Abstract
The effects of CO2 enrichment on the growth and glucosinolate (GS) concentrations in the bolting stem of Chinese kale (Brassica alboglabra L.) treated with three nitrogen (N) concentrations (5, 10, and 20 mmol/L) were investigated. Height, stem thickness, and dry weights of the total aerial parts, bolting stems, and roots, as well as the root to shoot ratio, significantly increased as CO2 concentration was elevated from 350 to 800 μl/L at each N concentration. In the edible part of the bolting stem, 11 individual GSs were identified, including 7 aliphatic and 4 indolyl GSs. GS concentration was affected by the elevated CO2 concentration, N concentration, and CO2×N interaction. At 5 and 10 mmol N/L, the concentrations of aliphatic GSs and total GSs significantly increased, whereas those of indolyl GSs were not affected, by elevated atmospheric CO2. However, at 20 mmol N/L, elevated CO2 had no significant effects on the concentrations of total GSs and total indolyl GSs, but the concentrations of total aliphatic GSs significantly increased. Moreover, the bolting stem carbon (C) content increased, whereas the N and sulfur (S) contents decreased under elevated CO2 concentration in the three N treatments, resulting in changes in the C/N and N/S ratios. Also the C/N ratio is not a reliable predictor of change of GS concentration, while the changes in N and S contents and the N/S ratio at the elevated CO2 concentration may influence the GS concentration in Chinese kale bolting stems. The results demonstrate that high nitrogen supply is beneficial for the growth of Chinese kale, but not for the GS concentration in bolting stems, under elevated CO2 condition.
Keywords: Carbon dioxide (CO2), Brassica alboglabra, Nitrogen (N), Growth, Bolting stem, Aliphatic glucosinolates, Indolyl glucosinolates, Carbon/nitrogen ratio (C/N), Nitrogen/sulfur ratio (N/S)
INTRODUCTION
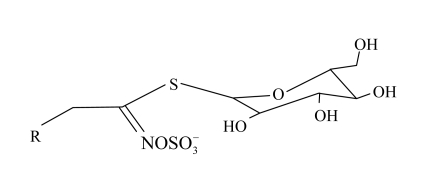
Epidemiological studies show that there is a negative relationship between Brassicaceae vegetable intake and the risk of a number of cancers (Wattenberg, 1993; Kohlmeier and Su, 1997; Price et al., 1998). Recently, it has been widely recognized that some of the cancer-chemoprotective activities in these vegetables are attributable to their contents of glucosinolates (GSs) (Zhao et al., 1992; Wattenberg, 1993; Tawfiq et al., 1995; Fahey et al., 1997; Rosa et al., 1997; Holst and Williamson, 2004) (Fig.1). GSs are amino acid-derived secondary compounds, a characteristic of dicotyledonous plants. So far, more than 20 GSs have been identified in Brassicaceae family (Rodman, 1991). They can be grouped into aliphatic, aromatic, and indolyl GSs according to the amino acid, from which they are derived (Louda and Mole, 1991; Halkier and Du, 1997). Besides the health-promoting properties, GSs also play an important role in plant defense against insects and herbivores, and are utilized as special flavors in the food industry (Fenwick et al., 1983; Chew, 1988; Gijzen et al., 1989; Baik et al., 2003).
Fig. 1.
General structure of glucosinolates (GSs)
It is widely accepted that GS content is affected by environmental factors including climatic conditions, nutritional availability, and agronomic practices, in addition to genetic characteristics (Fenwick et al., 1989). Presently, cancer incidence and global climate change are major topical issues. Because GSs exhibit cancer-chemoprotective activities, many researches are focused on the effect of climatic conditions on changes in GS content in vegetables (Schreiner, 2005). The rise in atmospheric carbon dioxide (CO2) concentration is one of the most prominent climatic changes in recent decades (IPCC, 2007). Climate simulations indicate that the atmospheric CO2 concentration is expected to reach 700 μl/L by the end of this century, which is double as much as the current CO2 concentration (Caswell, 2004). Increasing atmospheric CO2 concentration may affect natural ecosystems by directly influencing plant growth and photochemistry due to an increased photosynthetic rate, especially in C3 plants (Islam et al., 1996; Kim et al., 2001; Das et al., 2002). In spite of increased plant growth under elevated CO2 concentrations (Bazzaz, 1990; Mooney et al., 1991; Amthor, 2001), aerial plant parts accumulate generally less nitrogen (N), and carbon (C)/N ratio increases (Baxter et al., 1994; Epron et al., 1996), which could influence plant secondary metabolites synthesis and concentration. GSs, as N- and C-containing secondary metabolites, might be affected by atmospheric CO2 enrichment owing to changes in the plant’s C supply and N content (Cotrufo et al., 1998). Moreover, Habash et al. (1995) observed that synthesis of the amino acid precursors of GSs from triosephosphates increased at an elevated CO2 concentration. However, Karowe et al.(1997) reported that total foliar GS content in mustard decreased significantly under elevated CO2 conditions. This conflict might reflect a species-specific response to elevated CO2 concentration (Karowe et al., 1997). An increasing number of studies indicate that N availability can have a large impact on the plant response to elevated CO2 concentration (Kimball et al., 1995; 2002). Moreover, N application is one of the most important nutrient factors that significantly affect GS synthesis and content (Schnug, 1989; Zhao et al., 1994; Ahmad et al., 2007). In oilseed rape (Brassica napus L.), the GS content in the seed decreased with the higher N supply in sulfur (S)-deficient soil, but increased in S-sufficient soil (Zhao et al., 1994). However, in broccoli sprouts (Brassica oleracea var. italica), N fertilization has a negative effect on GS content, even at a very low concentration (Aires et al., 2006).
Therefore, it is logical to take N nutrition into account when investigating the effects of CO2 enrichment on GS content. At present, limited information is available on the effect of elevated atmospheric CO2 in combination with N availability on GS content in brassicaceous vegetables. It is not clear whether GS content changes consistently at different N levels. In this study, we determined the interactive effects of elevated CO2 concentration and N availability on the contents of individual and total GSs in the edible part of the bolting stem of Chinese kale, which is a nutritionally healthy vegetable belonging to the Brassicaceae (Cruciferae) family and rich in GSs, and has spread quickly in southeastern China, Taiwan region, and Japan since the last decade (He et al., 2002).
