Abstract
Background
Puberty is an extremely important phase in the physical and psychosocial development of the adolescent.
Methods
Selective literature review.
Results
The diagnosis of abnormal puberty requires thorough knowledge of normal pubertal development and of the variations of normal puberty as well as its pathology. Variations of normal pubertal development can be expected, by definition, to occur at a frequency of roughly 3%. A detailed history is the first step in the diagnostic evaluation of a normal variant or an abnormal puberty. Further evaluation includes laboratory testing (estradiol, testosterone, and the results of a GnRH test, among others) and imaging studies (x-ray of the left hand and wrist, ultrasonography of the gonads, magnetic resonance imaging). Treatment is directed at both the acute and the long-term consequences of precocious, markedly delayed, or absent pubertal development.
Conclusions
Disorders of pubertal development should be recognized early, correctly diagnosed by a pediatric endocrinologist, and appropriately treated.
Keywords: child and adolescent health, puberty, disorders of pubertal development, hypogonadism
Puberty is a sensitive phase of physical, mental, and social development for both girls and boys. A thorough acquaintance with the normal course of puberty is necessary. Any deviation from it, though it will be viewed with great anxiety by the young patient, can represent either a normal or pathological variant of pubertal development. The physician should be able to provide the young patient with accurate information and see him or her through the process of puberty in a reassuring manner.
If a normal variant is present, the treating physician can help the patient and his or her parents with thorough counseling. Rarely there is a need for a time-consuming and expensive diagnostic evaluation. If the child’s pubertal development is pathological, the cause of the pubertal disorder must be found by specific diagnostic testing, and the necessary treatment must be initiated.
The learning objectives of this article are to acquaint the reader thoroughly with
normal pubertal development and its temporal course;
normal variants and pathological disorders of pubertal development, and their etiologies.
This article is based on a selective review of the literature. No clinical guidelines on this subject are available.
Normal puberty
Hormonal control of puberty.
Puberty begins with activation of the hypothalamic-pituitary-gonadal system.
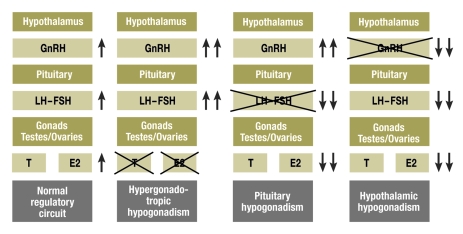
The hypothalamic-pituitary-gonadal axis undergoes an active phase during fetal and neonatal development and then enters a resting phase that lasts for the rest of childhood till puberty. Puberty begins with an activation of the hypothalamic-pituitary-gonadal system (figure). The influence of the hypothalamic hormone GnRH (gonadotropin releasing hormone), the gonadotropins LH (luteinizing hormone) and FSH (follicle-stimulating hormone), and the sex steroids estradiol or testosterone brings about the manifestations of puberty, both external (breast development, genital enlargement) and internal (uterus, ovaries, testes). Pubic hair develops independently of the activation of the hypothalamic-pituitary-gonadal pathways, largely through the effect of androgens secreted by the adrenal glands (adrenarche).
Figure.
Schematic representation of the hypothalamic-pituitary-gonadal pathways.
T, testosterone; E2, estradiol
The onset of puberty.
Puberty begins in girls with enlargement of the mammary glands, in boys with an increase of testicular volume.
The different phases of external pubertal development in girls are conventionally designated as Tanner stages B1 through B5 for breast development and PH1 through PH6 for pubic hair growth. For a detailed description of the Tanner stages, the reader can refer to standard textbooks of pediatrics and pediatric endocrinology (1, 2). Tables 1 and 2 show the timing of the various stages in girls and boys, respectively.
Table 1. Chronological age at the onset of pubertal development in girls (Tanner stages). Mean, standard deviation (SD), and normal range of the initial development of signs of puberty (–2SD to +2SD)*1.
| Parameter | Mean | SD | Normal range (years) | |
| (years) | (years) | (–2SD to +2SD) | ||
| PH 2 | 10.4 | 1.2 | 8.0 | 12.6 |
| B 2 | 10.9 | 1.2 | 8.5 | 13.3 |
| PHV | 12.2 | 1.0 | 10.2 | 14.2 |
| Menarche | 13.4 | 1.1 | 11.2 | 15.6 |
*1modified from (3)
B = breast development; PH = development of pubic hair; PHV = peak height velocity, i.e., the time when longitudinal growth during puberty is fastest
Table 2. Chronological age at the onset of pubertal development in boys (Tanner stages). Mean, standard deviation (SD), and normal range of the initial development of signs of puberty (–2SD to +2SD).*1.
| Parameter | Mean | SD | Normal range (years) | |
| (years) | (years) | (–2SD to +2SD) | ||
| G 2 | 11.2 | 1.5 | 8.2 | 14.2 |
| Testicular volume (≥ 3 mL) | 11.9 | 0.9 | 10.1 | 13.7 |
| PH 2 | 12.2 | 1.5 | 9.2 | 15.2 |
| Spermarche | 13.4 | — | 11.7 | 15.3 |
| PHV | 13.9 | 0.8 | 12.3 | 15.5 |
The mammary gland grows from a breast bud that can be palpated under the nipple (Tanner stage B2) to a fully developed female breast (Tanner stage B4 or B5) over a period of 3.6 years, on average (3, 4).
