Abstract
Background
For an increasing number of patients with severe symptomatic aortic stenosis, advanced age and comorbidity make the risk of surgery unacceptably high. In such cases, catheter-based techniques for aortic valve implantation are a new therapeutic option. In this paper, we describe the initial results obtained at the German Heart Center, Munich, with a new technique of this kind.
Methods
From June 2007 to September 2008, 152 patients underwent transcatheter aortic valve implantation at the German Heart Center, Munich (121 transfemorally, 26 transapically, and 5 through other sites of access). In this technique, a stent-mounted valve is crimped onto a catheter and then positioned and deployed in the aortic annulus under fluoroscopic control.
Results
The 30-day mortality was 11.8% in this group of patients at high risk. The more common post-procedural complications were third-degree atrioventricular block leading to pacemaker implantation (31/152, 20%), vascular complications (25/152, 16%), and cerebrovascular events (8/152, 5%). Six months after the procedure, the patients had recovered clinically to a considerable extent, and the implanted prostheses exhibited good hemodynamic function.
Conclusions
The technical feasibility of catheter-based aortic valve implantation has been demonstrated at multiple centers around the world. Its indications still need to be refined on the basis of the short- and long-term results of the randomized and observational studies that are currently in progress. It is already apparent that catheter-based aortic valve implantation can bring about clinical improvement in patients who are deemed ineligible for open surgery.
Keywords: aortic stenosis, aortic surgery, cardiac valve replacement, minimally invasive treatment, catheterization
Calcifying aortic stenosis is the most common acquired valvular disease in Western countries; its prevalence increases with age (1). Surgical replacement of the aortic valve is the treatment of choice for high-grade, symptomatic aortic stenosis (2). The therapeutic benefit with respect to relief of symptoms, and with respect to survival, is well documented (2). Nonetheless, in a steadily aging population, cardiac surgery bears an unacceptably high risk for an increasing number of patients with high-grade aortic stenosis because of advanced age and major comorbidity. For these reasons, as many as 60% of all patients with high-grade aortic stenosis currently do not undergo surgical valve replacement, even though their symptoms are severe and their prognosis with conservative treatment is poor (3).
Less invasive, transcatheter techniques for aortic valve implantation have been developed in recent years in order to provide these patients an adequate form of treatment with an acceptably low risk. Briefly, a stent-mounted bioprosthesis is crimped onto a catheter, and then positioned and deployed in the aortic annulus under fluoroscopic control. As the native aortic valve remains in place, these techniques are referred to as transcatheter aortic valve implantation, in contrast to surgical aortic valve replacement, in which the native valve is excised.
The procedure is performed on the beating heart without the use of a heart-lung machine and can even be performed without general anaesthesia and ventilation in some patients. Thus, the main sources of surgical risk for these elderly, multimorbid patients can be avoided: the major operative trauma of sternotomy, the consequences of extracorporeal circulation, and the long duration of anesthesia and postoperative mechanical ventilation.
The technical feasibility of transcatheter valve implantation has been demonstrated in case reports for both of the types of aortic valvular prosthesis that are currently approved for use (4– 7). These well-documented pioneer efforts were followed by the first reports of the use of transcatheter valve implantation in larger groups of patients (8– 10).
In this article, we will discuss the various techniques currently in use, all of which are now being performed at the German Heart Center in Munich. Furthermore, we will discuss the results that have been obtained to date, with follow-up times of up to six months, in the context of the available literature and the current recommendations of the medical societies.
Methods
Patients
Transcatheter aortic valve implantations were performed on 152 patients at the German Heart Center, Munich, from June 2007 to September 2008. These patients either had a specific contraindication to conventional surgical aortic valve replacement, such as severe, extensive calcification of the ascending aorta, or else they were very old and had major comorbidities (table). During the same time interval, 108 patients were seen and evaluated for transcatheter treatment at our institution in whom the procedure was not possible for anatomical reasons or who declined to undergo it.
