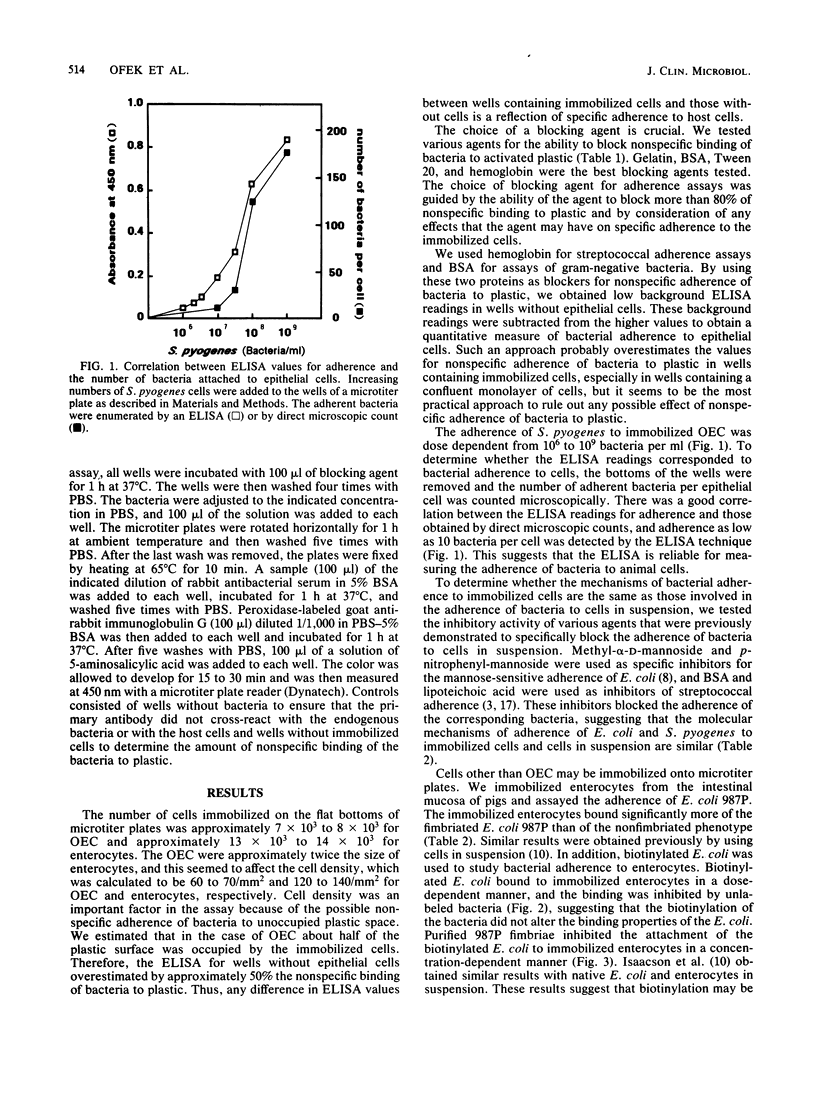
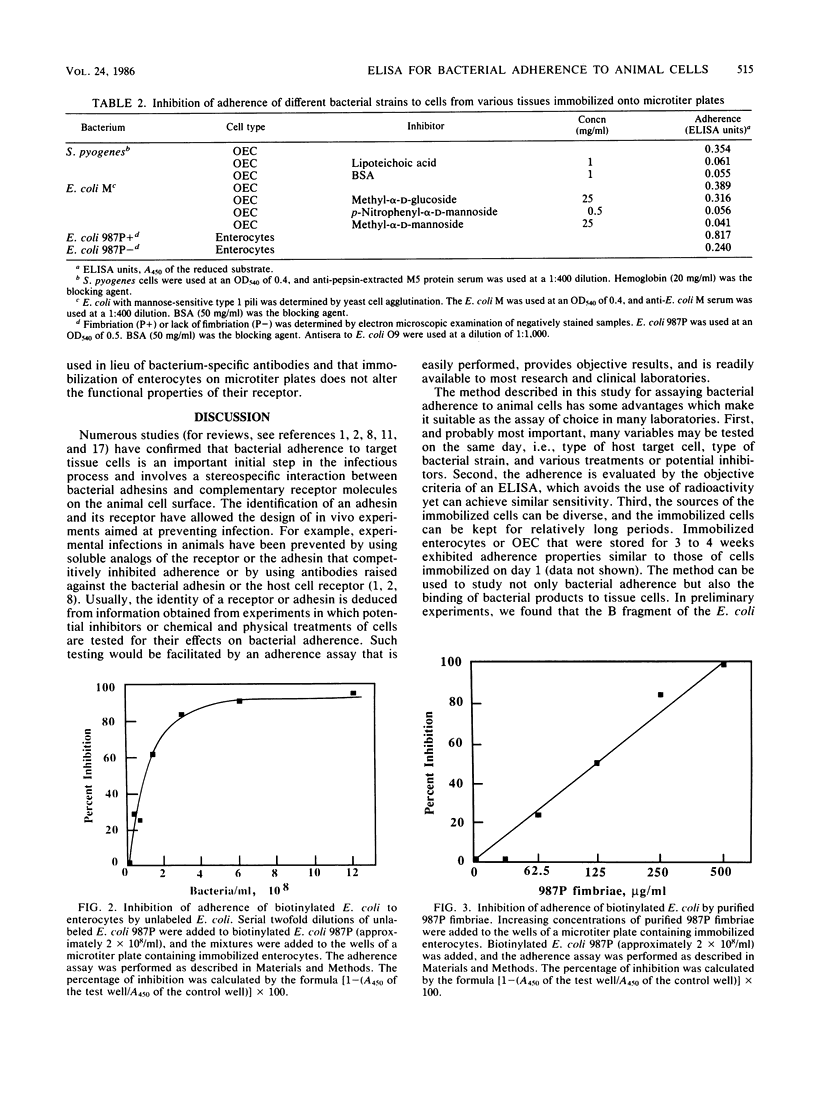
Abstract
Epithelial cells scraped from human oral mucosa and from pig intestines were immobilized onto the flat bottom surfaces of microtiter plates to study the adherence of various bacterial species to host cells. Bacterial adherence was quantitated either by an enzyme-linked immunosorbent assay technique with specific antibacterial serum as the first antibody followed by peroxidase-conjugated second antibody or by using biotinylated bacteria and avidin-peroxidase as the detecting agent. Unlabeled Escherichia coli and purified E. coli 987P fimbriae inhibited the adherence of biotinylated E. coli to immobilized enterocytes. The adherence of a mannose-sensitive strain of E. coli to immobilized oral epithelial cells was inhibited by mannose derivatives. The adherence of fimbriated E. coli 987P to immobilized enterocytes was approximately four times higher than the adherence of a nonfimbriated variant of the same strain. The adherence of Streptococcus pyogenes to oral cells was detected in the range of 10 to 150 bacteria per cell and was inhibited by lipoteichoic acid and albumin. The data suggest that the putative receptors which bind bacteria on the immobilized cells retain a functional form similar to that of native cells in suspension. The proposed adherence assay is easy to perform, allows the detection of specific adherence of test bacteria, and provides objective quantitation of adherence with a sensitivity of 10 bacteria per cell. Most importantly, the assay allows the testing of many variables in the same day.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Courtney H. S., Simpson W. A., Beachey E. H. Relationship of critical micelle concentrations of bacterial lipoteichoic acids to biological activities. Infect Immun. 1986 Feb;51(2):414–418. doi: 10.1128/iai.51.2.414-418.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. B., Ofek I., Beachey E. H. Heterogeneity of type-specific and cross-reactive antigenic determinants within a single M protein of group A streptococci. J Exp Med. 1980 May 1;151(5):1026–1038. doi: 10.1084/jem.151.5.