Abstract
As with Usher syndrome observed in humans, the two main phenotypes of the tubby mouse are progressive hearing loss and retinal degeneration. Yet, the mechanism underlying the tub-related cochlear degeneration is still unclear. The reduction/oxidation (redox) imbalance in the cell is related to many kinds of diseases. This study examined expressions of thioredoxin (Trx) and Trx reductase (TrxR), an important redox system in the cell, and the related upstream and downstream proteins of the Trx/TrxR in the tubby mouse cochlea. This report also examined the therapeutic effect of sulforaphane (SF) on the cochlear degeneration, which showed a protective effect on the tub-related retinal degeneration in our previous report. The results showed that the tub-mutation resulted in a significant suppression of Trx and TrxR expressions. Expression level of Nrf2 (NFE2 related factor 2), a transcription factor that regulates expression of Trx and TrxR and others, was also suppressed in the tubby mouse cochlea. Furthermore, a lowered level of activated extracellular signal-regulated kinase (p-ERK) was observed in the tubby mouse cochlea. In contrast, caspase-3 expression and activity were enhanced in the tubby mouse, suggesting apoptotic cell death. The tub-related molecular alterations in the cochlea were prevented by chronic treatment with SF. As a result, the SF-treatment significantly delayed the tub-related cochlear degeneration. Other unknown proteins may contribute to tubby-related degeneration because Nrf2 regulates many other antioxidants besides Trx/TrxR and sulforaphane did not prevent cochlear degeneration completely although it completely prevented alterations of Nrf2 and Trx/TrxR.
Keywords: Tubby mice, Age-related hearing loss, Thioredoxin, Extracellular signal-regulated kinase, Caspase, Sulforaphane
1. Introduction
The tubby strain of obese mice arose spontaneously in a mouse colony at the Jackson Laboratory (Coleman and Eicher, 1990). It is an autosomal recessive mutation, mapping to mouse chromosome 7 (North et al., 1997). The mutation has been associated with a G → T transversion, which abolishes a donor splice site in the gene and results in a larger transcript containing the unspliced intron (Noben-Trauth et al., 1996). The tubby phenotype is characterized by late-onset weight gain accompanied by progressive cochlear and retinal degeneration (Ohlemiller et al., 1995, 1997). The combination of these phenotypes resembles human syndromes, such as Usher’s (retinal and cochlear degeneration), Bardet-Biedl, and Alstrom’s (obesity and sensory deficits). The tubby is a loss-of-function mutation of the tub gene and that loss of the tub gene is sufficient to give rise to the full spectrum of tubby phenotypes (Stubdal et al., 2000).
A progressive hearing loss was reported in the tubby mouse after 3 weeks of age (Heckenlively et al., 1995; Ikeda et al., 1999). A slightly delayed cochlear degeneration was also reported (Ohlemiller et al., 1995, 1997). The mutation caused outer hair cell (OHC) loss in the extreme basal region (hook area) by 1 month of age and the damaged area expanded to the apical turn by about 6 months of age. The inner hair cells (IHCs) were affected in the hook region by about 6 months of age (Ohlemiller et al., 1995, 1997). Yet, the mechanism underlying the death of auditory hair cells in the tubby mouse is still unclear.
Reduction/oxidation (redox) imbalance is related to many kinds of diseases (Fujino et al., 2006). Thioredoxin (Trx) and Trx reductase (TrxR), with NADPH, compose an important redox system in the cell (Buchanan et al., 1994; Fujino et al., 2006; Holmgren, 1995). Trx is characterized by a redox active site with the sequence of -Trp-Cys-Gly-Pro-Cys-Lys-. The two cysteine residues within the redox active center provide the sulfhydryl groups involved in reducing activity. The Trx can be oxidized by free radicals, such as reactive oxygen species (ROS). The oxidized form Trx (Trx-S2), containing a disulfide bridge in the active site, is reduced to a dithiol (Trx-(SH)2) by TrxR in the presence of NADPH. Besides functioning as an antioxidant, Trx has also been identified as an interacting partner or a physiological inhibitor of ASK1 (apoptosis signal-regulating kinase 1), which is a mitogen-activated protein kinase kinase kinase (MAP3K), and activation of which initiates cellular stress response signaling cascades including JNK and p38 pathways (Ichijo et al., 1997; Roberts and Der, 2007). The reduced form of Trx binds to and inhibits ASK1 and the subsequent activation of JNK/p38 activities and apoptotic processes (Saitoh et al., 1998). In contrast, oxidization of Trx by ROS releases Trx from ASK1 leading to JNK and p38 activities and then caspase activation and apoptotic cell death.
Nuclear factor erythroid 2 (NFE2)-related factor-2 (Nrf2) is a transcription factor that regulates the expression of Trx and TrxR as well as other antioxidants via the antioxidant response element (ARE) in the nuclear DNA (Copple et al., 2008). In the absence of cellular stress, Nrf2 is tethered within the cytosol by an inhibitory partner, Keap1. ROS accumulation or activation of extracellular signal-regulated kinase (ERK) releases Nrf2 from the complex. Translocation of the Nrf2 from cytosol to the nucleus activates ARE-regulated gene expression (Copple et al., 2008).
Sulforaphane (SF), a naturally occurring isothiocyanate (Zhang et al., 1992), showed a protective effect on the tub-related retinal degeneration observed in our previous report (Kong et al., 2007). The SF-treatment was repeatedly reported to enhance Trx and TrxR expressions (Kong et al., 2007; Tanito et al., 2005; Zhang et al., 2003). This experiment was designed to determine expressions of Trx, TrxR, and the related upstream/downstream proteins in the tubby mouse. This experiment also explores the therapeutic effect of SF in preventing tub-related cochlear degeneration.
