Summary
Antrochoanal polyp was described by Professor Gustav Killian, in 1906, giving a specificity among polyposis; it represents 4-6% of all nasal polyps and displays both analogies and differences with bilateral nasal polyposis. Antrochoanal polyp is a benign lesion originating from the mucosa of the maxillary sinus, growing through the accessory ostium into the middle meatus and, thereafter, protruding posteriorly to the choana and nasopharynx. Incomplete excision of antrochoanal polyp almost always leads to recurrence. The Authors, therefore, provocatively question? Whether the antrochoanal polyp is a benign tumour or not? The Authors analyse the largest series of antrochoanal polyps present in the literature and report on a series of 200 patients treated consecutively at the ENT Clinic at the University of Florence, Italy. Clinical-aetiological data related to these 200 patients, treated between January 1988 and April 2006, have been analysed. Evaluation of the data presents some analogies and some disagreement with results from other series. In conclusion, based on the data obtained, it is tempting to suggest that the antrochoanal polyp develops from an increase in pressure in the Highmoro antrum due to a phlogistic-anatomical alteration at ostio-meatal complex/middle meatus level, in patients with a pre-existing silent antral cyst, subsequently forced to herniation outside, through the accessory ostium.
Keywords: Antrochoanal polyp, Antral cyst, Accessory ostium, Maxillary ostium obstruction
Riassunto
Il polipo antrocoanale fu descritto dal Professor Gustav Killian nel 1906, che gli attribuì una specificità tra le varie forme di poliposi. Esso rappresenta il 4-6% di tutti i polipi nasali ed ha analogie e differenze con le poliposi nasali bilaterali. Si tratta di una lesione benigna che origina dalla mucosa del seno mascellare, si accresce attraverso l’ostio accessorio verso il meato medio e successivamente si estende posteriormente verso la coana e il rinofaringe. L’escissione incompleta del polipo antrocoanale è destinata ad una sicura recidiva. Per questa ragione ci siamo chiesti in modo provocatorio se il polipo antrocoanale sia da considerare un tumore benigno o meno. Abbiamo studiato la più ampia casistica di polipi antrocoanali presenti in letteratura composta da 200 pazienti trattati consecutivamente presso la Clinica Otorinolaringoiatrica dell’Università di Firenze, analizzando molti dati clinici ed eziologici di questi 200 pazienti curati tra il gennaio 1988 e l’aprile 2006. La valutazione dei nostri dati presenta alcune analogie e alcune differenze con i risultati di altre casistiche. Sulla base dei dati in nostro possesso e sulle nostre considerazioni possiamo ipotizzare che i polipi antrocoanali si sviluppino a causa di un aumento pressorio all’interno dell’antro di Highmoro, causato da alterazioni flogistico-anatomiche a livello del complesso ostio-meatale/meato medio, in pazienti con una preesistente cisti antrale silente, successivamente forzata ad erniare attraverso l’ostio mascellare accessorio.
Introduction
In 1691, Fredrik Ruysch, the famous Dutch anatomist, described two cases of nasal polyps arising in the Highmoro antrum 1.
In 1891, Zuckerkandl from Graz, described the case of a polyp arising from the maxillary sinus and coming out through a wide accessory ostium 2.
Professor Gustav Killian, from Freiburg, in his paper “The origin of Choanal polypi”. published in The Lancet on July 14, 1906, was the first to describe antrochoanal polyp (ACP) giving a specificity among nasal polyposis: “Choanal polypi are usually unilateral and solitary. They have a peculiar pear-shaped form. In the thick part of the polypus, there is usually a large cystic space. This may be prolonged well into the stalk or the stalk may consist of nothing more than the thin wall of a cyst. Microscopically, these polyps differ very little from the common nasal polyps. Choanal polyps must, in fact, all be in the form of a constricted sac of which one half is in the antrum and the other in the nose and nasopharynx. Thus a maxillary part is distinguished from a naso-pharyngeal part. By means of inflation or irrigation of the antrum through the accessory ostium, a polypus is occasionally driven out of the cavity into the nose” 3. According to an interesting statement in the literature, Palfyn, in 1753 4, had already observed such a case.
