Abstract
Objective: To evaluate effects of epigallocatechin-3-gallate (EGCG) on the viability, membrane properties, and zinc distribution, with and without the presence of Zn2+, in human prostate carcinoma LNCaP cells. Methods: We examined changes in cellular morphology and membrane fluidity of LNCaP cells, distribution of cellular zinc, and the incorporated portion of EGCG after treatments with EGCG, Zn2+, and EGCG+Zn2+. Results: We observed an alteration in cellular morphology and a decrease in membrane fluidity of LNCaP cells after treatment with EGCG or Zn2+. The proportion of EGCG incorporated into liposomes treated with the mixture of EGCG and Zn2+ at the ratio of 1:1 was 90.57%, which was significantly higher than that treated with EGCG alone (30.33%). Electron spin resonance (ESR) studies and determination of fatty acids showed that the effects of EGCG on the membrane fluidity of LNCaP were decreased by Zn2+. EGCG accelerated the accumulation of zinc in the mitochondria and cytosol as observed by atomic absorption spectrometer. Conclusion: These results show that EGCG interacted with cell membrane, decreased the membrane fluidity of LNCaP cells, and accelerated zinc accumulation in the mitochondria and cytosol, which could be the mechanism by which EGCG inhibits proliferation of LNCaP cells. In addition, high concentrations of Zn2+ could attenuate the actions elicited by EGCG.
Keywords: Epigallocatechin-3-gallate (EGCG), Zinc, LNCaP, Membrane fluidity
INTRODUCTION
Tea catechin has chemopreventive potential in cancers, especially in prostate cancer. Oral green tea catechin is safe and effective in the treatment of pre-malignant lesions of prostate cancer (Bettuzzi et al., 2006). Epigallocatechin-3-gallate (EGCG), the major polyphenolic constituent present in green tea, showed significant inhibitory action on the growth of prostate cancer cells in vitro and tumor models (Liao et al., 1995; Gupta et al., 2000; Chung et al., 2001; Vayalil and Katiyar, 2004). However, the exact mechanism by which EGCG brings about its anti-proliferative action on cancer cells is not clear. It is believed that the 67-kDa laminin receptor (67LR) is the primary target of EGCG (Tachibana et al., 2004; Fujimura et al., 2005). Furthermore, membrane androgen receptor could be one potential target of EGCG, which is expressed by prostate cancer but not by non-neoplastic cells (Kampa et al., 2002; 2005; Stathopoulos et al., 2003). It is likely that agents that act on laminin and androgen receptors could induce the polymerization of action cytoskeleton and the redistribution of microfilaments (Kampa et al., 2002), and cause apoptosis (Kampa et al., 2005). Very few studies have been performed to date on the action of EGCG on prostate cancer cell membrane.
Recently, several reports have focused on the interactions of catechin with metal ions (Reznichenko et al., 2006; Yu et al., 2005; Hayakawa et al., 2004; Chen et al., 2007). Catechin induces redox reactions or chelates with metal ions, especially transitional metal ions, which lead to bioactivity changes of catechin. Zinc ion (Zn2+) plays a significant role in the pathogenesis of prostate cancer. Prostate gland is rich in zinc (Yaman et al., 2005). Zn2+ inhibits the growth of prostate cancer cells and acts as an anti-tumor agent (Uzzo et al., 2006; Franklin and Costello, 2007), but this has been disputed. In a case-control study, it has been reported that high zinc intake (≥15.7 mg/d) increases the risk of prostate cancer (Gallus et al., 2007). In view of this, it is important to know the interaction between EGCG and Zn2+ and how their interaction modifies the risk of prostate cancer.
Our previous studies revealed that biological activities of EGCG are altered in the presence of Zn2+, Cu2+, and Cd2+, and the results rely on (1) the relative concentrations of EGCG and metal ions, and (2) the order of their additions that might have led to the formation of complexes between EGCG and metal ions, which could lead to changes in the generation of free radicals within the cells (Yu et al., 2005; 2007; Chen et al., 2007). Nevertheless, there is little information available in literature about the interactions between EGCG and metal ions, especially with regard to their action on cell membrane of prostate cancer cells. Hence, we studied the effects of EGCG and Zn2+ on viability, membrane properties, and zinc distribution of LNCaP cells.
MATERIALS AND METHODS
Chemicals
Purified EGCG (>98%) and egg phosphatidylcholine (egg PC, approximately 99%), and the spin labels 5-doxyl stearic acid (5-SASL) and 16-doxyl stearic acid (16-SASL) were purchased from Sigma (St. Louis, MO, USA). Human prostate cancer cells (LNCaP) were obtained from Shanghai Institute of Cell Biology, Chinese Academy of Sciences. All other chemicals were of extra-pure grade or analytical grade unless otherwise specified.
Cell culture
LNCaP cells were cultured in F-12 nutrient mixture medium (GIBCO, Invitrogen Corporation) supplemented with 10% (v/v) fetal bovine serum and 100 U/ml penicillin-streptomycin in a humidified 5% CO2 incubator at 37 °C. The cells were incubated for an additional 24 h prior to treatment. After treatment with EGCG and Zn2+ at various concentrations, the cells were incubated for a further 24 h. All experiments were repeated at least thrice, each time in duplicate.
3-[4,5-Dimethythiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assays
We performed MTT assays to determine the effects of EGCG and Zn2+ on the growth of LNCaP cells. Cells were plated in 96-well tissue culture plates and were treated with varying doses of EGCG and Zn2+ for 24, 36, and 48 h. After completion of the treatment, cells were washed with phosphate buffered saline (PBS), and 20 μl of 5 mg/ml MTT was added. Then the cells were incubated for 4 h at 37 °C to allow the formation of formazan precipitate, which subsequently was dissolved in dimethyl sulfoxide (DMSO). The absorbance in each well was then measured with a microplate reader (Thermal Lab System, Finland) at 490 nm, and the viability of cells in each well was presented as the percentage of control cells.
In addition, we investigated the mixture of EGCG and Zn2+ on the growth of LNCaP cells. Herein, we chose a settled concentration of EGCG (80 μmol/L) and different concentrations of Zn2+, with EGCG and Zn2+ added in different orders. The treated cells were incubated for 24 h at 37 °C, and their viability was determined by MTT assay.
Microscopic images of LNCaP cells adhering on the plate
LNCaP cells were equally cultured in 6-well plates (20 mm×20 mm) for 24 h and then exposed to EGCG (80 μmol/L), Zn2+ (80 μmol/L), and EGCG+Zn2+ (80 μmol/L+80 μmol/L) for another 24 h, and the cells without treatment were considered as control. After exposure, microscopic images of LNCaP cells adhering on the plate were made through an inverted microscope under magnification 20× (XDS-1B, Chongqing, China).
