pup-derived tissue (Table 1). However, CXCL12 responses were observed only in the young animals (Fig. 1a and b). GTPγS incorporation in pup-derived cortical membranes was dose-dependent, reaching the maximum stimulation at 1 nM (Fig. 1a).
Table 1.
CXCL12-stimulated [35S]GTPγS binding in the cortex of P2–P7 and adult rats (CPM±SEM)
| Basal | DAMGO 1 μM |
CXCL12 0.01 nM |
CXCL12 0.1 nM |
CXCL12 1 nM |
CXCL12 10 nM |
CXCL12 100 nM |
|
|---|---|---|---|---|---|---|---|
| P2–P7 | 1,280±58.34 | 1,795±140.9 | 1,089±178.2 | 1,389±117.8 | 1,569±97.48 | 1,485±81.92 | 1,543±84.93 |
| Adult | 5,425±212.5 | 6,725±287.7 | 4,568±259.2 | 5,265±205 | 5,254±223.2 | 5,415±169.3 | 5,189±208.3 |
Fig. 1.
Effects of CXCL12 and DAMGO on [35S]GTPγS incorporation in brain homogenates from pups and adult rats. Brain cortices of P2–P7 pups (a) or adult animals (b) were exposed to CXCL12 or DAMGO as indicated. Graphs represent the mean ratio of agonist-stimulated GTP binding over basal (n=3) ± SEM. *p<0.01 and **p<0.05 versus basal; Dunnett's multiple comparison test after ANOVA. The bottom panel (c) shows data from adult hippocampus homogenates treated with different concentrations of CXCL12. DAMGO or morphine was used as positive controls (inset), as no response to CXCL12 was observed. p<0.01, morphine 1, 0.1, 0.001 μM versus basal; p<0.05, morphine 0.3, 0.03, 0.003 μM versus basal; p<0.05, DAMGO 10 and 1 μM versus basal; Dunnett's multiple comparison test after ANOVA
To determine whether the lack of CXCL12-induced response in adult animals was restricted to the cortex, we also studied the effect of the chemokine in the hippocampus, an area expressing high levels of CXCR4 (Lavi et al. 1997). Tissue homogenates (0.5 mg/ml) were exposed to various concentrations of CXCL12 (0.01–100 nM); for comparison, DAMGO- and morphine-induced responses (0.0003-10 μM) were also monitored. In analogy to the previous findings, these experiments showed that CXCL12 failed to stimulate GTPγS binding to G proteins in the adult hippocampus (Fig. 1c).
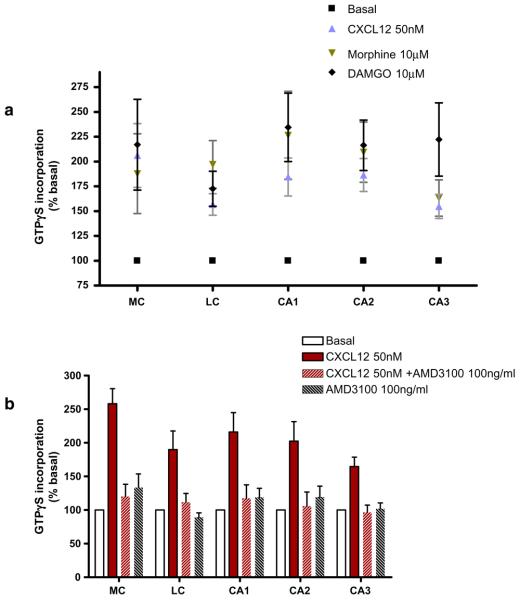
Next, we studied the regional distributions of G-protein activation induced by CXCL12, using GTPγS autoradiography. Coronal sections from rats' brain at different ages were processed for [35S]GTPγS binding followed by quantitative analysis (Fig. 2). The concentration of CXCL12 in these studies was 50 nM, as preliminary experiments showed that CXCL12 concentrations higher than 10 nM were necessary to obtain maximum stimulation of the binding of [35S]GTPγS in the different areas of rat brain, as also noted by others (Albrecht et al. 1998). DAMGO (10 μM) was used as a positive control of μ-OR activation. The affinity of the peptide for μ-OR is three orders of magnitude higher than for δ-OR (Toll et al. 1998; Zhao et al. 2003), and it has been shown that DAMGO at concentrations up to 10 μM produces no measurable [35S] GTPγS binding in cells expressing only δ-OR (Toll et al. 1998; Alt et al. 2002). The data show that, similarly to the homogenized tissue, CXCL12 stimulates [35S]GTPγS binding in brain sections of newborn rats (P4–P7), but it had no effect in the adult brain, as shown in Fig. 2. In agreement with previous studies, DAMGO and morphine stimulated GTPγS binding in all the areas we studied, both in young and adult animals, although their effect was more pronounced in younger animals (Fig. 2).