Ultrasonography reveals an increase in uterine volume, at first without, and then with, a visible layer of uterine mucosa ("mucosal reflex"). The ovaries develop follicular cysts. Multicystic ovaries with more than six cysts can already be seen in the early stages of puberty (5). The first menstrual period (menarche) occurs at an average age of 13.4 years, according to the longitudinal data obtained by Largo et al. (3). In 2006, the German Health Interview and Examination Survey for Children and Adolescents (Kinder- und Jugendgesundheitssurvey, KiGGS), using the status quo method, found the median age at menarche to be 12.8 years (6). The mean age at menarche is highly correlated within families, between monozygotic twins, and within ethnic groups (7). Emotional factors and the nutritional state are also important. At first, the menstrual periods are irregular and consist mainly of anovulatory cycles; in 80% of girls, the menstrual periods become regular and ovulatory within five years after the menarche.
The timing of menarche and spermarche.
Menarche and spermarche occur at a mean age of 13.4 years.
In boys, pubertal development begins with an increase in testicular size, which can be gauged using the Prader orchidometer (8); the normal values for testicular size during puberty are as given by Zachmann (9). Over the further course of puberty, pubic hair appears (Tanner stages PH1 through PH6), as does facial hair, the voice breaks, and penis size increases (Tanner stages G1 through G5) (4) (table 2).
Spermatozoa first become detectable in specimens of boys’ spontaneously produced morning urine at a mean age of 13.4 years (spermarche) (10). As the testes become larger in the ensuing years, the maturation of spermatogenesis is completed.
Psychosocial and psychopathological aspects of pubertal development
Major mental and emotional changes accompany the physical changes of puberty. A basic structuring of the personality (identity) takes place; the child separates itself emotionally from its parents and experiences social orientation outside the family. These mental and emotional maturational processes do not necessarily occur in close parallel with the young person’s physical development.
Psychosocial aspects of puberty.
The mental and emotional changes of puberty include basic structuring of the personality (identity), separation of self from parents, and social orientation outside the family.
During puberty, the body comes to be perceived differently. Feelings of self-worth or self-doubt can arise, as can feelings of shame and emotional vulnerability. An increasingly strong pursuit of autonomy leads the young person to retreat into his or her own living space and establish a separation from other family members, despite a persistent dependency on them. Social orientation to the peer group takes place. The manner in which the individual copes with these developments is influenced by the perceptions of others. Family members, friends, teachers, and the entire social environment no longer see a child, but rather an adolescent. These processes often manifest themselves in the adolescent’s behavior as emotional lability or insecurity, or else as rebelliousness or aggression. In the phase of social maturation, the adolescent breaks away from the family, assumes personal responsibility and social roles, and contends with the larger society’s norms and values (11). These extensive changes can occur without any problem, but they can also lead to mental and emotional disturbances, particularly in the setting of normal variants or pathological types of early or late pubertal development. An early or late beginning of somatic pubertal development makes it difficult for the individual to come to terms with the stress of "being different" in the company of his or her peers. With their increasing height and muscle mass, boys who go through puberty earlier than usual physically resemble grown men before their peers (12, 13). On the other hand, late pubertal development can lead transiently to a negative self-image. Emotional disturbances such as expansive behavior and alcohol and drug consumption may arise as potential compensating mechanisms.
Other physical changes, such as the weight gain accompanying puberty, can lead to emotional disturbances because they conflict with the Western ideal of slimness. This development can cause especially girls to suffer from eating disorders (anorexia nervosa, bulimia) or depression (13, 14).
Normal variants of puberty
The diagnostic evaluation of disorders of puberty necessarily involves the differentiation of pathological disorders from the normal variants of early or late puberty (table 3). Normal variants include constitutional delay or acceleration of growth and puberty (pubertas tarda, pubertas accelerata), early breast development (premature thelarche), early development of pubic hair (premature pubarche), and pubertal gynecomastia.
Table 3. The diagnosis and differential diagnosis of normal variants and pathological types of puberty.
| Early normal or pathological puberty | Late normal or pathological puberty | ||
| Pathological entity | Normal variant | Normal variant | Pathological entity |
| True precocious puberty | Mammary gland hyperplasia of the newborn | (Pubertas tarda) | Hypergonadotropic hypogonadism |
| Precocious pseudopuberty | Premature thelarche | (Pubertas tarda) | Hypogonadotropic hypogonadism |
| Premature adrenarche | Premature pubarche | (Pubertas tarda) | |
| Constitutional or idiopathic acceleration of growth and puberty | Constitutional or idiopathic delay of growth and puberty | ||
Normal variants.
Normal variants include constitutionally delayed or accelerated growth and puberty, premature thelarche, premature pubarche, and pubertal gynecomastia.
In constitutional delay of growth and puberty (pubertas tarda), the spontaneous onset of puberty occurs more than two standard deviations later than normal mean age of onset—or, alternatively, later than the 97th percentiles—for the Tanner stage in question (see tables 1 and 2) (15, 16). For example, in a girl with delayed puberty, breast development to Tanner stage B2 occurs when she is more than 13.3 years old; in a boy with pubertas tarda, a testicular volume of 3–4 mL is first reached when he is more than 13.7 years old. Growth is markedly slower than usual for age, and height falls from the patient’s previously maintained percentile to a lower one. The patient’s adult height is often (17), but not always (18), in the target height range. A suspected diagnosis of constitutional delay of growth and puberty can be definitively confirmed only when puberty and the pubertal growth spurt finally do occur spontaneously, more than two standard deviations later than the normal mean age.
The mirror image of constitutional delay of growth and puberty is constitutional acceleration of growth and puberty. The onset of puberty (breast development, testicular enlargement) occurs more than two standard deviations earlier than the normal mean age of onset. The hypothalamic-pituitary-gonadal axis is activated earlier than usual, but not before age 8 in girls (breast development) or before age 9 in boys (testicular enlargement) (differential diagnosis: precocious puberty).