Three years after Gustav Killian, Brown Kelly, in 1909, observing a frequent association between antral cyst and the presence of a wide accessory ostium, demonstrated a correlation between these conditions 5.
ACPs represent 4-6% of all nasal polyps 6. Nasal bilateral polyposis is a clinical condition found in ~1-4% of the Caucasian population 7.
Some analogies and some differences between ACP and bilateral nasal polyposis can be found, namely:
In bilateral nasal polyposis and in ACP, the male/female ratio is 2:1 6 8.
Larsen and Tos found that the mean age at presentation of nasal polyposis was 50 years and for ACP 27 years 9. ACPs are more frequent in children. Chen et al. 10 found that 28% of ACPs occurred in children. One third of all nasal polyps in children are ACP 11.
In the literature, only one case of ACP, occurring in members of a same family (two brothers), has been described. On the contrary, it is well documented that simple nasal polyps can occur in several members of a same family 7.
ACPs are usually unilateral with only 3 bilateral cases having been reported in the literature 12–14. On the contrary, nasal polyps are usually bilateral.
Il-5 has a chemotactic effect for eosinophils, and its synthesis is increased in bilateral nasal polyposis when compared to ACP 15.
Bilateral nasal polyposis stems from the ethmoid cells; ACP originates from the maxillary sinus 16. Stammberger & Hawke found that ACP left the sinus through an accessory ostium in 70% of the cases studied 17, but probably this occurs in 100%.
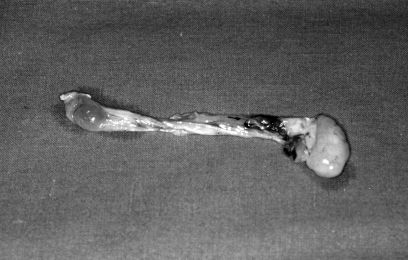
Macroscopically, ACP is composed of a cystic part filling the maxillary sinus and a solid part emerging through an enlarged maxillary accessory ostium 18, (Fig. 1). Bilateral ethmoidal nasal polyps never present cystic cavities.
Fig. 1.
Macroscopically the antrochoanal polyp is composed of a cystic part (filling the maxillary sinus) and a solid part coming out through the maxillary ostium.
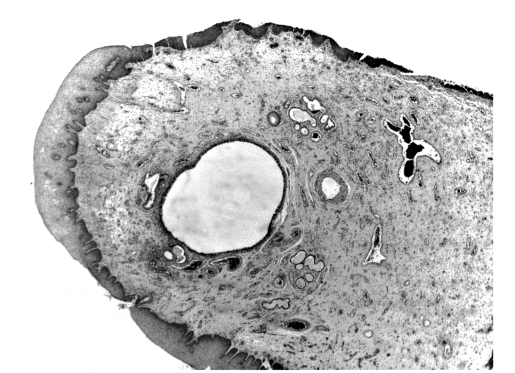
Microscopically, the antral part of an ACP polyp shows a central cystic cavity (Fig. 2) surrounded by a homogeneous oedematous stroma with few cells. The surfaces of the polyp are covered by a respiratory epithelium 19.
Fig. 2.
An original histological feature shows the presence of cystic frame in an antrochoanal polyp.
The cysts are often collapsed with an endothelial lining.
This endothelial lining is best revealed with Ulex Europeas, a marker that is specific for lymphatic endothelium 20.
It is important to stress out that no histological differences were observed between maxillary intramural cysts and the cystic part of ACP 18.
Occasionally, ACP may display pseudosarcomatous changes 21.
ACP shows clear histological differences when compared to bilateral ethmoidal polyps, consisting of a lower inflammatory infiltrate and a lower eosinophil infiltrate 22.
The more common manifestation of ACP is unilateral nasal obstruction (especially during the expiratory phase), but may sometimes be (20-25% of cases) bilateral, depending upon the blockage of the nasopharynx 23.
