Abstract
Low temperature stress during germination and early seedling growth is an important constraint of global production of maize. The effects of seed priming with 0.25%, 0.50%, and 0.75% (w/v) chitosan solutions at 15 °C on the growth and physiological changes were investigated using two maize (Zea mays L.) inbred lines, HuangC (chilling-tolerant) and Mo17 (chilling-sensitive). While seed priming with chitosan had no significant effect on germination percentage under low temperature stress, it enhanced germination index, reduced the mean germination time (MGT), and increased shoot height, root length, and shoot and root dry weights in both maize lines. The decline of malondialdehyde (MDA) content and relative permeability of the plasma membrane and the increase of the concentrations of soluble sugars and proline, peroxidase (POD) activity, and catalase (CAT) activity were detected both in the chilling-sensitive and chilling-tolerant maize seedlings after priming with the three concentrations of chitosan. HuangC was less sensitive to responding to different concentrations of chitosan. Priming with 0.50% chitosan for about 60~64 h seemed to have the best effects. Thus, it suggests that seed priming with chitosan may improve the speed of germination of maize seed and benefit for seedling growth under low temperature stress.
Keywords: Seed priming, Chitosan, Low temperature stress, Germination, Physiological changes, Maize
INTRODUCTION
Low temperature is one of the most important limiting factors in the productivity of plants. Maize (Zea mays L.) is a thermophilic crop. Low temperature frequently causes injuries to maize seed germination and seedling growth, thus being detrimental to early spring planting (Parera and Cantliffe, 1994). Previous reports indicated that the most conspicuous changes occurred in cellular membranes and enzymes under low temperature stress conditions (Zhou and Leul, 1998; Lukatkin, 2003).
Seed priming has been shown to improve seed performance under sub-optimal temperature conditions (Lin and Sung, 2001). Priming increases the environmental range suitable for germination, and provides faster and synchronous seedling emergence (McDonald, 1999). Common priming methods such as polyethylene glycol (PEG) treatment are not suitable for large scale cereal crop production because farmers in developing countries could not cover the costs. PEG priming is frequently applied to vegetable seeds in laboratory and field experiments. Other priming methods such as drum priming need special equipment, and some methods are not easy to operate or not cheap enough to be used in large scale (Hu et al., 2005).
Chitosan is an abundant and comparatively cheap organic compound in China. It is a large cationic polysaccharide mainly obtained from waste materials from seafood processing. Chitosan treatment of wheat seeds induced resistance to certain disease and improved seed quality (Reddy et al., 1999). Seed soaked with chitosan increased the energy of germination, germination percentage, lipase activity, and gibberellic acid (GA3) and indole acetic acid (IAA) levels in peanut (Zhou et al., 2002). Seed coated with chitosan may accelerate seed germination and improve the tolerance to stress condition of hybrid rice seedlings (Ruan and Xue, 2002) and restrain the growth and reproduction of sclerotinia rot in carrot (Cheah et al., 1997). Seed priming with two different acidic chitosan solutions improved the vigor of maize seedlings (Shao et al., 2005). Thus, it seems that chitosan is a promising material for seed treatments. In the present study, the effects of seed priming with different concentrations of chitosan solutions were investigated. For better understanding the results, chilling-tolerant and chilling-sensitive maize inbred lines were used to determine germination and the seedling growth in relation to physiological changes under low temperature stress after the seed priming.
MATERIALS AND METHODS
Materials
Seeds of two maize (Zea mays L.) inbred lines, ‘HuangC’ (chilling-tolerant) and ‘Mo17’ (chilling-sensitive) (Zheng et al., 2006), from Zhangye Seed Company (Gansu, China) were used. Their original moisture contents were 12.3% and 11.7%, respectively.
Seed priming
Chitosan solutions (Yuhuan Chemicals, Zhejiang, China) of 0.25%, 0.50%, and 0.75% (w/v) concentrations (pH 5.1) were used for seed priming. Four replicates of 50 seeds each were used for each treatment. Fifty seeds were placed on a 3-layer blotter wetted with 12 ml chitosan solution in a 12-cm diameter petri dish. The petri dishes were sealed with parafilm. The seeds were primed at 15 °C in darkness for 58 h (0.25%) and 64 h (0.50% and 0.75%) for Mo17, and 48 h (0.25%) and 60 h (0.50% and 0.75%) for HuangC, respectively. Following the priming, seeds were dried back to their original moisture contents at room temperature. The un-priming dry seeds were used as control (CK).
