Abstract
Site-directed mutagenesis (SDM) has been a very important method to probe the function-structure relationship of proteins. In this study, we introduced an easy-to-use, polymerase chain reaction (PCR)-based SDM method for double-stranded plasmid DNA, with a designed restriction site to ensure simple and efficient mutant screening. The DNA sequence to be mutated was first translated into amino acid sequence and then the amino acid sequence was reversely translated into DNA sequence with degenerate codons, resulting in a large number of sequences with silent mutations, which contained various restriction endonuclease (RE) sites. Certain mutated sequence with an appropriate RE site was selected as the target DNA sequence for designing a pair of mutation primers to amplify the full-length plasmid via inverse PCR. The amplified product was 5′-phosphorylated, circularized, and transformed into an Escherichia coli host. The transformants were screened by digesting with the designed RE. This protocol uses only one pair of primers and only one PCR is conducted, without the need for hybridization with hazardous isotope for mutant screening or subcloning step.
Keywords: Site-directed mutagenesis (SDM), Restriction endonuclease, Mutant screening
Site-directed mutagenesis (SDM) has a variety of applications and is extensively used in molecular biology. Over the last three decades, various SDM methods have been described (Nagy et al., 2004; Zheng et al., 2004; Seyfang and Jin, 2004; An et al., 2005; Wei et al., 2004; Jin et al., 2007; Heckman and Pease, 2007; Tseng et al., 2008; Li et al., 2008; Chapnik et al., 2008) and some commercial SDM kits based on these techniques are available. The SDM techniques can be grouped into two major categories: polymerase chain reaction (PCR)-based and non-PCR-based. The PCR-based SDM methods are used more frequently than the non-PCR-based methods. Rabhi et al.(2004) have introduced an inverse PCR-based SDM method with forward and reverse primers to amplify the full-length plasmid. The blunt-ended amplification products are 5′-phosphorylated, self-ligated, and transformed into Escherichia coli. The design of this protocol is straightforward and the procedures are brief. However, hybridization has to be conducted for mutant screening because the difference between the original sequence and the target sequence is only one or a few base pairs, which makes the most common screening method, i.e., restriction digestion, not applicable. The laborious hybridization step with hazardous isotope deters researchers from adopting this simple mutagenesis method. Here we present a novel mutagenesis strategy, designed restriction endonuclease-assisted mutagenesis (DREAM), which introduces one or more restriction endonuclease cleavage site(s) into proximity of the mutation site without altering the target amino acid sequence, so as to simplify the mutant screening and make the method more practical.
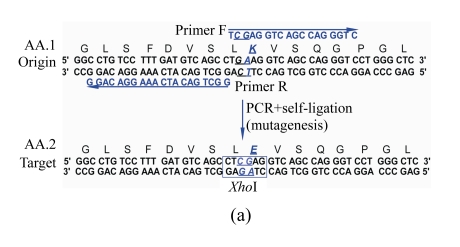
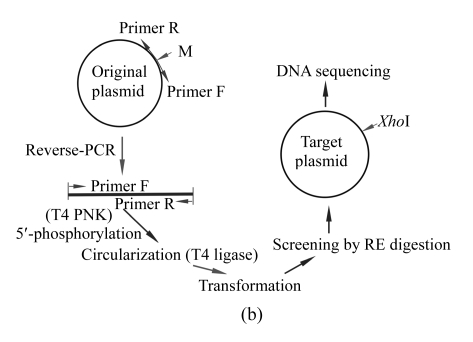
The principle of the suggested SDM method is outlined in Fig.1. For an amino acid sequence to be mutated, its target sequence is reversely translated into DNA sequences using degenerate codons, resulting in many silently mutated sequences containing various restriction endonuclease cleavage sites. Certain sequence with an appropriate restriction site is selected as the target DNA sequence for designing mutagenic primers. The full-length plasmid DNA was amplified by inverse PCR with a high-fidelity DNA polymerase and the amplified product was 5′-phosphorylated by T4 polynucleotide kinase and then self-ligated. After transformation into an E. coli host, the transformants were readily screened by digesting with the introduced restriction endonuclease.