MATERIALS AND METHODS
Plant growth
Seeds of Chinese kale (Brassica alboglabra L. var. Sijicutiao) were sown in vermiculite and germinated in a greenhouse with computer-controlled growth conditions on Huajiachi campus of Zhejiang University, Hangzhou, China. The growth conditions in the greenhouse were constant day/night temperature of 23/18 °C and the natural photoperiod. After two weeks, the seedlings were irrigated with nutrient solution containing 5 mmol N/L. Four weeks later, healthy seedlings in which the third true leaf had emerged were transplanted to 1.8-L pots containing nutrient solution with 10 mmol N/L by being fixed in a foam cavity with sponge. Each pot contained two seedlings and was covered with black plastic foil to prevent algal growth and evaporation. All the pots were transferred to four growth chambers with 65% relative humidity, constant day/night temperature of 23/18 °C, and 500 μmol/(m2·s) photosynthetically-active radiation for 16 h/d. One week after transplanting, plants were treated with 5.0 mmol N/L (low N concentration), 10 mmol N/L (medium N concentration), or 20 mmol N/L (high N concentration) (Chen et al., 2005), and were grown under either ambient CO2 [(350±20) μl/L, denoted as A] or elevated CO2 [(800±20) μl/L, denoted as E). The N was supplied as NH4NO3 and the basic nutrient solution contained 1 mmol/L K2HPO4, 4 mmol/L KCl, 3 mmol/L CaCl2, 2 mmol/L MgSO4·7H2O, 36 μmol/L ethylene diamine tetraacetic acid (EDTA)-Fe, 46.4 μmol/L H3BO3, 9.07 μmol/L MnCl2·4H2O, 0.765 μmol/L ZnSO4·7H2O, 0.3 μmol/L CuSO4·5H2O, and 0.09 μmol/L H2MoO4·H2O (Hoagland and Arnon, 1938). CO2 was supplied from gas tanks for the elevated CO2 concentration treatment. There were two replicate chambers per CO2 concentration and three replicate pots per N treatment in each chamber. The position of every pot was rotated randomly when the solutions were renewed every three days. To avoid a potential chamber effect, the pots were switched with those from the other chamber with the same CO2 concentration every week. The culture solutions were continuously aerated and adjusted to pH 6.0 using diluted NaOH or HCl every day until harvest. After treated for 35 d, the selected growth parameters were measured, and every plant was separated into different parts, weighed, and lyophilized. The bolting stems were ground into a powder and stored in a desiccator at −20 °C prior to C, N, S, and GS analyses.
Extract preparation for glucosinolate analysis
Extracts for GS analysis were prepared according to the method of Kiddle et al.(2001) with some modifications. Triplicate samples (0.1 g) of freeze-dried powder were each weighed in 5 ml tubes, and crude GSs were extracted with 1.5 ml 70% (v/v) methanol at 75 °C for 10 min in a water bath. The mixture was centrifuged at 5000×g for 10 min at 4 °C and the supernatant was decanted into another tube. The extraction was repeated twice from residues using the same procedure. The three supernatants were combined to give a final extract volume of 5 ml. 2 ml of each GS extract was added to a mini-column filled with diethylaminoethanol (DEAE) Sephadex A-25 (80 mg as dry matter) (170170-01, Amersham Biosciences, Sweden) activated with 0.5 mol/L pyridine acetate, and desulfated by sulfatase (S9626, Sigma-Aldrich Co., MO, USA). After reaction at room temperature overnight (16 h), the desulfated glucosinolates (desulfoGSs) were eluted with 2 ml deionized water and stored at −20 °C prior to high-performance liquid chromatography (HPLC) analysis. 2-PropenylGS (sinigrin, S1647, Sigma-Aldrich Co., MO, USA) was used as an external standard for GS quantitative analysis.
High performance liquid chromatography
The desulfated extract (20 μl) was analyzed by HPLC (Beckman Coulter System Gold HPLC, Beckman, USA) using a Hypersil ODS2 column (250 mm×4.6 mm, 5 μm; Elite, China) with a Beckman Ultrasphere ODS guard column (45 mm×4.6 mm, 5 μm; Beckman, USA). The wavelength of the ultraviolet detector was set at 227 nm. The mobile phase was a mixture of deionized water (A) and acetonitrile (B) and ran at a flow rate of 1 ml/min. The elution program consisted of a linear gradient from 0 to 20% (B) in 18 min and constant 20% (B) for a further 16 min, then the column was eluted with 100% (B) for 5 min and equilibrated with 0 (B) for 6 min prior to the injection of the next sample (Macfarlane-Smith and Griffiths, 1988).
Mass spectrometry analysis
The separated compounds were identified according to the mass spectrometry (MS) data obtained by a liquid chromatography-mass spectrometry data (LC-MSD) system (Agilent 1100 LC/MSD, Agilent Co., USA). The conditions used for the electrospray source were ionspray mode, positive; capillary voltage, 4 kV; nebulizer pressure, 42 184.8 Pa; fragment voltage, 100 V; curtain gas, nitrogen; drying gas flow, 13 L/min; desolvation gas temperature, 350 °C. Each individual desulfoGS was identified according to their (M+H)+, (M+Na)+, (M+K)+, and (M-glucosyl+H)+ in the MS.
Analyses of bolting stem carbon, nitrogen, and sulfur contents
Bolting stem C content was determined by titration after digestion by H2SO4 and potassium permanganate (Lu, 1999). Bolting stem N content was determined titrimetrically using the Kjeldahl procedure with salicylic acid, sodium thiosulfate, and zinc as catalysts (Pruden et al., 1985). For measuring the S content, 0.1 g aliquot of the ground materials was digested with HNO3 and HClO4. The S content was determined using an inductively coupled plasma atomic emission spectrometer (ICP-MS; Agilent, 7500a, USA) (Lu, 1999).
Statistical analysis
Two-way analysis of variance (ANOVA) was performed to determine the main effects (N and CO2 treatments) and their interactions with a significance level of P<0.05. The normality of data and the homogeneity of variances were verified by Shapiro-Wilk test and Bartlett test, respectively, before using ANOVA. Differences between means were analyzed by Fisher’s protected least significant difference (LSD) procedure. All statistical analysis procedures were performed by using SPSS for Windows version 12.0 (SPSS, Chicago, IL, USA).
RESULTS
Plant growth
Elevated CO2 concentration significantly increased plant height, stem thickness, dry weights of the total aerial parts, bolting stems, and roots, and the root-to-shoot ratio, compared with those in the ambient CO2 treatment (Table 1). Regardless of N concentration, the height, stem thickness, dry weights of the total aerial parts, bolting stems, and roots, and root-to-shoot ratio increased by 15.64%, 11.79%, 11.91%, 15.03%, 16.34%, and 3.90%, respectively, with elevated CO2 concentration. Nitrogen levels also significantly affected each growth parameter (Table 1). The 10 mmol N/L solution significantly increased the height, stem thickness, and dry weights of the total aerial parts, bolting stems, and roots, compared with those in the 5 mmol N/L solution in both CO2 regimes. However, there was no significant difference between the 10 and 20 mmol N/L solution treatments for the above parameters in both CO2 conditions, except that height and dry weight of the total aerial parts differed significantly between the two N treatments at the elevated CO2 concentration. The root-to-shoot ratio did not differ significantly among the three N concentrations at the ambient CO2 concentration, but there was a significant difference between the 5 and 10 mmol/L N concentrations at the elevated CO2 concentration. Moreover, there were significant CO2×N interactions for plant height (P<0.01) and dry weights of the total aerial parts (P<0.01) and roots (P<0.05), but not for bolting stem thickness, dry weight of the bolting stem, or root-to-shoot ratio (Table 1).
Table 1.