Measurement of germination and physiological characteristics of seedlings
After priming, seed germination tests were carried out. Fifty seeds each for each treatment were placed in a plastic germination box (12 cm×18 cm) with sand bed, and each experiment was replicated four times. Then, seeds were incubated in a germination chamber at 25 °C under alternating cycle of 12 h illumination and 12 h darkness for 5 d (Zheng et al., 2006), followed by low temperature stress at 5 °C for 3 d. After that, the seedlings were transferred to 25 °C for recovering growth for 3 d. Seeds were considered germinated when there was a visible coleoptile protrusion through the sand bed. The germinated seeds were counted daily for 11 d. Then, the germination percentage was calculated on Day 11. The germination index and mean germination time were calculated as GI=∑(Gt/Tt) and MGT=∑(Gt×Tt)/∑Gt, respectively (Zhang et al., 2007; Kaya et al., 2008), where Gt is the number of germinated seeds on Day t, Tt is time corresponding to Gt in days.
Root length and shoot height were measured manually with a ruler and dry weights of shoot and root were determined after drying at 80 °C for 24 h. Relative electrolyte leakage as a measurement for membrane permeability was determined according to the method of Li (2001). Leaves of seedlings were cut into segments of about 5 mm. Each replication (0.2 g fresh weight (FW)) was collected and washed with deionized water, and then placed in the test tube with 10 ml deionized water and covered with a plug. After incubation at 25 °C for 6 h, the relative electrolyte leakage of the leaves was measured using a conductometer (DDS-11A, Shanghai, China), and the value was named E 1. Subsequently, the test tube was kept in water at 100 °C for 30 min, and then cooled to 25 °C, and the second relative electrolyte leakage was measured as E 2. The relative electrolyte leakage of deionized water was named E 0. The relative electrolyte leakage (REL) was calculated as follows: REL(%)=(E 1−E 0)/(E 2−E 0)×100. The concentrations of malondialdehyde (MDA), proline, and soluble sugar in leaves were measured using thiobarbituric acid (TBA) reaction method, colorimetric method, and anthrone colorimetric method, respectively (Li, 2001). Leaf peroxidase (POD) activity was determined by guaiacol method (Zhu and Zhong, 1990), and leaf catalase (CAT) activity was determined by the method described by Fu and Huang (2001). All measurements mentioned above were made after seed germination for 8 d (at 25 °C under alternating cycle of 12 h illumination and 12 h darkness for 5 d, followed by low temperature stress at 5 °C for 3 d) on 20 randomly selected seedlings per measurement.
Statistical analysis
All data were analyzed by means of analysis of variance (ANOVA) using Statistical Analysis System (SAS) software; percentage data were arcsin-transformed before analysis according to
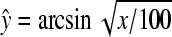 . .
|
Significant level P=0.05 was used.
RESULTS
Effects of seed priming with chitosan on germination
There were no significant differences in germination percentage among treatments irrespective of variety (Table 1). All the three concentrations of chitosan significantly increased the GI and reduced the MGT when compared with controls in both lines tested. The GI and the MGT of 0.50% chitosan priming treatment were significantly higher and lower than those of 0.75% chitosan treatment, respectively, but were not significantly different from those of 0.25% chitosan treatment in Mo17; however, there were no significant differences among the three priming treatments with chitosan in HuangC.
Table 1.
Effects of seed priming with various concentrations of chitosan on germination percentage (GP), germination index (GI), and mean germination time (MGT) of maize after germination for 11 d including 3 d of low temperature stress and 3 d of recovering growth
| Variety | Treatment | GP (%) | GI | MGT (d) |
| Mo17 | CK | 98a | 68.22c | 7.17a |
| 0.25% chitosan | 97a | 77.57ab | 6.91c | |
| 0.50% chitosan | 98a | 80.09a | 6.89c | |
| 0.75% chitosan | 96a | 73.23b | 7.01b | |
| HuangC | CK | 97a | 69.09b | 7.13a |
| 0.25% chitosan | 98a | 81.18a | 6.89b | |
| 0.50% chitosan | 99a | 80.63a | 6.88b | |
| 0.75% chitosan | 97a | 80.67a | 6.85b | |
Different letter(s) following the values indicated significant difference (P=0.05, LSD) among treatments within the same variety
Effects of seed priming with chitosan on seedling growth
All priming treatments with chitosan significantly increased the shoot height and shoot dry weight compared with the control in Mo17 (Table 2). Seed priming with 0.50% and 0.25% chitosan significantly increased the root dry weight and the root length as compared with the control, respectively. The most pronounced effect was recorded in 0.50% concentration.