Delayed puberty.
When growth and puberty are constitutionally delayed, puberty spontaneously begins at a time that is more than two standard deviations later than the mean time of breast development in girls or increase in testicular volume in boys.
In girls with premature thelarche, breast tissue develops early—sometimes in the first two years of life, sometimes later, and occasionally even in the neonatal period—and then persists until the true onset of puberty (19). Premature thelarche is an incomplete form of pubertal development without any other signs of early puberty, such as a pubertal growth spurt or marked acceleration of skeletal age. In rare cases, premature thelarche undergoes a transition to precocious puberty (20).
Premature pubarche is a further normal variant of pubertal development in which pubic and/or axillary hair develop in a girl before age 8 or in a boy before age 9 (21). The serum levels of adrenal androgens are normal, and there is no androgen-induced growth spurt. The skeletal age corresponds to the chronological age or is only mildly accelerated. Premature pubarche is to be distinguished from premature adrenarche with elevated levels of adrenal androgens, the adrenogenital syndrome, and an androgen-secreting tumor.
Some degree of pubertal gynecomastia can be observed in about 50% to 90% of boys (22). In most cases, breast tissue is palpable only as a small induration under the nipple that regresses spontaneously in 6 to 18 months. Treatment is not indicated.
In rare cases, the gynecomastia is more pronounced and persists longer, so that severe psychosocial problems ensue that may necessitate medical treatment, e.g., with tamoxifen (23), or surgical intervention. Only in exceptional cases is pubertal gynecomastia due to increased estrogen production or other hormonal causes, chromosomal anomalies such as Klinefelter syndrome (47, XXY), or medications such as spironolactone. Pubertal gynecomastia is to be distinguished from lipomastia in an overweight male adolescent.
The pathology of early puberty
Pathological conditions in which puberty occurs early are divided into gonadotropin-dependent disorders (true precocious puberty) and gonadotropin-independent disorders (precocious pseudopuberty). A further possibility to be considered is pathological adrenal dysfunction leading to the isolated, premature appearance of pubic hair (premature adrenarche) (table 3).
Gonadotropin-dependent early puberty (true precocious puberty)
By definition, true precocious puberty is initiated by premature activation of the hypothalamic-pituitary-gonadal axis. Its prevalence is estimated at 1:5000 to 1:10 000. It is five to ten times more common in girls than in boys (24, 25).
Pubertal gynecomastia.
Pubertal gynecomastia of varying extent is seen in 50% to 90% of boys.
Pulsatile GnRH secretion begins as in normal puberty. In girls, breast tissue develops under estrogenic influence. In boys, the testes enlarge first, and then the penis enlarges under the influence of testosterone. The clinical course resembles that of normal pubertal development. Pubic hair usually appears later, as a result of stimulation by androgens derived from the adrenal gland and from the testes or ovaries.
An early pubertal growth spurt occurs at about the same time as the external signs of puberty appear. Because of the simultaneous acceleration of skeletal development, the individual’s final height is often reduced, sometimes to the point of short stature, i.e., height below the third percentile.
The diagnosis of true precocious puberty must be considered when the initial signs of puberty appear in a girl under age 8 or a boy under age 9. The diagnosis is then confirmed by the clinical findings, including the Tanner stages and growth spurt, acceleration of skeletal growth, the results of GnRH testing, and the findings of gonadal and uterine ultrasonography. In the GnRH test, the stimulated LH/FSH quotient at 30 minutes is greater than 1. The estradiol level (in girls) or the testosterone level (in boys) is markedly elevated for chronological age, but corresponds to the current pubertal stage.
In girls, ultrasonography reveals a multicystic ovary with more than 6 follicles of diameter 4 mm or more (5). The uterine volume increases, and endometrium is produced (a "uterine mucosal reflex" is visible).
The cause of precocious puberty remains unidentified in 80% of girls and 40% of boys. Aside from these idiopathic cases, precocious puberty can also result from organic lesions in the hypothalamic and pituitary area, primarily in boys (e1). Hypothalamic hamartoma, glioma, astrocytoma, and germinoma can cause precocious puberty; it can also occur in children with internal hydrocephalus or other lesions of the central nervous system, such as an earlier episode of meningitis or traumatic brain injury or prior radiotherapy to the head. Magnetic resonance imaging of the brain should be performed in order to search for a possible organic cause.
Pathological forms.
Pathological forms of puberty include gonadotropin-dependent precocious puberty, gonadotropin-independent precocious pseudopuberty, and premature adrenarche.
In true precocious puberty, treatment is indicated because of the major psychosocial stress on the affected child resulting from the very early appearance of signs of puberty, the frequent (and generally wrong) assumption by others that the child possesses a correspondingly early mental and emotional "maturity," and the risk of reduced adult height due to disproportional acceleration of skeletal age. The treatment involves the administration of GnRH analogs that suppress the effects of the elevated gonadotropins LH and FSH through down-regulation of the pituitary GnRH receptors (24). Physical examinations during treatment reveal the slowing or cessation or sometimes even a return of the prepubertal stage; on biochemical testing, the gonadotropins LH and FSH as well as the sex steroids estrogen or testosterone are detectable only in very low concentrations, or not at all. In doubtful cases, a GnRH test can be performed during the trough just before the next scheduled GnRH injection, in order to determine whether the gonadotropins have been adequately suppressed. If basal and/or stimulated LH and FSH are measured in higher concentrations, then GnRH analogs should be given at a higher dose or at shorter intervals. The pubertal stage, height, and skeletal age of the patient should be monitored over the course of treatment (24).