Effects of elevated CO2 concentration on height, stem thickness, dry weights of aerial parts, bolting stems, and roots, and root-to-shoot ratio of Chinese kale at three nitrogen (N) concentrations
| CO2 (μl/L) | N (mmol/L) | Height (cm) | Stem thickness (cm) | Dry weight (g/plant) |
Root-to-shoot ratio | ||
| Total aerial part | Bolting stem | Root | |||||
| 350 | 5 | 26.13±3.00d | 0.72±0.02d | 4.84±0.10e | 1.63±0.10d | 0.45±0.03d | 0.093±0.006c |
| 10 | 29.13±2.59c | 0.82±0.09bc | 6.30±0.12c | 2.24±0.08b | 0.61±0.02b | 0.096±0.005abc | |
| 20 | 28.25±1.98cd | 0.81±0.04c | 6.27±0.07c | 2.29±0.13b | 0.60±0.02b | 0.095±0.004bc | |
| 800 | 5 | 32.44±2.22b | 0.77±0.04cd | 5.38±0.17d | 1.96±0.07c | 0.51±0.03c | 0.095±0.004bc |
| 10 | 34.88±1.48a | 0.88±0.08ab | 6.97±0.13b | 2.53±0.16a | 0.70±0.02a | 0.100±0.002a | |
| 20 | 29.25±1.58c | 0.89±0.07a | 7.14±0.16a | 2.60±0.10a | 0.71±0.03a | 0.100±0.007ab | |
| Source of variance | |||||||
| CO2 | *** | ** | *** | *** | *** | * | |
| N | *** | *** | *** | *** | *** | * | |
| CO2×N | ** | NS | ** | NS | * | NS | |
Data followed by the same superscript letter(s) indicate no significant difference at the P<0.05 level. Values are the mean±SD. Significance levels indicated by two-way ANOVA: NS, not significant
Significance levels indicated by two-way ANOVA: P<0.05;
Significance levels indicated by two-way ANOVA: P<0.01;
Significance levels indicated by two-way ANOVA: P<0.001
Glucosinolate content
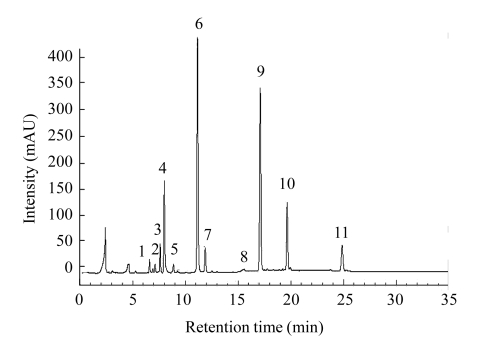
A typical HPLC profile of desulfoGSs in the bolting stem is shown in Fig.2. Eleven individual GSs were identified according to the elution order from the HPLC column and confirmed by electrospray ionization mass spectrometry (ESI-MS) analysis based on their MS data. All GSs were identified by analyzing the chemical structure of the aglucone chain R and described according to the trivial names that have been popularly used for decades (Fig.1 and Table 2). Seven aliphatic GSs comprising glucoiberin, progoitrin, sinigrin, glucoraphanin, glucoalyssin, gluconapin, and glucoerucin, and four indolyl GSs consisting of 4-hydroxyglucobrassicin, glucobrassicin, 4-methoxyglucobrassicin, and neoglucobrassicin, were identified.
Fig. 2.
Typical HPLC elution profile of desulfated glucosinolates in bolting stem of Chinese kale
Peak numbers refer to GSs listed in Table 2
Table 2.
Desulfated glucosinolates identified in bolting stems of Chinese kale
| No.* | Retention time (min) | Side-chain structure | Trivial name | Desulfated molecular weight | Response factor† |
| 1 | 6.58 | CH3-SO-(CH2)3- | Glucoiberin | 343 | 1.07 |
| 2 | 7.10 | CH2=CHCH(OH)CH2- | Progoitrin | 309 | 1.09 |
| 3 | 7.59 | CH2=CHCH2- | Sinigrin | 279 | 1.00 |
| 4 | 7.98 | CH3-SO-(CH2)4- | Glucoraphanin | 357 | 1.07 |
| 5 | 9.24 | CH3-SO-(CH2)5- | Glucoalyssin | 371 | 1.07 |
| 6 | 11.13 | CH2=CH-(CH2)2- | Gluconapin | 293 | 1.11 |
| 7 | 11.86 | Indole-(4-OH)-3-CH2- | 4-Hydroxyglucobrassicin | 384 | 0.28 |
| 8 | 15.54 | CH3-S-(CH2)4- | Glucoerucin | 341 | 1.00‡ |
| 9 | 17.08 | Indole-3-CH2- | Glucobrassicin | 368 | 0.29 |
| 10 | 19.64 | Indole-(4-OCH3)-3-CH2- | 4-Methoxyglucobrassicin | 398 | 0.25 |
| 11 | 24.86 | Indole-(OCH3)-3-CH2- | Neoglucobrassicin | 398 | 0.20 |
Numbering is based on the elution order of desulfated glucosinolates from HPLC;
The response factors relative to the standard sinigrin were experimentally determined with HPLC by the International Organization for Standardization (ISO 9167-1) in 1992 for individual GS content in rapeseed;
Not yet determined by the ISO
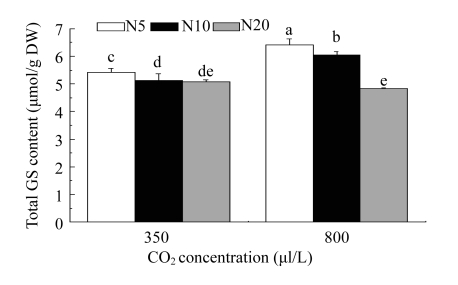
The major GSs were gluconapin, sinigrin, glucoraphanin, and glucoiberin, which constituted about 54.48%, 8.80%, 8.63%, and 5.93%, respectively, of the total GS concentration on average. The proportions of the other seven individual GSs were less than 5% on average. The total GS concentration in each treatment was expressed as the sum of the 11 identified individual GS species (Fig.3). The total GS content ranged 4.82~6.41 μmol/g DW (dry weight). CO2 concentration significantly affected the concentrations of total GSs, total aliphatic GSs, and all individual aliphatic GSs except glucoerucin, but not the concentrations of total or individual indolyl GSs (Table 3 and Fig.3). Under elevated CO2, the total GS concentration increased in the 5 and 10 mmol N/L treatments by 15.59% and 18.01%, respectively, compared with those at ambient CO2. However, elevated CO2 did not affect the total GS concentration at 20 mmol N/L. In the 5 and 10 mmol N/L solution treatments, all individual aliphatic GSs increased under elevated CO2 compared with the ambient CO2 treatment, whereas individual indolyl GSs were not affected by the elevated CO2 concentration. However, in the 20 mmol N/L treatment under the elevated CO2 condition, the total aliphatic GS concentration decreased significantly (P<0.01), while there was no significant effect on total indolyl GS content (P>0.05), resulting in a slight decrease in total GS concentration (P>0.05). Nitrogen concentration also significantly affected the concentrations of total GSs, total aliphatic GSs, individual aliphatic GSs except glucoerucin, total indolyl GSs, and individual indolyl GSs except 4-methoxyglucobrassicin (Tables 3 and 4). The 5 mmol N/L treatment increased the total aliphatic GS concentration compared with the 10 and 20 mmol N/L treatments, but there was no significant difference in the effects of the 10 and 20 mmol N/L treatments at ambient CO2. However, under the elevated CO2 concentration, the total aliphatic GS concentration decreased significantly with increased N supply. Total indolyl GS concentration increased in the 20 mmol/L N treatment compared with that in the 5 mmol N/L treatment at both CO2 concentrations, whereas the difference between the 10 and 20 mmol N/L treatments was not significant at the ambient CO2 concentration but was significant under the elevated CO2 concentration. Moreover, there were significant CO2×N interactions for total GS concentration (P<0.001), total aliphatic GS concentration (P<0.001), and concentrations of all individual aliphatic GSs (P<0.01) except glucoerucin, but not for the concentrations of total indolyl GSs or individual indolyl GSs (P>0.05) except neoglucobrassicin.