Table 2.
Effects of seed priming with various concentrations of chitosan on shoot height (SH) and root length (RL), shoot and root dry weights (SDW and RDW) of maize seedlings after germination for 8 d including 3 d of low temperature stress
| Variety | Treatment | SH (cm) | RL (cm) | SDW (mg/shoot) | RDW (mg/root) |
| Mo17 | CK | 7.23c | 12.47b | 16.03c | 18.15b |
| 0.25% chitosan | 10.80a | 13.48a | 19.23b | 17.75b | |
| 0.50% chitosan | 10.61a | 12.49ab | 21.43a | 21.05a | |
| 0.75% chitosan | 9.32b | 12.69ab | 19.67b | 17.90b | |
| HuangC | CK | 10.50b | 15.26b | 26.13b | 15.60b |
| 0.25% chitosan | 12.99a | 18.61a | 32.40a | 18.33ab | |
| 0.50% chitosan | 12.65a | 17.53ab | 35.07a | 20.43a | |
| 0.75% chitosan | 12.88a | 19.17a | 32.53a | 17.57ab | |
Different letter(s) following the values indicated significant difference (P=0.05, LSD) among treatments within the same variety
For HuangC, seed priming with all the three concentrations of chitosan significantly increased the shoot height and shoot dry weight compared with the control, and there were no significant differences among the three treatments with chitosan (Table 2). The three priming treatments with chitosan increased root length, and the increase of root length by priming with 0.25% and 0.75% chitosan reached a significant level compared with the control. Seed priming with 0.50% chitosan significantly increased the root dry weight compared with the control, and there were no significant differences among 0.25% and 0.75% chitosan treatments and the control in both lines.
Effects of seed priming with chitosan on relative permeability of plasma membrane and MDA concentration
The relative permeability of the plasma membrane after the 0.50% and 0.75% chitosan treatments was significantly lower than that of the control in Mo17 (Table 3). For HuangC, only the relative permeability of plasma membrane of 0.50% chitosan treatment was significantly lower than that of the control.
Table 3.
Effects of seed priming with various concentrations of chitosan on relative permeability of plasma membrane (RPPM) and MDA concentration (C MDA) of maize seedlings after germination for 8 d including 3 d of low temperature stress
| Variety | Treatment | RPPM | CMDA (nmol/g FW) |
| Mo17 | CK | 0.364a | 4.08a |
| 0.25% chitosan | 0.258ab | 3.74ab | |
| 0.50% chitosan | 0.207b | 3.64ab | |
| 0.75% chitosan | 0.198b | 3.36b | |
| HuangC | CK | 0.207a | 5.55a |
| 0.25% chitosan | 0.184ab | 4.67b | |
| 0.50% chitosan | 0.172b | 4.35b | |
| 0.75% chitosan | 0.188ab | 4.25b | |
Different letter(s) following the values indicated significant difference (P=0.05, LSD) among treatments within the same variety
All the three priming treatments with chitosan reduced MDA concentration and the decrease of MDA by priming with 0.75% chitosan reached a significant level compared with the control in Mo17 (Table 3). For HuangC, the MDA concentrations of all the three treatments with chitosan were significantly lower than that of the control, and there were no significant differences among the three treatments.
Effects of seed priming with chitosan on concentrations of soluble sugar and proline
Priming treatments with 0.25% and 0.50% chitosan significantly increased the soluble sugar concentration compared with the control in Mo17 (Table 4). Seed priming treatment with 0.50% chitosan significantly increased proline concentration compared with the control and 0.75% chitosan treatment, and there was no significant difference between 0.25% and 0.50% chitosan treatments in Mo17.
Table 4.