Table. Preoperative patient characteristics.
| Patients overall | N = 152 | |
| Mean age at time of surgery | 81 ± 7 years | |
| Number (percentage) of female patients | n = 87 (57%) | |
| Mortality risk (mean; logistic EuroSCORE, www.euroscore.org) | 24 ± 15% | |
| Aortic valve orifice area | 0.65 ± 0.19 cm2 | |
| Maximal gradient across aortic valve | 79 ± 25 mm Hg | |
| Mean gradient across aortic valve | 49 ± 17 mm Hg | |
| Coronary heart disease | n = 79 (52%) | |
| Previous PTCA/stent | n = 28 (18%) | |
| Previous bypass surgery | n = 24 (16%) | |
| Pulmonary hypertension (systolic pulmonary artery pressure >60 mm Hg) | n = 41 (27%) | |
| Chronic obstructive pulmonary disease | n = 36 (24%) | |
| Renal failure (creatinine >1.5 mg/dL) | n = 30 (20%) | |
| Previous cardiac surgery | n = 29 (19%) | |
| Bypass surgery | n = 23 (15%) | |
| Valvular surgery | n = 4 (3%) | |
| Combined/other | n = 2 (1%) | |
| Previous stroke | n = 15 (10%) | |
PTCA = percutaneous transluminal coronary angioplasty
Prostheses
Two types of aortic valve prosthesis are now available for transcatheter implantation, both of which were granted CE certification ("Conformité Européenne") in 2007. Both of them are ten to twenty times as expensive as conventionally implantable aortic valve prostheses.
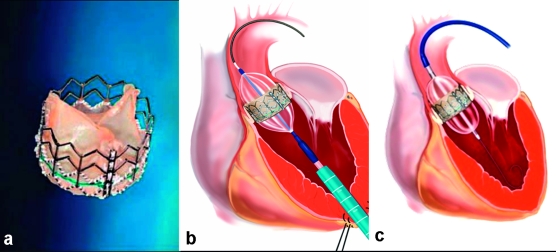
The Edwards-Sapien prosthesis (figure 1) is a bovine pericardial bioprosthesis mounted on a balloon-expandable steel stent. Positioning of this prosthesis requires particular care for implantation height, as too high implantation may impair coronary flow. The delivery catheter through which the folded prosthesis is brought into the aortic position (22 to 24 French, depending on prosthesis size) via a retrograde transfemoral approach can be bent while it is being advanced in order to pass more easily through the aortic arch. The Edwards-Sapien prosthesis is available for both transarterial and transapical implantation.
Figure 1.
(a) Edwards-Sapien prosthesis
(b) transapical placement
(c) transfemoral placement
(Reprinted with the kind permission of Edwards Lifesciences, Irvine, California)
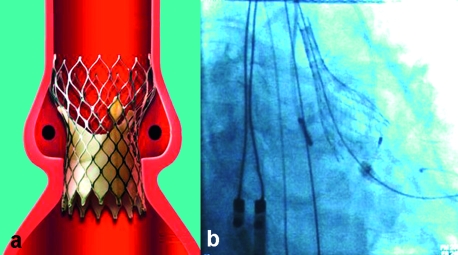
The CoreValve prosthesis (figure 2) is a porcine pericardial valve mounted in a self-expandable nitinol stent. The broader distal end of the stent enables it to be anchored more tightly in the ascending aorta. The physical properties of nitinol allow self-expansion of the prosthesis during deployment. Thus, unlike the Edwards-Sapien prosthesis, the CoreValve prosthesis unfolds without the aid of a balloon. The stent framework of the prosthesis does not impair coronary perfusion. The delivery catheter of the CoreValve prosthesis is of 18 French size. The CoreValve prosthesis, too, is available for both transarterial and (very soon also) transapical implantation. The Edwards-Sapien and CoreValve prostheses are both currently available in two sizes.
Figure 2.