Fig. 3.
Comparison of the total GS concentration in bolting stems of Chinese kale grown at ambient CO2 (350 μl/L) and elevated CO2 (800 μl/L) concentrations under three nitrogen (N) concentrations
N5: 5 mmol N/L; N10: 10 mmol N/L; N20: 20 mmol N/L. Columns with the same letter(s) indicate no significant difference at the P<0.05 level. The bars represent the standard error
Table 3.
Effect of CO2 concentration on the individual and total aliphatic GS concentrations in bolting stems of Chinese kale at three nitrogen (N) concentrations
| CO2 (μl/L) | N (mmol/L) | GS concentration (μmol/g DW) |
|||||||
| GIB | PRO | GRA | SIN | GAL | GNP | GRU | Total | ||
| 350 | 5 | 0.17±0.02d | 0.13±0.02c | 0.15±0.01d | 0.51±0.02b | 0.10±0.01b | 3.62±0.11b | 0.05±0.01b | 4.71±0.12c |
| 10 | 0.31±0.01c | 0.14±0.01c | 0.46±0.08c | 0.40±0.08bc | 0.10±0.01b | 2.89±0.12d | 0.06±0.01ab | 4.36±0.22d | |
| 20 | 0.34±0.01b | 0.25±0.01b | 0.88±0.06a | 0.35±0.02c | 0.23±0.03a | 2.17±0.03e | 0.04±0.01b | 4.27±0.08d | |
| 800 | 5 | 0.46±0.01a | 0.38±0.07a | 0.18±0.02d | 0.64±0.12a | 0.21±0.05a | 3.85±0.14a | 0.07±0.03a | 5.79±0.14a |
| 10 | 0.35±0.03b | 0.16±0.04c | 0.49±0.03c | 0.70±0.04a | 0.15±0.02b | 3.39±0.17c | 0.06±0.00ab | 5.31±0.09b | |
| 20 | 0.33±0.02bc | 0.25±0.03b | 0.57±0.02b | 0.35±0.00c | 0.22±0.02a | 2.16±0.03e | 0.04±0.01b | 3.93±0.01e | |
| Source of variance | |||||||||
| CO2 | *** | *** | ** | *** | ** | *** | NS | *** | |
| N | NS | *** | *** | *** | *** | *** | NS | *** | |
| CO2×N | *** | *** | *** | ** | ** | ** | NS | *** | |
GIB: glucoiberin; PRO: progoitrin; GRA: glucoraphanin; SIN: sinigrin; GAL: glucoalyssin; GNP: gluconapin; GRU: glucoerucin. Data followed by the same superscript letter(s) indicate no significant difference at P<0.05 level. Values are mean±SD. Significance levels indicated by two-way ANOVA: NS, not significant; * P<0.05
Significance levels indicated by two-way ANOVA: P<0.01;
Significance levels indicated by two-way ANOVA: P<0.001
Table 4.
Effect of CO2 concentration on the individual and total indolyl GS concentrations in bolting stems of Chinese kale at three nitrogen (N) concentrations
| CO2 (μl/L) | N (mmol/L) | GS concentration (μmol/g DW) |
||||
| 4HGB | GBS | 4MGB | NGBS | Total | ||
| 350 | 5 | 0.01±0.00bc | 0.18±0.04b | 0.26±0.03a | 0.24±0.01b | 0.70±0.06cd |
| 10 | 0.02±0.01a | 0.25±0.01a | 0.25±0.03a | 0.25±0.03b | 0.77±0.07bc | |
| 20 | 0.02±0.00a | 0.28±0.00a | 0.27±0.01a | 0.25±0.01b | 0.81±0.01ab | |
| 800 | 5 | 0.01±0.00c | 0.14±0.04b | 0.25±0.03a | 0.23±0.02b | 0.62±0.07d |
| 10 | 0.02±0.00ab | 0.25±0.01a | 0.25±0.03a | 0.24±0.04b | 0.75±0.05bc | |
| 20 | 0.02±0.00a | 0.28±0.01a | 0.27±0.01a | 0.33±0.02a | 0.89±0.04a | |
| Source of variance | ||||||
| CO2 | NS | NS | NS | NS | NS | |
| N | *** | *** | NS | ** | *** | |
| CO2×N | NS | NS | NS | * | NS | |
4HGB: 4-hydroxyglucobrassicin; GBS: glucobrassicin; 4MGB: 4-methoxyglucobrassicin; NGBS: neoglucobrassicin. Data followed by the same superscript letter(s) indicate no significant difference at P<0.05 level. Values are mean±SD. Significance levels indicated by two-way ANOVA: NS, not significant
Significance levels indicated by two-way ANOVA: P<0.05;
Significance levels indicated by two-way ANOVA: P<0.01;
Significance levels indicated by two-way ANOVA: P<0.001
Bolting stem carbon, nitrogen, and sulfur contents
With elevated CO2, the C content in the bolting stem increased in the 5, 10, and 20 mmol N/L treatments by 11.38%, 13.62%, and 10.46%, respectively, relative to the ambient CO2 concentration (Table 5). The effect of CO2 on C content was strongly significant (P<0.001), but the effects of N concentration and CO2×N interactions were not significant (P>0.05). The N content in bolting stem increased significantly with increasing N concentration in the nutrient solution under the same CO2 regime. In contrast, the N content decreased in the three N treatments under the enriched CO2 concentration compared with that of the ambient CO2 treatment (Table 5). The decreases in N content in the 5, 10, and 20 mmol N/L treatments were 10.97%, 13.27%, and 4.59%, respectively. There were significant CO2×N interactions for the C/N ratio (Table 5). The C/N ratio at the three N concentrations all increased under the elevated CO2 concentration by 25.13%, 31.20%, and 15.73%, respectively. There were significant N concentration (P<0.01) and CO2 concentration (P<0.001) effects on S content, but CO2×N interactions were not significant for S content. Under the elevated CO2 concentration, S content decreased in all of the N treatments. Because of the decreases in both N and S contents, the N/S ratio changed. The N/S ratio was significantly affected by CO2 concentration (P<0.05), N concentration (P<0.001), and CO2×N interactions (P<0.01). Under the elevated CO2 concentration, the N/S ratio decreased significantly in the 5 and 10 mmol N/L treatments (P<0.05), but the decrease in N and S contents at the ambient and elevated CO2 concentrations did not differ significantly in the 20 mmol N/L treatment.