Analysis of cell membrane fluidity by electron spin resonance labeled using 5- and 16-SASL
Stearic acid spin labels (5- and 16-SASL) were used as a probe to quantify membrane fluidity of prostate cancer cells. After stimulation, the suspension of LNCaP cells in PBS was labeled with 5- and 16-SASL at a final concentration of 100 μmol/L and then incubated for 30 min at 37 °C with gentle shaking to incorporate the spin labels into the membranes. The labeled cells were washed three times with PBS to remove unbounded spin label and re-suspended in PBS. Finally, 20 μl of suspension was transferred to a glass capillary for electron spin resonance (ESR) experiments. All ESR experiments were performed at 9.852 GHz using a Bruker ESR A300 spectrometer (Bruker, Germany) and were operating at a center field strength of 3509 G with scan range of 60 G, modulation amplitude of 4.0 G, and microwave power of 20 mW. Samples were run in three times and the typical examples are shown in the figures.
Determination of composition of fatty acids by gas chromatography-mass spectrometry (GC-MS)
After various treatments, LNCaP cells were harvested and suspended in pre-cool Tris-HCl buffer (pH 7.4), and broken by Ultrasonic Cell Disruption System (JY92-II, China). The samples were centrifuged at 12 000 r/min for 15 min at 4 °C and washed with the Tris-HCl buffer thrice. Total lipids from prostate cancer cells were extracted by 5% (w/v) hydrochloric acid/methanol. The extracted lipids were transesterified by sequential saponification and esterification (Knapp, 1979), a reaction that was performed under a nitrogen atmosphere to avoid oxidation of polyunsaturated fatty acids. Electron impact ionization was performed with Agilent 5973 (USA) GC-MS system equipped with a 25 m×0.2 mm i.d.×0.33 μm HP-1 column. The column head pressure was set to 60 kPa and the injection volume was 1 μl with a splitting ratio of 1:10. After injection, the column temperature was programmed at 3 °C/min from 160 to 220 °C, then 5 °C/min from 220 to 240 °C.
Measurement of EGCG incorporated into liposomes
Egg PC (100 mg) was dissolved in a small amount of chloroform. The solution was put in a round-bottomed flask and the solvent evaporated off with a rotary evaporator. The thin film of egg PC on the inner surface of the flask was dried with a vacuum pump. Then 10 ml Tris-HCl buffer (pH 7.4) was poured into the flask and the mixture was transferred into a 50-ml plastic centrifuge tube. The sealed tube was sonicated for 10 min. During this sonication step the phase of liposome was changed into small unilamellar vesicles. The liposome solution was diluted 10 times with Tris-HCl buffer. EGCG solution and EGCG+Zn2+ solution were added to the liposome solution and the mixture was vortexed in a centrifuge tube. After 20 min of incubation at 20 °C, the mixture was centrifuged at 18 000×g for 5 min. The EGCG content in the supernatant was determined by HPLC (LC-2010A, Shimadzu, Japan). Concentration of EGCG was estimated from the calibration curves prepared with the standard solution. The proportion (%) of EGCG incorporated into the lipid bilayers of each treatment was calculated as follows:
| (1−nnon-incorporated/nadded)×100%, |
where n non-incorporated is amount of EGCG non-incorporated and n added is amount of EGCG added.
Distribution of zinc in LNCaP cells
After treatment, the cells were harvested and broken by Ultrasonic Cell Disruption System (JY92-II, China). The broken cell pellets were used to prepare other subcellular organelles as follows. The broken cell pellets were precipitated in a refrigerated centrifuge at 3 000 r/min for 10 min. The supernatant was decanted from the nuclear fraction and centrifuged at 9 000 r/min for 10 min to get the mitochondrial fraction. Subsequently, the solution was centrifuged at 20 000 r/min for 30 min to isolate the lysosomal fraction from the cytosol together with microsomes. The nuclear fraction, mitochondrial fraction, and lysosomal fraction were washed twice with cold PBS and suspended in cold PBS. The distribution of zinc in PC-3 cells was analyzed by atomic absorption spectrometer (AAS800, Perkin-Elmer, USA). All the experiments were replicated three times.
Statistical analysis
Results are expressed as the mean±SD of at least three independent replications of each experiment. Statistical significance was determined by pair t-test analysis using Origin7.5 software for windows. Mean values were considered significantly different at P<0.05 or P<0.01.
RESULTS
Viability of LNCaP cells in the presence of EGCG and Zn2+
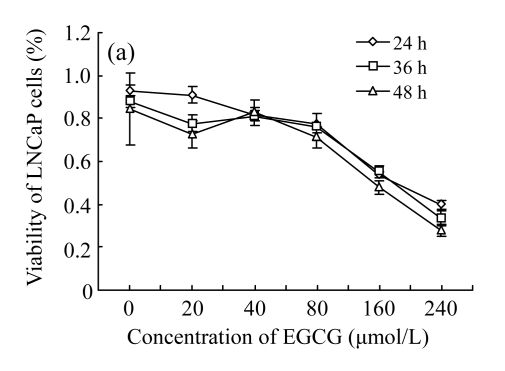
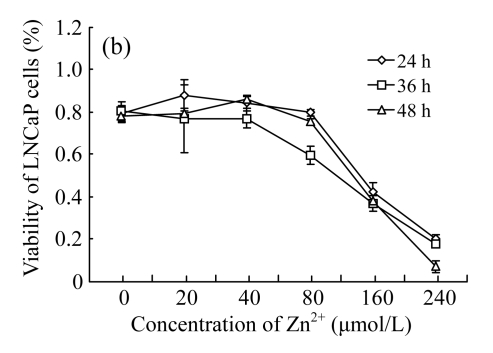
As shown in Fig.1, treatments with low concentrations (<80 μmol/L) of EGCG and Zn2+ had no significant effects on the growth of LNCaP cells. However, treatments with high concentrations (>80 μmol/L) resulted in a loss of cell viability, with a sharp decrease at the concentrations of 160~240 μmol/L. Furthermore, a time-dependent manner was found under the treatments of high concentrations, with cell viability decreasing most at 48 h, less 36 h, and least at 24 h. The results indicate that the treatments with EGCG and Zn2+ at high concentrations suppressed the growth of LNCaP cells in concentration- and time-dependent manners (Fig.1).
Fig. 1.
