Abstract
Breastfeeding among methadone-maintained women is frequently challenged because of unclear guidelines regarding this practice. Previous research has confirmed that concentrations of methadone in breastmilk in the neonatal period are low. Currently unknown are the concentrations of methadone in breastmilk among women who breastfeed for longer periods of time. The purpose of this research is to examine concentrations of methadone in the plasma and breastmilk of women who breastfeed their infants beyond the neonatal period. Four methadone-maintained women provided blood and breastmilk samples up to 6 months postpartum. The concentrations of methadone in blood and breastmilk were low, contributing to the recommendation of breastfeeding for some methadone-maintained women.
INTRODUCTION
The American Academy of Pediatrics recommends breastmilk as the preferred feeding for all infants with few exceptions.1 The population of infants exposed to methadone in utero, at risk for multiple health and developmental disabilities,2 stands to substantially benefit from the well-known advantages of breastmilk. Yet methadone-maintained women have low rates of lactation due to difficulties presented by care providers, mothers, and infants.3 Research to date has shown that concentrations of methadone in breastmilk during the neonatal period are low and unlikely to have any effect on the breastfed infant.4-11 Previous work by this group has shown that the concentrations of methadone in breastmilk are low, unrelated to maternal methadone dose, and increase over time in the first 30 days of life.11 Absent from these reports are discussions regarding concentrations of methadone in breastmilk among women who choose longer-term lactation.
SUBJECTS AND METHODS
Subjects
Five methadone-maintained lactating women provided breastmilk and plasma specimens monthly, up to 6 months, for as long as breastmilk was a daily part of their infant's diet. Subjects were in their late twenties (mean age = 27.8 years, SD 5.4); three were Caucasian, and two were African-American. All but one smoked cigarettes daily (three of them 1 pack per day, one ½ pack per day). Two subjects were primiparous. One infant was male, and birth weights were appropriate for gestational age (mean birth weight = 3,197.0 g, SD 322.7). Specimens were taken at any time of day coincident with a pediatric clinic visit, scheduled as close as possible after the infant's monthly birthdate. Paired samples of foremilk and hindmilk were collected from the same breast, identified as the breast opposite that used at the last feeding, using an electric breast pump. Foremilk was collected prior to the infant's feeding; hindmilk was collected from the same breast after the infant had ceased feeding from that breast. Maternal plasma specimens were collected simultaneously. Infant plasma was collected at any time blood was routinely drawn during the pediatric health care maintenance visit. Time of last maternal methadone dose and other medication intake was recorded for each participant. Maximal infant ingestible dose was calculated using the maximal concentration of methadone in breastmilk and mean infant milk intake established for same age infants in previously published research.12 Informed, written consent was obtained for all participants. The research was approved by the local governing institutional review board.
Laboratory analysis
Breastmilk specimens containing methadone and metabolites were obtained from methadone-maintained breastfeeding mothers and stored in polypropylene vials at −20°C until time of analysis. Preparation of breast milk specimens included methanolic protein precipitation followed by solid-phase extraction (SPE). Extracts were analyzed by a validated liquid chromatography atmospheric pressure chemical ionization tandem mass spectrometry (LC-APCI-MS/MS) method. Briefly, 0.5-mL aliquots of breastmilk were mixed with internal standard and chilled methanol to precipitate proteins, followed by centrifugation. The organic supernatants were partially dried under nitrogen and subjected to SPE. Eluates were evaporated to dryness under nitrogen and reconstituted in mobile phase and analyzed for methadone on an LCQ Deca XP ion trap mass spectrometer (ThermoFinnigan, San Jose, CA). Identification and quantification of methadone were based on selected reaction monitoring. The limit of quantification (LOQ) was 10 ng/mL with a linear dynamic range of 10−500 ng/mL. Extraction efficiency was >97% with inter- and intra-day imprecision <20%.
Maternal blood was collected in lithium heparin tubes, mixed well, centrifuged prior to separation of plasma, and frozen at −20°C in polypropylene tubes until analysis. Plasma specimens were analyzed by gas chromatography mass spectrometry (Agilent, Dover, DE) following SPE using minor chromatographic and extraction modifications of the method of Galloway and co-workers.13,14 The LOQ for methadone was 5.0 ng/mL, and the range of linearity was 5−2,000 ng/mL. Intra- and inter-day imprecision was <20%.
Infant blood was collected in lithium heparin Vacutainer® tubes (BD Diagnostics, Franklin Lakes, NJ), mixed, and centrifuged at 500 g to separate plasma. Infant plasma was stored at −20°C in polypropylene tubes until time of analysis. Plasma specimens (200 μL) were diluted with 600 μL of acetonitrile, mixed, and centrifuged at 3,130 g for 5 minutes to pellet the precipitated protein. Supernatants were dried completely under nitrogen, reconstituted with 200 μL of water, and analyzed by a validated LC-APCI-MS/MS method. Identification and quantification of methadone were based on selected reaction monitoring. The LOQ for methadone in infant plasma was 1 ng/mL with a linear dynamic range of 1−500 ng/mL. Extraction efficiency was greater than 87.5% with inter- and intra-day imprecision <20%.