Table 5.
Effect of CO2 concentration on the carbon (C), nitrogen (N), and sulfur (S) contents, C/N ratio, and N/S ratio in bolting stems of Chinese kale at three N concentrations
| CO2 (μl/L) | N (mmol/L) | C content (%) | N content (%) | S content (%) | C/N ratio | N/S ratio |
| 350 | 5 | 30.88±0.81b | 3.58±0.05e | 0.81±0.01a | 8.62±0.10c | 4.42±0.09d |
| 10 | 30.87±1.00b | 4.44±0.08c | 0.85±0.02ab | 6.95±0.17d | 5.21±0.15b | |
| 20 | 31.81±0.37b | 5.06±0.11a | 0.83±0.02b | 6.29±0.18e | 6.09±0.19a | |
| 800 | 5 | 34.39±0.12a | 3.19±0.03f | 0.77±0.02b | 10.79±0.12a | 4.16±0.12e |
| 10 | 35.08±0.14a | 3.85±0.08d | 0.81±0.02c | 9.12±0.43b | 4.77±0.11c | |
| 20 | 35.13±0.47a | 4.82±0.10b | 0.77±0.02c | 7.28±0.25d | 6.27±0.17a | |
| Source of variance | ||||||
| CO2 | *** | *** | *** | *** | * | |
| N | NS | *** | ** | *** | *** | |
| CO2×N | NS | * | NS | ** | ** | |
Data followed by the same superscript letter(s) indicate no significant difference at P<0.05 level. Values are mean±SD. Significance levels indicated by two-way ANOVA: NS, not significant
Significance levels indicated by two-way ANOVA: P<0.05;
Significance levels indicated by two-way ANOVA: P<0.01;
Significance levels indicated by two-way ANOVA: P<0.001
DISCUSSION
Little is known about the interactive effect of CO2 enrichment and N availability on GS content in vegetables. In the present study, the effect of an elevated CO2 concentration at three different N concentrations (5, 10, and 20 mmol/L) on GS content in the bolting stem of Chinese kale was investigated. We observed that the CO2 concentration and N concentration in the nutrient solution showed significant interactive effects on the height and dry weights of the aerial parts, bolting stems, and roots. The maximum stem thickness and dry weights of the aerial parts, bolting stems, and roots were obtained at 20 mmol N/L under the elevated CO2 condition, which confirms the importance of N availability in determining the vegetable’s response to elevated CO2. Similar conclusions have also been reported for the grass Bromus mollis, rice, wheat, and tomato seedlings (Larigauderie et al., 1988; Kim et al., 2001; Kobayashi et al., 2001; Li et al., 2007).
In this study, 11 individual GSs were detected in bolting stems of Chinese kale, of which the major GSs were gluconapin, sinigrin, glucoiberin, and glucoraphanin. These results are in agreement with a previous study on Chinese kale (La et al., 2008). Glucoraphanin, sinigrin, glucoiberin, and glucobrassicin are reported to be the most important GSs for the hydrolysis products serving as the most powerful agents protecting human and animal cells against carcinogenesis (Bones and Rossiter, 1996; Fahey et al., 1997; Nilsson et al., 2006). We detected all the GSs in bolting stem, and the concentrations of sinigrin, glucoiberin, and glucobrassicin were similar to those reported for broccoli (Kushad et al., 1999; Padilla et al., 2007). A high concentration of progoitrin in vegetables is a latent problem because it causes goiter and other harmful effects on animals, such as depressed growth, poor egg production, and liver damage (Heaney and Fenwick, 1995). However, there is no evidence that Brassica consumption has any goitrogenic effects on humans (Mithen et al., 2000) and also there is no normative limit issued yet for progoitrin concentration in vegetables. Fortunately, the concentration of progoitrin detected in the edible part of Chinese kale was relatively low, ranging 0.12~0.37 μmol/g DW.
The elevated CO2 concentration increased the total GS concentration as a result of a strong increase in aliphatic GSs, whereas there was no significant effect on the concentrations of indolyl GSs at 5 or 10 mmol N/L, compared with those at the ambient CO2 concentration. This is in agreement with a previous study on broccoli inflorescences (Schonhof et al., 2007). However, at 20 mmol N/L, the difference between the ambient and elevated CO2 treatments was not significant.
To our knowledge, no corresponding previous study has reported on the interactive effect of CO2 and N concentrations on GS content. Bryant et al.(1983) advanced the carbon/nutrient balance hypothesis to predict the change of N- and C-containing compounds under elevated CO2 conditions. These authors pointed out that, under an elevated CO2 concentration, because of the increased C supply and N limitation, the concentrations of N- and C-containing compounds increased. Studies on the changes in condensed tannin, soluble phenolic polymer, and GS concentrations in the oilseed rape (Brassica napus) leaves under CO2 enrichment are consistent with this hypothesis (Peñuelas and Estiarte, 1998; Himanen et al., 2008). However, some studies of the effect of CO2 enrichment on GS content are not consistent with the prediction of the carbon/nutrient balance hypothesis (Karowe et al., 1997; Reddy et al., 2004; Schonhof et al., 2007). In the present study, under the elevated CO2 condition, the C content in bolting stem of Chinese kale increased while the N content decreased in all of the three N treatments, which resulted in an increase in the C/N ratio; however, GS concentration did not change consistently with the increase in C/N ratio. Clearly, the change in C/N ratio was not a reliable predictive tool for the change in GS composition and concentration in Chinese kale bolting stems.
N and S are not only two essential elements that are constituents of amino acids, but also the main factors that affect GS content in bolting stems of Chinese kale. Under the elevated CO2 concentration, besides the decrease in N content, S content simultaneously decreased in all of the N treatments. However, in broccoli inflorescences, N content decreased while the S content was unaffected by a rise in CO2 concentration because of the unchanged fresh and dry weights of broccoli between CO2 regimes (Schonhof et al., 2007). In young pedunculate oak (Quercus robur L.) trees, sulfate uptake was enhanced under CO2 enrichment (650 μl/L) (Seegmüller et al., 1996), which indicated that there are genotypic differences in the sulfate absorption response to elevated atmospheric CO2 concentration. In the present study, because of the changes in N and S contents, the N/S ratio decreased in the 5 and 10 mmol N/L treatments under the elevated CO2 concentration, but was not affected significantly by the 20 mmol N/L solution because of the similar change in N and S contents under enriched CO2 concentration. Hesse et al.(2004) reported that decrease in the N/S ratio due to diminished N content caused increased synthesis of sulfurous cysteine as a precursor of methionine. Moreover, cysteine and methionine act as effective sulfur donors in thiohydroxamate formation in the syntheses of aliphatic and indolyl GSs (Mikkelsen et al., 2002). However, in our study, at the 5 and 10 mmol N/L concentrations, the aliphatic GS concentration increased with the decrease in N/S ratio, but indolyl GS concentration was not affected by the reduced N/S ratio under the elevated CO2 concentration, which agreed with previous results of the effect of elevated CO2 on the GS content of broccoli inflorescences (Schonhof et al., 2007). Although indolyl GS concentrations were unchanged under the elevated CO2 concentration, indolyl GS content increased with the increasing N supply, which is in agreement with the findings of Shattuck and Wang (1993) and Kim et al.(2002).