Cytotoxicity of EGCG and Zn2+ on LNCaP cells by MTT assay (n=7)
(a) Treatments with different concentrations of EGCG only; (b) Treatments with different concentrations of Zn2+ only
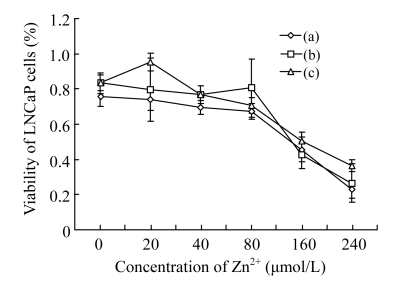
Subsequently, we chose the settled 80 μmol/L EGCG and several concentrations of Zn2+ into the cells to investigate the interactions of EGCG with Zn2+ on the growth of LNCaP cells. We found that the viability of LNCaP cells was hardly changed when Zn2+ concentrations were increased from 0 to 80 μmol/L (Fig.2). But after Zn2+ concentrations were over 80 μmol/L, the viability of LNCaP cells was significantly increased. Herein, we also investigated the effects of EGCG with Zn2+ in different addition orders. We found that the effect of Zn2+ added before EGCG was better than those of Zn2+ added after EGCG and added at the same time at high concentrations of Zn2+ (>80 μmol/L).
Fig. 2.
Cytotoxicity of the interactions of EGCG with Zn2+ on LNCaP cells by MTT assay (n=7)
(a) After different concentrations of Zn2+ were added, LNCaP cells were incubated for 2 h at 37 °C, and then mixed with 80 μmol/L EGCG for 24 h; (b) After 80 μmol/L EGCG was added, LNCaP cells were incubated for 2 h at 37 °C, then mixed with different concentrations of Zn2+ for 24 h; (c) After 80 μmol/L EGCG and different concentrations of Zn2+ were added at the same time, LNCaP cells were incubated for 24 h at 37 °C
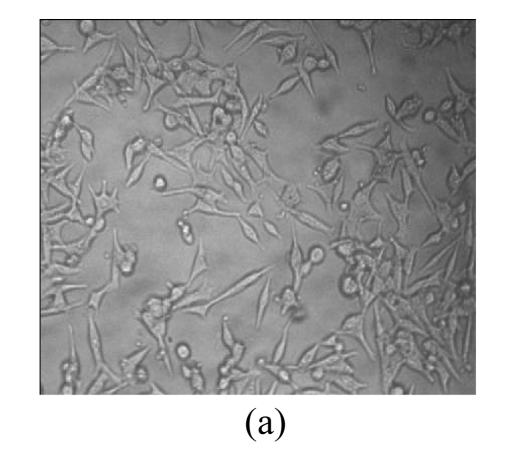
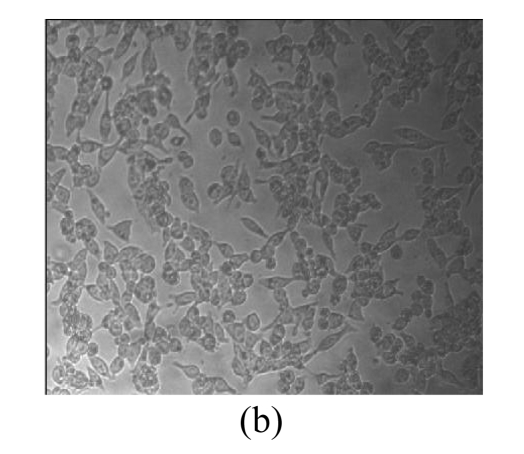
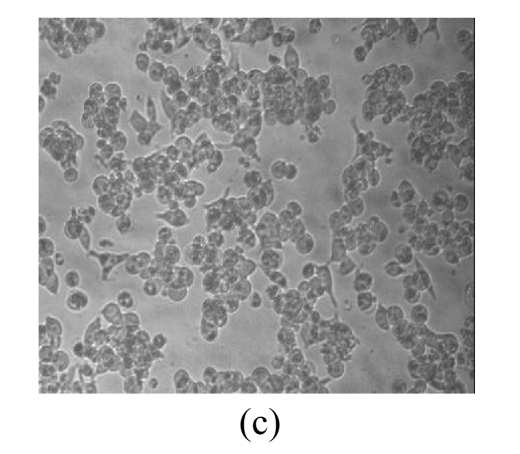
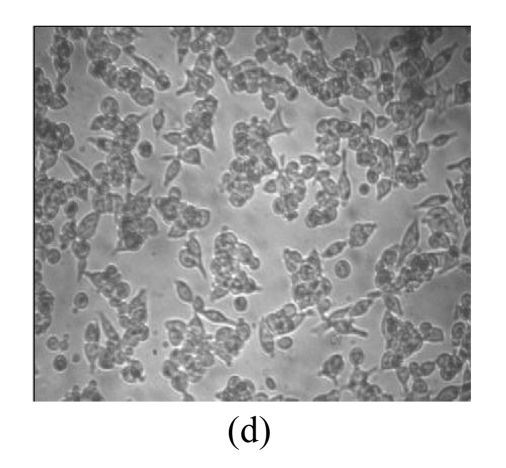
Morphological variations of LNCaP cells
Morphological variations of LNCaP cells treated with EGCG, Zn2+, and mixture of EGCG and Zn2+ were observed through an inverted microscope. As shown in Fig.3, normal LNCaP cells adhered on the plate uniformly with a compressed shape. When exposed to 80 μmol/L EGCG, 80 μmol/L Zn2+, and mixture of EGCG and Zn2+ for 24 h, LNCaP cells appeared obviously morphological variations, became round in shape, and clustered on the plate. Very few cells remained compressed under the treatments with EGCG and EGCG+Zn2+, and the suspended dead cells were found in nutrient mixture medium (data not shown). The morphological variations indicated the cytotoxic capacity of EGCG and Zn2+ on LNCaP cells. EGCG and Zn2+ could change cell morphology, and thus influence the survival of LNCaP cells.
Fig. 3.
Microscopic images of LNCaP cells after exposure for 24 h
(a) Normal LNCaP cells; (b) LNCaP cells treated with 80 μmol/L EGCG; (c) LNCaP cells treated with 80 μmol/L Zn2+; (d) LNCaP cells treated with the mixture of 80 μmol/L EGCG and 80 μmol/L Zn2+
Effects of the interactions of EGCG with Zn2+ on the membrane fluidity of LNCaP cells
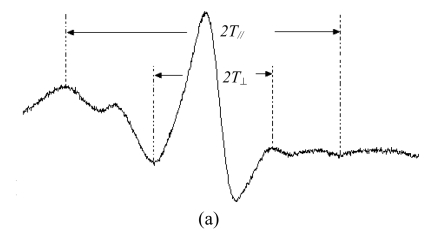
The typical ESR spectra obtained from 5- and 16-SASL in the membranes of prostate cancer cells are given in Fig.4. In the membrane, SASL undergoes anisotropic rotational motion. Actually, 5-SASL is positioned closer to the surface of the membrane and 16-SASL is near the center of membrane bilayer. The ESR spectra of n-doxyl stearic acid spin labels in the membranes are further characterized by calculating the values of the order parameter S. As depicted in Table 1, EGCG and Zn2+ perturbed the hydrocarbon chains near the surface of the membranes, and the increase in S value for 5-SASL indicated the reduction of membrane fluidity in LNCaP cells (P<0.05). However, no significant differences were noted between the control and the cells treated with the mixture of EGCG and Zn2+. From the order parameter, in the coexist system of EGCG and Zn2+, the S value was lower than that in the EGCG treatment, and the interaction of EGCG with Zn2+ decreased the effect of EGCG on the membrane fluidity.