RESULTS
Results from four subjects who had complete analysis of specimens and comprised the final sample are presented in Table 1. Day 30 data for the four subjects have been previously reported within a larger sample.11 One woman (subject number 1) was maintained on a selective serotonin reuptake inhibitor throughout the study period; no other women required chronic medications other than methadone, and all denied relapse to licit or illicit drug use or alcohol consumption. None of the infants had significant health or developmental concerns in the first 6 months of life as determined by the pediatric health care history. Sampling times were variable, from time of trough maternal methadone level (the hours prior to daily methadone dosing) to peak (1−3 hours after oral methadone dosing). Despite variable sampling times and methadone doses, concentrations of methadone in breastmilk and maternal plasma were low, consistent with previous research. Maximal infant ingestible dose of methadone was calculated for each subject at 30 days, and for subject number 4 at each month, because the concentration of methadone in breastmilk was obtained at the approximate time of peak maternal methadone levels for each. The calculated ingestible doses at each time period were low, less than ⅓ mg per day.
Table 1.
Breastmilk Methadone Concentrations, Pre- and Post-Feed, Maternal Plasma Methadone Concentrations, and Maximal Ingestible Infant Dose
| ID | Day | Dose (mg) | Hours post-dose | BM Pre/Post (ng/mL) | P (ng/mL) | Max dose (mg/day) |
|---|---|---|---|---|---|---|
| 1 | 30 | 105 | 3.0 | 315.6/347.0 | 803.0 | 0.27 |
| 60 | 105 | 23.5 | 45.2/174.6 | 407.0 | ||
| 90 | 105 | 1.5 | 183.2/299.5 | 521.0 | ||
| 120 | 110 | 3.0 | 292.1/206.9 | 573.0 | ||
| 2 | 30 | 80 | 3.0 | 198.7/244.9 | 325.0 | 0.19 |
| 60 | 80 | 22.5 | 389.3/247.4 | 397.0 | ||
| 90 | 70 | 24.0 | 153.7/27.1 | 182.0 | ||
| 3 | 30 | 65 | 3.0 | 407.1/359.5 | 651.0 | 0.31 |
| 60 | 60 | 19.0 | 186.9/345.8 | 482.0 | ||
| 90 | 60 | 23.0 | 374.4/277.6 | 373.0 | ||
| 120 | 25 | 19.0 | 138.6/161.9 | 149.0 | ||
| 4 | 30 | 60 | 3.0 | 188.7/164.6 | 514.0 | 0.15 |
| 60 | 60 | 2.5 | 133.4/233.9 | 589.0 | 0.16 | |
| 90 | 60 | 2.5 | 180.9/402.2 | – | 0.30 | |
| 120 | 60 | 1.0 | 260.5/198.6 | 692.0 | 0.19 | |
| 150 | 60 | 1.0 | 183.8/281.4 | 595.0 | 0.24 | |
| 180 | 60 | 1.0 | 210.2/283.9 | 653.0 | 0.22 |
ID, subject ID number; Day, approximate day of infant life; Dose, maternal methadone dose at the time of breastmilk and plasma sampling; Hours post-dose, number of hours after single daily oral maternal methadone dose; BM Pre/Post, breastmilk methadone concentration pre-feed/post-feed (foremilk/hindmilk); P, maternal plasma methadone concentration at the time of breastmilk sampling; Max dose, maximal ingestible infant dose methadone via breastmilk per day.
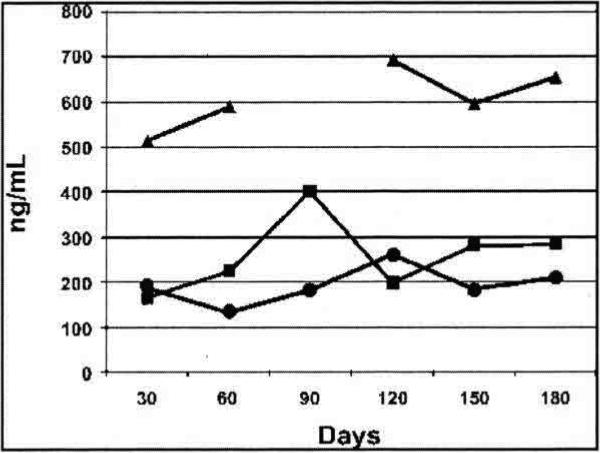
Maternal plasma and breast milk specimens from the fifth subject were unavailable for analysis because of laboratory error. For this subject alone, infant plasma was obtained at 1 year. At this time the child was breastfed during the nighttime only, and had been last breastfed approximately 12 hours previously. The child's plasma methadone concentration was 2.0 ng/mL. This child had no significant health or developmental concerns during the first year. For one subject (number 4), methadone dose and breastmilk/plasma sampling times remained relatively consistent (at approximate times of peak maternal plasma levels) for 6 months; her results are presented in Figure 1.