2. Materials and methods
2.1. Animals and genotype analysis
Homozygous mutant tubby mice (tub/tub) were purchased from the Jackson Laboratory (Bar Harbor, ME), and bred with wild type C57BL/6J (wt/wt) mice in the University of Oklahoma Health Sciences Center animal facilities. The obtained heterozygous tubby mice (tub/wt) were used as breeding parents. All animals were born and raised in a 12-h-on and 12-h-off cyclic light environment. The animal facilities are registered with the US Department of Agriculture and are inspected semiannually by the members of the Institutional Animal Care and Use Committee (IACUC) serving the University of Oklahoma Health Sciences Center. All procedures regarding the use and handling of animals were reviewed and approved by the IACUC. For genotype analysis, we used the Jackson Laboratory protocol (Kong et al., 2006, 2007). Briefly, genomic DNA was purified by proteinase K digestion and 2-propanol extraction from tail snips for PCR. Primer sequences used were 5′-CCGTGTCACACAGGCTTCT-3′ (forward); and 5′-CTGGGCACCATGCGTACA-3′ (reverse). PCR conditions consisted of denaturation at 94 °C for 1.5 min, then 35 cycles of 94 °C, and 55 °C, and 72 °C for 30 s, a final extension at 72 °C for 2 min, and 10 °C for 10 min. The PCR products were digested with the SmlI restriction enzyme (New England BioLabs, Inc., Beverly, MA) at 55 °C for 2 h and then separated by 5% MetaPhor agarose gel (FMC, BioProducts, Rockland, ME) electrophoresis. In the wt/wt mice, the 101 bp PCR products were undigested. In the tub/tub mice, the 101 bp PCR products were cut into 52 bp and 49 bp products. In the tub/wt mice, some PCR products were cut into 52 bp and 49 bp products and some were undigested (see Fig. 1). A total of 169 mice (including wt/wt, tub/wt/, and tub/tub) were used in this study. Animal number in each experiment in each group was present in the caption for each figure.
Fig. 1.
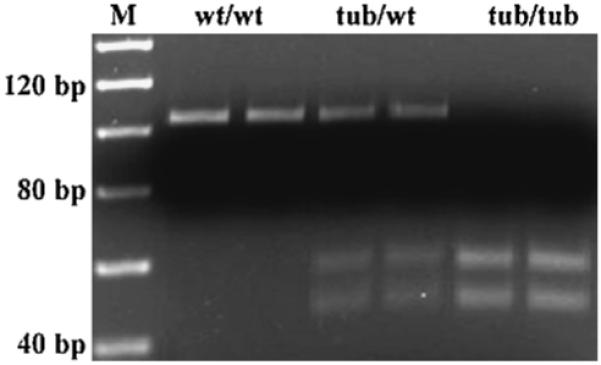
Genotypes of the mice used in this study. Wild type (wt/wt): characterized with 101 bp PCR product; homozygous mutant type (tub/tub): characterized with 52 bp and 49 bp 2 digested PCR products; heterozygous type (tub/wt): characterized with all of the 3 undigested and digested products.
2.2. Sulforaphane (SF) treatment
Mice at postnatal day 10 (P10) were injected intraperitoneally (i.p.) with SF (S8046, LKT Laboratories Inc., St. Paul, MN) at a dose of 50 mg/kg body weight (dissolved in phosphate buffered saline (PBS)), or PBS alone for 20 days (P10-P30, 1/day) as controls. The SF-dose was chosen based on our previous study in which the SF-treatment prevented the tub-related molecular alterations and delayed degeneration in the retina (Kong et al., 2007).
2.3. Hair cell counting
Animals were anesthetized with CO2 and then decapitated and the cochleae were removed immediately. The round and oval windows, as well as the apex of the cochlea were opened to facilitate perfusion. The cochleae were perfused with the incubation solution containing 0.05% tetranitro blue tetrazolium (TNBTZ), 0.05 M sodium succinate, and 0.05 M phosphate buffer and then incubated in the solution for 1 h at 37 °C. TNBTZ is an electron acceptor that, on reduction, precipitates as an insoluble and highly colored formazan. Succinate dehydrogenese (SDH) in the cell oxidizes sodium succinate and provides electrons for the reduction of the electron acceptor. Thus, the cell is colored. The stained cochleae were fixed in 10% buffered formalin for 2 days. After fixation, the cochleae were decalcified in 7% EDTA (ethylenediamine tetraacetic acid) solution. The basilar membranes with the organs of Corti were dissected out and mounted on slides. Hair cells within each section of 500-μm-length on the basilar membrane were counted under a light microscope and the cell numbers were compared to the control numbers obtained in the wild type mice and expressed as % of loss. Hair cell losses were plotted as a function of cochlear length (cochleogram).
2.3.1. Semiquantitative reverse transcription polymerase chain reaction (RT-PCR)
RT-PCR was performed as previously described (Kong et al., 2007; Zhou et al., 2005). Briefly, mice were decapitated after anesthesia with CO2 inhalation and the cochleae were removed immediately. The cochleae were frozen in the liquid nitrogen and stored in a -80 °C freezer before RNA extraction. Total RNA from two cochleae of each mouse was extracted using TRIzol reagent (Invitrogen Corp., Carlsbad, CA) according to the manufacturer’s protocol. The concentration of RNA was quantified by reading the optical density at 260 nm on spectrophotometer (Bio-Rad, Hercules, CA). cDNA was synthesized using The First-Strand Synthesis System for RT-PCR kit (Invitrogen Corp., Carlsbad, CA). PCR conditions consisted of a denaturation at 94 °C for 2 min, then 26-30 cycles of 94 °C for 30 s, 55-58 °C for 15 s, 72 °C for 30 s, and the final extension at 72 °C for 10 min. The PCR products and DNA marker were loaded on agarose gel (1-2% in 1 × TAE buffer, Invitrogen Corp., Carlsbad, CA) and electrophoresed at 105 V for 0.5-1 h. The PCR products on agarose gel were visualized by staining with 0.5 μg/mL ethidium bromide and the gel was photographed under UV light. Gray scale of the band was measured, averaged within each group, and compared between groups. Internal control housekeeping gene, 18S, was run simultaneously. The primer sequences used for PCR were as follows (forward and reverse):
18S: (accession no. NR_003278): 5′-GTAGTGACGAAAAATAACAATACA-3′ and 5′-TGCTGGCACCAGACTTGCCCTCCA-3′
Trx (accession no. X77585): 5′-CAAATGCATGCCGACCTTCCAGTT-3′ and 5′-TGGCAGTTGGGTATAGACTCTCCA-3′
TrxR (accession no. NM_015762): 5′-TGTAATGGTGCGGTCCATTCTCCT-3′ and 5′-TTGTGGATTGAGCAGT-CACCCTGA-3′
Nrf2 (accession no. U20532): 5′-AGTTCTCGCTGCTCGGACTA-3′ and 5′-AGGCATCTTGTTTGGGAATG-3′
Caspase-3 (accession no. NM_009810): 5′-TGGCAACGGAATTCGACTCCTTCT-3′ and 5′-TGAGCATGGACACAATA-GACGGGA-3′
2.3.2. Quantitative real-time PCR analysis
Gene expression in the cochlear samples was also performed using the real-time PCR as described previously (Kong et al., 2007; Yu et al., 2004; Zhou et al., 2005). Total RNA isolation and cDNA synthesis were described above. All PCR reactions were in a final volume of 30 μl comprised of SYBR Green PCR mix (Invitrogen Corp., Carlsbad, CA), 600 μM forward and reverse primers, and 1 ng of synthesized cDNA. Real-time PCR was performed in 96-well plates using a Bio-Rad iCycler (Bio-Rad, Hercules, CA). The following PCR cycle parameters were used: polymerase activation for 15 min at 95 °C, 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 60 s. Expression of the target genes (Trx, TrxR, caspase-3) in each sample was normalized to the expression level of 18 s rRNA of the sample. The normalized gene expression was compared to the expression in the control animals and expressed as fold change over the control. Four steps were followed. (1) Determination of the threshold cycle (Ct) of the target gene and 18 s rRNA; (2) Calculation of Ct difference (ΔCt) between 18 s and the target gene in each sample; (3) Subtraction of ΔCt of the control group from treated animal (ΔΔCt); (4) Calculation of the relative gene expression: Fold increase = 2(-ΔΔCt.