Fig. 1.
Schematic diagram of the site-directed mutagenesis (SDM) method
(a) Designing of target DNA sequence. To introduce an appropriate restriction site into the target sequence, the target amino acid sequence is reverse-translated into a silently mutated sequence. Mutagenic primers for inverse PCR to amplify the full-length plasmid are selected according to silently mutated DNA sequence. Origin: the original DNA sequence to be mutated; Target: the designed target DNA sequence with a designed restriction site for rapid mutant screening; AA.1: amino acid sequence deduced from the original DNA sequence; AA.2: the target amino acid sequence. The mutated nucleotides are underlined, and the designed restriction site (XhoI) is indicated by an open box; (b) Flow chart of the mutagenesis procedures. PNK: polynucleotide kinase; RE: restriction endonuclease; PCR: polymerase chain reaction
To demonstrate the validity of the suggested method, we designed a point mutation in plasmid pcDNA3.1-pIgR, which is approximately 8 kb in size and expresses human polymeric immunoglobulin receptor (pIgR) by the cytomegalovirus (CMV) early promoter and enhancer. We performed the SDM to introduce an amino acid substitution (Lys to Glu) in pIgR cDNA on this plasmid (Fig.1). To simplify the restriction site designing, we made use of a free online tool WatCut (http://watcut.uwaterloo.ca/watcut/watcut/template.php?act=silent_new) to display all the silently mutated sequences containing restriction sites. From the displayed sequences, we chose a sequence containing an XhoI site as our target sequence. Based on this sequence, we designed a pair of inverse PCR primers (Fig.1). The sequences of the forward and reverse primers are 5′-TCG AGG TCA GCC AGG GTC-3′ and 5′-GGC TGA CAT CAA AGG ACA GG-3′, respectively. Since the inverse PCR was conducted with a high-fidelity DNA polymerase with proofreading 3′-5′ exonuclease activity, there were no 3′-A tails generated. So the primers were designed by directly copying the target sequence without overlapping region included (Fig.1).
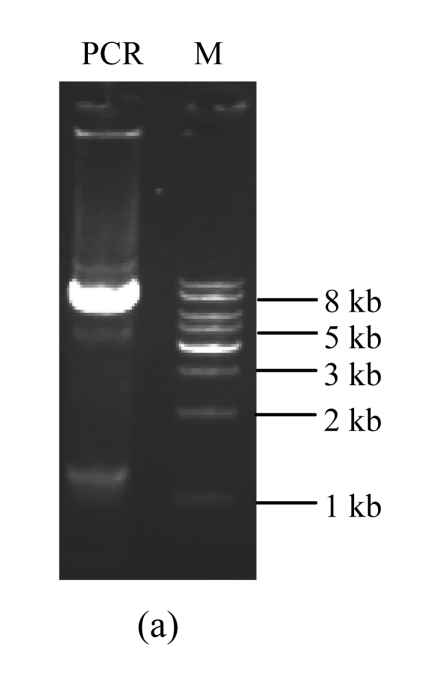
A standard PCR reaction was then carried out with a 50-μl total reaction mixture containing 1× HF PCR buffer (Mg2+ Plus, Invitrogen), 200 μmol/L dNTPs, 200 nmol/L forward and reverse primers, 1 ng template DNA (pcDNA3.1-pIgR), and 1 U Phusion™ high-fidelity DNA polymerase (New England BioLabs). PCR reaction was carried out with a GeneAmp PCR system 2400 (Perkin Elmer, Foster City, CA, USA). The PCR parameters were as follows: pre-denaturation at 98 °C for 30 s; followed by 35 cycles of denaturation at 98 °C for 10 s, annealing at 65 °C for 20 s, and polymerization at 72 °C for 150 s; and a final extension step at 72 °C for 10 min. The PCR products were then separated on 1% (w/v) agarose gel electrophoresis (Fig.2a) and recovered using a glass milk DNA extraction kit (BioDev, Beijing, China). A phosphorylation reaction of the PCR products was carried out at 37 °C for 30 min in 50-μl total reaction mixture containing 1× T4 polynucleotide kinase buffer (50 mmol/L Tris-HCl, 10 mmol/L MgCl2, and 5 mmol/L DL-dithiothreitol (DTT)), 5 U T4 polynucleotide kinase (PNK, New England BioLabs), and 200 μmol/L adenosine triphosphate (ATP). After an inactivation step at 70 °C for 5 min to inactivate the T4 polynucleotide kinase, the PCR products were circularized using 350 U T4 DNA ligase (Takara) at 12 °C for 16 h. 10 μl of the ligation mixture was then used to transform competent E. coli Top10 (Invitrogen) and the transformation mixture was plated on a Luria-Bertani (LB) agar plate containing 100 μg/ml ampicillin and incubated at 37 °C.