Other clinical manifestations are rhinorrhoea, bleeding, snoring, foreign body sensation, halitosis, headache, post nasal drip and loss of sense of smell.
There are occasional reports of cases starting with epistaxis 24, polyp strangulation 25, spontaneous amputation 26, dyspnoea and dysphagia 27, with extension to the mouth producing dysphagia and speech disorders 28, obstructive sleep apnoea and cachexia 29.
Nasal endoscopy and computed tomography (CT) represent the golden standard in the diagnosis of ACP.
During nasal anterior rhinoscopy or nasal endoscopy, ACP appears as a bright, white, mass in the middle meatus and nasal cavity, with a stalk rising to the accessory ostium and sometimes, during the examination of the oral cavity, as a white egg shape mass behind the uvula.
By using CT, the diagnosis of ACP is made when a mass fills the maxillary sinus growing through the accessory or natural ostium into the middle meatus and the posterior choana 19. CT distinctively reveals a hypo-attenuating mass occupying the maxillary sinus and extending through the middle meatus into the nasal cavity and sometimes also extending posteriorly toward the choana 30.
Magnetic resonance imaging (MRI) shows hypointense T1, and enhanced T2 signals. When intravenous gadolinium is administered, the cystic part of the polyp is enhanced in the peripheral area 31.
The differential diagnosis is between ACP and tumours such as angiofibroma, olfactory neuroblastoma, meningo-encefalocele or hemangioma. Inverted papilloma is a typically unilateral lesion which must be differentiated from ACP.
Other bone-destroying diseases such as lymphoma, Wegener granulomatosis or rhabdomyosarcoma should also be considered 32.
Surgery is the only feasible treatment. Several surgical techniques have been described in the literature.
In the past, the Caldwell-Luc technique was used.
The Caldwell-Luc approach offers good exposure and ensures complete removal of the polyp and the associated antral mucosa 11. However, the Caldwell-Luc procedure may have possible effects including cheek anaesthesia, cheek swelling and carries risks to the developing teeth in children 33.
Functional endoscopic sinus surgery (FESS) is, currently, the gold standard technique 34.
The accepted treatment for ACP is removal by FESS and this technique consists of resection of the nasal part of the polyp and its cystic antral part with its attachment to the maxillary wall via the middle meatus 17.
The lower part of the uncinate process is removed and then the maxillary ostium widened. If the polyp is too large for en bloc removal, the intra-nasal portion of the ACP is cut with scissors. Endoscopes (45°–70°–120°) are used to identify the maxillary antrum and the point of origin of the cystic part of ACP. The antral portion of an ACP should be removed, together with the base of its origin, to minimize post-operative recurrence 16.
The use of a microdebrider may be indicated, as complementary to endoscopic surgery 16.
The polyps usually arise from the posterior, inferior, lateral or medial walls of the maxillary antrum and only in rare cases from the anterior wall 22.
Identifying and removing the origin of the polyp in the maxillary antrum together with the main bulk of the polyp, are cornerstones to successful treatment of ACPs 35.
Another surgical technique has been described by El-Guindy & Mansour 36. ACP are treated by combining endoscopic middle meatal surgery and transcanine sinuso-scopy. More recently, Hong et al. described this technique with powered instrumentation 16: the antral cystic part of the ACP polyp is removed by a trocar inserted through the canine fossa. The cannula of the trocar is removed, leaving the sheath in the canine fossa. Under endoscopic control via the middle meatus, a microdebrider is inserted into the sinus and the antral portion of the ACP is resected by powered instrumentation.
The combined endoscopic and transcanine approach is suggested in the management of ACPs originating from the lateral and anterior wall of the antrum and the transnasal endoscopic approach for other localizations 16 35.
The success rate was 76.9% in the transnasal endoscopic approach and 100% in the combined endoscopic and transcanine approach 35.
Material and Methods
Herein, we report on the largest series of ACP so far described in the literature: 200 patients treated and observed at follow-up at the ENT Department of Florence University between January 1988 and April 2006.