Fig. 4.
Typical ESR spectra of 5-SASL (a) and 16-SASL (b) in the membranes of LNCaP cells at 25 °C
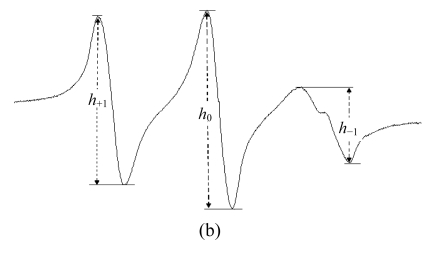
The spectra were recorded at 9.852 GHz at 25 °C, microwave power of 20 mW, modulation amplitude of 4.0 G, center field strength of 3509 G, and scan range of 60 G. The order parameters (S) were calculated from the ESR spectra by using the equation: S=0.5407(T //−T ⊥)/a 0, where a 0=(T //+2T ⊥)/3. The rotation correlation time (t) was calculated from the ESR spectra by using the equation: t=6.51×10−10ΔH 0[(h 0/h −1)−(h 0/h +1)]S, where ΔH 0 was the center line width. T //, T ⊥, h 0, h −1, and h +1 were measured from the ESR spectra as indicated in the figure
Table 1.
The values of order parameter (S) and correlation rotation time (t) in LNCaP cells labeled with 5- and 16-SASL
| Treatment |
S |
t | |
| 5-SASL | 16-SASL | ||
| Control | 0.467±0.0025 | 0.195±0.0013 | 7.85±0.11 |
| EGCG | 0.493±0.0017* | 0.204±0.0008* | 10.42±0.18* |
| Zn2+ | 0.500±0.0027* | 0.207±0.0012* | 8.64±0.14* |
| EGCG+Zn2+ | 0.468±0.0064 | 0.202±0.0007 | 9.53±0.06* |
Significant difference from control: P<0.05
Because the position of the spin label was shifted toward the center region in the membranes, the similar trend of the order parameter was obtained for 16-SASL as described in 5-SASL. Based on these results, it could be inferred that EGCG reduced the membrane fluidity of LNCaP cells, which was much depressed when treated with the mixture of EGCG and Zn2+.
The effective rotation correlation time t is another empirical ESR parameter that is often used to characterize the dynamics of 16-SASL in the membranes. The t value for 16-SASL reflects the micro viscosity of the membrane bilayer near the center, and the higher t value predicts the greater micro viscosity and the lower membrane fluidity. All treatments exerted a significantly increased effect on the t value compared with the control in LNCaP cells (P<0.05), while the t value was decreased by interactions of EGCG and Zn2+. In essence, EGCG perturbed the hydrocarbon chain relatively close to the membrane surface and the membrane center in fluid phase membranes of prostate cancer cells, the disordering effects were enhanced by EGCG at 80 μmol/L, and the presence of Zn2+ could attenuate the effects.
Effects of the interactions of EGCG with Zn2+ on the unsaturation of fatty acids of LNCaP cells
Many factors could influence the cell membrane fluidity. Unsaturation of fatty acids is one such factor. Hence, we studied the fatty acid composition of the membranes of prostate cancer cells, and found that three major methyl esters of fatty acids, namely, hexadecanoic acid, 9-octadecenoic acid (Z)-, and octadecanoic acid, are present in LNCaP cells (Table 2). The ratio of unsaturated/saturated (U/S, unsaturation of fatty acids) is known to affect cell membrane fluidity; the higher the U/S ratio, the higher the membrane fluidity. As shown in Table 2, the U/S value was depressed by EGCG or Zn2+ treatment, which indicates a decrease in the relative content of unsaturated fatty acids in prostate cancer cells. It was likely that EGCG and Zn2+ decreased the membrane fluidity of prostate cancer cells by reducing the membrane content of unsaturated fatty acids possibly through the decrease of unsaturated fatty acids synthesis. However, Zn2+ could attenuate this impact of EGCG.
Table 2.
The composition and unsaturation of fatty acids in LNCaP cells in each treatment
| Treatment | Composition (%) |
U/S | ||
| Hexadecanoic acid, methyl ester | 9-Octadecenoic acid (Z)-, methyl ester | Octadecanoic acid, methyl ester | ||
| Control | 40.875±0.7115 | 26.918±1.1095 | 22.622±1.502 | 0.347±0.0195 |
| EGCG | 50.687±2.102 | 11.656±0.5585 | 32.501±3.0155 | 0.135±0.008* |
| Zn2+ | 38.202±2.067 | 9.738±0.9490 | 27.011±0.6455 | 0.149±0.011* |
| EGCG+Zn2+ | 49.878±1.433 | 22.941±0.8790 | 22.812±1.133 | 0.315±0.0135 |
Determinations of fatty acids were programmed by GC-MS. The column head pressure was set to 60 kPa and the injection volume was 1 μl with a splitting ratio of 1:10. After injection, the column temperature was programmed at 3 °C/min from 160 to 220 °C, and then 5 °C/min from 220 to 240 °C.