Effects of seed priming with various concentrations of chitosan on the concentrations of soluble sugar (C SS) and proline (C P) of maize seedlings after germination for 8 d including 3 d of low temperature stress
| Variety | Treatment | CSS (mg/g FW) | CP (μg/g FW) |
| Mo17 | CK | 18.37b | 15.58b |
| 0.25% chitosan | 22.74a | 18.73ab | |
| 0.50% chitosan | 22.46a | 21.61a | |
| 0.75% chitosan | 20.41ab | 17.61b | |
| HuangC | CK | 32.47b | 13.14b |
| 0.25% chitosan | 36.20a | 14.84a | |
| 0.50% chitosan | 37.09a | 15.60a | |
| 0.75% chitosan | 39.18a | 15.38a | |
Different letter(s) following the values indicated significant difference (P=0.05, LSD) among treatments within the same variety
For HuangC, all treatments with chitosan significantly increased the concentrations of soluble sugar and proline compared with the control, but there were no significant differences among all the three treatments with chitosan (Table 4).
Effects of seed priming with chitosan on POD and CAT activities
In Mo17, the POD activity of the 0.25% chitosan treatment was higher than that of the control but higher concentrations of chitosan did not significantly affect POD activity (Table 5). There was no significant effect of chitosan treatment on the CAT activity of this variety either.
Table 5.
Effects of seed priming with various concentrations of chitosan on POD and CAT activities of maize leaves after germination for 8 d including 3 d of low temperature stress
| Variety | Treatment | POD activity (U/(g FW·min)) | CAT activity (U/(g FW·min)) |
| Mo17 | CK | 1063b | 16.0a |
| 0.25% chitosan | 1259a | 15.3a | |
| 0.50% chitosan | 1216ab | 19.3a | |
| 0.75% chitosan | 1108ab | 19.0a | |
| HuangC | CK | 732ab | 16.5b |
| 0.25% chitosan | 606b | 19.5a | |
| 0.50% chitosan | 849a | 21.0a | |
| 0.75% chitosan | 732ab | 24.0a | |
Different letter(s) following the values indicated significant difference (P=0.05, LSD) among treatments within the same variety
In HuangC, seed priming with 0.50% chitosan had no significant effect on POD activity, but significantly increased CAT activity in comparison with the control (Table 5). Between the three chitosan treatments there was no significant difference in CAT activity.
DISCUSSION
Priming is used to control the hydration level within seeds so that the metabolic activity necessary for germination can occur, but radicle emergence is still prevented. There are many factors associated with the effects of seed priming, but the concentrations of priming solutions as well as the time and temperature during priming were crucial (Khan, 1992). Sometimes, priming effects and conditions varied with varieties (Adegbuyi et al., 1981; Khan, 1992). In the present study, we found that the combinations of priming time and concentration were different between the two maize lines, Mo17 and HuangC.
Seeds of the two inbred maize lines showed different responses to chitosan priming under coldstress. Among the three different concentrations, priming with 0.50% chitosan had the best enhancement effects for chilling-sensitive Mo17, while no significantly different effects among the three concentration treatments were observed for chilling-tolerant HuangC. This difference may be related to the differences in antioxidant enzyme activities, proline concentration, and plasma membrane permeability in these two maize inbred lines (Gao et al., 2006).
Low temperature induces damage to cell membranes and also affects physiological functions of plants. Relative permeability of the plasma membranes has been used to illustrate the damage degree of low temperature stress (Lukatkin, 2003). The lower relative permeability of plasma membrane under low temperature stress means less damage from low temperature stress. Priming with chitosan reduced the relative permeability of the plasma membranes of the two lines under low temperature stress, indicating that chitosan priming alleviated the chilling injury of the seedlings. Similar observations have been made previously by seed film coated with a cold-tolerant agent on maize subjected to low temperature stress (Li et al., 2004).
Proline and soluble sugar are small molecules, which are synthesized after suffering intimidation action (Hu et al., 2006). These molecules are strongly hydrophilic, and alleviate stress damage in plant cells by reducing the water potential and keeping the activities of some biological macromolecules (Rathinasabapathi, 2000). Accumulation of organic compounds such as proline in the cytoplasm also plays an important role in osmotic adjustment in plants (Watanabe et al., 2000). After sand priming, seed germination and seedling growth of alfalfa were improved, and soluble sugar and proline concentrations under high salt concentration stress were also increased (Hu et al., 2006). Similar effects were also achieved in this experiment, which suggests that priming with chitosan improved chilling tolerance in maize seeds.