The following clinical-aetiological data were analysed:
sex;
age;
Ige-mediated allergy;
asthma association;
association with chronic infectious rhino-sinusitis;
anatomical nasal features;
surgery;
recurrences;
histology.
Results
Sex: there were 128 males (64%) and 72 females (36%), thus a male preponderance.
Age: age range 5-81 years, mean age 40 years, median age 29 years. Only 8 patients were < 10 years old (4%).
IgE-mediated allergy: in 70 patients allergies were revealed by skin tests (35%) and 50 (25%) showed allergy to perennial allergens.
Asthma association: 6 patients had associated asthma (3%).
Chronic rhino-sinusitis: a previous history of chronic infectious rhino-sinusitis was found in 20 (10%) patients.
-
Nasal anatomical features: of these 200 ACP patients, 100 had ACP in the left nasal cavity (50%), 97 (48.5%) in the right and 3 bilateral (1.5%).
110 patients had septal deviation (55%), 42 (21%) inferior turbinate hypertrophy, and 14 (7%) concha bullosa. Only, 44 patients were without nasal anatomical defects.
It is important to emphasize that the presence of an anatomic alteration, such as septal deviation or turbinate alteration is the only significant correlating factor (p < 0.001) found when evaluating the present series (Chi-square test).
-
Surgery: in 4 cases (children < 7 years), only polypectomy was performed (2%), in 24, Caldwell-Luc operation and polypectomy (before 1993) (12%), in 172 (86%), FESS, together with septum correction in 67 (33.5%).
No major complications occurred.
-
Recurrences: all 200 patients were observed at follow-up with nasal endoscopy.
Recurrence was detected in 4 (2%) patients, all of which in children < 7 years of age in whom only polypectomy was performed and the recurrences were observed within 18 months; thereafter FESS was performed without recurrence.
Histology: all polyps were described as inflammatory polyps at histological examination.
Discussion
In 1988, Berg et al. suggested that ACP could arise from an antral cyst 18.
It is important to note that antral cysts are present in 8-10% of the population 18.
It has also been suggested that the development of the cystic part of ACP can be caused by acinar mucous gland obstruction as a result of chronic phlogosis (allergic or infectious) 10.
Piquet et al. hypothesized that these cysts arise from lymphatic duct stenosis following inflammation of the sinus mucosa 20.
Intramural cysts are a frequent finding in young patients. Intramural cysts, occcurring early in life, may emerge from tooth channels through which the permanent teeth have migrated 37.
It is important to bear in mind that concomitant development of antral cysts and maxillary ostium obstruction could be caused by the same chronic inflammatory (allergic or infectious) condition 38.
On the basis of these observations and evaluating the data obtained, the obvious question is: What could be responsible for causing antral cyst herniation through the accessory maxillary ostium into the nose giving rise to an ACP?
Thereafter, a pathogenetic hypothesis can be suggested.
First of all, it is possible that the same chronic inflammatory (allergic or infectious) factors, could cause not only the development of an antral cyst and mucosal oedema but also swelling at the ostio-meatal complex/middle meatus level.
Therefore, the simultaneous complete closure of the accessory maxillary ostium, obstructed by the medial surface of an antral cyst and the partial obstruction of maxillary natural ostium could be possible.
The air, penetrating the sinus through the narrowed natural ostium (Fig. 3a) would be unable to be released during breathing out on account of the mucosal oedema or swelling at ostio-meatal complex/middle meatus level. According to the Bernoulli theory, the rate of flow is higher at the stricture, creating a pressure drop perpendicular to the wall of the stricture, thus complete obstruction of the natural ostium during breathing out is more likely to occur. The simultaneous obstruction of the natural and accessory ostium contribute to the increase in the pressure level in the Highmoro antrum (Fig. 3b).
Fig. 3a.