(a) Diagram of the CoreValve prosthesis in the aortic root (reprinted with the kind permission of CoreValve, Irvine, California)
(b) Release of the CoreValve prosthesis in the beating heart
Preoperative evaluation
In addition to the studies that are routinely performed before aortic valve replacement, patients about to undergo transcatheter aortic valve implantation are evaluated with CT scanning of the chest, abdomen, and pelvis. These studies enable precise determination of the size of the aortic valve annulus, so that the most suitable type of prosthesis and the appropriate prosthesis size can be chosen. The arterial vessels are also evaluated with respect to their diameter, course, calcifications, stenosis, and previous interventions, so that the optimal approach can be planned for the individual patient. A transthoracic or transesophageal echocardiogram provides supplementary information.
Access sites
Transcatheter aortic valve implantation can be performed through a variety of access sites, all of which are currently in use at the German Heart Center in Munich.
1. Transarterial, retrograde transcatheter aortic valve implantation: (a) Transfemoral transcatheter valve implantation (N = 121; n = 117, CoreValve; n = 4, Edwards-Sapien). Transfemoral access was obtained either by percutaneous puncture (n = 83) followed by closure with an arterial closure device or else by surgical cut-down of a femoral artery (n = 38). The applicability of this approach is limited by the anatomy of the vessels in the inguinal area, and of the aorta. The CoreValve system requires that the luminal diameter of the peripheral artery be at least 6.5 mm, while the corresponding minimum diameter for the Edwards-Sapien system is 7 mm. Contraindications to the transfemoral approach include dissection or severe tortuosity of the iliac vessels or the aorta, e.g. due to scoliosis, and previous bypass surgery or stenting of the peripheral vessels or of the abdominal aorta.
(b) Transcatheter valve implantation via the subclavian artery (N = 3, all performed with the CoreValve): This approach can be considered for patients with severely diseased peripheral vessels not suitable for valve implantation. The left subclavian artery is to be preferred, because the angle of implantation by way of the right subclavian artery is unfavorable. In this approach, too, the vessel must be at least as wide as the minimum diameter specified above (see [a]). We have treated three patients with this approach, two through the left subclavian artery and one through the right subclavian artery. The artery is surgically exposed through a 4–5 cm incision below the clavicle.
(c) Alternative approach through the ascending aorta (N = 2, both performed with the CoreValve): In two patients that could be treated neither transarterially nor transapically, the authors implanted a CoreValve prosthesis directly through the ascending aorta. An upper partial sternotomy was performed, and the prosthesis was implanted via aortic puncture.
2. Transapical, antegrade transcatheter aortic valve implantation (N = 26; n = 5, CoreValve; n = 21, Edwards-Sapien): If a transarterial implantation cannot be performed for any of the above reasons, the valvular prosthesis is implanted through the left ventricular apex. A left mini-thoracotomy is performed, and the left ventricular apex is exposed in the fifth intercostal space. The prosthesis is brought into annular position through the left ventricular apex which is secured by pledgeted purse-string sutures.
Implantation of the prostheses
All patients were treated under general anaesthesia and mechanical ventilation in order to assure stable hemodynamic conditions and to enable immediate intervention in case of complications. The procedures were performed in a hybrid operating room with a permanently installed angiography unit. Thus, we had optimal radiological visualization of the placement of the valvular prosthesis and were at the same time fully equipped for surgery in case any complication should arise that might need immediate surgical treatment.
A balloon valvuloplasty of the stenotic aortic valve is always performed before the prosthetic valve is implanted. A balloon catheter is placed either transarterially or transapically by way of the delivery catheter and then expanded under tachycardic ventricular stimulation (so-called rapid pacing, at 160–180 beats per minute). Next, the catheter bearing the crimped valvular prosthesis is introduced. The CoreValve prosthesis is deployed stepwise on the beating heart; the Edwards-Sapien prosthesis is expanded with a balloon, again under tachycardic ventricular stimulation. The native aortic valve remains in situ and is pushed to the edge of the aortic root by the expanding prosthesis.