Besides the effect of N on GS concentration and composition, plant species is thought to be taken into account as they experience increasing atmospheric CO2 levels. For broccoli, which is a cultivated derivative of B. oleracea, aliphatic GS concentration increased while indole GS concentration decreased under elevated CO2 conditions (Schonhof et al., 2007). In a recent study, elevated CO2 (720 μl/L) increased the concentrations of total aliphatic GSs and aromatic GSs and decreased the indole GS concentration in leaves of both transgenic and wild-type oilseed rapes (Brassica rapa subsp. oleifera) (Himanen et al., 2008). However, in Arabidopsis thaliana CO2 enrichment did not significantly influence the GS concentration (Bidart-Bouzat et al., 2005). Karowe et al.(1997) suggested that responses in GS content to elevated CO2 concentration appeared to be species-specific, as in the same experiment total GS concentrations in both young and old mustard (Brassica juncea) leaves decreased, whereas GS concentrations in radish (Raphanus sativus L.) and turnip (Brassica rapa subsp. rapa) appeared to be unaffected by CO2 enrichment (724 μl/L). Furthermore, Reddy et al.(2004) and Bidart-Bouzat et al.(2005) observed that the significant changes in individual GS concentrations were not consistent among cultivars of oilseed rape and Arabidopsis thaliana under elevated CO2 conditions, supporting the hypothesis that, in general, the response to elevated CO2 differs among cultivars. Moreover, in Chinese kale bolting stems, the concentrations of aliphatic GS glucoerucin and all individual indolyl GSs were not affected by the elevated CO2 concentration, indicating that the concentrations of individual GSs within a specific GS group (i.e., aliphatic, aromatic, or indolyl) did not change consistently, which agreed with the conclusion that the response to elevated CO2 also depends on the individual GS type (Bidart-Bouzat et al., 2005).
CONCLUSION
The elevated CO2 concentration promoted the growth of Chinese kale in the three N treatments and a high N concentration is beneficial for the growth of Chinese kale. GS concentration was affected by CO2 concentration, N concentration, and CO2×N interactions. Under the elevated CO2 concentration, total GS concentration increased as a result of the increase in aliphatic GS concentration in the 5 and 10 mmol N/L treatments, but there was no significantly difference in the 20 mmol N/L treatment, compared with GS concentration under the ambient CO2 concentration. The maximum total GS concentration was recorded in the 5 mmol N/L treatment under the elevated CO2 concentration. Because of the increase in C content and decrease in N and S contents, the C/N ratio was increased significantly at each N level and N/S ratio decreased significantly in the 5 and 10 mmol N/L treatments. However, changes in the C/N ratio were not a reliable predictor of changes in GS concentration in Chinese kale bolting stems. Changes in N and S contents and the N/S ratio could contribute to the change in GS concentration in the bolting stem of Chinese kale. These results indicate that at an elevated CO2 concentration high N availability promoted the growth of Chinese kale, but reduced the total GS content in bolting stems.
Footnotes
Project (No. 2007CB109305) supported by the National Basic Research Program (973) of China
References
- 1.Ahmad G, Jan A, Arif M, Jan MT, Khattak RA. Influence of nitrogen and sulfur fertilization on quality of canola(Brassica napus L.) underrainfed conditions. J Zhejiang Univ Sci B. 2007;8(10):731–737. doi: 10.1631/jzus.2007.B0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aires A, Rosa E, Carvalho R. Effect of nitrogen and sulfur fertilization on glucosinolates in the leaves and roots of broccoli sprouts (Brassica oleracea var. italica) J Sci Food Agric. 2006;86(10):1512–1516. doi: 10.1002/jsfa.2535. [DOI] [Google Scholar]
- 3.Amthor JS. Effects of atmospheric CO2 concentration on wheat yield: review of results from experiments using various approaches to control CO2 concentration. Field Crops Res. 2001;73(1):1–34. doi: 10.1016/S0378-4290(01)00179-4. [DOI] [Google Scholar]
- 4.Baik HY, Juvik JA, Jeffery EH, Wallig MA, Kushad M, Klein BP. Relating glucosinolate content and flavor of broccoli cultivars. J Food Sci. 2003;68(3):1043–1050. doi: 10.1111/j.1365-2621.2003.tb08285.x. [DOI] [Google Scholar]
- 5.Baxter R, Ashenden TW, Farrar JF. Effects of elevated carbon dioxide on three grass species from montane pasture. II. Nutrient uptake, allocation and efficiency of use. J Exp Bot. 1994;45(9):1267–1278. doi: 10.1093/jxb/45.9.1267. [DOI] [Google Scholar]
- 6.Bazzaz FA. The response of natural ecosystems to the rising global CO2 levels. Annu Rev Ecol Syst. 1990;21(1):167–196. doi: 10.1146/annurev.es.21.110190.001123. [DOI] [Google Scholar]
- 7.Bidart-Bouzat MG, Mithen R, Berenbaum MR. Elevated CO2 influences herbivory-induced defense responses of Arabidopsis thaliana . Oecologia. 2005;145(3):415–424. doi: 10.1007/s00442-005-0158-5. [DOI] [PubMed] [Google Scholar]
- 8.Bones AM, Rossiter JT. The myrosinase glucosinolates system, its organization and biochemistry. Plant Physiol. 1996;97(1):194–208. doi: 10.1111/j.1399-3054.1996.tb00497.x. [DOI] [Google Scholar]
- 9.Bryant JP, Chapin FS, Klein DR. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos. 1983;40(3):357–368. doi: 10.2307/3544308. [DOI] [Google Scholar]
- 10.Caswell H. Advances in Ecological Research. New York: Academic Press; 2004. [Google Scholar]
- 11.Chen RY, Liu HC, Song CZ, Sun GW. Effect of nitrogen nutrient on the growth and quality of Chinese kale. Trans CSAE. 2005;21(S):143–146. (in Chinese) [Google Scholar]
- 12.Chew FS. Biologically Active Natural Products. American Chemical Society Symposium; Washington DC. 1988. [Google Scholar]
