Abstract
We recently showed an increase in vascular endothelial growth factor (VEGF), decrease in angiopoietin-1 (Ang-1) and unaltered Ang-2 expression by the villous placenta with advancing baboon pregnancy. Moreover, placental VEGF expression was increased by estrogen in early pregnancy. In the present study, we determined whether placental Ang-1 and Ang-2 are regulated by estrogen. Ang-1 and Ang-2 mRNA and protein were determined by RT-PCR and immunocytochemistry in the placenta of baboons on day 60 of gestation (term is 184 days) after administration of estrogen precursor androstenedione on days 25-59 or on day 54 after acute estradiol administration. Chronic androstenedione treatment increased serum estradiol levels 3-fold (P<0.001) and decreased (P<0.05) villous cytotrophoblast Ang-1 mRNA to a level (0.36 ± 0.08 relative to 18S rRNA) that was one-third of that in untreated animals (0.98 ± 0.26). Within 2 h of estradiol administration, cytotrophoblast Ang-1 mRNA was decreased to a level (0.24 ± 0.05) one-fifth (P<0.05) of that in untreated animals (1.14 ± 0.23). However, Ang-2 mRNA levels were unaltered. Ang-1, Ang-2 and estrogen receptors α and β protein were localized within villous cytotrophoblasts providing a mechanism for estrogen action at this site. In summary, estrogen increased VEGF, decreased Ang-1, and had no effect on Ang-2 expression within placental cytotrophoblasts during early baboon pregnancy. We propose that the estrogen-dependent differential regulation of these angioregulatory factors underpins the unique pattern of neovascularization established within the villous placenta during primate pregnancy.
Keywords: Placenta, Trophoblast, Angiopoietin, Estrogen, Baboon
Introduction
A new vascular system is developed via vasculogenesis, angiogenesis and vascular remodeling within the villous placenta during human pregnancy. Angiogenesis and vascular remodeling are orchestrated by coordinated interactions of stimulatory and inhibitory factors (Ferrara and Davis-Smyth, 1997; Hanahan, 1997; Iruela-Arispe et al., 1997). The most widely recognized angiostimulatory factor, vascular endothelial growth factor (VEGF), binds to its KDR/flk-1 and flt-1 receptors and stimulates endothelial cell proliferation, permeability, migration and assembly into capillary tubes (Ferrara and Davis-Smyth, 1997; Ferrara, 2004). Two other proteins, angiopoietin-1 (Ang-1) and Ang-2, acting via the Tie-2 receptor, work in concert with VEGF to control vessel maturation, remodeling and stabilization (Hanahan, 1997; Maisonpierre et al., 1997; Yancopoulos et al., 2000; Eklund and Olsen, 2006; Brindle et al., 2006). Ang-1 promotes survival and reorganization of endothelial cells and recruitment and association of pericytes/vascular smooth muscle cells with endothelial cells to mature and stabilize newly-formed blood vessels. In contrast, Ang-2, by exerting an antagonistic action on the Ang-1/Tie-2 receptor signal, causes vessel destabilization, loosening of the vessel wall, and suppression of endothelial cell contact with peri-endothelial mural cells rendering endothelial cells more accessible to VEGF, potentially to further promote angiogenesis (Maisonpierre et al., 1997; Lobov et al., 2002; Visconti et al., 2002; Eklund and Olsen, 2006).