Significant difference from the control: P<0.05
Effects of EGCG and the interactions of EGCG with Zn2+ on the amount of EGCG incorporated into liposomes
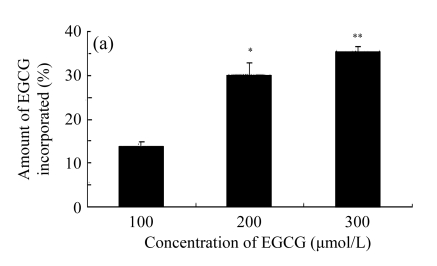
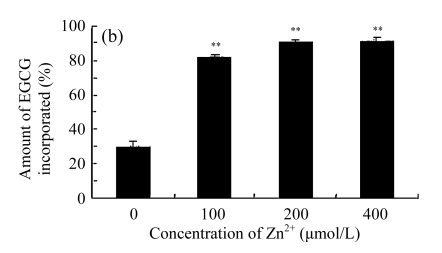
In order to investigate the effects of EGCG, Zn2+, and EGCG+Zn2+ on the cell membrane, we studied the incorporation of EGCG into liposomes. The liposomes are very similar to biological membranes. Results show that EGCG was incorporated into lipid bilayers of liposomes in a dose-dependent manner (Fig.5a). The amount of EGCG incorporated into the liposomes was increased in the presence of Zn2+ (Fig.5b), reflecting the balance of the affinity of these compounds for lipid bilayers of liposomes. We tested the effect of different ratios of EGCG to Zn2+ (2:1, 1:1, 1:2) on the amount of EGCG incorporated in the liposomes. It was noted that in the presence of Zn2+, the amount of EGCG incorporated into the lipid bilayers of the liposomes was significantly increased compared with EGCG alone (Fig.5b). Treatment of 1:1 or 1:2 of EGCG to Zn2+ did not display any significant difference in the amount of EGCG incorporated into the lipid bilayers of the liposomes, but a ratio of 1:1 of EGCG to Zn2+ gave the best results.
Fig. 5.
Effects of EGCG and Zn2+ on the amount of EGCG incorporated into the liposomes
(a) Different concentrations of EGCG were added into the liposomal solution; (b) 200 μmol/L EGCG and different concentrations of Zn2+ (0, 100, 200, 400 μmol/L) were added into the liposomal solution. Then the liposomal solution was incubated at 20 °C for 20 min, centrifuged and determined by HPLC. The ratio (%) was calculated by dividing the amount of EGCG incorporated by the amount of EGCG added. Data were expressed as mean±SE of at least three times. Significant difference from the minimum concentration: * P<0.05, ** P<0.01
Distribution of zinc in LNCaP cells
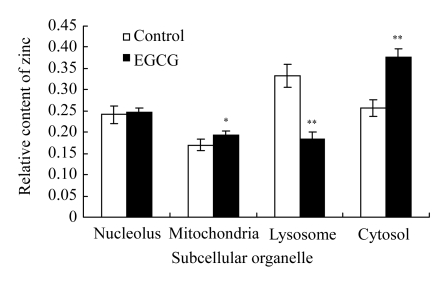
Under the condition of no extracellular zinc, we examined the distribution of zinc in the subcellular organelles of LNCaP cells in the presence of EGCG. Irrefutable and overwhelming clinical and experimental evidence now exists that altered cellular and mitochondrial zinc accumulation and associated changes in citrated-related metabolism are essential to the development and progression of prostate malignancy (Singh et al., 2006; Costello et al., 2005). As shown in Fig.6, the treatment with 80 μmol/L EGCG produced a significant increase in the relative contents of zinc in the mitochondria and cytosol (P<0.01), whereas there was an apparent decrease in lysosome.
Fig. 6.
Content of Zn2+ in the subcellular organelles of LNCaP cells
The subcellular organelles were isolated by a refrigerated centrifuge and measured by electrothermal atomic absorption spectrometry. Cells were treated with 80 μmol/L EGCG for 24 h and cells without treatment were considered as control. Data were expressed as mean±SE of at least three times. Significant difference from minimum concentration: * P<0.05, ** P<0.01
From our results, it was evident that Zn2+ was transported from the lysosome to cytosol and mitochondria in the presence of EGCG in LNCaP cells. Zinc/metabolic transformation in LNCaP cells had important implications on cellular bioenergetics, cell growth and proliferation, lipodenesis, and other metabolic-associated malignant activities.
DISCUSSION
Prostate cancer represents the most common malignancy for chemoprevention studies because of its particularly long latency period and high rate of mortality and morbidity. Many anti-tumor agents against prostate cancer cells have been developed, but their unacceptable systemic toxicity to normal tissues frequently limits their usage in clinics. At the time of clinical diagnosis in humans most prostate cancers represent themselves as a mixture of androgen-sensitive (LNCaP) and androgen-insensitive cells. Prostate cancer in the early stage is androgen dependent. Therefore, studying chemopreventive effect of EGCG in the LNCaP cells and its pathways has been useful in understanding the molecular mechanism involved in tumor initiation as well as the properties of LNCaP cells. Natural chemicals show great potential in the development of novel chemotherapeutics against cancer. EGCG, one such natural chemical present in green tea, has gained much attention for its cancer-chemopreventive properties. Importantly, at physiologically attainable concentrations EGCG kills cancer cells through apoptosis but has no effect on normal cells (Ahmad et al., 1997; 2000; Chen et al., 1998). In the present work, MTT assays demonstrated that treatments with EGCG and Zn2+ at high concentrations significantly inhibited the proliferation of LNCaP cells (Fig.1). After exposed to EGCG, Zn2+, and EGCG+Zn2+, visible cellular morphological changes were noted in LNCaP cells (Fig.3). It was documented that the growth of LNCaP had been significantly halted, which could be due to the effect of EGCG on the cell membrane of LNCaP cells. This is supported by the results of the studies done using ESR. In the present study, it was observed that EGCG, Zn2+, and EGCG+Zn2+ decreased LNCaP cell membrane fluidity (P<0.05). Since fluid properties of biological membranes were essential for numerous cell functions including cell growth, solute transport, signal transduction, and membrane-associated enzymatic activities (Sunshine and McNamee, 1994; Salinas et al., 2005; Balint et al., 2005), it is possible that even mild alterations in membrane fluidity could cause aberrant function and set in motion pathological processes (Cooper, 1977; de Gómez Dumm et al., 2001). However, it should be noted that changes in membrane fluidity need not necessarily render them ‘leaky’ since transport or translocation of compounds across cell membranes is a different physiological process, which is not directly related to the horizontal fluidity of the membrane.
Changes in cell membrane fluidity depend to a large extent on the content of unsaturation of fatty acids in the phospholipid fraction of membranes (Thewke et al., 2000; Turk et al., 2004). It was noted that PC-3 cells treated with EGCG or Zn2+ contained low levels of unsaturated fatty acids and showed a decrease in membrane fluidity. This information reaffirmed that interactions with cell membrane for treatment with EGCG or Zn2+ facilitated the decrease of membrane fluidity to result in anti-proliferation of LNCaP cells. Catechins or zinc ions are known to induce oxidative damage through the generation of reactive oxygen species (Oikawa et al., 2003; Bishop et al., 2007; Kanadzu et al., 2006). Hence, it is likely that the generation of reactive oxygen species induced by catechins and zinc ions peroxidized membrane unsaturated fatty acids, which could lead to a decrease in membrane fluidity (Teresa et al., 2002).