The treatment of true precocious puberty should be terminated when it is time for normal puberty to begin, and when it can be expected that the patient will attain an optimal adult height. The decision to end treatment should be taken by general agreement between the physician, the child, and the parents. Puberty will then run its course spontaneously, the duration of puberty depending on the stage that had already been attained by the time the treatment for precocious puberty was discontinued. The follow-up studies on treated patients have not revealed any treatment-related disturbances of the hypothalamic-pituitary-gonadal axis (e2, e3). When treatment is begun early, the patient’s adult height lies in the range predicted before therapy was begun (e4).
Gonadotropin-independent early puberty (precocious pseudopuberty)
Precocious pseudopuberty arises, by definition, before and independently of the maturation of the hypothalamic-pituitary-gonadal axis (e5). The appearance of secondary sexual characteristics is due to the increased production of female or male hormones. Estrogens induce isosexual pseudopuberty in girls and heterosexual pseudopuberty in boys; conversely, androgens induce isosexual pseudopuberty in boys and heterosexual pseudopuberty in girls. The hypothalamic-pituitary-gonadal axis is suppressed by the abnormally elevated secretion of androgens or estrogens.
Precocious pseudopuberty has many different causes. It can be due to external factors, such as the therapeutic or accidental ingestion of estrogens or androgens, or a large number of other conditions. These include tumors, disturbances of steroid biosynthesis, and congenital syndromes (e5).
The time of diagnosis.
The diagnosis of precocious puberty should be considered when girls develop the first signs of puberty before age 8, or boys before age 9.
Hormone-secreting tumors of the central nervous system, the adrenal gland, the liver or other organs can be responsible for the development of precocious pseudopuberty. Germ-cell tumors secrete human chorionic gonadotropin (hCG), which, in turn, stimulates the LH-receptors of the testes (for example), which then produce testosterone. Tumors of this type can arise in the gonads, the central nervous system (pineal and pituitary glands), the liver, the retroperitoneal space, or the posterior mediastinum, which are the sites of origin of the sex-determining cells during embryonic development (e6).
Adrenal tumors can produce androgens as well as cortisol and thereby induce iso- or heterosexual precocious pseudopuberty in addition to the clinical signs of Cushing syndrome (e7).
Leydig cell tumors must also be considered in the differential diagnosis of precocious pseudopuberty. Physical examination often, though not always, reveals a palpable asymmetry of testicular size. In unclear cases, ultrasonography, magnetic resonance imaging, and/or testicular biopsy must be performed.
A special case is that of familial, gonadotropin-independent precocious pseudopuberty ("familial testotoxicosis"), a disorder in which an activating mutation of the LH receptor causes early Leydig cell maturation and increased testosterone production (e8). The disease is transmitted in an autosomal dominant inheritance pattern affecting boys only. In the affected boys, signs of puberty appear at the age of 1 to 4 years.
The etiology of precocious pseudopuberty.
Precocious pseudopuberty has many different causes. It can be due to external factors, tumors, syndromes, or disturbances of steroid biosynthesis in the adrenal glands.
Another very special case is that of McCune-Albright syndrome, which is characterized by three clinically evident phenomena (e9). First, the characteristic, jagged café-au-lait spots become visible on the skin. Later, polyostotic fibrous dysplasia of the bones develops, leading to pathological fractures that can result in severe osseous deformities. Puberty occurs early as the result of repeatedly arising ovarial cysts, which can cause withdrawal bleeding even in early childhood.
Patients with McCune-Albright syndrome have a somatic mutation in the alpha subunit of the G protein, leading to continuous activation of adenylate cyclase and thereby to the pathological secretion of a variety of different hormones. In addition to precocious pseudopuberty, the patient may suffer from hyperthyroidism, Cushing syndrome, and increased growth hormone secretion.
Hereditary disorders of adrenal steroid biosynthesis are transmitted in an autosomal recessive pattern. The associated enzyme defect in androgen, glucocorticoid, and/or mineralocorticoid synthesis produces a clinical condition that presents with premature adrenarche (e10). The enzyme defect can be diagnosed by means of a steroid profile and/or ACTH test in combination with molecular genetic analysis. 21-hydroxylase deficiency is the most common cause, leading to the simple virilizing form of the adrenogenital syndrome. A 3ß-hydroxysteroid dehydrogenase defect should be included in the differential diagnosis.
Pathological absence of puberty
When puberty does not occur spontaneously, no development of secondary sexual characteristics is observed. If pubic hair develops, this is usually due to the secretion of adrenal hormones and does not imply activation of the hypothalamic-pituitary-gonadal axis. The concentrations of the pituitary hormones LH and FSH are low when the disturbance has its origin in the hypothalamus or pituitary gland (hypogonadotropic hypogonadism); they are high when the cause is ovarian or testicular failure (hypergonadotropic hypogonadism). In either case, the level of the gonadal hormone, estradiol or testosterone, is low. Follicular maturation or sperm production does not take place.
Hormonal status.
In precocious pseudopuberty, the androgen or estrogen level is elevated, while the gonadotropins LH and FSH are suppressed.
Further diagnostic evaluation is needed if no breast development has yet occurred in a girl aged 14.5 years (mean + 3 standard deviations) (table 1) or if the testes have not reached a size of 3 mL or more in a boy aged 14.6 years (mean + 3 standard deviations) (table 2). Even at this late age, the major differential diagnosis is constitutional delay of growth and puberty.