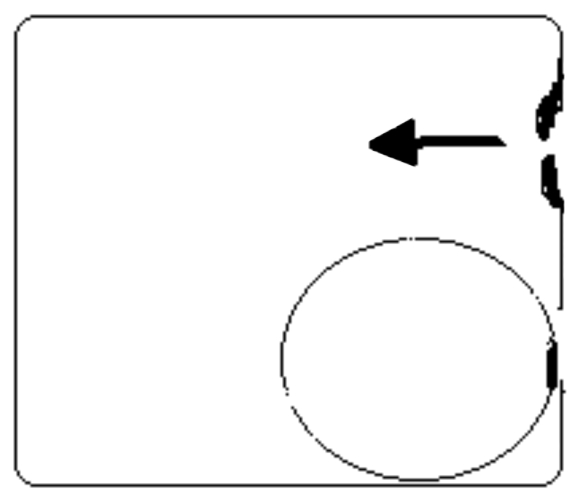
Closure of the accessory ostium obstructed by the medial surface of a pre-existent silent antral cyst make easy the increasing pressure level in maxillary sinus.
Fig. 3b.
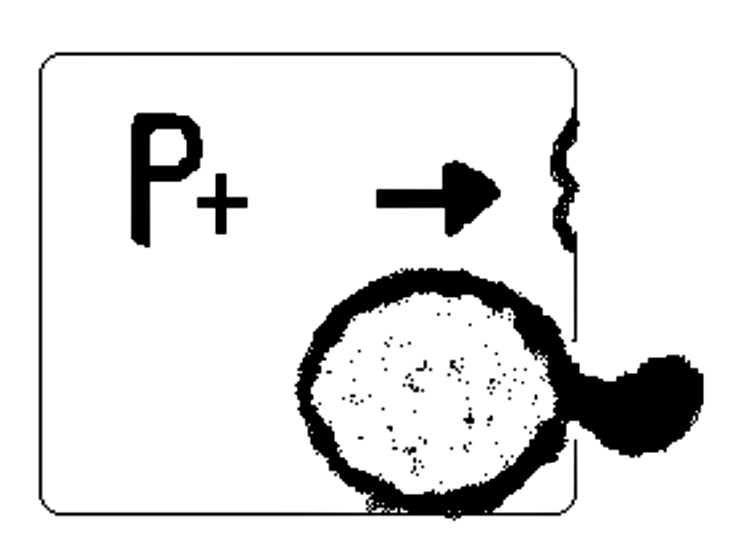
Due to the partial obstruction of maxillary ostium the air, penetrating the sinus through the natural ostium would not be able to exit during expiration.
Thus due to the increased pressure level in the maxillary sinus, an intramural cyst could be forced to herniate outside, through the accessory ostium, thus giving rise to ACP formation (Fig. 3).
Fig. 3c.
The intramural cyst would be forced to herniate outside, through the accessory ostium, giving rise to ACP formation.
This mechanism will probably be enhanced by all the anatomical features which may lead to changes in the pressure gradient between the middle meatus and Highmoro antrum level (septal deviation or spur, alteration of uncinate process, hypertrophy of inferior turbinate and bulla etmoidalis, or concha bullosa).
In our series, 83% of cases present anatomical disorders, 110 patients had septal deviation (55%), 42 (21%) inferior turbinate hypertrophy, and 14 (7%) concha bullosa.
Conclusions
The present report refers to the largest series of ACP described in the literature: 200 patients treated and observed at follow-up at the ENT Department of Florence University between January 1988 and April 2006.
The most important data emerging from the study were:
the presence of nasal disorders of an anatomical nature (83% of cases) represents the only statistically significant factor correlated to the presence of ACP in our review (p < 0.001);
35% present a clinical history of allergy and 10% a clinical history of infectious chronic rhinosinusitis;
only 8 patients were < 10 years old (4%);
3 cases were bilateral.
One hundred years after the first detailed description by Killian, we have hypothesized a pathogenetic origin.
Development of ACP could be due to an increased pressure level in the Highmoro antrum caused by a inflammatory-anatomical alteration at ostio-meatal complex/middle meatus level, in a patient with a pre-existing silent antral cyst, subsequently forced to herniate outside, through the accessory ostium.