Since EGCG elicited the decrease of cell membrane fluidity, we studied the influence of Zn2+ on incorporation of EGCG into liposomes. The results demonstrate that after an initial interaction of EGCG with the cell membrane, EGCG could enter into LNCaP cells. Interactions of EGCG with Zn2+ remarkably increased the incorporation of EGCG into liposomes (Fig.5b). However, it was noted that, interactions between EGCG and Zn2+ decreased the effects of EGCG as evident from the results obtained with ESR and fatty acid analysis of the cell membranes. Because the ratio of EGCG to Zn2+ is 1:1, this could partly be due to the high concentrations of Zn2+ and structural characters of EGCG. The D-ring hydroxyl groups of EGCG occupy the first coordination sphere around metal ion to form a diolate combination ring, while B-ring hydroxyl groups have weak interactions with metal ions (Navarro et al., 2005). As suggested by previous reports (Hashimoto et al., 1999; Kajiya et al., 2001), the number of hydroxyl groups on the B-ring in EGCG structure, the presence of a galloyl moiety, and the steric characters on the D-ring could affect its affinity for the lipid bilayers. As a result of this, incorporation of EGCG into the lipid bilayers of the liposomes is enhanced, which could lead to the formation of another ion channels after complexing of EGCG with Zn2+, which is expected to induce structural variation and influence the effects of EGCG.
Trace elements have been important in the etiology of cancer. The prostate gland contains high concentration of zinc. Zn2+ plays a significant role in the pathogenesis of prostate cancer. Catechin is a very important compound in green tea and several reports have recently focused on its interaction with metal ions and suggested that interactions of EGCG with metal ions provided a new insight in the role of EGCG in preventing prostate cancer. It is very important to investigate the interaction of EGCG with Zn2+ for chemoprevention of prostate cancer. Hence, we employed the higher concentrations of EGCG and Zn2+ than their physiologically relevant concentrations in the present work.
The present study also demonstrates that the effect of EGCG on LNCaP cells was attenuated in the presence of extracellular zinc at the concentration ratio 1:1. It is known that the biological behavior of EGCG could be influenced by metal ions, especially transition metal ions (e.g., iron, zinc, and copper ions) (Chen et al., 2006; Hayakawa et al., 2004). EGCG chelates metal ions, which is an important factor that affects the bioactivity of EGCG. EGCG has two rings, B- and D-rings, which have exactly the same local structure that could potentially participate in forming complexes with metals. Recent studies showed that EGCG-metal ion complex has a role in the initiation and development of cancer (Furukawa et al., 2003; Azam et al., 2004). It is likely that the D-ring hydroxyl groups could occupy the first coordination sphere around metal ion, whereas the B-ring hydroxyl group may have a secondary effect on such a complex formation (Navarro et al., 2005). Thermo-gravimetric and atomic absorption spectrophotometric studies indicate that the 3-OH-4-oxo and 3-OH-4-OH moieties are the most likely binding sites of flavonoids for metals. Accordingly, catechin could form 1:1 complex with either Zn2+or Cu2+, which can be monitored through the linearization algorithms as competitive with pre-concentration of the metal ions on the mercury electrode (Esparza et al., 2005). Kagaya et al.(2002) reported that it was the formation of zinc-EGCG complex that enhanced effect of zinc on hepatoprotectivity of EGCG in isolated rat hepatocytes. These observations give a hint that the biological behavior of EGCG was changed in the presence of extracellular zinc, due maybe to the formation of zinc-EGCG complex.
In prostate cancer, the malignant cells lose the ability of zinc accumulation. A growing body of evidence has suggested that the loss of this unique capacity to retain high levels of zinc is an important factor in the development and progression of malignant prostate cells (Tsui et al., 2006). EGCG entering into cells was able to affect the distribution of cellular zinc (Fig.6). The result indicates that EGCG could increase zinc accumulation of the mitochondria and cytosol in LNCaP cells. The regulation and maintenance of a “normal” concentration and distribution of cellular zinc are essential to the function, metabolism, growth, proliferation, and survival of cells. In general, all the cytosolic Zn2+ existed bound to either immobile macromolecules (~90%) or to mobile low molecular weight ligands (~10%). The former would not provide Zn2+ for delivery into the mitochondria. Therefore, the cytosolic pool of low molecular Zn2+ ligands (Zn-ligands) was the source of zinc for incorporation into and interaction with the mitochondria (Costello et al., 2005). The cellular accumulation of zinc was an inhibitory effect on proliferation of these prostate cells, which results from its induction of apoptosis (Liang et al., 1999; Feng et al., 2000; 2002). Zinc caused an increase in the cellular level of Bax, and enhanced the Bax/BCL-2 ratio, which was an initiating apoptotic signal in cells (Franklin and Costello, 2007; Feng et al., 2003). This effect resulted from a direct action of zinc on the mitochondrial release of cytochrome c followed by caspase activation and cascading apoptotic events (Feng et al., 2000; 2002). In contrast, most reports with other mammalian cells describe a role of zinc in the inhibition of apoptosis (Truong-Tran et al., 2000; Nodera et al., 2001). The most likely reason for this is that the levels of zinc to which the cells were exposed were lower and exposed time of cells was shorter than that of the present work. This, in part, could account for the difference in the zinc effects on apoptosis.
CONCLUSION
In the present study, we demonstrated that EGCG interacted with cell membrane, decreased the membrane fluidity of LNCaP cells, and accelerated zinc accumulation in the mitochondria and cytosol, which was attenuated by high concentrations of Zn2+. These results may suggest a mechanism by which EGCG inhibits proliferation of LNCaP cells.