Hypogonadism, whether it is tertiary (hypothalamic), secondary (pituitary), or primary (gonadal), can be either congenital (e11) or acquired (figure). The classic example of congenital hypothalamic hypogonadism is Kallmann syndrome, in which hypogonadism is characteristically accompanied by hyposmia or anosmia (box 1, rightmost column in the figure). Kallmann syndrome is due to an impairment of the normal migration of the GnRH neurons from the region of the olfactory nerve to the ventral hypothalamus. Mutations in the KAL1 gene on the short arm of the X chromosome (Xp22.3) are responsible for the X-chromosomal recessive form, while mutations in the FGFR1 (fibroblast growth factor receptor 1) gene on the short arm of chromosome 8 (8p11.2–p11.1) are responsible for the autosomal dominant form (e12). In addition, mutations in the prokineticin-2 gene, the prokineticin-2 receptor gene, and the nasal embryonic LHRH factor (NELF) gene have been described as rare causes of Kallmann syndrome (e12– e14) (box 1).
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
Which of the following is a normal variant of pubertal development?
Premature adrenarche
True precocious puberty
Pubertas tarda
Precocious pseudopuberty
Kallmann syndrome
Question 2
Which of the following clinical findings generally indicates the presence of true precocious puberty?
Development of the first signs of puberty in a girl aged 8 or older
Development of the first signs of puberty in a boy aged 9 or older
Unilateral testicular enlargement in a boy aged 11 or older
Onset and continuation of breast development in a girl under age 8
Decreasing velocity of growth in a boy in whom puberty has begun
Question 3
Which of the following biochemical laboratory parameters indicates the presence of true precocious puberty in a six-year-old girl?
An elevated estradiol level and suppressed LH and FSH levels
A stimulated LH-FSH quotient greater than 1 in the GnRH test
Isolated elevation of DHEAS levels
Low estradiol levels
Elevated hCG levels
Question 4
What would you do if you found isolated, bilateral mammary gland enlargement in a neonate?
Nothing
Gonadal ultrasonography
Skeletal age determination
GnRH testing
HCG testing
Question 5
Which of the following characterizes hypergonadotropic hypogonadism?
Low LH and FSH levels
Elevated testosterone or estradiol levels
Anosmia, as in Kallmann syndrome
An increase in testicular volume before age 5
Elevated LH and FSH levels in adolescence and/or adulthood
Question 6
Which of the following data or findings are consistent with the diagnosis of a normal variant of pubertal development?
The appearance of signs of puberty at a time corresponding to the 10th to 90th percentile for normal pubertal development
Androgen levels corresponding to Tanner stage 2 of puberty
The appearance of signs of puberty in a six-year-old girl
The appearance of signs of puberty at a time within two standard deviations of the mean for normal pubertal development
The appearance of signs of puberty at a time later than two standard deviations from the mean for normal pubertal development
Question 7
What is the first sign of pubertal development in boys?
Increasing size of the penis
Deepening voice
Acne vulgaris
Increasing size of the testes
Spermarche
Question 8
What is the first sign of pubertal development in girls?
A growth spurt
Deepening voice
Breast development
Vaginal secretion
Acne vulgaris
Question 9
What is meant by "premature thelarche"?
The isolated appearance of pubic hair
A normal variant of pubertal development
Premature development of the breasts in association with a "pubertal" growth spurt
A preliminary stage of precocious puberty
Breast development beginning at age 12
Question 10
Which of the following can cause precocious pseudopuberty?
A hormone-secreting tumor of the central nervous system
Pituitary hypoplasia
Activation of the hypothalamic-pituitary-gonadal axis
Klinefelter syndrome (47, XXY)
Ullrich-Turner syndrome (45, X)
Hypogonadotropic hypogonadism can also manifest itself as a disturbance of GnRH secretion without any abnormality of the sense of smell (e15– e16). Mutations in the GnRH receptor account for about 6% to 13% of all autosomal recessive forms of hypogonadotropic hypogonadism (e17). Further hypothalamic causes of absent puberty are listed in box 1. Inactivating mutations in the GPR54 (G-coupled protein receptor 54) gene are located on chromosome 19 (19p13.3) (e18).
The term "functional hypothalamic hypogonadism" refers to a usually reversible dysfunction of the hypothalamic-pituitary-gonadal axis that can arise in the setting of anorexia nervosa, during situations of severe stress, or when the affected person participates in very intense physical activity, including sport.
Pathology of absent puberty.
Hypogonadism is divided into hypogonadotropic hypogonadism (of hypothalamic or pituitary origin) and hypergonadotropic hypogonadism.
A number of different disorders can be the cause of secondary (pituitary) hypogonadotropic hypogonadism (see "pituitary hypogonadism" in the figure and box 2). Congenital developmental abnormalities of the pituitary gland usually cause a complex deficiency of multiple pituitary hormones; therefore, the diagnosis of hypogonadotropic hypogonadism is often preceded by that of a growth hormone deficiency, pituitary hypothyroidism, and/or pituitary ACTH deficiency. The associated genetic defects affect the transcription factors HESX1, PROP1, LHX3, LHX4, and others, which are responsible for the normal development of the pituitary gland during embryogenesis. Magnetic resonance imaging may reveal hypoplasia or aplasia of the adenohypophysis, a rudimentary or absent pituitary stalk primordium, and/or ectopy of the neurohypophysis.
Box 1. Hypothalamic causes of absent puberty.