Postoperative care and follow-up
All patients were transferred postoperatively to the cardiac surgical intensive care unit and extubated approximately two hours after the procedure. Continuous ECG monitoring is mandatory for at least three days, because atrioventricular block may arise suddenly even several days after implantation. Lifelong inhibition of platelet aggregation with acetylsalicylic acid (100 mg/day) is recommended for both types of prosthesis; when a CoreValve prosthesis is used, the patient is additionally given clopidogrel (75 mg/day) for six months. The patients were followed up with
echocardiographic functional testing of the prosthesis before hospital discharge (97% complete data),
a telephone interview 30 days later (99% complete data),
a follow-up appointment 6 months later with repeated echocardiography or
a telephone interview, if the patient could not come for a follow-up appointment (91% complete data).
Statistical evaluation
The data are given in percentages, or else as mean ± standard deviation for normally distributed parameters. Differences between mean values of pressure gradients, NYHA (New York Heart Association) classifications, and subjectively quantified health status were tested with Student’s t-test for paired variables. Survival was plotted on Kaplan-Meier survival curves. The statistical evaluation was performed with the SPSS 16.0 software package (German-language version).
Results
Clinical results
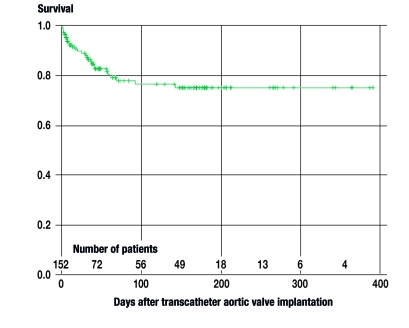
150 of the 152 attempted transcatheter valve implantations were successfully completed. One patient sustained a rupture of the ascending aorta, whereupon the procedure was immediately converted to an open surgical aortic valve replacement. In another patient, a supravalvular dislocation of the prosthesis occurred, so that, again, conversion to an open surgical procedure was necessary. Both of these patients were women. Four patients had cardiac depression intraoperatively, necessitating connection to a heart-lung machine; all four could be stabilized on the machine and were then successfully weaned from it. In this cohort of patients, all of whom were at high risk, the 30-day mortality was 11.8% (18/152; 8 of cardiac/valvular causes, 9 non-cardiac, 1 unknown). Twelve patients died later on in their course (3 of valvular causes, 9 non-cardiac; see figure 3). The most common postoperative complications were third-degree atrioventricular block necessitating pacemaker implantation (31/152, 20%), vascular complications (25/152, 16%), and cerebrovascular events (8/152, 5%).
Figure 3.
Kaplan-Meier survival curve after transcatheter valve implantations performed at the German Heart Center, Munich; 30-day survival, 88.2% (134 of 152 patients)
Before the implantation of an aortic valve prosthesis, 97% of patients were in NHYA stage III or IV; 30 days after the procedure, 86% were in stage I or II, and 83% still were in stage I or II six months after the procedure (p<0.001). The patients were asked to rate their own health status on a subjective scale ranging from 0% (worst possible condition) to 100% (best possible condition); the average values that they gave were 52% preoperatively, 67% at 30 days, and 64% at 6 months (p<0.001).
Hemodynamics
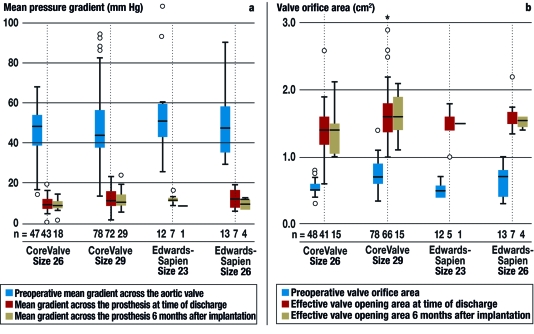
The mean echocardiographically determined pressure gradient across the prosthetic valve was 12 ± 4 mm Hg at the time of discharge and 11± 4 mm Hg six months later. The effective orifice area was 1.56 ± 0.4 cm2 at the time of discharge and 1.54 ± 0.3 cm2 six months later (figure 4). The frequency of paravalvular leaks of grade 2 or higher was 11% at the time of discharge and 7% six months later.