- 13.Cotrufo MF, Ineson P, Scott A. Elevated CO2 reduces the nitrogen concentration of plant tissues. Glob Change Biol. 1998;4(1):43–54. doi: 10.1046/j.1365-2486.1998.00101.x. [DOI] [Google Scholar]
- 14.Das M, Pal M, Zaidi PH, Raj A, Sengupta UK. Stage sensitivity of mung bean (Vigna radiata L. Wilczek) to an elevated level of carbon dioxide. J Agron Crop Sci. 2002;188(4):219–224. doi: 10.1046/j.1439-037X.2002.00556.x. [DOI] [Google Scholar]
- 15.Epron D, Liozon R, Mousseau M. Effects of elevated CO2 concentration on leaf characteristics and photosynthetic capacity of beech (Fagus sylvatica) during the growing season. Tree Physiol. 1996;16:425–432. doi: 10.1093/treephys/16.4.425. [DOI] [PubMed] [Google Scholar]
- 16.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA. 1997;94(19):10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenwick GR, Heaney RK, Mullin WJ. Glucosinolates and their breakdown products in food and food plants. CRC Crit Rev Food Sci Nutr. 1983;18(2):123–201. doi: 10.1080/10408398209527361. [DOI] [PubMed] [Google Scholar]
- 18.Fenwick GR, Heaney RK, Mawson R. Toxicants of Plant Origin. Florida: CRC Press; 1989. [Google Scholar]
- 19.Gijzen M, McGregor I, Seguin-Swartz G. Glucosinolate uptake by developing rapeseed embryos. Plant Physiol. 1989;89(1):260–263. doi: 10.1104/pp.89.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habash DZ, Paul MJ, Parry MA, Keys AJ, Lawlor DW. Increased capacity for photosynthesis in wheat grown at elevated CO2: the relationship between electron-transport and carbon metabolism. Planta. 1995;197(3):482–489. doi: 10.1007/BF00196670. [DOI] [Google Scholar]
- 21.Halkier BA, Du L. The biosynthesis of glucosinolates. Trends Plant Sci. 1997;2(11):425–431. doi: 10.1016/S1360-1385(97)90026-1. [DOI] [PubMed] [Google Scholar]
- 22.He HJ, Chen H, Schnitzler WH. Glucosinolate composition and contents in Brassica vegetables. Sci Agric Sinica. 2002;35(2):192–197. (in Chinese) [Google Scholar]
- 23.Heaney RK, Fenwick GR. Natural toxins and protective factors in Brassica species, including rapeseed. Nat Toxins. 1995;3(4):233–237. doi: 10.1002/nt.2620030412. [DOI] [PubMed] [Google Scholar]
- 24.Hesse H, Nikiforova V, Gakiere B, Hoefgen R. Molecular analysis and control of cysteine biosynthesis: integration of nitrogen and sulphur metabolism. J Exp Bot. 2004;55(401):1283–1292. doi: 10.1093/jxb/erh136. [DOI] [PubMed] [Google Scholar]
- 25.Himanen SJ, Nissinen A, Auriola S, Poppy GM, Stewart CN, Holopainen JK, Nerg AM. Constitutive and herbivore-inducible glucosinolate concentrations in oilseed rape (Brassica napus) leaves are not affected by Bt Cry1Ac insertion but change under elevated atmospheric CO2 and O3 . Planta. 2008;227(2):427–437. doi: 10.1007/s00425-007-0629-5. [DOI] [PubMed] [Google Scholar]
- 26.Hoagland DR, Arnon DI. The Water Culture Method for Growing Plants Without Soil, Circ 347. Berkley: California Agricultural Experiment Station; 1938. [Google Scholar]
- 27.Holst B, Williamson G. A critical review of the bioavailability of glucosinolates and related compounds. Nat Prod Rep. 2004;21(3):425–447. doi: 10.1039/b204039p. [DOI] [PubMed] [Google Scholar]
- 28.IPCC (Intergovernmental Panel on Climate Change) Cambridge: Cambridge University Press; Climate Change 2007. 2007
- 29.Islam MS, Matsui T, Yoshida Y. Effect of carbon dioxide enrichment on physico-chemical and enzymatic changes in tomato fruits at various stages of maturity. Sci Hortic. 1996;65(2-3):137–149. doi: 10.1016/0304-4238(95)00867-5. [DOI] [Google Scholar]
- 30.Karowe DN, Seimens DH, Mitchell-olds T. Species-specific response of glucosinolate content to elevated atmospheric CO2 . J Chem Ecol. 1997;23(11):2569–2582. doi: 10.1023/B:JOEC.0000006667.81616.18. [DOI] [Google Scholar]
- 31.Kiddle G, Bennett RN, Botting NP, Davidson NE, Robertson AAB, Wallsgrove RM. High performance liquid chromatography separation of natural and synthetic desulfoglucosinolates and their chemical validation by spectroscopic, NMR and CI-MS methods. Phytochem Anal. 2001;12(4):226–242. doi: 10.1002/pca.589. [DOI] [PubMed] [Google Scholar]
- 32.Kim HY, Lieffering M, Miura S, Kobayashi K, Okada M. Growth and nitrogen uptake of CO2-enriched rice under field conditions. New Phytol. 2001;150(2):223–229. doi: 10.1046/j.1469-8137.2001.00111.x. [DOI] [Google Scholar]
- 33.Kim SJ, Matsuo T, Watanabe M, Watanabe Y. Effect of nitrogen and sulphur application on the glucosinolate content in vegetable turnip rape (Brassica rapa L.) Soil Sci Plant Nutr. 2002;48(1):43–49. [Google Scholar]
- 34.Kimball BA, Pinter JPJ, Garcia RL, LaMorte RL, Wall GW, Hunsaker DJ, Wechsung G, Wechsung F, Kartschall T. Productivity and water use of wheat under free-air CO2 enrichment. Global Change Biol. 1995;1(6):429–442. doi: 10.1111/j.1365-2486.1995.tb00041.x. [DOI] [Google Scholar]
- 35.Kimball BA, Zhu JG, Cheng L, Kobayashi K, Bindi M. Responses of agricultural crops to free-air CO2 enrichment. J Appl Ecol. 2002;13(10):1323–1338. (in Chinese) [PubMed] [Google Scholar]
- 36.Kobayashi K, Lieffering M, Kim HY. Structure and Function in Agroecosystem Design and Management. Florida: CRC Press; 2001. [Google Scholar]
- 37.Kohlmeier L, Su L. Cruciferous vegetable consumption and colorectal cancer risk: meta-analysis of the epidemiological evidence. FASEB J. 1997;11(3):2141. [Google Scholar]
- 38.Kushad MM, Brown AF, Kurilich AC, Juvik JA, Klein BP, Wallig MA, Jerrery EH. Variation of glucosinolates in vegetables crops of Brassica oleracea . J Agric Food Chem. 1999;47(4):1541–1548. doi: 10.1021/jf980985s. [DOI] [PubMed] [Google Scholar]
- 39.La GX, Fang P, Li YJ, Wang Y. Determination of desulpho-glucosinolates in bolting stems of Chinese kale by liquid chromatography-mass spectrometry. Journal of Zhejiang University (Agric Life Sci) 2008;34(5):557–563. doi: 10.3785/j.issn.1008-9209.2008.05.012. (in Chinese) [DOI] [Google Scholar]