2.4. Western blot analysis
Western blot analysis was performed as described previously (Kong et al., 2006, 2007; Li et al., 2006; Yu et al., 2004; Zhou et al., 2005). Briefly, the two cochleae from each animal were removed quickly after decapitation and homogenized in ice-cold Tissue lysis buffer (Geno Technology, Inc, St. Louis, MO, USA) and then incubated on ice for 20 min. The lysates were centrifuged at 12,000 × g for 15 min and the supernatants were collected. Equal amounts of protein samples (20 μg) were loaded on to a 12% sodium dodecyl sulfate polyacrylamide gel and electrophoresed at 100 V. The isolated proteins were transferred onto a nitrocellulose membrane (Bio-Rad). The membrane was first blocked in 5% non-fat dry milk solution for 1 h at room temperature and then incubated with one of the following primary antibodies at 4 °C overnight: (1) rabbit polyclonal anti-Trx antibody (1:2000, Redox Bioscience, Kyoto, Japan); (2) rabbit polyclonal anti-TrxR antibody (1:1000, Abcam Inc., Cambridge, MA); (3) rabbit polyclonal anti-Nrf2 antibody (1:200, Santa Cruz Biotechnology, Inc., Santa Cruz, CA); (4) rabbit polyclonal anti-caspase-3 antibody (1:200, Santa Cruz Biotechnology, Inc., Santa Cruz, CA); (5) rabbit polyclonal anti-ERKs (1:1000) and anti-pERKs antibodies (1:1000 and 1:500 respectively, Cell Signaling Technology, Beverly, MA). After hybridization with the primary antibody, the membrane was incubated with the horseradish peroxidase-conjugated secondary antibody (1:5000 dilution; Amersham Biosciences, Buckinghamshire, UK) for 1 h at room temperature. Protein bands were detected with enhanced chemiluminescence Western blotting reagents (Amersham Biosciences, Buckin-ghamshire, UK). Gray scale of the band was measured averaged within each group, and compared between groups. All the primary and secondary antibodies were diluted in Tris buffered saline containing 0.1% Tween 20 and 2.5% non-fat dry milk. Equivalent sample loading was confirmed by staining the membrane with Coomassie brilliant blue R-250 (CBB).
2.5. Caspase-3 activities assays
The two cochleae from each animal were removed after decapitation, homogenized, and then centrifuged for 15 min at 12,000 × g. The supernatants were collected. Measurements of activities of caspase-3 in the samples were determined using commercially available kits according to the manufacturer’s manuals (Cat#: KHZ0021, BioSource International, Inc.). Briefly, 300 μg protein extract was added in the reaction buffer containing 200 μM caspase-specific substrate conjugated to p-nitroaniline (pNA) and incubated at 37 °C for 2 h. The amount of free pNA representing caspase-3 activity was measured by spectrometry at 405 nm.
2.6. Statistical analysis
To compare cochlear location-dependent differences of hair cell losses between groups, a two-way ANOVA was performed using GraphPad Prism software (v. 5.01). Significance of differences of protein expressions and activities was determined by t-test. A p value <0.05 was considered to be statistically significant.
3. Results
3.1. Progressive hair cell loss in tubby mice
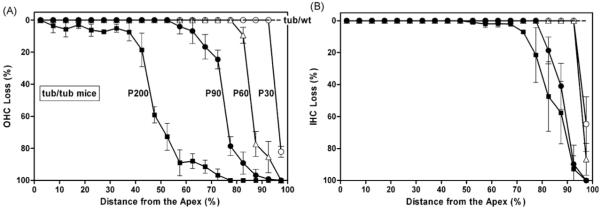
Fig. 2 presents OHC loss (A) and IHC loss (B) in tub/tub mice as a function of cochlear length (cochleogram). The degeneration started from the basal turn and expanded progressively with time towards the apical turn. By 1 month of age, both OHC loss and IHC loss occurred in the hook area (open circles). By 2 months of age, all OHCs in the basal 10% of region were missing. By 3 months of age, almost all OHCs in the basal quarter of region were gone. After 6 months, all OHCs in the basal half area were destroyed. However, IHC degeneration appeared to develop more slowly than the OHC. There was no hair cell loss observed in tub/wt mice.
Fig. 2.
Cochleograms showing progressive OHC losses (A) and IHC losses (B) in tub/tub mice. There was no hair cell loss in the tub/wt mice. Data are expressed as mean ± S.D. (n = 4 in each group).
3.2. Expression levels of Trx and TrxR in the tubby mouse cochlea
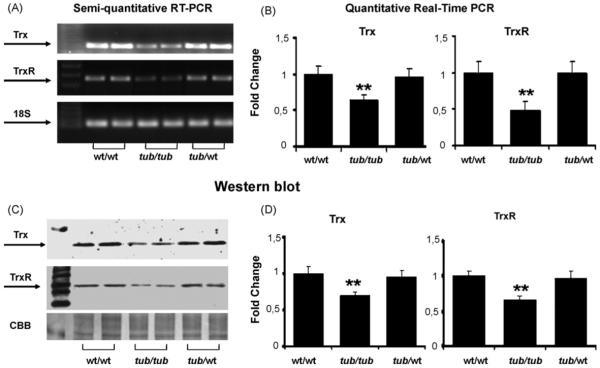
Fig. 3 presents expression levels of Trx and TrxR in the cochlea in wt/wt, tub/tub, and tub/wt mice at P30, at which only a few hair cells in the hook area were missing (see Fig. 2). RT-PCR showed suppressed gene expressions of Trx and TrxR, especially TrxR, in the tub/tub mice compared to both the wt/wt and the tub/wt mice (Fig. 3A). Quantitative measurements by using real-time PCR measurement showed that reductions of the gene expressions were significantly different (p < 0.01) between groups (Fig. 3B). Protein levels of Trx and TrxR in the tub/tub mice, measured by Western blot, were also significantly lower than that in the wt/wt and the tub/wt groups (p < 0.01, Fig. 3C and D). Expressions of Trx and TrxR in the tub/wt mice did not show significant differences from those in the wt/wt mice (p > 0.05).