-
Migration disorder of the GnRH neurons (Kallmann syndrome) due to mutations in (e12):
the KAL1 gene (chromosome Xp22.3)
the fibroblast growth factor receptor 1 (FGFR-1) gene (chromosome 8p11.2–p11.1)
the prokineticin 2 gene (e13)
the prokineticin 2 receptor gene
nasal embryogenic LHRH factor (NELF)
Disturbances of GnRH secretion without anosmia or hyposmia (e19)
Mutations of the GnRH receptor gene
Mutations of the leptin gene
Mutations of the leptin receptor gene
Mutations of the G-coupled protein receptor 54 gene (GPR54) (e18)
The pituitary hormones LH and FSH consist of a common alpha-subunit and a specific beta-subunit. Mutations in the latter or in the receptors for LH and FSH cause hypogonadotropic hypogonadism (e19).
Moreover, radiotherapy of the head for the treatment of leukemia and brain tumors can cause either secondary or tertiary hypogonadism.
Kallmann syndrome.
The classic type of hypothalamic hypogonadism, in which this disturbance is combined with anosmia, is called Kallmann syndrome.
The classic congenital gonadal disorders associated with hypergonadotropic hypogonadism are chromosomal anomalies such as Ullrich-Turner syndrome (45, X) in girls and Klinefelter syndrome (47, XXY) in boys (45) (see "hypergonadotropic hypogonadism" in the figure). Acquired forms are due to autoimmune diseases, radiotherapy, or chemotherapy. These patients have significantly elevated LH and FSH levels because of the lack of the negative feedback mechanism that normally results from the estrogens or from testosterone, as well as from the inhibins A and B, and that normally inhibits secretion of the hypothalamic-pituitary hormones LH and FSH. On clinical examination, the patient is found to be in the prepubertal Tanner stages B1 and G1, with testicular volume less than 3 mL. Gonadal ultrasonography reveals a small uterus without a mucosal reflex. The secretion of adrenal steroids is unimpaired in most cases, and pubic hair therefore appears in the normal fashion.
Any type of hypogonadism can arise either as the complete form of the disease, in which all signs of puberty are absent, or as an incomplete form, in which partial functioning of the hypothalamic-pituitary-gonadal pathway is reflected in some degree (usually incomplete) of external pubertal development. In some cases, such disturbances can be difficult to differentiate from constitutional delay of growth and puberty (pubertas tarda).
Classic disorders.
The classic forms of hypergonadotropic hypogonadism are Ullrich-Turner syndrome (45, X) in girls and Klinefelter syndrome (47, XXY) in boys.
The treatment of hypogonadism.
The treatment of hypogonadism begins with a low-dose substitution therapy with estrogens/gestagens in girls or testosterone in boys.
The treatment of hypogonadism consists of substitution therapy with estrogens/gestagens or with testosterone. It begins with low doses of estrogens or testostereone, which are slowly raised to the full substitution dose while the clinical findings are monitored (development of secondary sexual characteristics). For further details, the reader is referred to textbooks of pediatric (2) and adult endocrinology (e21).
Box 2. Pituitary causes of absent puberty.
-
Developmental disorders of the pituitary gland due to mutations in
the HESX 1 gene
the PROP 1 gene
the PIT 1 gene
the LHX 3 gene
the LHX 4 gene
Mutations of the β-LH or β-FSH gene (e14)
Mutations of the LH or FSH receptor gene
-
Tumors of the hypothalamic-pituitary region
Craniopharyngioma
Germinoma
Langerhans cell histiocytosis
Prolactinoma
Adenoma
Radiotherapy of the hypothalamic-pituitary region
-
Congenital midline defects
Solitary maxillary median central incisor syndrome
Agenesis of the corpus callosum
Further Information on CME.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education.
Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Chambers of Physicians of the German federal states (Länder). CME points of the Chambers of Physicians can be acquired only through the Internet by the use of the German version of the CME questionnaire within 6 weeks of publication of the article, i.e., by 5 June 2009. See the following website: www.aerzteblatt.de/cme.
Participants in the CME program can manage their CME points with their 15-digit "uniform CME number" (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the www.aerzteblatt.de website under "meine Daten" ("my data"), or upon registration. The EFN appears on each participant’s CME certificate.
The solutions to the following questions will be published in volume 25/2009. The CME unit "Heart Valve Surgery Today" (volume 13/2009) can be accessed until 8 May 2009.
For volume 21/2009 we plan to offer the topic "The Differential Diagnosis of Food Intolerance."