VEGF, Ang-1, Ang-2, and the Tie-2 receptor are expressed by cytotrophoblast, syncytiotrophoblast, and vascular cells within the human placenta (Sharkey et al., 1993; Clark et al, 1996; Goldman-Wohl et al., 2000; Wulff et al., 2003; Bussolati et al., 2000). Relatively little is known, however, about the regulation of these angioregulatory factors within the villous placenta during human pregnancy. We recently showed that the rise in estrogen levels of advancing baboon pregnancy was associated with an increase in VEGF (Hildebrandt et al., 2001), a decrease in Ang-1 and absence of change in Ang-2 (Babischkin et al., 2007) mRNA and protein expression by placental villous cytotrophoblasts. Moreover, we showed that placental villous cytotrophoblast VEGF expression and vessel density were increased by prematurely elevating estrogen levels in early baboon gestation (Albrecht et al., 2004; Robb et al., 2004), and proposed that estrogen promotes placental VEGF expression and angiogenesis. Therefore, it is possible that estrogen also regulates placental Ang-1 and Ang-2 expression during baboon pregnancy. In the present study, we assessed this possibility by determining mRNA levels of Ang-1 and Ang-2 in isolated cell fractions of, and protein localization of Ang-1, Ang-2 as well as estrogen receptors α and β by immunocytochemistry within, the villous placenta in baboons in which estrogen levels were chronically or acutely elevated during early gestation when estrogen is normally low.
Materials and Methods
Animals
Female baboons (Papio anubis), originally obtained from the Southwest Foundation for Biomedical Research (San Antonio, TX) and weighing 13-15 kg, were used in this study. Baboons were housed individually in large primate cages and fed standard laboratory primate chow (Harlan Primate Diet, Madison, WI) and fresh fruit twice daily, multiple vitamins daily, and water ad libitum. Females were paired with male baboons for a period of 5 days at the time of ovulation as estimated by menstrual cycle records and turgescence of external sex skin. Pregnancy was confirmed by ultrasonography and day 1 of pregnancy was designated as the day preceding deturgescence. Baboons were cared for and used strictly in keeping with USDA regulations and the NIH Guide for the Care and Use of Laboratory Animals (National Academy Press, 1996). The present study was approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine.
Chronic study
To determine the effect of chronically elevating estrogen early in gestation on placental Ang-1 and -2 expression, placentas were obtained by cesarean section on day 60 of gestation (length of gestation is 184 days) from halothane-anesthetized baboons untreated (n = 7) or treated daily on days 25-59 with androstenedione (30 mg/day sc in 1.0 ml sesame oil, n = 7). Because androstenedione is readily converted to estrogen within the primate trophoblast (Walsh, 1985; Waddell et al., 1992), we reasoned that chronic administration of androstenedione would yield a physiological production/distribution of estrogen locally within the placenta. Baboons were sedated with ketamine HCl (10 mg/kg body weight, im) at 1- to 2-day intervals on days 25-59 and 2-4 ml blood was collected from a peripheral saphenous vein and from a uterine vein at the time of cesarean section. Serum estradiol and progesterone levels were determined by chemiluminescent RIA (Immulite, Diagnostic Products Corp., Los Angeles, CA), as described previously (Albrecht et al., 2000).
Acute study
To assess the effect of an acute increase in estrogen, placentas were obtained via cesarean section on day 54 of gestation from baboons: (a) 2 h after a bolus iv injection of 17β-estradiol (1 μg/kg body weight in 1 ml 5% ethanol: saline) administered via an antecubital vein to produce a rapid elevation in estrogen levels and sc insertion of silastic capsules containing 17β-estradiol to sustain estrogen in increased level (n = 4); (b) 2 h after a bolus iv injection of 5% ethanol:saline vehicle alone (n = 2); or (c) untreated (n = 4). The estradiol capsule implants were employed to eliminate repetitive iv injection and to maintain estrogen at a relatively constant level over the 2 h treatment period. Estradiol treated, vehicle treated and untreated baboons were initially sedated with ketamine and then anesthetized with halothane for purposes of placental removal via cesarean section. Because Ang-1/Ang-2 data were similar in baboons untreated or treated for 2 h with saline results were combined. Blood samples (2 ml) were obtained from a maternal saphenous vein at 15 min intervals during the 2 h acute study period and serum estradiol concentrations determined by RIA.