Footnotes
Project (No. 30470198) supported by the National Natural Science Foundation of China
References
- 1.Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst. 1997;89(24):1881–1886. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad N, Gupta S, Mukhtar H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor kappaB in cancer cells versus normal cells. Arch Biochem Biophys. 2000;376(2):338–346. doi: 10.1006/abbi.2000.1742. [DOI] [PubMed] [Google Scholar]
- 3.Azam S, Hadi N, Khan NU, Hadi SM. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: implications for anticancer properties. Toxicol in Vitro. 2004;18(5):555–561. doi: 10.1016/j.tiv.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Balint E, Grimley PM, Gan Y, Zoon KC, Aszalos A. Plasma membrane biophysical properties linked to the antiproliferative effect of interferon-alpha. Acta Microbiol Immunol Hung. 2005;52(3-4):407–432. doi: 10.1556/AMicr.52.2005.3-4.12. [DOI] [PubMed] [Google Scholar]
- 5.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66(2):1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 6.Bishop GM, Dringen R, Robinson SR. Zinc stimulates the production of toxic reactive oxygen species (ROS) and inhibits glutathione reductase in astrocytes. Free Radic Biol Med. 2007;42(8):1222–1230. doi: 10.1016/j.freeradbiomed.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Yu H, Shen S, Yin J. Role of Zn2+ in epigallocatechin gallate affecting the growth of PC-3 cells. J Trace Elem Med Biol. 2007;21(2):125–131. doi: 10.1016/j.jtemb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Chen YM, Wang MK, Huang PM. Catechin transformation as influenced by aluminum. J Agric Food Chem. 2006;54(1):212–218. doi: 10.1021/jf051926z. [DOI] [PubMed] [Google Scholar]
- 9.Chen ZP, Schell JB, Ho CT, Chen KY. Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett. 1998;129(2):173–179. doi: 10.1016/S0304-3835(98)00108-6. [DOI] [PubMed] [Google Scholar]
- 10.Chung LY, Cheung TC, Kong SK, Fung KP, Choy YM, Chan ZY, Kwok TT. Induction of apoptosis by green tea catechins in human prostate cancer DU145 cells. Life Sci. 2001;68(10):1207–1214. doi: 10.1016/S0024-3205(00)01020-1. [DOI] [PubMed] [Google Scholar]
- 11.Cooper RA. Abnormalities of cell-membrane fluidity in the pathogenesis of disease. N Engl J Med. 1977;297(7):371–377. doi: 10.1056/NEJM197708182970707. [DOI] [PubMed] [Google Scholar]
- 12.Costello LC, Franklin RB, Feng P. Mitochondrial function, zinc, and intermediary metabolism relationships in normal prostate and prostate cancer. Mitochondrion. 2005;5(3):143–153. doi: 10.1016/j.mito.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Gómez Dumm NT, Giammona AM, Touceda LA, Raimondi C. Lipid abnormalities in chronic renal failure patients undergoing hemodialysis. Medicina (B Aires) 2001;61(2):142–146. [PubMed] [Google Scholar]
- 14.Esparza I, Salinas I, Santamara C, Garcia-Mina JM, Fernandez JM. Electrochemical and theoretical complexation studies for Zn and Cu with individual polyphenols. Anal Chim Acta. 2005;543(1-2):267–274. doi: 10.1016/j.aca.2005.04.029. [DOI] [Google Scholar]
- 15.Feng P, Liang JY, Li TL, Guan ZX, Zou J, Franklin R, Costello LC. Zinc induces mitochondria apoptogenesis in prostate cells. Mol Urol. 2000;4(1):31–36. [PubMed] [Google Scholar]
- 16.Feng P, Li TL, Guan ZX, Franklin RB, Costello LC. Direct effect of zinc on mitochondrial apoptogenesis in prostate cells. Prostate. 2002;52(4):311–318. doi: 10.1002/pros.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng P, Li TL, Guan ZX, Franklin RB, Costello LC. Effect of zinc on prostatic tumorigenicity in nude mice. Ann N Y Acad Sci. 2003;1010(1-2):316–320. doi: 10.1196/annals.1299.056. [DOI] [PubMed] [Google Scholar]
- 18.Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch Biochem Biophys. 2007;463(2):211–217. doi: 10.1016/j.abb.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujimura Y, Yamada K, Tachibana H. A lipid raft-associated 67 kDa laminin receptor mediates suppressive effect of epigallocatechin-3-O-gallate on FcepsilonRI expression. Biochem Biophys Res Commun. 2005;336(2):674–681. doi: 10.1016/j.bbrc.2005.08.146. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa A, Oikawa S, Murata M, Hiraku Y, Kawanishi S. (−)-Epigallocatechin gallate causes oxidative damage to isolated and cellular DNA. Biochem Pharmacol. 2003;66(9):1769–1778. doi: 10.1016/S0006-2952(03)00541-0. [DOI] [PubMed] [Google Scholar]
- 21.Gallus S, Foschi R, Negri E, Talamini R, Franceschi S, Montella M, Ramazzotti V, Tavani A, Dal Maso L, La Vecchia C. Dietary zinc and prostate cancer risk: a case-control study from Italy. Eur Urol. 2007;52(4):1052–1056. doi: 10.1016/j.eururo.2007.01.094. [DOI] [PubMed] [Google Scholar]
- 22.Gupta S, Ahmad N, Nieminen AL, Mukhtar H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (−)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol Appl Pharmacol. 2000;164(1):82–90. doi: 10.1006/taap.1999.8885. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto T, Kumazawa S, Nanjo F, Hara Y, Nakayama T. Interaction of tea catechins with lipid bilayers investigated with liposome systems. Biosci Biotechnol Biochem. 1999;63(12):2252–2255. doi: 10.1271/bbb.63.2252. [DOI] [PubMed] [Google Scholar]
- 24.Hayakawa F, Ishizu Y, Hoshino N, Yamaji A, Ando T, Kimura T. Prooxidative activities of tea catechins in the presence of Cu2+ . Biosci Biotechnol Biochem. 2004;68(9):1825–1830. doi: 10.1271/bbb.68.1825. [DOI] [PubMed] [Google Scholar]
- 25.Kagaya N, Kawase M, Maeda H, Tagawa Y, Nagashima H, Ohmori H, Yagi K. Enhancing effect of zinc on hepatoprotectivity of epigallocatechin gallate in isolated rat hepatocytes. Biol Pharm Bull. 2002;25(9):1156–1160. doi: 10.1248/bpb.25.1156. [DOI] [PubMed] [Google Scholar]
- 26.Kajiya K, Kumazawa S, Nakayama T. Steric effects on interaction of tea catechins with lipid bilayers. Biosci Biotechnol Biochem. 2001;65(12):2638–2643. doi: 10.1271/bbb.65.2638. [DOI] [PubMed] [Google Scholar]
- 27.Kampa M, Papakonstanti EA, Hatzoglou A, Stathopoulos EN, Stournaras C, Castanas E. The human prostate cancer cell line LNCaP bears functional membrane testosterone receptors that increase PSA secretion and modify actin cytoskeleton. FASEB J. 2002;16(11):1429–1431. doi: 10.1096/fj.02-0131fje. [DOI] [PubMed] [Google Scholar]