Solutions to the CME questionnaire in volume 9/2009:
Schrem H, Barg-Hock H, Strassburg CP, Schwarz A, Klempnauer J: Aftercare for Patients With Transplanted Organs: 1c, 2d, 3d, 4a, 5b, 6d, 7e, 8a, 9b, 10e
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
The authors declare that they have no conflict of interest as defined by the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Lentze MJ SJ, Schulte FJ, Spranger J., Pädiatrie . In: Lentze MJ SJ, Schulte FJ, Spranger J, editors. Springer Verlag; 2007. International readers might want to refer to the following English-language textbook: Sperling MA: Pediatric Endocrinology, 3rd edition. Philadelphia London Toronto Montreal Sydney Tokyo: W. Saunders Company; 2008. [Google Scholar]
- 2.Stolecke H. Endokrinologie des Kindes- und Jugerndalters. Berlin Heidelberg New York: Springer-Verlag; 1997. International readers might want to refer to the following English-language textbook: Kronenberg HM, Melmed S, Polonsky KS, Larsen PR: Williams Textbook of Endocrinology, 11th edition. Philadelphia London Toronto Montreal Sydney Tokyo: W. Saunders Company; 2008. [Google Scholar]
- 3.Largo RH PA. Pubertal development in Swiss girls. Helv Paediatr Acta. 1983;38(3):229–243. [PubMed] [Google Scholar]
- 4.Largo RH PA. Pubertal development in Swiss boys. Helv Paediatr Acta. 1983;38(3):211–228. [PubMed] [Google Scholar]
- 5.Traggiai C, Stanhope R. Disorders of pubertal development. Best Practice & Research Clinical Obstetrics & Gynaecology. 2003;17(1):41–56. doi: 10.1053/ybeog.2003.0360. [DOI] [PubMed] [Google Scholar]
- 6.Kahl H SRA, Schlaud M. Sexuelle Reifung von Kindern und Jugendlichen in Deutschland. Ergebnisse des Kinder- und Jugendgesundheitssurveys (KiGGS) Bundesgesundheitsbl-Gesundheitsforsch-Gesundheitsschutz. 2007;50:677–685. doi: 10.1007/s00103-007-0229-3. [DOI] [PubMed] [Google Scholar]
- 7.Parent A-S, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon J-P. The Timing of Normal Puberty and the Age Limits of Sexual Precocity: Variations around the World, Secular Trends, and Changes after Migration 10.1210/er.2002-0019. Endocr Rev. 2003;24(5):668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 8.Prader A. Testicular size: assessment and clinical importance. Triangle. 1966;7:240–243. [PubMed] [Google Scholar]
- 9.Zachmann M PA, Kind HP, Häfliger H, Budliger H. Testicular volume during adolescence. Cross-sectional and longitudinal studies. Helv Paediatr Acta. 1974;29:61–72. [PubMed] [Google Scholar]
- 10.Nielsen CT SN, Richardson DW, Hunter WM, et al. Onset of the release of spermatozoa (spermarche) in boys in relation to age, testicular growth, pubic hair, and height. J Clin Endocrinol Metab. 1986;62(3):532–535. doi: 10.1210/jcem-62-3-532. [DOI] [PubMed] [Google Scholar]
- 11.Grob A JU. Erwachsen werden: Entwicklungspsychologie des Jugendalters. Beltz PVU. 2003 [Google Scholar]
- 12.Cohane GH PHJ. Body image in boys: a review of the literature. Int J Eat Disord. 2001;29:373–379. doi: 10.1002/eat.1033. [DOI] [PubMed] [Google Scholar]
- 13.Pinquart M. Krisen im Jugendalter. Monatsschr Kinderheilkd. 2003;151:43–47. [Google Scholar]
- 14.Stattin H MD. Pubertal maturation in female development. Hillsdale New Jersey: Erlbaum; 1990. [Google Scholar]
- 15.Reiter EO LP. Delayed puberty. Adoles Med. 2002;13:101–118. [PubMed] [Google Scholar]
- 16.Traggiai C, Stanhope R. Delayed puberty. Best Practice & Research Clinical Endocrinology & Metabolism. 2002;16(1):139–151. doi: 10.1053/beem.2001.0186. [DOI] [PubMed] [Google Scholar]
- 17.Cools BLM RR, Op De Beck L, Du Caju MVL. Boys with a simple delayed puberty reach their target height. Horm Res. 2008;70:209–214. doi: 10.1159/000137663. [DOI] [PubMed] [Google Scholar]
- 18.Wehkalampi K VK, Laine T, Dunkel L. Progressive reduction of relative height in childhood predicts adult stature below target height in boys with constituional delay of growth and puberty. Horm Res. 2007;68:99–104. doi: 10.1159/000101011. [DOI] [PubMed] [Google Scholar]
- 19.Klein KO MV, Brown-Dawson JM, Larmore KA, Cabezas P, Cortinez A. Estrogen levels in girls with premature thelarche compared with normal prepubertal girls as determined by an ultrasensitive recombinant cell bioassay. J Pediatr. 1999;134(2):190–192. doi: 10.1016/s0022-3476(99)70414-2. [DOI] [PubMed] [Google Scholar]
- 20.Pasquino AM, Pucarelli I, Passeri F, Segni M, Mancini MA, Municchi G. Progression of premature thelarche to central precocious puberty. The Journal of Pediatrics. 1995;126(1):11–14. doi: 10.1016/s0022-3476(95)70492-2. [DOI] [PubMed] [Google Scholar]
- 21.Ibanez L, DiMartino-Nardi J, Potau N, Saenger P. Premature Adrenarche-Normal Variant or Forerunner of Adult Disease? 10.1210/er.21.6.671. Endocr Rev. 2000;21(6):671–696. doi: 10.1210/edrv.21.6.0416. [DOI] [PubMed] [Google Scholar]