Fig. 3.
Expressions of Trx and TrxR showing the tub-related deficits of the proteins. (A) Gene expressions determined with RT-PCR; (B) gene expression measured with real-time PCR; (C) representative Western blots; (D) measurements of the Western blot bands. The expressions of the proteins were measured at P30. For gene expression measurements, 18S rRNA was used as the internal control. For protein assay, CBB was used as the control. The measurements are expressed as mean ± S.D. (n = 4 in each group). *p < 0.05; **p < 0.01.
3.3. SF therapeutic effect on Trx/TrxR deficit in the tubby mouse cochlea
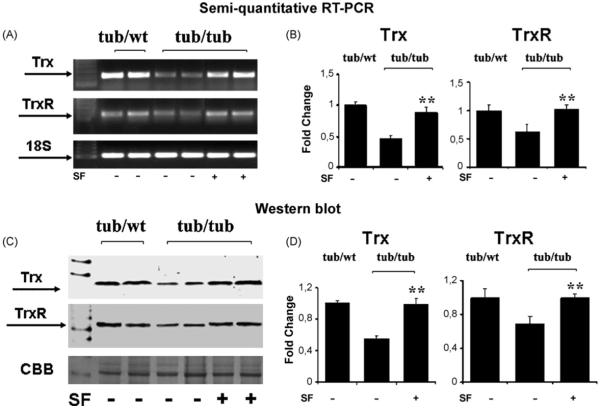
Tub/tub mice were treated with daily injections with SF (50 mg/kg, i.p.) or PBS for 20 days (P10-P30) and expressions of Trx and TrxR at both mRNA level and protein level were determined at P30.Fig. 4 presents gene expressions (A and B) and protein levels (C and D) of Trx and TrxR in the tub/tub and tub/wt mice. As in Fig. 3, both gene expressions and protein levels in the tub/tub mice were lower than that in the tub/wt mice. The suppressed expressions of Trx and TrxR in the tub/tub mice were prevented by the SF-treatment (p < 0.01).
Fig. 4.
The SF-protective effect on expressions of Trx and TrxR in the tub/tub mice. (A and B) Gene expressions measured with RT-PCR; (C and D) protein levels measured with Western blot. For gene expression measurements, 18S rRNA was used as the control. For protein assay, CBB was used as the control. SF treatment: daily intraperitoneally injection with SF (50 mg/kg in PBS) or PBS alone for 20 days from P10. The cochleae were harvested on P30 and the measurements are expressed as mean ± S.D. (n = 4 in each group). *p < 0.05; **p < 0.01.
3.4. Nrf2 and ERK expression levels in the tubby mouse cochlea and the effect of SF treatment
Fig. 5 presents expression levels of Nrf2 in the tubby mouse cochlea. The gene expression at P30 in the tub/tub mice was significantly down-regulated (p < 0.05) compared to the tub/wt mice. The tub-related down-regulation of Nrf2 gene expression was significantly prevented (p < 0.05, see Fig. 5A and B) by SF treatment (50 mg/kg/day, i.p., from P10 to P30). The SF-treatment also slightly enhanced Nrf2 gene expression in the tub/wt mice, but the difference was not significant. Similarly, Nrf2 protein levels measured at P60 and P90 in the cochlea in the tub/tub mice were lower than that in the tub/wt mice. The SF-treatment prevented the tub-related Nrf2 reduction (p < 0.01, see Fig. 5C and D).
Fig. 5.
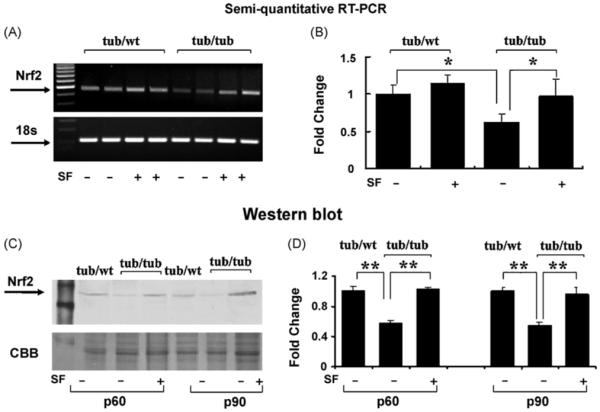
Suppressed expression of Nrf2 in the tub/tub mice and the SF-protective effect. (A and B) Gene expression measured with RT-PCR at P30; (C and D) protein level measurements by Western blot at P60 and P90. For gene expression measurements, 18S rRNA was used as the internal control. For protein assay, CBB was used as the control. SF treatment: daily intraperitoneally injection with SF (50 mg/kg in PBS) or PBS alone for 20 days from P10. The measurements are expressed as mean ± S.D. (n = 4 in each group). *p < 0.05; **p < 0.01.
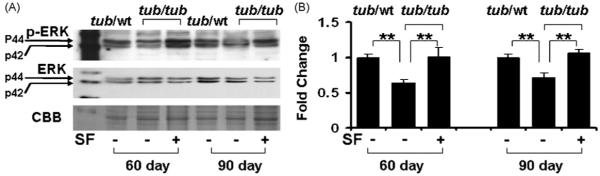
Fig. 6 presents protein levels of ERK1/2 and pERK1/2 in the cochlea measured at P60 and P90. The total ERK proteins did not show consistent tub-related or SF-related changes (Fig. 6A). However, the activated ERK (pERK) proteins showed a tub-related reduction at both P60 and P90 (p < 0.01) and the reduction was prevented by the SF treatment (p < 0.01).
Fig. 6.
Suppressed ERK activation in the tub/tub mice and the SF-protective effect. (A) Representative Western blots; (B) measurements of p-ERKs (activated ERK). CBB was used as the control. The total ERKs are also presented, showing no or less change with the treatment. SF treatment: daily intraperitoneally injection with SF (50 mg/kg in PBS) or PBS alone for 20 days from P10. The cochleae were harvested at P60 and P90. The measurements are expressed as mean ± S.D. (n = 4 in each group). *p < 0.05; **p < 0.01.