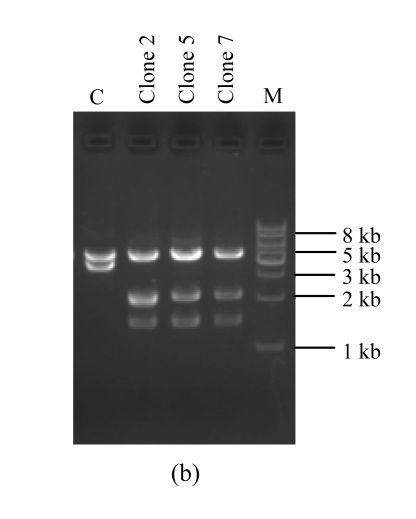
Fig. 2.
Amplification and screening of target plasmid
DNAs were subjected to 1% (w/v) agarose gel electrophoresis and visualized with ethidium bromide staining. (a) Amplification of the full-length target plasmid; (b) Restriction analysis of the target plasmids. Recombinant plasmids and the original plasmid as a control are digested by designed endonuclease XhoI combined with BglII. PCR: the amplified products; M: DNA molecular marker (1 kb ladder); C: the original plasmid pcDNA3.1-pIgR as a control
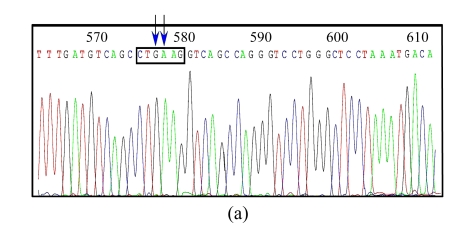
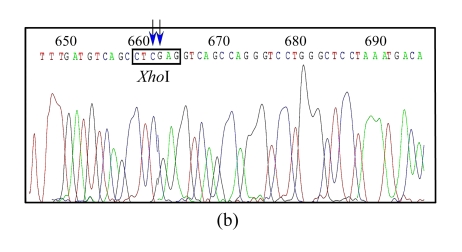
Ten colonies were picked up randomly and the plasmid DNA was extracted and digested by the designed restriction enzyme XhoI along with another enzyme BglII. Of the 10 plasmids, 3 displayed the desired electrophoresis pattern (Fig.2b). DNA sequencing analysis confirmed that all of the three clones contained the correctly mutated DNA sequence (Fig.3b). To check whether unwanted mutations were generated by PCR in the pIgR genes and in the regulation elements, we sequenced a region of about 3600 bp of a clone, which encompasses the whole pIgR open reading frame, the CMV early promoter and enhancer, and the bovine growth hormone (BGH) polyadenylation site. The results show that no additional mutations were caused by PCR.
Fig. 3.