Figure 4.
Hemodynamic function of the different types and sizes of valves that can be implanted through catheters;
(a) mean gradient,
(b) valve orifice area
Discussion
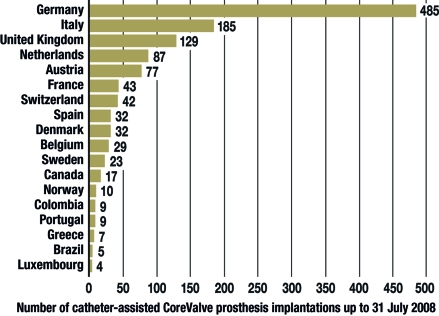
Since Alain Cribier performed the first successful transcatheter aortic valve implantation in a human in 2002 (11), the technique has undergone further development. It is now being evaluated in a small number of hospitals as a therapeutic option for patients who, for a variety of reasons, cannot undergo conventional surgery. More than 2500 patients around the world have undergone transcatheter aortic valve implantation to date in the setting of feasibility and safety studies (I-REVIVE, RECAST, REVIVAL-1, TRAVERCE, the Siegburg "First-in-Man" trial [12], and the 18F-Safety trial) (figure 5). In Europe and the USA, multiple safety and feasibility studies of the CoreValve prosthesis and an FDA (Food and Drug Administration) approval study of the Edwards-Sapien valve are currently in progress (see http://www.clinicaltrials.gov/ct2/search). No results of these studies have yet been published.
Figure 5.
The number of CoreValve prostheses implanted worldwide as of 31 July 2008 (reprinted with the kind permission of CoreValve, Irvine, California)
The results that have been published so far from clinical trials involving 22 to 86 patients (8– 10, 13, 14) allow only preliminary conclusions to be drawn about a number of unresolved issues that will, in the future, be critical in determining the proper clinical role of transcatheter valve implantation.
First of all, the survival advantage putatively conferred by the new technique has not yet been scientifically documented. The 30-day mortality of transcatheter aortic valve implantation is 9% to 18%, according to the literature published to date (8– 10, 13, 14), and currently stands at 11.8% in the authors’ own clinical experience. These figures are lower than the expected mortality of conventional aortic valve replacement in a comparable group of patients, which has been estimated at 24% with the aid of risk scoring. The EuroScore (www.euroscore.org), however, probably does not fully reflect the true risk for a highly selected patient group (15). Comparisons with the mortality after surgical aortic valve replacement, which is 3–4% overall and 7.9% for patients over age 80 at the German Heart Center, Munich, are problematic because patients treated with the transcatheter technique are a selected group of persons at high risk. Ultimately, the survival advantage of transcatheter valve implantation can only be conclusively demonstrated with a randomized study, as is now being carried out for the Edwards-Sapien prosthesis.
Second, long-term follow-up data are lacking. Once these are collected, the potential valve-associated complications and the durability and function of the new prostheses will have to be compared with the corresponding results after conventional, biological aortic valve replacement. In the studies published to date, mean pressure gradients of 9 to 11 mm Hg and effective orifice areas of 1.6 to 1.7 cm2 at the time of discharge have been described (8– 10, 13, 14), while only one author has reported data at 6 months: gradient 7 mm Hg, effective orifice area 1.5 cm2 (14).
In our experience, the hemodynamic function of the prosthesis remains highly satisfactory six months after implantation (figure 4). The mean pressure gradients among our own patients (12 ± 4 mm Hg at the time of discharge and 11± 4 mm Hg six months later) are comparable to those of conventionally implanted bioprostheses (10 to 15 mm Hg, see Ref. [16]). Nonetheless, data with longer follow-up times are indispensable if the indication for transcatheter aortic valve implantation is to be expanded to patients under age 75.