The primary aim of FESS will be complete removal of the polyp (both nasal and cystic parts).
Furthermore, on the basis of our hypothesis, we suggest performing a wide middle meatotomy connecting the natural and accessory ostium and, last but not least, to correct all the predisposing factors of an anatomical nature, such as septal deviations, spurs, turbinate hypertrophy or concha bullosa.
It would also be important to perform the Prick test and radioallergosorbent test as well as nasal cytology in all ACP patients, in order to prescribe appropriate medical treatment in allergic patients and in patients with chronic bacterial rhinosinusitis.
References
- 1.Ruysch F. Observation um anatomica chirurgicaram anturca. 1691. [Google Scholar]
- 2.Zuckerkandl E. Normale und pathologische Anatomie der Nasenholme. Vienna 1892. [Google Scholar]
- 3.Killian G. The origin of choanal polypi. Lancet 1906;2:81-2. [Google Scholar]
- 4.Palfyn J. Anatomie chirurgicale. Paris 1753. [Google Scholar]
- 5.Brown K. Nasal antral polyposis. Lancet 1909;1:89-91. [Google Scholar]
- 6.Sirola R. Choanal polyps. Acta Otolaryngol 1965;64:42-8. [PubMed] [Google Scholar]
- 7.Montague ML, McGarry GW. Familial antrochoanal polyposis – a case report. Eur Arch Otorhinolaryngol 2004;261:507-8. [DOI] [PubMed] [Google Scholar]
- 8.Settipane GA, Chafee FII. Nasal polyps in asthma and rhinitis. A review of 6037 patients. J Allergy Clin Immunol 1977;59:17-21. [DOI] [PubMed] [Google Scholar]
- 9.Larsen PL, Tos M. Origin of nasal polyposis. Laryngoscope 1991;101:305-12. [DOI] [PubMed] [Google Scholar]
- 10.Chen JK, Schloss MD, Azouz ME. Antrochoanal polyps: 10-year retrospective study in the pediatric population with a review of the literature. J Otolaryngol 1989;18:168-72. [PubMed] [Google Scholar]
- 11.Schramm VL, Efferon MZ. Nasal polyps in children. Laryngoscope 1980;90:1488-95. [PubMed] [Google Scholar]
- 12.Myatt HM, Cabrera M. Bilateral antrochoanal polyps in a child; a case report. J Laryngol Otol 1996;110:272-4. [DOI] [PubMed] [Google Scholar]
- 13.Basu SK, Bandyopadhyay SN, Bora H. Bilateral antrochoanal polyps. J Laryngol Otol 2001;115:561-2. [DOI] [PubMed] [Google Scholar]
- 14.Ylmaz YF, Titiz A, Ozcam M, Tezer MS, Ozlugedik S, Unal A. Bilateral antrochoanal polyps in an adult: a case report. B-ENT 2007;397-9. [PubMed] [Google Scholar]
- 15.Bachert C, Van Cauwenberge PB. Inflammatory mechanisms in chronic sinusitis. Acta Otorhinolaryngol Belg 1977;51:209-17. [PubMed] [Google Scholar]
- 16.Hong SK, Min YG, Kim CN, Byun SW. Endoscopic removal of antral part of antrochoanal polyp by powered instrumentation. Laryngoscope 2001;111:1774-8. [DOI] [PubMed] [Google Scholar]
- 17.Stammberger H, Hawke M. Essentials of functional endoscopic sinus surgery. St Louis: Mosby 1993. p. 103-5. [Google Scholar]
- 18.Berg O, Carenfelt C, Silfversward C, Sobin A. Origin of the choanal polyp. Arch Otolaryngol Head Neck Surg 1988;114:1270-1. [DOI] [PubMed] [Google Scholar]