DNA sequence analysis of the original and target plasmids
(a) The original plasmid DNA sequence with arrows to show the nucleotides to be mutated; (b) The target plasmid with arrows to show the target nucleotides and an open box to show the designed restriction site XhoI
In summary, we have developed a novel SDM strategy that makes use of the degenerate amino acid codons to introduce a restriction enzyme cleavage site into the proximity of the mutation site for easy and rapid mutant screening, thus eliminating the necessity for arduous hybridization screening using hazardous isotopes. The whole mutagenesis procedure is simple and no commercial kits are needed. Since the mutagenesis is carried out directly on double stranded plasmid DNA, the resulted plasmid is fit for direct use without any further subcloning. This mutagenesis protocol can not only introduce point mutations, but also be used to generate insertions and deletions. To perform this PCR-based SDM, a high-fidelity thermostable DNA polymerase should be used to avoid unwanted mutations that could be resulted from DNA polymerization with error-prone thermostable DNA polymerase like Taq. We recommend the New England BioLabs Phusion™ DNA polymerase, because it has a very low mutation rate (4.4×10−7 bp−1), which is 50 times lower than that of the Taq polymerase and 6 times lower than that of the Pfu polymerase. The very high fidelity of Phusion™ DNA polymerase may account for the results that there were no unwanted mutations in our experiments. Other virtues of Phusion™ polymerase include high speed and high yield, which makes it easy to amplify a DNA of up to 20 kb according to the manufacturer’s manual. Since most plasmids are shorter than 20 kb, this SDM protocol could be applied virtually to all plasmids.
Footnotes
Project supported by the Hi-Tech Research and Development (863) Program of China (No. 2007AA02Z151) and the National Natural Science Foundation of China (No. 30872223)
References
- 1.An Y, Ji J, Wu W, Lv A, Huang R, Wei Y. A rapid and efficient method for multiple-site mutagenesis with a modified overlap extension PCR. Appl Microbiol Biotechnol. 2005;68(6):774–778. doi: 10.1007/s00253-005-1948-8. [DOI] [PubMed] [Google Scholar]
- 2.Chapnik N, Sherman H, Froy O. A one-tube site-directed mutagenesis method using PCR and primer extension. Anal Biochem. 2008;372(2):255–257. doi: 10.1016/j.ab.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Heckman KL, Pease LR. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat Protoc. 2007;2(4):924–932. doi: 10.1038/nprot.2007.132. [DOI] [PubMed] [Google Scholar]
- 4.Jin C, Cai X, Ma H, Xue Y, Yao J, Yao X. An efficient site-directed mutagenesis method for ColE1-type ori plasmid. Anal Biochem. 2007;363(1):151–153. doi: 10.1016/j.ab.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Qiu Y, Shen Y, Ding C, Liu P, Zhou J, Ma Z. Splicing together different regions of a gene by modified polymerase chain reaction-based site-directed mutagenesis. Anal Biochem. 2008;373(2):398–400. doi: 10.1016/j.ab.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Nagy ZB, Felfoldi F, Tamas L, Puskas LG. A one-tube, two-step polymerase chain reaction-based site-directed mutagenesis method with simple identification of the mutated product. Anal Biochem. 2004;324(2):301–303. doi: 10.1016/j.ab.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Rabhi I, Guedel N, Chouk I, Zerria K, Barbouche MR, Dellagi K, Fathallah DM. A novel simple and rapid PCR-based site-directed mutagenesis method. Mol Biotechnol. 2004;26(1):27–34. doi: 10.1385/MB:26:1:27. [DOI] [PubMed] [Google Scholar]
- 8.Seyfang A, Jin JH. Multiple site-directed mutagenesis of more than 10 sites simultaneously and in a single round. Anal Biochem. 2004;324(2):285–291. doi: 10.1016/j.ab.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Tseng WC, Lin JW, Wei TY, Fang TY. A novel megaprimed and ligase-free, PCR-based, site-directed mutagenesis method. Anal Biochem. 2008;375(2):376–378. doi: 10.1016/j.ab.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Wei D, Li M, Zhang X, Xing L. An improvement of the site-directed mutagenesis method by combination of megaprimer, one-side PCR and DpnI treatment. Anal Biochem. 2004;331(2):401–403. doi: 10.1016/j.ab.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Zheng L, Baumann U, Reymond JL. An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res. 2004;32(14):e115. doi: 10.1093/nar/gnh110. [DOI] [PMC free article] [PubMed] [Google Scholar]