Furthermore, there is evidence that neurological complications arise less commonly after transapical than after transarterial implantation of the prosthetic valve. In our own group of patients, 6.4% of those that underwent transarterial implantation sustained a neurological event (5 infarctions in the territory of the middle cerebral artery with hemiparesis, 1 cerebellar infarction with complete neurological recovery, and 2 patients who failed to wake up after the intervention—corresponding figures from the literature: 4% to 10% [9, 10]), while no patient that underwent transapical implantation sustained any neurological complication (corresponding figures from the literature: 0% to 3.5% [8, 14]). If these findings are borne out by further studies on a larger number of patients, then the choice of approach for transcatheter aortic valve implantation will, in future, have to take each patient’s individual neurological risk profile into account.
Once these questions are answered, the long-term goal will be to establish clear-cut indications for the transcatheter procedures. At the German Heart Center, Munich, we consider performing a transcatheter procedure in any patient over 75 years old or who would be a high-risk candidate for surgery, as judged both clinically and by the EuroScore (>20%), or if conventional, open surgical valve replacement is definitively contraindicated by, e.g., a porcelain aorta. Our attitude in this respect is consistent with the recommendations recently formulated by the European Association of Cardio-Thoracic Surgery, the European Society of Cardiology, and the European Association of Percutaneous Cardiovascular Interventions (17). These recommendations state that transcatheter aortic valve implantation should only be considered for patients with high-grade, symptomatic aortic stenosis who would be at high operative risk. The degree of risk is supposed to be estimated primarily by clinical judgment, supplemented by quantitative risk scores (EuroScore >20%, STS-Score >10% [STS, Society of Thoracic Surgeons]) (17). The American Heart Association has released a statement to the effect that transcatheter techniques should not be used in patients who are "good surgical candidates" (18), i.e., patients whose operative risk is not elevated.
Moreover, in the recommendations mentioned above, it is emphasized that the ideal place to perform a transcatheter procedure is a hybrid operating room (17), such as is available at the German Heart Center, Munich, and in other institutions. As is stated in the recommendations of the European Association of Cardio-Thoracic Surgery, the European Society of Cardiology, and the European Association of Percutaneous Cardiovascular Interventions, only a hybrid operating room can simultaneously provide both high-quality imaging and the availability of immediate surgical intervention when necessary, including the use of a heart-lung machine (17). In the future, specialized interdisciplinary teams will have to be trained in the performance of these procedures. The borders between cardiac surgery, cardiology, and interventional radiology will become increasingly blurred.
Overview
The technical feasibility of the new methods of transcatheter aortic valve implantation has been demonstrated at multiple centers around the world. The indications for these procedures will have to be further refined in future on the basis of the short- and long-term findings of both randomized and observational studies. The results we have obtained so far at the German Heart Center, Munich, indicate a notable clinical improvement of patients considered inoperable by conventional, open methods, as well as highly satisfactory hemodynamic function of the valvular prostheses six months after their implantation.
Key messages.
For an increasing number of patients with high-grade aortic stenosis, the risk of surgical aortic valve replacement is very high because of advanced age and major comorbidity.
For this group of patients, catheter-assisted aortic valve implantation is a new, curative mode of treatment.
In catheter-assisted aortic valve implantation, an aortic valve prosthesis folded to fit inside a catheter is placed in the aortic position under radiological guidance and then unfolded. Valvular prostheses can be placed by means of catheters either transarterially (usually via femoral puncture) or through the cardiac apex.
The technical feasibility of this method has already been demonstrated at a number of centers around the world.