Fig. 2.
Effects of CXCL12 and DAMGO on [35S]GTPγS incorporation in brain slices from pups and adult rats. Coronal brain sections were treated with different agonist concentrations as indicated in the graphs. Autoradiographs (both top and bottom panels) show images from brain slices treated with vehicle (a), CXCL12 (b), Morphine (c), or DAMGO (d). Analysis was performed in different brain areas as shown in the middle picture (from Paxinos and Watson Atlas; medial cortex, MC; lateral cortex, LC; and hippocampus (fields CA1, CA2, and CA3). Data are expressed as mean ± SEM of n=4 animals per group. Statistics for pups: basal vs CXCL12, 50 nM p<0.001; basal vs morphine, 10 μM, p<0.01; basal vs DAMGO, 10 μM, p<0.001 for each area reported in graph. Newman–Keuls Multiple comparison test after ANOVA. Statistics for adult rats: basal vs CXCL12, 50 nM, p>0.05; basal vs morphine, 10 μM; basal vs DAMGO, 10 μM, p<0.001, CXCL12 vs morphine, 10 μM, CXCL12 vs DAMGO, 10 μM, p<0.001 for each area reported in graph, Newman–Keuls multiple comparison test after ANOVA
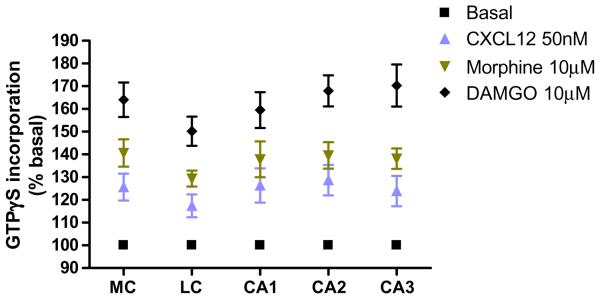
We then sought to establish more precisely the age at which the CXCR4 response disappears. Therefore, we measured the CXCL12-stimulated GTPγS binding in the brain of P8–P14 rats. As for the previous experiments, tissue slices were incubated with 50 nM CXCL12, 10 μM morphine, or 10 μM DAMGO. During the second week of postnatal life, the stimulation induced by CXCL12 was reduced compared to the first postnatal week, but was still significantly higher than basal level (Fig. 3a). The effect of CXCL12 is the result of the activation of CXCR4, as indicated by experiments with AMD3100, a specific and potent CXCR4 antagonist. Indeed, CXCL12-induced GTPγS binding was almost completely inhibited by preincubation of the tissue slices with AMD3100 (100 ng/ml) in all the areas (Fig. 3b). Finally, we studied the [35S]GTPγS incorporation in the brain of the rats in their third postnatal week (P15-P21). As previously, brain sections were incubated with 50 nM CXCL12, 10 μM morphine, or 10 μM DAMGO for the indicated time. We found that CXCR4-induced GTPγS binding was drastically reduced in these animals compared to the first week of life; CXCL12 stimulation gradually decreased and became statistically different compared to DAMGO or morphine (Fig. 4). The effect of the chemokine was completely lost at P21 (data not shown).
Fig. 3.
Effect of CXCL12 and μ-opioids agonists on [35S] GTPγS binding in brain slices from P8–P14 rats. Brains of 8-to 14-days old pups were treated with opioids or with CXCL12 (graph a) or with CXCL12 in the presence or absence of AMD3100 (graph b). Analysis was performed in different brain areas as indicated in Fig. 2. Data are expressed as mean ± SEM of n=3 animals per group. Statistics for (a): basal vs CXCL12, 50 nM, p<0.001; basal vs morphine, 10 μM; basal vs DAMGO, 10 μM, p<0.001; CXCL12 vs DAMGO, 10 μM, p<0.05; CXCL12 vs morphine, 10 μM, p>0.05 for each area reported in graph. Newman– Keuls multiple comparison test after ANOVA. Statistics for (b): basal vs CXCL12, p<0.001; CXCL12 vs CXCL12 + AMD3100, p<0.001; CXCL12 vs AMD3100, p<0.001 for each area reported in graph. Newman–Keuls multiple comparison test after ANOVA
Fig. 4.