Cell membrane stability was affected by lipid peroxidation caused by active oxygen species under various stress conditions (Fu and Huang, 2001), and the concentration of MDA was an indicator of lipid peroxidation in plant cells (Feng et al., 2003). The decline of the MDA concentrations in the two primed maize lines under low temperature stress suggested that seed priming with chitosan reduced lipid peroxidation. MDA was produced when polyunsaturated fatty acids in the membrane underwent peroxidation (Meloni et al., 2003). The MDA accumulation was reduced after seed film was coated with a cold-tolerant agent in maize under low temperature stress (Li et al., 2004), and the decline of the MDA accumulation was achieved after sand priming in alfalfa seedlings under salt stress condition (Hu et al., 2006). Our observations are consistent with these earlier reports.
The accumulation of MDA may be related to the POD and CAT enzyme activities, the lesser degree of membrane damage (as indicated by low MDA concentration), and the higher activities of antioxidative enzymes (Meloni et al., 2003). Chilling conditions may cause an increase in reactive oxygen species (ROS), starting oxidative damage to the membrane system of plants (Wise, 1995). Cooperation of protective enzymes such as POD and CAT could eliminate ROS and keep a homeostasis between producing and cleaning of ROS and reduce the level of free radicals. Finally, injury to cells could be declined or avoided (Hu et al., 2006). The three concentrations of chitosan used here increased the POD activity of Mo17 and significantly increased CAT activity of HuangC. It seems that for the chilling-sensitive maize seedlings, POD plays a key role in protecting seedlings from low temperature stress, and for the chilling-tolerant maize seedlings, CAT plays a key role.
In conclusion, this study shows that the chitosan priming increased the chilling tolerance of maize seedlings demonstrated by improving germination speed and shoot and root growth and maintaining membrane integrity and higher activities of antioxidative enzymes. The 0.50% chitosan seems to be a suitable concentration for seed priming. However, seed priming effect for other crops warrants further study.
Footnotes
Project supported by the Major Science and Technology Special Project (priority subject) of Zhejiang Province (No. 2008C12005-1) and the Key Project of Education Department of Zhejiang Province (No. 20070147), China
References
- 1.Adegbuyi E, Cooper SR, Don R. Osmotic priming of some herbage grass seed using polyethylene glycol (PEG) Seed Science and Techology. 1981;9(3):867–878. [Google Scholar]
- 2.Cheah LH, Page BBC, Shepherd R. Chitosan coating for inhibition of sclerotinia rot of carrots. New Zealand Journal of Crop Horticultural Science. 1997;25(1):89–92. [Google Scholar]
- 3.Feng ZZ, Guo AH, Feng ZW. Amelioration of chilling stress by triadimefon in cucumber seedlings. Plant Growth Regulation. 2003;39(3):277–283. doi: 10.1023/A:1022881628305. [DOI] [Google Scholar]
- 4.Fu J, Huang B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environmental and Experimental Botany. 2001;45(2):105–114. doi: 10.1016/S0098-8472(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 5.Gao CH, Hu J, Zheng YY, Zhang S. Antioxidant enzyme activities and proline content in maize seedling and their relationships to cold endurance. Chinese Journal of Applied Ecology. 2006;17(6):1045–1050. (in Chinese) [PubMed] [Google Scholar]
- 6.Hu J, Zhu ZY, Song WJ, Wang JC, Hu WM. Effects of sand priming on germination and field performance in direct-sown rice (Oryza sativa L.) Seed Science and Technology. 2005;33(1):243–248. [Google Scholar]
- 7.Hu J, Xie XJ, Wang ZF, Song WJ. Sand priming improves germination and growth of alfalfa under high-salt concentration stress. Seed Science and Technology. 2006;34(1):199–204. [Google Scholar]
- 8.Kaya M, Kaya G, Kaya MD, Atak M, Saglam S, Khawar KM, Ciftci CY. Interaction between seed size and NaCl on germination and early seedling growth of some Turkish cultivars of chickpea (Cicer arietinum L.) Journal of Zhejiang University SCIENCE B. 2008;9(5):371–377. doi: 10.1631/jzus.B0720268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan AA. Preplant physiological seed conditioning. Horticultural Reviews. 1992;13(13):131–181. [Google Scholar]