- 40.Larigauderie A, Hilbert DW, Oechel WC. Effect of CO2 enrichment and nitrogen availability on resource acquisition and resource allocation in a grass, Bromus mollis . Oecologia. 1988;77(4):544–549. doi: 10.1007/BF00377272. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Zhou JM, Duan ZQ, Du CW, Wang HY. Effect of CO2 enrichment on the growth and nutrient uptake of tomato seedlings. Pedosphere. 2007;17(3):343–351. doi: 10.1016/S1002-0160(07)60041-1. [DOI] [Google Scholar]
- 42.Louda S, Mole S. Herbivores: Their Interactions with Secondary Plant Metabolites. 2nd Ed. New York: Academic Press; 1991. [Google Scholar]
- 43.Lu RK. Analytical Methods of Soil Agro-chemistry. Beijing: Chinese Agriculture Science and Technology Press; 1999. (in Chinese) [Google Scholar]
- 44.Macfarlane-Smith WH, Griffiths DW. A time-course study of glucosinolates in the ontogeny of forage rape (Brassica napus L.) J Sci Food Agric. 1988;43(2):121–134. doi: 10.1002/jsfa.2740430203. [DOI] [Google Scholar]
- 45.Mikkelsen MD, Petersen B, Olsen C, Halkier BA. Biosynthesis and metabolic engineering of glucosinolates. Amino Acids. 2002;22(3):279–295. doi: 10.1007/s007260200014. [DOI] [PubMed] [Google Scholar]
- 46.Mithen RF, Dekker M, Verkerk R, Rabot S, Johnson IT. The nutritional significance, biosynthesis and bioavailability of glucosinolates in human foods. J Sci Food Agric. 2000;80(7):967–984. doi: 10.1002/(SICI)1097-0010(20000515)80:7<967::AID-JSFA597>3.0.CO;2-V. [DOI] [Google Scholar]
- 47.Mooney HA, Drake BG, Luxmoore RJ, Oechei WC, Pitelka LF. Predicting ecosystem responses to elevated CO2 concentrations. Bioscience. 1991;41(2):96–104. doi: 10.2307/1311562. [DOI] [Google Scholar]
- 48.Nilsson J, Olsson K, Engqvist G, Ekvall J, Olsson M, Nyman M, Kesson B. Variation in the content of glucosinolates, hydroxycinnamic acids, carotenoids, total antioxidant capacity and low-molecular-weight carbohydrates in Brassica vegetables. J Sci Food Agric. 2006;86(4):528–538. doi: 10.1002/jsfa.2355. [DOI] [Google Scholar]
- 49.Padilla G, Cartea ME, Velasco P, de Haro A, Prdás A. Variation of glucosinolates in vegetable crop of Brassica rapa . Phytochemistry. 2007;68(4):536–545. doi: 10.1016/j.phytochem.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 50.Peñuelas J, Estiarte M. Can elevated CO2 affect secondary metabolism and ecosystem function? Trends Ecol Evol. 1998;13(1):20–24. doi: 10.1016/S0169-5347(97)01235-4. [DOI] [PubMed] [Google Scholar]
- 51.Price KR, Casuscelli F, Colquhoun IJ, Rhodes MJC. Composition and content of flavonol glycosides in broccoli florets (Brassica oleracea) and their fate during cooking. J Sci Food Agric. 1998;77(4):468–472. doi: 10.1002/(SICI)1097-0010(199808)77:4<468::AID-JSFA66>3.0.CO;2-B. [DOI] [Google Scholar]
- 52.Pruden G, Kalembasa SJ, Jenkinson DS. Reduction of nitrate of prior to Kjeldahl digestion. J Sci Food Agric. 1985;36(2):71–73. doi: 10.1002/jsfa.2740360203. [DOI] [Google Scholar]
- 53.Reddy GVP, Tossavainen P, Nerg AM, Holopainen JK. Elevated atmospheric CO2 affects the chemical quality of Brassica plants and the growth rate of the specialist Plutella xylostella, but not the generalist, Spodoptera littoralis . J Agric Food Chem. 2004;52(13):4185–4191. doi: 10.1021/jf049358v. [DOI] [PubMed] [Google Scholar]
- 54.Rodman JE. A taxonomic analysis of glucosinolate producing plants, part 1: Phenetics. Syst Bot. 1991;16(4):598–618. doi: 10.2307/2418864. [DOI] [Google Scholar]
- 55.Rosa E, Heaney RK, Fenwick GR, Portas CAM. Glucosinolates in crop plants. Hort Rev. 1997;19:99–215. [Google Scholar]
- 56.Schnug E. Double low oilseed rape in West Germany, sulphur nutrition and glucosinolate levels. Aspects Appl Biol. 1989;23:67–82. [Google Scholar]
- 57.Schonhof I, Kläring HP, Krumbein A, Schreiner M. Interaction between atmospheric CO2 and glucosinolates in Broccoli. J Chem Ecol. 2007;33(1):105–114. doi: 10.1007/s10886-006-9202-0. [DOI] [PubMed] [Google Scholar]
- 58.Schreiner M. Vegetable crop management strategies to increase the quantity of phytochemicals. Eur J Nutr. 2005;44(2):85–94. doi: 10.1007/s00394-004-0498-7. [DOI] [PubMed] [Google Scholar]
- 59.Seegmüller S, Schulte M, Herschbach C, Rennenberg H. Interactive effects of mycorrhization and elevated atmospheric CO2 on sulphur nutrition of young pedunculate oak (Quercus robur L.) trees. Plant Cell Environ. 1996;19(4):418–426. doi: 10.1111/j.1365-3040.1996.tb00333.x. [DOI] [Google Scholar]
- 60.Shattuck VI, Wang W. Nitrogen dioxide fumigation alters the glucosinolate and nitrate levels in pak choy (Brassica campestris ssp. Chinensis) Sci Horticul. 1993;56(2):87–100. doi: 10.1016/0304-4238(93)90010-N. [DOI] [Google Scholar]
- 61.Tawfiq N, Heaney RK, Pulumb JA, Fenwick GR, Musk SR, Williamson G. Dietary glucosinolates as blocking agents against carcinogenesis: glucosinolate breakdown products assessed by induction of quinine reductase activity in murine hepa1c1c7 cells. Carcinogenesis. 1995;16(5):1191–1194. doi: 10.1093/carcin/16.5.1191. [DOI] [PubMed] [Google Scholar]
- 62.Wattenberg LW. Food and Cancer Prevention: Chemical and Biological Aspects. London: Royal Society of Chemistry; 1993. [Google Scholar]
- 63.Zhao F, Evans EJ, Bilsborrow PE, Schnug E, Syers JK. Correction for protein content in the determination of the glucosinolate content of rapeseed by the XRF method. J Sci Food Agric. 1992;58(3):431–433. doi: 10.1002/jsfa.2740580319. [DOI] [Google Scholar]
- 64.Zhao F, Evans EJ, Bilsborrow PE, Schnug E, Syers JK. Influence of nitrogen and sulphur on the glucosinolate profiles of rapeseed (Brassica napus L.) J Sci Food Agric. 1994;64(3):295–304. doi: 10.1002/jsfa.2740640309. [DOI] [Google Scholar]