- 19.Maldonado M, Martines A, Alobid I, Mullol J. The antrochoanal polyp. Rhinology 2004;43:178-82. [PubMed] [Google Scholar]
- 20.Piquet JJ, Chevalier D, Leger GP, Rouquette I, Leconte-Houcke M. Microchirurgie endo-nasale du polype antro-choanal. Acta Otorhinolaryngol Belg 1992;46:267-71. [PubMed] [Google Scholar]
- 21.Heck WE, Hallberg OE, Williams HL. Antrochoanal polyps. Arch Otolaryngol 1950;52:538-8. [DOI] [PubMed] [Google Scholar]
- 22.Min YG, Chung JW, Shin JS, Chi JG. Histologic structure of antrochoanal polyps. Acta Otolaryngol 1995;115:543-7. [DOI] [PubMed] [Google Scholar]
- 23.Kamel R. Endoscopic transnasal surgery in antrochoanal polyps. Arch Otolaryngol Head Neck Surg 1990;116:841-3. [DOI] [PubMed] [Google Scholar]
- 24.Robson AK, Barker CS, Whittet HB. Epistaxis as an unusual presentation of an antrochoanal polyp. J Laryngol Otol 1990;104:643-4. [DOI] [PubMed] [Google Scholar]
- 25.Ole-Lengine L, Manni JJ. A strangulation antrochoanal polyp. A case report. J Laryngol Otol 1993;107:342-3. [DOI] [PubMed] [Google Scholar]
- 26.Rashid AM, Soosay G, Morgan D. Unusual presentation of a nasal (antrochoanal) polyp. Br J Clin Pract 1994;48:108-9. [PubMed] [Google Scholar]
- 27.Grewal DS, Sharma BK. Dyspnea and dysphagia in a child due to an antrochoanal polyp. Auris Nasus Larynx 1984;11:25-8. [DOI] [PubMed] [Google Scholar]
- 28.Sharma HS, Daud ARA. Antrochoanal polyp “a rare paediatric emergency”. Int J Pediatr Otorhinolaryngol 1997;41:65-70. [DOI] [PubMed] [Google Scholar]
- 29.Salib RJ, Sarlek SA, Dutt SN, Pearman K. Antrochoanal polyp presenting with obstructive sleep apnea and cachexia. Int J Pediatr Otorhinolaryngol 2000;54:163-6. [DOI] [PubMed] [Google Scholar]
- 30.Weissman JL, Tabor EK, Curtin HD. Sphenochoanal polyps: Evaluation with CT and MRI imaging. Radiology 1991;178:145-8. [DOI] [PubMed] [Google Scholar]
- 31.Vuysere S, Hermans R, Marchal G. Sinochoanal polyp and its variant, the angiomatous polyp; MRI findings. Eur Radiol 2001;11:55-8. [DOI] [PubMed] [Google Scholar]
- 32.Grainger AJ, Zammit-Maempel I. Antrochoanal polyps in children. Eur Radiol 2001;11:347. [DOI] [PubMed] [Google Scholar]
- 33.Woolley AL, Clary RA, Lusk RP. Antrochoanal polyps in children. Am J Otolaryngol 1996;17:368-73. [DOI] [PubMed] [Google Scholar]
- 34.Vleming M, De Vries N. Endoscopic sinus surgery for antrochoanal polyps. Rhinology 1991;29:77-8. [PubMed] [Google Scholar]
- 35.Ta-Jen Lee, Shiang-Fu Huang. Endoscopic sinus surgery for antrochoanal polyps in children. Otolaryngol Head Neck Surg 2006;135:688-92. [DOI] [PubMed] [Google Scholar]
- 36.El-Guindy A, Mansour MH. The role of transcanine surgery in antrochoanal polyps. J Laryngol Otol 1994;108:1055-7. [DOI] [PubMed] [Google Scholar]
- 37.Berg O, Carenfelt C, Sobn A. On the diagnosis and pathogenesis of intramural maxillary cysts. Acta Otolaryngol (Stockh) 1989;108:464-8. [DOI] [PubMed] [Google Scholar]
- 38.Cook PR, Davis WE, McDonald R, McKinsey JP. Antrochoanal polyposis: A review of 33 cases. Ear Nose Throat J 1993;72:401-12. [PubMed] [Google Scholar]