3.5. Expression levels and activities of caspase-3 in the tubby mouse cochlea and the effect of SF treatment
Fig. 7 presents gene expressions (A), protein levels (B), and activities of caspase-3 (C) in the tubby mouse cochlea. Gene expression of caspase-3 in the tub/tub mice was significantly up-regulated compared to the tub/wt mice (p < 0.05) at P30. The tub-related up-regulation of gene expression of caspase-3 was significantly prevented by SF treatment (50 mg/kg/day, i.p., from P10 to P30, p < 0.05, Fig. 7A). Similarly, protein levels of caspase-3 in the tub/tub mice were higher than those in the tub/wt mice and the over expressed caspase-3 was prevented by the SF treatment (Fig. 7B). The caspase-3 activities in the tub/tub mice were higher than those in the tub/wt mice at P90 (p < 0.01). The hyperactivity of caspase-3 in the tub/tub mice was significantly prevented by the SF treatment (p < 0.05, Fig. 7C).
Fig. 7.
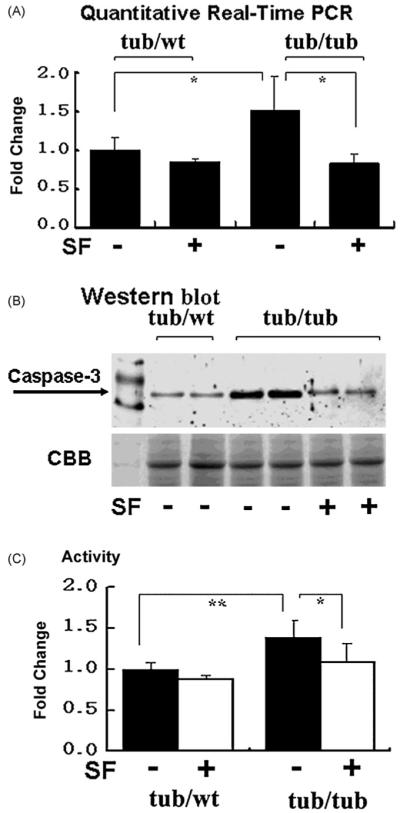
Over expression and hyperactivity of caspase-3 in the tub/tub mice and the SF-protective effect. (A) Gene expressions of caspase-3 measured at P30. Data are expressed as mean ± S.D. (n = 4 in each group); (B) Western blots at P30; (C) activities of caspase-3 measured at P90. Data are expressed as mean ± S.D. (n = 3in each group). SF treatment: 50 mg/kg, 1/day for 20 days starting from P10. *p < 0.05; **p < 0.01.
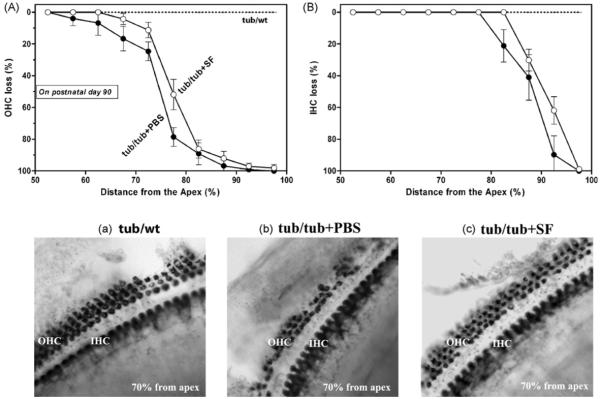
3.6. Hair cell losses in tub/tub mice receiving SF treatment
Fig. 8 presents OHC losses (A) and IHC losses (B) as a function of cochlear length in tub/tub mice at P90. The SF-treated group (open circles, 50 mg/kg/day, i.p., from P10 to P30) showed less OHC losses and less IHC losses compared to the PBS-treated group (filled circles). Two-way ANOVA analysis showed that the differences were significant (p < 0.0001). Images showing auditory hair cells from a cochlear location of 70% from the apex in a tub/wt mouse (a), a tub/tub mouse (b), and a tub/tub mouse treated with SF (c).
Fig. 8.
Protective effect of SF treatment on cochlear degeneration in tub/tub mice. (A) OHC loss; (B) IHC loss as a function of cochlear length. (a), (b), and (c) are representative images from the cochlear location of 70% from the apex in a tub/wt mouse, tub/tub mouse, and a tub/tub mouse treated with SF. SF treatment: daily intraperitoneally injection at a dose of 50 mg/kg or PBS for 20 days from P10. The cochleae were removed for hair cell counting on P90 and the hair cell losses (%) were expressed as mean ± S.D. (n =4 in each group).
4. Discussion
4.1. The tub-related cochlear degeneration
Cochlear degeneration in the tub/tub mouse started from the basal turn after about 30 postnatal days and then expanded gradually towards the apical turn with time. To cochlear stresses induced by various kinds of ototoxic agents, such as carbon monoxide (Liu and Fechter, 1995), the basal turn is known to be more vulnerable than the apical turn. Two recent observations confirmed an increased level of antioxidants, such as superoxide dismutase (SOD) and glutathione, towards the apical turn (Sha et al., 2001; Ying and Balaban, 2008). Thus, the tub-related cochlear degeneration may simply result from the oxidative stress due to the loss-of-function mutation.
4.2. Molecular alterations underlying the cochlear degeneration in the tubby mouse
This study showed that the tubby mutation resulted in a significant suppression of Trx and TrxR expression. Trx, a 12 kDa protein ubiquitously expressed in all living cells, is characterized by an active site sequence: -Trp-Cys-Gly-Pro-Cys-Lys-. The active site is localized in a protrusion of the protein’s three-dimensional structure. The two cysteine residues (Cys) provide the sulfhydryl groups involved in Trx-dependent reducing activity. The oxidized Trx (Trx-S2) by ROS, containing a disulfide bridge in the active site, is reduced to Trx-(SH)2 by the flavoenzyme TrxR with the presence of NADPH. Thus, the Trx family (Trx, TrxR, NADPH) plays an important role in ROS elimination as an important redox system existing in the cell (Fujino et al., 2006; Nakamura et al., 1994; Schallreuter and Wood, 1986; Spector et al., 1988). The cell is known to produce ROS during normal cellular activities, especially along the mitochondrial respiratory chain (Balaban et al., 2005). About 0.2% of total oxygen consumption is estimated to funnel to ROS generation (Staniek and Nohl, 2000; St-Pierre et al., 2002). ROS are normally eliminated by antioxidants including Trx system (Balaban et al., 2005). Over production due to over stimulation in the cochlea, such as intense noise exposure, lead to accumulation of ROS (Ohinata et al., 2000; Ohlemiller et al., 1999; Rao et al., 2001; Yamane et al., 1995a,b). Accumulation of ROS may also result from lack of antioxidants, caused by exposure to ototoxic reagents, such as cisplatin (Rybak and Somani, 1999; Rybak et al., 2000). The low level Trx/TrxR in the tub/tub mouse cochlea may lead to an elevation of ROS, which may directly oxidize macromolecules, such as proteins, lipids, and nucleic acids, leading to cell death or activate stress-activated protein kinase pathways, such as JNK and p38, leading to apoptosis (Balaban et al., 2005).