- 22.Narula HS CH. Gynecomastia. Endocrinol Metab Clin North America. 2007;36:497–519. doi: 10.1016/j.ecl.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Sarah EL, Faught KA, Jennifer V, Margaret LL. Beneficial effects of raloxifene and tamoxifen in the treatment of pubertal gynecomastia. The Journal of Pediatrics. 2004;145(1):71–76. doi: 10.1016/j.jpeds.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 24.Prete G C-SA-C, Trivin C, Brauner R. Idiopathic central precocious puberty in girls: presentation factors. BMC Pediatrics. 2008;8 doi: 10.1186/1471-2431-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carel J-C LJ. Precocious Puberty. N Engl J Med. 2008;358:2366–2377. doi: 10.1056/NEJMcp0800459. [DOI] [PubMed] [Google Scholar]
- e1.Kaplowitz P. Clinical Characteristics of 104 Children Referred for Evaluation of Precocious Puberty. J Clin Endocrinol Metab. 2004;89:3644–3650. doi: 10.1210/jc.2003-031532. [DOI] [PubMed] [Google Scholar]
- e2.Heger S, Partsch C-J, Sippell WG. Long-Term Outcome after Depot Gonadotropin-Releasing Hormone Agonist Treatment of Central Precocious Puberty: Final Height, Body Proportions, Body Composition, Bone Mineral Density, and Reproductive Function. J Clin Endocrinol Metab. 1999;84:4583–4590. doi: 10.1210/jcem.84.12.6203. [DOI] [PubMed] [Google Scholar]
- e3.Heger S, Müller M, Ranke M, Schwarz H-P, Waldhauser F, Partsch C-J, et al. Long-term GnRH agonist treatment for female central precocious puberty does not impair reproductive function. Molecular and Cellular Endocrinology. Puberty: A Sensor of Genetic and Environmental Interactions throughout Development. 2006:254–255. doi: 10.1016/j.mce.2006.04.012. 217-220. [DOI] [PubMed] [Google Scholar]
- e4.Partsch C-J, Heger S, Sippell WG. Management and outcome of central precocious puberty. Clinical Endocrinology. 2002;56:129–148. doi: 10.1046/j.0300-0664.2001.01490.x. [DOI] [PubMed] [Google Scholar]
- e5.Eugster E. Peripheral precocious puberty causes and mangement. Horm Res. 2009;69 S1:64–67. doi: 10.1159/000178041. [DOI] [PubMed] [Google Scholar]
- e6.Aoyama H, Shirato H, Ikeda J, Fujieda K, Miyasaka K, Sawamura Y. Induction Chemotherapy Followed by Low-Dose Involved-Field Radiotherapy for Intracranial Germ Cell Tumors. J Clin Oncol. 2002;20:857–865. doi: 10.1200/JCO.2002.20.3.857. [DOI] [PubMed] [Google Scholar]
- e7.Lee PD WR, Green O. Virilizing adrenocortical tumors in childhood: eight cases and a review of the literature. Pediatrics. 1985;76:437–444. [PubMed] [Google Scholar]
- e8.Reiter EO NE. Testotoxicosis: current viewpoint. Pediatric Endocrinol Rev. 2005;3:77–86. [PubMed] [Google Scholar]
- e9.Zacharin The spectrum of McCune Albright syndrome. Pediatric Endocrinol Rev. 2007;4(Suppl 4):412–418. [PubMed] [Google Scholar]
- e10.New MI. Nonclassical 21-Hydroxylase Deficiency. J Clin Endocrinol Metab. 2006;91:4205–4214. doi: 10.1210/jc.2006-1645. [DOI] [PubMed] [Google Scholar]
- e11.Bhagavath B, M.D., Layman L, M.D. The Genetics of Hypogonadotropic Hypogonadism. Semin Reprod Med. 2007;(4):272–286. doi: 10.1055/s-2007-980221. [DOI] [PubMed] [Google Scholar]
- e12.Fechner A FS, McGovern P. A review of Kallmann syndrome: genetics, pathophysiologgy, and clinical mangement. Obstet Gynecol Surv. 2008;63:189–194. doi: 10.1097/OGX.0b013e3181641278. [DOI] [PubMed] [Google Scholar]
- e13.Leroy C, Fouveaut C, Leclercq S, Jacquemont S, Boullay HD, Lespinasse J, et al. Biallelic mutations in the prokineticin-2 gene in two sporadic cases of Kallmann syndrome. Eur J Hum Genet. 2008;168:865–868. doi: 10.1038/ejhg.2008.15. [DOI] [PubMed] [Google Scholar]
- e14.Lofrano-Porto A, Barra GB, Giacomini LA, Nascimento PP, Latronico AC, Casulari LA, et al. Luteinizing Hormone Beta Mutation and Hypogonadism in Men and Women. N Engl J Med. 2007;357:897–904. doi: 10.1056/NEJMoa071999. [DOI] [PubMed] [Google Scholar]
- e15.Bhagavath B, Podolsky RH, Ozata M, Bolu E, Bick DP, Kulharya A, et al. Clinical and molecular characterization of a large sample of patients with hypogonadotropic hypogonadism. Fertility and Sterility. 2006;85:706–713. doi: 10.1016/j.fertnstert.2005.08.044. [DOI] [PubMed] [Google Scholar]
- e16.Quinton R, Duke VM, Robertson A, Kirk JMW, Matfin G, de Zoysa PA, et al. Idiopathic gonadotrophin deficiency: genetic questions addressed through phenotypic characterization. Clinical Endocrinology. 2001;55:163–174. doi: 10.1046/j.1365-2265.2001.01277.x. [DOI] [PubMed] [Google Scholar]
- e17.Bhagavath B, Ozata M, Ozdemir IC, Bolu E, Bick DP, Sherins RJ, et al. The prevalence of gonadotropin-releasing hormone receptor mutations in a large cohort of patients with hypogonadotropic hypogonadism. Fertility and Sterility. 2005;84:951–957. doi: 10.1016/j.fertnstert.2005.04.029. [DOI] [PubMed] [Google Scholar]
- e18.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr., Shagoury JK, et al. The GPR54 Gene as a Regulator of Puberty. N Engl J Med. 2003;349(17):1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- e19.Themmen APN, Huhtaniemi IT. Mutations of Gonadotropins and Gonadotropin Receptors: Elucidating the Physiology and Pathophysiology of Pituitary-Gonadal Function. Endocr Rev. 2000;21:551–583. doi: 10.1210/edrv.21.5.0409. [DOI] [PubMed] [Google Scholar]
- e20.Bojesen A GC. Klinefelter syndrome in clinical practice. Nature Clinical Practice Urology. 2007;4:192–204. doi: 10.1038/ncpuro0775. [DOI] [PubMed] [Google Scholar]
- e21.Allolio B SH. München Wien Baltimore: Urban & Schwarzenberg; 1996. Praktische Endokrinologie. [Google Scholar]