Effect of CXCL12 and μ-opioids agonists on [35S]GTPγS binding in P15–P21 rat brain slices. Brains of 15- to 21-day-old pups were used in this study. Analysis was performed in different brain areas as indicated previously. Data are expressed as mean ± SEM of n=3 animals per group (basal vs CXCL12, 50 nM, p<0.001; basal vs morphine, 10 μM; basal vs DAMGO, 10 μM, p<0.001; CXCL12 vs morphine, 10 μM; CXCL12 vs DAMGO, 10 μM, p<0.001, for each area reported in graph. Newman–Keuls multiple comparison test after ANOVA)
Discussion
The primary goal of this study was to determine whether CXCR4 and μOR are co-expressed and functionally coupled to G proteins in specific regions of the rat brain, as this would suggest that the two receptors can potentially interact in vivo. This information is necessary to direct our future set of experiments that aim to determine whether in vivo morphine treatment can inhibit CXCR4 function by altering its coupling to G proteins.
The data presented in this paper show that functional CXCR4 and μOR can be studied in the brain of animals younger than 3 weeks, using the GTPγS binding assay. During this period of time the functional expression of the opioid and chemokine receptor seems to overlap. Areas of higher stimulation are the medial and lateral cortex, and the hippocampus; other stimulated regions include the amygdala and basal ganglia (not shown). Responses to CXCL12 and the μOR agonists gradually decrease as the animals mature, which—at least, in the case of CXCR4—can be explained by the reduced expression of this receptor in the differentiated rat brain (Tissir et al. 2004; Berger et al. 2007). However, a higher basal level of G protein activation could be responsible for the diminished agonist response observed in the adult brain. Other experimental approaches might be more appropriate for studying the CXCR4-μOR interaction in adult animals. For instance, the antibody-capture GTPγS assay (Milligan 2003) could represent a more sensitive alternative to the traditional assay used in the present study. Although understanding the reason(s) of such reduction is interesting, it is not within the immediate scope of our study as the younger animals would be more suitable for our in vivo experiments—due to the well-characterized role of CXCR4 in the developing brain (Ma et al. 1998; Zou et al. 1998) as opposed to the adult brain. Our results are in agreement with findings from other investigators, such as those from Berger et al. (2007), who reported that the adult pattern of CXCR4 is established at 4 weeks in mice. Similarly, Tissir et al. (2004) reported that CXCR4 expression decreases from day P0 to P21. However, it is important to note that expression of both CXCR4 and its ligand have been demonstrated in adult brain in various species, including rodents and human (Stumm et al. 2002; Tran et al. 2004). Recent neuroanatomical and functional studies in rats have demonstrated a role of the CXCL12/CXCR4 system in regulation of neuroendocrine functions in the adult brain (Banisadr et al. 2002, 2003; Callewaere et al. 2006).
The data reported in this paper indicate that, in the pups, CXCL12, morphine, and DAMGO stimulation are comparable both in terms of regional distribution and maximal responses. Moreover, GTPγS incorporation was generally higher in the lateral cortex, independent of the agonist. The experiments with AMD3100 show that the CXCL12-induced response is exclusively mediated by CXCR4. This is an important piece of evidence as recent studies have identified a novel receptor for CXCL12 (CXCR7), whose expression in the CNS is still unclear (Balabanian et al. 2005; Burns et al. 2006), whereas a cleavage product of the chemokine can activate CCR3 (Vergote et al. 2006).
In conclusion, this study represents an initial step toward the in vivo characterization of the interaction between chemokine and opioid receptors, namely CXCR4 and μOR, in the brain, which could provide useful information about the mechanisms implicated in the alteration of chemokine receptor function in HIV+ drug users. Based on our previous studies with cultured neurons, we hypothesized that opioids may affect CXCR4 activation at a very early stage, such as the coupling of the receptor to the G protein. Preliminary experiments in our laboratory suggest that this might be the case in vivo, as CXCL12-induced GTPγS binding was inhibited by in vivo morphine treatment (Burbassi and Meucci, unpublished). For these experiments, animals were treated with morphine (20 mg/kg, single injection, s.c.), killed after 6 h and processed for in vitro assay with CXCL12 as indicated previously. This also suggests that opioids may act as physiological regulator of CXCR4 function independently of CXCR4 expression. Thus, one could speculate that opiates may physiologically inhibit CXCR4 activity in the adult brain and/or that drug abuse may impair chemokine function and contribute to neuronal injury.