Besides as an antioxidant, Trx also regulates JNK and p38 signaling pathways via ASK1 (apoptosis signal-regulating kinase 1). ASK1 is a mitogen-activated protein kinase kinase kinase (MAP3K), activating both JNK and p38 cascades (Baines and Molkentin, 2005). The reduced form Trx binds to and inhibits ASK1 and subsequently prevents JNK and p38 activities and subsequent apoptosis. Under conditions of oxidative stress, ROS oxidize Trx, leading to dissociation of Trx from ASK1 and then activation of JNK and p38 pathways (Fujino et al., 2006). In the tubby mouse cochlea, the Trx level is low. Thus, many ASK1 molecules may be free, leading to activation of JNK and p38 pathways and then apoptotic cell death. Indeed, while Trx/TrxR expressions in the tubby mouse cochlea were suppressed, caspase-3 expression and activity were enhanced. Activity of caspase-3 is known to lead to apoptotic cell death.
Expression of Trx and TrxR in the tub/tub mouse appeared to be determined by their upstream proteins, Nrf2, a transcription factor regulating various kinds of antioxidants including Trx and TrxR via antioxidant response element (ARE). While Trx and TrxR expressions were down-regulated in the tub/tub mouse cochlea, a lowered level of Nrf2 expression was observed. Under quiescent conditions, Nrf2 is typically sequestered by Keap1, an inhibitory partner of Nrf2, in the cytoplasm, where it is degraded (Copple et al., 2008). Under conditions of oxidative stress, however, Nrf2 is able to evade Keap1-mediated repression, accumulate within the nucleus, and transactivate ARE, leading to expression of antioxidants (Copple et al., 2008). In the tubby mouse, the low concentration of Nrf2 can lead to a low level expression of Trx and TrxR and other antioxidants leading to an accumulation of ROS leading to apoptotic cell death.
The abnormal Nrf2 expression in the tubby mouse also resulted from its upstream protein. The activated ERK1/2 (pERK1/2) level was lower in the tub/tub mouse than in the tub/wt mouse, although the total ERK level did not show a tub-related change. It is reported that the activated ERK may phosphorylate Nrf2 leading to its dissociation from Keap1 (Papaiahgari et al., 2004; Xu et al., 2006). However, it is still unclear how ERKs regulate transcription of Nrf2. There is evidence showing regulation of Nrf2 expression by ERK pathway. In our previous report, the SF-induced up-regulation of Nrf2 in the tubby mouse was blocked by ERK inhibition (Kong et al., 2007).
4.3. Therapeutic effect of sulforaphane
Interestingly, the tubby mutation-induced alterations in the cochlea, including hair cell death, were significantly prevented after treatment with SF, an isothiocyanate.
SF and two other isothiocyanates, phenethyl isothiocyanate and allyl isothiocyanate, are known to inhibit cancer cell growth and induce apoptosis (Brooks et al., 2001; Chen et al., 2002; Chiao et al., 2002; Singh et al., 2004; Srivastava et al., 2003; Xiao and Singh, 2002; Xiao et al., 2003; Xu et al., 2006). Instead of p53, which promotes expressions of Bax and caspase, the isothiocyanate-induced apoptosis could be mediated by ERK with unclear mechanisms (Xiao and Singh, 2002). ERK1/2 are known to be involved in cell survival. However, recent evidence suggests that the activation of ERK1/2 also contributes to cell death in some cell types and organs under certain conditions (Ou et al., 2007; Zhuang and Schnellmann, 2006).
It is generally accepted that isothiocyanates inhibit carcinogen-activating Phase I enzymes such as cytochrome P450 and induces cancer-protective Phase II enzymes such as TrxR (Conaway et al., 1996; Jakubikova et al., 2005; Prestera et al., 1993; Zhang et al., 2003). As described above, the tubby mutation affects activation of ERK1/2, which results in down-regulation of ARE-regulated genes leading to cell death. The SF treatment enhances activation of the ERKs, and thus prevents the tub-related down-regulated antioxidants and cell death. Our previous study in the tubby mouse retina showed that the SF-therapeutic effect was abolished after inhibition of ERK pathway (Kong et al., 2007).
Since the SF-treatment completely prevented the tub-related alterations of Nrf2 (see Fig. 5), which induces expressions of numerous antioxidants including Trx and TrxR, but only partially prevented the cochlear degeneration (see Fig. 8), other proteins or pathways may be involved in the tub-related cochlear degeneration.
In summary the tubby mutation affects activation of ERK1/2, resulting in down-regulation of Nrf2 and then ARE-regulated expressions of antioxidants including Trx/TrxR redox system, leading to hyperactivity of caspase-3 and cell death. SF enhances ERK activation and then prevents the tub-related alterations.
Acknowledgments
This study was supported by NIH grant P20 RR017730 from COBRE program of the National Center for Research Resources, Foundation Fighting Blindness and an unrestricted grant from RPB to Dr. Wei Cao of the Department of Ophthalmology, University of Oklahoma Health Science Center. Dr. Cao passed away in October 2007, but he will live on in our hearts.