- 10.Li HS. Principles and Techniques of Plant Physiological Biochemical Experiment. Beijing: Higher Education Press; 2001. p. 278. (in Chinese) [Google Scholar]
- 11.Li LQ, Hu J, Zhu ZY, Nkeshimana J. The effects of seed film coating with cold-tolerant agents on physiology and biochemistry changes of supersweet corn in low temperature stress. Journal of Zhejiang University (Agriculture and Life Science) 2004;30(9):311–317. (in Chinese) [Google Scholar]
- 12.Lin JM, Sung JM. Pre-sowing treatments for improving emergence of bitter gourd seedlings under optimal and sub-optimal temperatures. Seed Science and Technology. 2001;29(1):39–50. [Google Scholar]
- 13.Lukatkin AS. Contribution of oxidative stress to the development of cold-induced damage to leaves of chilling-sensitive plants: 3. Injury of cell membranes by chilling temperatures. Russian Journal of Plant Physiology. 2003;50(2):243–246. doi: 10.1023/A:1022985500733. [DOI] [Google Scholar]
- 14.McDonald MB. Seed deterioration: physiology, repair and assessment. Seed Science and Technology. 1999;27(1):177–237. [Google Scholar]
- 15.Meloni DA, Oliva MA, Martinez CA, Cambraia J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environmental and Experimental Botany. 2003;49(1):69–76. doi: 10.1016/S0098-8472(02)00058-8. [DOI] [Google Scholar]
- 16.Parera CA, Cantliffe DJ. Presowing seed priming. Horticultural Reviews. 1994;16(16):109–141. [Google Scholar]
- 17.Rathinasabapathi B. Metabolic engineering for stress tolerance: installing osmoprotectant synthesis pathways. Annals of Botany. 2000;86(4):709–716. doi: 10.1006/anbo.2000.1254. [DOI] [Google Scholar]
- 18.Reddy MVB, Arul J, Angers P, Couture L. Chitosan treatment of wheat seeds induces resistance to Fusarium graminearum and improves seeds quality. Journal of Agricultural and Food Chemistry. 1999;47(3):67–72. doi: 10.1021/jf981225k. [DOI] [PubMed] [Google Scholar]
- 19.Ruan SL, Xue QZ. Effects of chitosan coating on seed germination and salt-tolerance of seedlings in hybrid rice (Oryza sativa L.) Acta Agronomica Sinica. 2002;28(6):803–808. (in Chinese) [Google Scholar]
- 20.Shao CX, Hu J, Song WJ, Hu WM. Effects of seed priming with chitosan solutions of different acidity on seed germination and physiological characteristics of maize seedling. Journal of Zhejiang University (Agriculture and Life Science) 2005;31(6):705–708. (in Chinese) [Google Scholar]
- 21.Watanabe S, Kojima K, Ide Y, Satohiko Sasaki S. Effects of saline and osmotic stress on proline and sugar accumulation in Populus euphratica in vitro. Plant Cell Tissue and Organ Culture. 2000;63(3):199–206. doi: 10.1023/A:1010619503680. [DOI] [Google Scholar]
- 22.Wise R. Chilling-enhanced photooxidation: the production, action and study of reactive oxygen species produced during chilling in the light. Photosynthesis Research. 1995;45(2):79–97. doi: 10.1007/BF00032579. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Hu J, Zhang Y, Xie XJ, Allen K. Seed priming with brassinolide improves lucerne (Medicago sativa L.) seed germination and seedling growth in relation to physiological changes under salinity stress. Australian Journal of Agricultural Research. 2007;58(8):811–815. doi: 10.1071/AR06253. [DOI] [Google Scholar]
- 24.Zheng YY, Hu J, Zhang S, Gao CH, Song WJ. Identification of chilling-tolerance in maize inbred lines at germination and seedling growth stages. Journal of Zhejiang University (Agriculture and Life Science) 2006;32(1):41–45. (in Chinese) [Google Scholar]
- 25.Zhou WJ, Leul M. Uniconazole-induced alleviation of freezing injury in relation to changes in hormonal balance, enzyme activities and lipid peroxidation in winter rape. Plant Growth Regulation. 1998;26(1):41–47. doi: 10.1023/A:1006004921265. [DOI] [Google Scholar]
- 26.Zhou YG, Yang YD, Qi YG, Zhang ZM, Wang XJ, Hu XJ. Effects of chitosan on some physiological activity in germinating seed of peanut. Journal of Peanut Science. 2002;31(1):22–25. (in Chinese) [Google Scholar]
- 27.Zhu G, Zhong W. Plant Physiological Experiment. Beijing: Peking University Press; 1990. p. 377. (in Chinese) [Google Scholar]