FIG. 1.
Subject number 4 peak pre- and post-feed breastmilk and plasma methadone concentration: pre-feed breastmilk methadone concentration (•), post-feed breastmilk methadone concentration (■), and maternal plasma methadone concentration (▴).
CONCLUSIONS
Little is known regarding the long-term effects of prolonged breastmilk consumption by children of methadone-maintained women. Results presented in this report confirm that, among women breastfeeding beyond the neonatal period, concentrations of methadone in human milk are small, and the potential exposure to the infant is low and unlikely to have any negative effect on the developing child. Results contribute to the recommendation of breastfeeding for women requiring methadone maintenance and the designation of methadone as a medication usually compatible with breastfeeding.15 Methadone was present in the plasma of one infant breastfed for 1 year, though at a low concentration. Limitations of this study include small sample size. Future research should focus on health and developmental outcomes of infants born to methadone-maintained women who breastfed for periods exceeding the neonatal period.
ACKNOWLEDGMENTS
The authors thank the subjects, without whose cooperation this work is not possible, and the staff at the Center for Addiction and Pregnancy, Baltimore, MD. This work is supported by NIH/NIDA grants K08 DA00495 and RO1 DA019934, awarded to L.M.J., and the NIH, NIDA Intramural Research Program.
REFERENCES
- 1.American Academy of Pediatrics, Work Group on Breastfeeding Breastfeeding and the use of human milk. Pediatrics. 2005;115:496–506. [Google Scholar]
- 2.Jansson LM, Velez M. Understanding and treating substance abusers and their infants. Infants Young Child. 1999;11:79–89. [Google Scholar]
- 3.Jansson LM, Velez M, Harrow C. Methadone maintenance and lactation: A review of the literature and current management guidelines. J Hum Lact. 2004;20:62–71. doi: 10.1177/0890334403261027. [DOI] [PubMed] [Google Scholar]
- 4.Kreek M, Schecter A, Gutjahr C. Analysis of methadone and other drugs in maternal and neonatal body fluids: Use in evaluation of symptoms in a neonate of mother maintained on methadone. Am J Drug Alcohol Abuse. 1974;1:409–419. doi: 10.3109/00952997409011033. [DOI] [PubMed] [Google Scholar]
- 5.Blinick G, Inturrisi CE, Jerez E, et al. Methadone assays in pregnant women and progeny. Am J Obstet Gynecol. 1975;121:617–621. doi: 10.1016/0002-9378(75)90461-5. [DOI] [PubMed] [Google Scholar]
- 6.Kreek M. Methadone disposition during the perinatal period in humans. Pharmacol Biochem Behav. 1979;11:7–13. [PubMed] [Google Scholar]
- 7.Pond S, Inturrisi CE, Jerez E, et al. Altered methadone pharmacokinetics in methadone-maintained pregnant women. J Pharmacol Exp Ther. 1985;233:1–6. [PubMed] [Google Scholar]
- 8.Geraghty B, Grahma EA, Logan B, et al. Methadone levels in breast milk. J Hum Lact. 1997;13:227–230. doi: 10.1177/089033449701300312. [DOI] [PubMed] [Google Scholar]
- 9.Wojnar-Horton R, Kristensen JH, Yapp P, et al. Methadone distribution and excretion into breast milk of clients in a methadone maintenance program. Br J Clin Pharmacol. 1997;44:543–547. doi: 10.1046/j.1365-2125.1997.t01-1-00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarthy J, Posey B. Methadone levels in human milk. J Hum Lact. 2000;16:115–120. doi: 10.1177/089033440001600206. [DOI] [PubMed] [Google Scholar]
- 11.Jansson L, Choo RE, Harrow C, et al. Concentrations of methadone in breast milk and plasma in the immediate perinatal period. J Hum Lact. 2007;23:184–190. doi: 10.1177/0890334407300336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neville MC, Keller R, Seacat J, et al. Studies in human lactation: milk volumes in lactating women during the onset of lactation and full lactation. Am J Clin Nutr. 1988;48:1375–1386. doi: 10.1093/ajcn/48.6.1375. [DOI] [PubMed] [Google Scholar]
- 13.Galloway F, Bellet N. Methadone conversion to EDDP during GC-MS analysis of urine samples. J Anal Toxicol. 1999;23:615–619. doi: 10.1093/jat/23.7.615. [DOI] [PubMed] [Google Scholar]
- 14.Alburges M, Huang W, Foltz RL, et al. Determination of methadone and its N-demethylation metabolites in biologic specimens by GC-PICI-MS. J Anal Toxicol. 1996;20:362–368. doi: 10.1093/jat/20.6.362. [DOI] [PubMed] [Google Scholar]
- 15.American Acadmey of Pediatrics, Committee on Drugs The transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108:776–789. doi: 10.1542/peds.108.3.776. [DOI] [PubMed] [Google Scholar]