Abbreviations
- ARE
antioxidant-responsive element
- ASK1
apoptosis signal-regulating kinase 1
- CBB
Coomassic brilliant blue R-250
- Ct
threshold cycle
- ERK
extracellular signal-regulated kinase
- IHC
inner hair cell
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- NADPH
reduced form of nicotinamide-adenine dinucleotide phosphate
- NFE2
nuclear factor E2
- Nrf2
NFE2-related factor 2
- OHC
outer hair cell
- PBS
phosphate buffered saline
- RT-PCR
reverse transcription polymerase chain reaction
- Redox
reduction/oxidation
- ROS
reactive oxygen species
- SDH
succinate dehydrogenese
- SF
sulforaphane
- TNBTZ
tetranitro blue tetrazolium
- Trx
thioredoxin
- TrxR
thioredoxin reductase
- tub/tub
homozygous mutant tubby mice
- tub/wt
heterozygous tubby mice
- wt/wt
wild type C57BL/6J mice
References
- Baines CP, Molkentin JD. Stress signaling pathways that modulate cardiac myocyte apoptosis. J. Mol. Cell Cardiol. 2005;38:47–62. doi: 10.1016/j.yjmcc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Brooks JD, Paton VG, Vidanes G. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidemiol. Biomarkers Prev. 2001;10:949–954. [PubMed] [Google Scholar]
- Buchanan BB, Schurmann P, Decottignies P, Lozano RM. Thioredoxin: a multifunctional regulatory protein with a bright future in technology and medicine. Arch. Biochem. Biophys. 1994;314:257–260. doi: 10.1006/abbi.1994.1439. [DOI] [PubMed] [Google Scholar]
- Chen YR, Han J, Kori R, Kong AN, Tan TH. Phenylethyl isothiocyanate induces apoptotic signaling via suppressing phosphatase activity against c-Jun N-terminal kinase. J. Biol. Chem. 2002;277:39334–39342. doi: 10.1074/jbc.M202070200. [DOI] [PubMed] [Google Scholar]
- Chiao JW, Chung FL, Kancherla R, Ahmed T, Mittelman A, Conaway CC. Sulforaphane and its metabolite mediate growth arrest and apoptosis in human prostate cancer cells. Int. J. Oncol. 2002;20:631–636. doi: 10.3892/ijo.20.3.631. [DOI] [PubMed] [Google Scholar]
- Coleman DL, Eicher EM. Fat (fat) and tubby (tub): two autosomal recessive mutations causing obesity syndromes in the mouse. J. Hered. 1990;81:424–427. doi: 10.1093/oxfordjournals.jhered.a111019. [DOI] [PubMed] [Google Scholar]
- Conaway CC, Jiao D, Chung FL. Inhibition of rat liver cytochrome P450 isozymes by isothiocyanates and their conjugates: a structure-activity relation-ship study. Carcinogenesis. 1996;17:2423–2427. doi: 10.1093/carcin/17.11.2423. [DOI] [PubMed] [Google Scholar]
- Copple IM, Goldring CE, Kitteringham NR, Park BK. The Nrf2-Keap1 defence pathway: Role in protection against drug-induced toxicity. Toxicology. 2008;246:24–33. doi: 10.1016/j.tox.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Fujino G, Noguchi T, Takeda K, Ichijo H. Thioredoxin and protein kinases inredox signaling. Semin. Cancer Biol. 2006;16:427–435. doi: 10.1016/j.semcancer.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Heckenlively JR, Chang B, Erway LC, Peng C, Hawes NL, Hageman GS, Roderick TH. Mouse model for Usher syndrome: linkage mapping suggests homology to Usher type I reported at human chromosome 11p15. Proc. Natl. Acad. Sci. U.S.A. 1995;92:11100–11104. doi: 10.1073/pnas.92.24.11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin structure and mechanism: conformational changes on oxidation of the active-site sulfhydryls to a disulfide. Structure. 1995;3:239–243. doi: 10.1016/s0969-2126(01)00153-8. [DOI] [PubMed] [Google Scholar]
- Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Zheng QY, Rosenstiel P, Maddatu T, Zuberi AR, Roopenian DC, North MA, Naggert JK, Johnson KR, Nishina PM. Genetic modification of hearing in tubby mice: evidence for the existence of a major gene (moth1) which protects tubby mice from hearing loss. Hum. Mol. Genet. 1999;8:1761–1767. doi: 10.1093/hmg/8.9.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubikova J, Sedlak J, Mithen R, Bao Y. Role of PI3K/Akt and MEK/ERK signaling pathways in sulforaphane- and erucin-induced phase II enzymes and MRP2 transcription G2/M arrest and cell death in Caco-2 cells. Biochem. Pharmacol. 2005;69:1543–1552. doi: 10.1016/j.bcp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Kong L, Li F, Soleman CE, Li S, Elias RV, Zhou X, Lewis DA, McGinnis JF, Cao W. Bright cyclic light accelerates photoreceptor cell degeneration in tubby mice. Neurobiol. Dis. 2006;21:468–477. doi: 10.1016/j.nbd.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Kong L, Tanito M, Huang Z, Li F, Zhou X, Zaharia A, Yodoi J, McGinnis JF, Cao W. Delay of photoreceptor degeneration in tubby mouse by sulfora-phane. J. Neurochem. 2007;101:1041–1052. doi: 10.1111/j.1471-4159.2007.04481.x. [DOI] [PubMed] [Google Scholar]
- Li C, Tang Y, Li F, Turner S, Li K, Zhou X, Centola M, Yan X, Cao W. 17beta-estradiol (betaE2) protects human retinal Muller cell against oxidative stress in vitro: evaluation of its effects on gene expression by cDNA microarray. Glia. 2006;53:392–400. doi: 10.1002/glia.20291. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fechter LD. MK-801 protects against carbon monoxide-induced hearing loss. Toxicol. Appl. Pharmacol. 1995;132:196–202. doi: 10.1006/taap.1995.1099. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Matsuda M, Furuke K, Kitaoka Y, Iwata S, Toda K, Inamoto T, Yamaoka Y, Ozawa K, Jodoi J. Adult T cell leukemia-derived factor/human thioredoxin protects endothelial F-2 cell injury caused by activated neutrophils or hydrogen peroxide. Immunol. Lett. 1994;42:75–80. doi: 10.1016/0165-2478(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Naggert JK, North MA, Nishina PM. A candidate gene for the mouse mutation tubby. Nature. 1996;380:534–538. doi: 10.1038/380534a0. [DOI] [PubMed] [Google Scholar]
- North MA, Naggert JK, Yan YZ, Noben-Trauth K, Nishina PM. Molecular characterization of TUB, TULP1, and TULP2, members of the novel tubby gene family and their possible relation to ocular diseases. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3128–3133. doi: 10.1073/pnas.94.7.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohinata Y, Miller JM, Altschuler RA, Schacht J. Intense noise induces formation of vasoactive lipid peroxidation products in the cochlea. Brain Res. 2000;878:163–173. doi: 10.1016/s0006-8993(00)02733-5. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Hughes RM, Mosinger-Ogilvie J, Speck JD, Grosof DH, Silverman MS. Cochlear and retinal degeneration in the tubby mouse. Neuroreport. 1995;6:845–849. doi: 10.1097/00001756-199504190-00005. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Hughes RM, Lett JM, Ogilvie JM, Speck JD, Wright JS, Faddis BT. Progression of cochlear and retinal degeneration in the tubby (rd5) mouse. Audiol. Neurootol. 1997;2:175–185. doi: 10.1159/000259242. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Wright JS, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol. Neurootol. 1999;4:229–236. doi: 10.1159/000013846. [DOI] [PubMed] [Google Scholar]