- 28.Kampa M, Nifli AP, Charalampopoulos I, Alexaki VI, Theodoropoulos PA, Stathopoulos EN, Gravanis A, Castanas E. Opposing effects of estradiol- and testosterone-membrane binding sites on T47D breast cancer cell apoptosis. Exp Cell Res. 2005;307(1):41–51. doi: 10.1016/j.yexcr.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 29.Kanadzu M, Lu Y, Morimoto K. Dual function of (−)-epigallocatechin gallate (EGCG) in healthy human lymphocytes. Cancer Lett. 2006;241(2):250–255. doi: 10.1016/j.canlet.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 30.Knapp DR. Handbook of Analytical Derivatization Reactions. New York, USA: Wiley Publishers; 1979. p. 164. [Google Scholar]
- 31.Liang JY, Liu YY, Zou J, Franklin RB, Costello LC, Feng P. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate. 1999;40(3):200–207. doi: 10.1002/(SICI)1097-0045(19990801)40:3<200::AID-PROS8>3.3.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao S, Umekita Y, Guo J, Kokontis JM, Hiipakka RA. Growth inhibition and regression of human prostate and breast tumors in athymic mice by tea epigallocatechin gallate. Cancer Lett. 1995;96(2):239–243. doi: 10.1016/0304-3835(95)03948-V. [DOI] [PubMed] [Google Scholar]
- 33.Navarro RE, Santacruz H, Inoue M. Complexation of epigallocatechin gallate (a green tea extract, EGCG) with Mn2+: nuclear spin relaxation by the paramagnetic ion. J Inorg Biochem. 2005;99(2):584–588. doi: 10.1016/j.jinorgbio.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Nodera M, Yanagisawa H, Wada O. Increased apoptosis in a variety of tissues of zinc-deficient rats. Life Sci. 2001;69(14):1639–1649. doi: 10.1016/S0024-3205(01)01252-8. [DOI] [PubMed] [Google Scholar]
- 35.Oikawa S, Furukawaa A, Asada H, Hirakawa K, Kawanishi S. Catechins induce oxidative damage to cellular and isolated DNA through the generation of reactive oxygen species. Free Radic Res. 2003;37(8):881–890. doi: 10.1080/1071576031000150751. [DOI] [PubMed] [Google Scholar]
- 36.Reznichenko L, Amit T, Zheng H, Avramovich-Tirosh Y, Youdim MB, Weinreb O, Mandel S. Reduction of iron-regulated amyloid precursor protein and beta-amyloid peptide by (−)-epigallocatechin-3-gallate in cell cultures: implications for iron chelation in Alzheimer’s disease. J Neurochem. 2006;97(2):527–536. doi: 10.1111/j.1471-4159.2006.03770.x. [DOI] [PubMed] [Google Scholar]
- 37.Salinas DG, De La Fuente M, Reyes JG. Changes of enzyme activity in lipid signaling pathways related to substrate reordering. Biophys J. 2005;89(2):885–894. doi: 10.1529/biophysj.104.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh KK, Desouki MM, Franklin RB, Costello LC. Mitochondrial aconitase and citrate metabolism in malignant and nonmalignant human prostate tissues. Mol Cancer. 2006;5(1):14. doi: 10.1186/1476-4598-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stathopoulos EN, Dambaki C, Kampa M, Theodoropoulos PA, Anezinis P, Delakas D, Delides GS, Castanas E. Membrane androgen binding sites are preferentially expressed in human prostate carcinoma cells. BMC Clin Pathol. 2003;3(1):1. doi: 10.1186/1472-6890-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sunshine C, McNamee MG. Lipid modulation of nicotinic acetylcholine receptor function: the role of membrane lipid composition and fluidity. Biochim Biophys Acta. 1994;1191(1):59–64. doi: 10.1016/0005-2736(94)90233-X. [DOI] [PubMed] [Google Scholar]
- 41.Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green tea polyphenol EGCG. Nat Struct Mol Biol. 2004;11(4):380–381. doi: 10.1083/nsmb743. [DOI] [PubMed] [Google Scholar]
- 42.Teresa G, Anna A, Maria P, Zofia J, Gerard B. Carp erythrocyte lipids as a potential target for the toxic action of zinc ions. Toxicol Lett. 2002;132(1):57–64. doi: 10.1016/S0378-4274(02)00066-8. [DOI] [PubMed] [Google Scholar]
- 43.Thewke D, Kramer M, Sinensky MS. Transcriptional homeostatic control of membrane lipid composition. Biochem Biophys Res Commun. 2000;273(1):1–4. doi: 10.1006/bbrc.2000.2826. [DOI] [PubMed] [Google Scholar]
- 44.Truong-Tran AQ, Ho LH, Chai F, Zalewski PD. Cellular zinc fluxes and the regulation of apoptosis/gene-directed cell death. J Nutr. 2000;130(5S):1459S–1466S. doi: 10.1093/jn/130.5.1459S. [DOI] [PubMed] [Google Scholar]
- 45.Tsui KH, Chang PL, Juang HH. Zinc blocks gene expression of mitochondrial aconitase in human prostatic carcinoma cells. Int J Cancer. 2006;118(3):609–615. doi: 10.1002/ijc.21411. [DOI] [PubMed] [Google Scholar]
- 46.Turk M, Méjanelle L, Sentjurc M, Grimalt JO, Gunde-Cimerman N, Plemenitas A. Salt-induced changes in lipid composition and membrane fluidity of halophilic yeast-like melanized fungi. Extremophiles. 2004;8(1):53–61. doi: 10.1007/s00792-003-0360-5. [DOI] [PubMed] [Google Scholar]
- 47.Uzzo RG, Crispen PL, Golovine K, Makhov P, Horwitz EM, Kolenko VM. Diverse effects of zinc on NF-kappaB and AP-1 transcription factors: implications for prostate cancer progression. Carcinogenesis. 2006;27(10):1980–1990. doi: 10.1093/carcin/bgl034. [DOI] [PubMed] [Google Scholar]
- 48.Vayalil PK, Katiyar SK. Treatment of epigallocatechin-3-gallate inhibits matrix metalloproteinases-2 and -9 via inhibition of activation of mitogen-activated protein kinases, c-jun and NF-kappaB in human prostate carcinoma DU-145 cells. Prostate. 2004;59(1):33–42. doi: 10.1002/pros.10352. [DOI] [PubMed] [Google Scholar]
- 49.Yaman M, Atici D, Bakirdere S, Akdeniz I. Comparison of trace metal concentrations in malign and benign human prostate. J Med Chem. 2005;48(2):630–634. doi: 10.1021/jm0494568. [DOI] [PubMed] [Google Scholar]
- 50.Yu HN, Shen SR, Xiong YK. Cytotoxicity of epigallocatechin-3-gallate to LNCaP cells in the presence of Cu2+ . J Zhejiang Univ Sci B. 2005;6(2):125–131. doi: 10.1631/jzus.2005.B0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu HN, Shen SR, Yin JJ. Effects of interactions of EGCG and Cd(2+) on the growth of PC-3 cells and their mechanisms. Food Chem Toxicol. 2007;45(2):244–249. doi: 10.1016/j.fct.2006.08.015. [DOI] [PubMed] [Google Scholar]