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean E. A., Isaacson R. E. In vitro adhesion of piliated Escherichia coli to small intestinal villous epithelial cells from rabbits and the identification of a soluble 987P pilus receptor-containing fraction. Infect Immun. 1982 Jun;36(3):1192–1198. doi: 10.1128/iai.36.3.1192-1198.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firon N., Ofek I., Sharon N. Carbohydrate specificity of the surface lectins of Escherichia coli, Klebsiella pneumoniae, and Salmonella typhimurium. Carbohydr Res. 1983 Aug 16;120:235–249. doi: 10.1016/0008-6215(83)88019-7. [DOI] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Fusco P. C., Brinton C. C., Moon H. W. In vitro adhesion of Escherichia coli to porcine small intestinal epithelial cells: pili as adhesive factors. Infect Immun. 1978 Aug;21(2):392–397. doi: 10.1128/iai.21.2.392-397.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- Korhonen T. K., Nurmiaho E. L., Ranta H., Edén C. S. New Method for isolation of immunologically pure pili from Escherichia coli. Infect Immun. 1980 Feb;27(2):569–575. doi: 10.1128/iai.27.2.569-575.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy B., Moon H. W., Isaacson R. E. Colonization of porcine intestine by enterotoxigenic Escherichia coli: selection of piliated forms in vivo, adhesion of piliated forms to epithelial cells in vitro, and incidence of a pilus antigen among porcine enteropathogenic E. coli. Infect Immun. 1977 Apr;16(1):344–352. doi: 10.1128/iai.16.1.344-352.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislawski L., Simpson W. A., Hasty D., Sharon N., Beachey E. H., Ofek I. Role of fibronectin in attachment of Streptococcus pyogenes and Escherichia coli to human cell lines and isolated oral epithelial cells. Infect Immun. 1985 Apr;48(1):257–259. doi: 10.1128/iai.48.1.257-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne G. M., Deneke C. F., Gorbach S. L. Hemagglutination and adhesiveness of toxigenic Escherichia coli isolated from humans. Infect Immun. 1979 Mar;23(3):690–699. doi: 10.1128/iai.23.3.690-699.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