- Ou YC, Yang CR, Cheng CL, Raung SL, Hung YY, Chen CJ. Indomethacin induces apoptosis in 786-O renal cell carcinoma cells by activating mitogen-activated protein kinases and AKT. Eur. J. Pharmacol. 2007;563:49–60. doi: 10.1016/j.ejphar.2007.01.071. [DOI] [PubMed] [Google Scholar]
- Papaiahgari S, Kleeberger SR, Cho HY, Kalvakolanu DV, Reddy SP. NADPH oxidase and ERK signaling regulates hyperoxia-induced Nrf2-ARE transcriptional response in pulmonary epithelial cells. J. Biol. Chem. 2004;279:42302–42312. doi: 10.1074/jbc.M408275200. [DOI] [PubMed] [Google Scholar]
- Prestera T, Holtzclaw WD, Zhang Y, Talalay P. Chemical and molecular regulation of enzymes that detoxify carcinogens. Proc. Natl. Acad. Sci. U.S.A. 1993;90:2965–2969. doi: 10.1073/pnas.90.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao DB, Moore DR, Reinke LA, Fechter LD. Free radical generation in the cochlea during combined exposure to noise and carbon monoxide: an electrophysiological and an EPR study. Hear. Res. 2001;161:113–122. doi: 10.1016/s0378-5955(01)00366-5. [DOI] [PubMed] [Google Scholar]
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Somani S. Ototoxicity. Amelioration by protective agents. Ann. N. Y. Acad. Sci. 1999;884:143–151. [PubMed] [Google Scholar]
- Rybak LP, Husain K, Morris C, Whitworth C, Somani S. Effect of protective agents against cisplatin ototoxicity. Am. J. Otol. 2000;21:513–520. [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of. apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallreuter KU, Wood JM. The role of thioredoxin reductase in the reduction of free radicals at the surface of the epidermis. Biochem. Biophys. Res. Commun. 1986;136:630–637. doi: 10.1016/0006-291x(86)90487-0. [DOI] [PubMed] [Google Scholar]
- Sha SH, Taylor R, Forge A, Schacht J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear. Res. 2001;155:1–8. doi: 10.1016/s0378-5955(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- Spector A, Yan GZ, Huang RR, McDermott MJ, Gascoyne PR, Pigiet V. The effect of H2O2 upon thioredoxin-enriched lens epithelial cells. J. Biol. Chem. 1988;263:4984–4990. [PubMed] [Google Scholar]
- Srivastava SK, Xiao D, Lew KL, Hershberger P, Kokkinakis DM, Johnson CS, Trump DL, Singh SV. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits growth of PC-3 human prostate cancer xenografts in vivo. Carcinogenesis. 2003;24:1665–1670. doi: 10.1093/carcin/bgg123. [DOI] [PubMed] [Google Scholar]
- Staniek K, Nohl H. Are mitochondria a permanent source of reactive oxygen species? Biochim. Biophys. Acta. 2000;1460:268–275. doi: 10.1016/s0005-2728(00)00152-3. [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- Stubdal H, Lynch CA, Moriarty A, Fang Q, Chickering T, Deeds JD, Fairchild-Huntress V, Charlat O, Dunmore JH, Kleyn P, Huszar D, Kapeller R. Targeted deletion of the tub mouse obesity gene reveals that tubby is a loss-of-function mutation. Mol. Cell Biol. 2000;20:878–882. doi: 10.1128/mcb.20.3.878-882.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanito M, Kwon YW, Kondo N, Bai J, Masutani H, Nakamura H, Fujii J, Ohira A, Yodoi J. Cytoprotective effects of geranylgeranylacetone against retinal photooxidative damage. J. Neurosci. 2005;25:2396–2404. doi: 10.1523/JNEUROSCI.4866-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Singh SV. Phenethyl isothiocyanate-induced apoptosis in p53-deficient PC-3 human prostate cancer cell line is mediated by extracellular signal-regulated kinases. Cancer Res. 2002;62:3615–3619. [PubMed] [Google Scholar]
- Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, Trump DL, Singh SV. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- Xu C, Yuan X, Pan Z, Shen G, Kim JH, Yu S, Khor TO, Li W, Ma J, Kong AN. Mechanism of action of isothiocyanates: the induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol Cancer Ther. 2006;5(8):1918–1926. doi: 10.1158/1535-7163.MCT-05-0497. [DOI] [PubMed] [Google Scholar]
- Yamane H, Nakai Y, Takayama M, Iguchi H, Nakagawa T, Kojima A. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur. Arch. Otorhinolaryngol. 1995a;252:504–508. doi: 10.1007/BF02114761. [DOI] [PubMed] [Google Scholar]
- Yamane H, Nakai Y, Takayama M, Konishi K, Iguchi H, Nakagawa T, Shibata S, Kato A, Sunami K, Kawakatsu C. The emergence of free radicals after acoustic trauma and strial blood flow. Acta. Otolaryngol. Suppl. (Stockh) 1995b;519:87–92. doi: 10.3109/00016489509121877. [DOI] [PubMed] [Google Scholar]
- Ying YL, Balaban C. Spatial distribution of manganese superoxide dismutase 2 (Mn SOD2) expression in rodent and primate spiral ganglion cells. Abst. Assoc. Res. Otolaryngol. 2008:264. doi: 10.1016/j.heares.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Yu X, Rajala RV, McGinnis JF, Li F, Anderson RE, Yan X, Li S, Elias RV, Knapp RR, Zhou X, Cao W. Involvement of insulin/phosphoinositide 3-kinase/Akt signal pathway in 17 beta-estradiol-mediated neuroprotection. J. Biol. Chem. 2004;279:13086–13094. doi: 10.1074/jbc.M313283200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc. Natl. Acad. Sci. U.S.A. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Svehlikova V, Bao Y, Howie AF, Beckett GJ, Williamson G. Synergy between sulforaphane and selenium in the induction of thioredoxin reductase 1 requires both transcriptional and translational modulation. Carcinogenesis. 2003;24:497–503. doi: 10.1093/carcin/24.3.497. [DOI] [PubMed] [Google Scholar]
- Zhou X, Li F, Kong L, Tomita H, Li C, Cao W. Involvement of inflammation, degradation, and apoptosis in a mouse model of glaucoma. J. Biol. Chem. 2005;280:31240–31248. doi: 10.1074/jbc.M502641200. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Schnellmann RG. A death-promoting role for extracellular signal-regulated kinase. J. Pharmacol. Exp. Ther. 2006;319:991–997. doi: 10.1124/jpet.106.107367. [DOI] [PubMed] [Google Scholar]