Acknowledgment
The authors wish to thank Nayla Chaijale, Jeegar Patel, and Nicholas Di Patrizio for helpful assistance during the initial phases of this study. This paper received grant support from NIH DA15014, NIH DA19808 to OM, and DK67648 to KJS.
Footnotes
This paper was presented at the 13th SNIP Meeting, Salt Lake City (Utah), April 11–14, 2007.
References
- Albrecht E, Samovilova NN, Oswald S, Baeger I, Berger H. Nociceptin (orphanin FQ): high-affinity and high-capacity binding site coupled to low-potency stimulation of guanylyl-5′-O-(gamma-thio)-triphosphate binding in rat brain membranes. J Pharmacol Exp Ther. 1998;286:896–902. [PubMed] [Google Scholar]
- Alt A, Clark MJ, Woods JH, Traynor JR. Mu and delta opioid receptors activate the same G proteins in human neuroblastoma SH-SY5Y cells. Br J Pharmacol. 2002;135:217–225. doi: 10.1038/sj.bjp.0704430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon KB, Harrison JK. Chemokines and their receptors in neurobiology: perspectives in physiology and homeostasis. J Neuroimmunol. 2000;104:92–97. doi: 10.1016/s0165-5728(99)00266-0. [DOI] [PubMed] [Google Scholar]
- Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Fontanges P, Haour F, Kitabgi P, Rostene W, Melik Parsadaniantz S. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur J Neurosci. 2002;16:1661–1671. doi: 10.1046/j.1460-9568.2002.02237.x. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Skrzydelski D, Kitabgi P, Rostene W, Parsadaniantz SM. Highly regionalized distribution of stromal cell-derived factor-1/CXCL12 in adult rat brain: constitutive expression in cholinergic, dopaminergic and vasopressinergic neurons. Eur J Neurosci. 2003;18:1593–1606. doi: 10.1046/j.1460-9568.2003.02893.x. [DOI] [PubMed] [Google Scholar]
- Bell JE, Arango JC, Robertson R, Brettle RP, Leen C, Simmonds P. HIV and drug misuse in the Edinburgh cohort. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S35–S42. doi: 10.1097/00126334-200210012-00003. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Assimacopoulos S, Grove EA, Miller RJ. The chemokine stromal cell-derived factor-1 regulates the migration of sensory neuron progenitors. J Neurosci. 2005;25:3995–4003. doi: 10.1523/JNEUROSCI.4631-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger O, Li G, Han SM, Paredes M, Pleasure SJ. Expression of SDF-1 and CXCR4 during reorganization of the postnatal dentate gyrus. Dev Neurosci. 2007;29:48–58. doi: 10.1159/000096210. [DOI] [PubMed] [Google Scholar]
- Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaere C, Banisadr G, Desarmenien MG, Mechighel P, Kitabgi P, Rostene WH, Melik Parsadaniantz S. The chemokine SDF-1/CXCL12 modulates the firing pattern of vasopressin neurons and counteracts induced vasopressin release through CXCR4. Proc Natl Acad Sci USA. 2006;103:8221–8226. doi: 10.1073/pnas.0602620103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahoe RM, Vlahov D. Opiates as potential cofactors in progression of HIV-1 infections to AIDS. J Neuroimmunol. 1998;83:77–87. doi: 10.1016/s0165-5728(97)00224-5. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Happe HK, Bylund DB, Murrin LC. Agonist-stimulated [35S] GTPgammaS autoradiography: optimization for high sensitivity. Eur J Pharmacol. 2001;422:1–13. doi: 10.1016/s0014-2999(01)01043-3. [DOI] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Buch S, Berger JR, Tyor WR, Nath A, Bruce-Keller AJ, Knapp PE. Molecular targets of opiate drug abuse in neuroAIDS. Neurotox Res. 2005;8:63–80. doi: 10.1007/BF03033820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu QX, Barry AP, Wang ZX, Connolly SM, Peiper SC, Greenberg ML. Evolution of the human immunodeficiency virus type 1 envelope during infection reveals molecular corollaries of specificity for coreceptor utilization and AIDS pathogenesis. J Virol. 2000;74:11858–11872. doi: 10.1128/jvi.74.24.11858-11872.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi E, Strizki JM, Ulrich AM, Zhang W, Fu L, Wang Q, O'Connor M, Hoxie JA, Gonzalez-Scarano F. CXCR-4 (Fusin), a co-receptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types, including microglia and neurons. Am J Pathol. 1997;151:1035–1042. [PMC free article] [PubMed] [Google Scholar]
- Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, DuboisDalcq M. Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42:139–148. doi: 10.1002/glia.10139. [DOI] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher CE, Selley DE, Childers SR. Relationship of mu opioid receptor binding to activation of G-proteins in specific rat brain regions. Biochem Pharmacolacol. 2000;59:1395–1401. doi: 10.1016/s0006-2952(00)00272-0. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Miller RJ, Meucci O. AIDS and the brain: is there a chemokine connection? Trends Neurosci. 1999;22:471–479. doi: 10.1016/s0166-2236(99)01408-3. [DOI] [PubMed] [Google Scholar]
- Milligan G. Principles: extending the utility of [35S]GTP gamma S binding assays. Trends Pharmacol Sci. 2003;24:87–90. doi: 10.1016/s0165-6147(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Patel JP, Sengupta R, Bardi G, Khan MZ, Mullen-Przeworski A, Meucci O. Modulation of neuronal CXCR4 by the micro-opioid agonist DAMGO. J Neurovirol. 2006;12:492–500. doi: 10.1080/13550280601064798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif NA, Hughes J. Discrete mapping of brain Mu and delta opioid receptors using selective peptides: quantitative autoradiography, species differences and comparison with kappa receptors. Peptides. 1989;10:499–522. doi: 10.1016/0196-9781(89)90135-6. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Childers SR. In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5′-[gamma-[35S]thio]-triphosphate binding. Proc Natl Acad Sci USA. 1995;92:7242–7246. doi: 10.1073/pnas.92.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Dworkin SI, Childers SR. Effects of chronic morphine administration on mu opioid receptor-stimulated [35S] GTPgammaS autoradiography in rat brain. J Neurosci. 1996;16:2684–2692. doi: 10.1523/JNEUROSCI.16-08-02684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Hollt V, Schulz S. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci. 2002;22:5865–5878. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Hollt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Wetzel MA, Zhang N, Steele AD, Kaminsky DE, Chen C, Liu-Chen LY, Bednar F, Henderson EE, Howard OM, Oppenheim JJ, Rogers TJ. Selective inactivation of CCR5 and decreased infectivity of R5 HIV-1 strains mediated by opioid-induced heterologous desensitization. J Leukoc Biol. 2003;74:1074–1082. doi: 10.1189/jlb.0203067. [DOI] [PubMed] [Google Scholar]
- Talbot JN, Happe HK, Murrin LC. Mu opioid receptor coupling to Gi/o proteins increases during postnatal development in rat brain. J Pharmacol Exp Ther. 2005;314:596–602. doi: 10.1124/jpet.104.082156. [DOI] [PubMed] [Google Scholar]
- Tissir F, Wang CE, Goffinet AM. Expression of the chemokine receptor Cxcr4 mRNA during mouse brain development. Brain Res Dev Brain Res. 2004;149:63–71. doi: 10.1016/j.devbrainres.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, Schwartz RW, Haggart D, O'Brien A, White A, Kennedy JM, Craymer K, Farrington L, Auh JS. Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res Monogr. 1998;178:440–466. [PubMed] [Google Scholar]
- Tran PB, Miller RJ. Chemokine receptors in the brain: a developing story. J Comp Neurol. 2003;457:1–6. doi: 10.1002/cne.10546. [DOI] [PubMed] [Google Scholar]
- Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J Neurosci Res. 2004;76:20–34. doi: 10.1002/jnr.20001. [DOI] [PubMed] [Google Scholar]
- van der Meer P, Ulrich AM, Gonzalez-Scarano F, Lavi E. Immunohistochemical analysis of CCR2, CCR3, CCR5, and CXCR4 in the human brain: potential mechanisms for HIV dementia. Exp Mol Pathol. 2000;69:192–201. doi: 10.1006/exmp.2000.2336. [DOI] [PubMed] [Google Scholar]
- Vergote D, Butler GS, Ooms M, Cox JH, Silva C, Hollenberg MD, Jhamandas JH, Overall CM, Power C. Proteolytic processing of SDF-1alpha reveals a change in receptor specificity mediating HIV-associated neurodegeneration. Proc Natl Acad Sci USA. 2006;103:19182–19187. doi: 10.1073/pnas.0604678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao GM, Qian X, Schiller PW, Szeto HH. Comparison of [Dmt1]DALDA and DAMGO in binding and G protein activation at mu, delta, and kappa opioid receptors. J Pharmacol Exp Ther. 2003;307:947–954. doi: 10.1124/jpet.103.054775. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]