The future role of this new mode of treatment for aortic stenosis will be determined by the results of large-scale randomized trials and observational studies.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Dr. Mazzitelli and Prof. Bauernschmitt have received consulting fees from the Edwards company. The remaining authors declare that they have no conflict of interest as defined by the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21:1220–1225. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 2.Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–e231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 3.Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg. 2006;82:2111–2115. doi: 10.1016/j.athoracsur.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 4.Grube E, Laborde JC, Zickmann B, et al. First report on a human percutaneous transluminal implantation of a self-expanding valve prosthesis for interventional treatment of aortic valve stenosis. Catheter Cardiovasc Interv. 2005;66:465–469. doi: 10.1002/ccd.20544. [DOI] [PubMed] [Google Scholar]
- 5.Lange R, Schreiber C, Gotz W, et al. First successful transapical aortic valve implantation with the Corevalve Revalving system: a case report. Heart Surg Forum. 2007;10:E478–E479. doi: 10.1532/HSF98.20071140. [DOI] [PubMed] [Google Scholar]
- 6.Sack S, Naber C, Kahlert P, et al. Percutaneous heart valve implantation in the aortic position. Herz. 2005;30:433–437. doi: 10.1007/s00059-005-2726-1. [DOI] [PubMed] [Google Scholar]
- 7.Webb JG, Chandavimol M, Thompson CR, et al. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation. 2006;113:842–850. doi: 10.1161/CIRCULATIONAHA.105.582882. [DOI] [PubMed] [Google Scholar]
- 8.Walther T, Simon P, Dewey T, et al. Transapical minimally invasive aortic valve implantation: multicenter experience. Circulation. 2007;116:I240–I245. doi: 10.1161/CIRCULATIONAHA.106.677237. [DOI] [PubMed] [Google Scholar]
- 9.Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol. 2007;50:69–76. doi: 10.1016/j.jacc.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 10.Webb JG, Pasupati S, Humphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation. 2007;116:755–763. doi: 10.1161/CIRCULATIONAHA.107.698258. [DOI] [PubMed] [Google Scholar]
- 11.Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106:3006–3008. doi: 10.1161/01.cir.0000047200.36165.b8. [DOI] [PubMed] [Google Scholar]
- 12.Grube E, Laborde JC, Gerckens U, et al. Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study. Circulation. 2006;114:1616–1624. doi: 10.1161/CIRCULATIONAHA.106.639450. [DOI] [PubMed] [Google Scholar]
- 13.Rodés-Cabau J DE, De LaRochellière R, Doyle D, et al. Feasibility and initial results of percutaneous aortic valve implantation including selection of the transfemoral or transapical approach in patients with severe aortic stenosis. Am J Cardiol. 2008;102:1240–1246. doi: 10.1016/j.amjcard.2008.06.061. [DOI] [PubMed] [Google Scholar]
- 14.Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of transcatheter insertion of a stented aortic valve by the left ventricular apex. Ann Thorac Surg. 2008;86:46–54. doi: 10.1016/j.athoracsur.2008.04.049. [DOI] [PubMed] [Google Scholar]
- 15.Grossi EA, Schwartz CF, Yu PJ, et al. High-risk aortic valve replacement: are the outcomes as bad as predicted? Ann Thorac Surg. 2008;85:102–106. doi: 10.1016/j.athoracsur.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Bleiziffer S, Eichinger WB, Hettich I, et al. Impact of patient-prosthesis mismatch on exercise capacity in patients after bioprosthetic aortic valve replacement. Heart. 2008;94:637–641. doi: 10.1136/hrt.2007.116673. [DOI] [PubMed] [Google Scholar]
- 17.Vahanian A, Alfieri O, Al-Attar N, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2008;29:1463–1470. doi: 10.1093/eurheartj/ehn183. [DOI] [PubMed] [Google Scholar]
- 18.Rosengart TK, Feldman T, Borger MA, et al. Percutaneous and minimally invasive valve procedures: a scientific statement from the American Heart Association Council on Cardiovascular Surgery and Anesthesia, Council on Clinical Cardiology, Functional Genomics and Translational Biology Interdisciplinary Working Group, and Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2008;117:1750–1767. doi: 10.1161/CIRCULATIONAHA.107.188525. [DOI] [PubMed] [Google Scholar]