Placental tissue
A minimum of 8 randomly selected sections of placental villous tissue (each approximately 5 mm3) were collected and either fixed in formalin and embedded in paraffin for immunocytochemistry or frozen and stored at −80°C for mRNA analysis. The remaining villous tissue was enzymatically digested to obtain enriched fractions of villous cytotrophoblasts and villous stromal cells as described by our laboratory (Henson et al., 1988) and Kliman et al (1986). Briefly, placental villous tissue was dispersed in 0.1% collagenase, 0.1% hyaluronidase and 0.02% DNase I or 0.125% trypsin (Sigma Chemical Co, St. Louis, MO). Enriched cytotrophoblast and villous stromal cell fractions were isolated via 5-70% Percoll (Amersham Biosciences, Piscataway, NJ) gradient centrifugation. Sufficient amounts of syncytiotrophoblast were not obtained on days 54 or 60 or villous stromal cells on day 54 for mRNA analysis.
RT-PCR
Ang-1 and Ang-2 mRNA levels were quantified by RT-PCR as described in our recent studies (Hildebrandt et al., 2001; Babischkin et al., 2007). Total RNA was extracted from villous cell fractions or whole villous tissue via guanidine isothiocyanate-cesium chloride gradient centrifugation and quantified by UV absorbance. Oligonucleotide primers were designed (Invitrogen, Carlsbad, CA) based on the human sequences for: Ang-1 (Davis et al., 1996), forward, 5′-GGGGGAGGTTGGACTGTAAT-3′, reverse, 5′-AGGGCACATTTGCACATACA-3′; Ang-2 (Maisonpierre et al., 1997), forward, 5′-GGATCTGGGGAGAGAGGAAC-3′, reverse, 5′-CTCTGCACCGAGTCATCGTA-3′; and 18S RNA (Torczynski et al., 1985), forward, 5′-TCAAGAACGAAAGTCGGAGG-3′, and reverse, 5′-GGACATCTAAGGGCATCACA-3′. Total RNA (75-300 ng) underwent reversed transcription using 200 U Moloney murine leukemia virus reverse transcriptase (Invitrogen), 250 ng random primers (Invitrogen), 1 mM dithiothreitol (Invitrogen), 40 U RNAguard ribonuclease inhibitor (Amersham) and 1 mM dNTPs (Invitrogen). Negative controls, in which either the reverse transcriptase enzyme or RNA was not added to the RT reaction, were performed to test for genomic DNA contamination. One or 5 μl of the RT reactions were then added to separate PCR reactions (final volume 50 μl) containing 1.25 U cloned Thermus aquaticus DNA polymerase (Amplitaq, Perkin-Elmer Corp/Cetus, Norwalk, CT), 0.2 mM dNTPs, and 10 pmol each of Ang-1, Ang-2, and 18S rRNA primer sets. PCR was performed in a programmable thermal cycler (MJ Research Inc., Cambridge, MA) for 33 (Ang-1), 28 (Ang-2), or 14-16 (18S rRNA) cycles with denaturing at 94°C, annealing at 60°C, extension at 72°C and final extension at 72°C. PCR products were fractionated on 2% agarose gel, visualized with ethidium bromide and scanned using Gel-Doc and Multi- Analyst software (Bio-Rad Laboratories, Hercules, CA). The intensities of amplified Ang-1 and Ang-2 mRNA products were assessed as the relative area under each sample band normalized to 18S rRNA. No difference in 18S rRNA levels was observed with treatment.
Immunocytochemistry
The localization of Ang-1, Ang-2 and estrogen receptor α and β was assessed by immunocytochemistry using methods employed in our laboratories (Hildebrandt et al, 2001; Billiar et al., 1997). Placental villous tissue was sectioned (5 μm), deparaffinized, rehydrated in graded concentrations of ethanol, boiled in 0.01 M Na citrate for 5 min for antigen retrival, and incubated in H2O2 to block endogenous peroxidase. Sections were incubated overnight at 4°C with antibodies to goat polyclonal human Ang-1 (1:80 dilution, R & D Systems, Minneapolis, MN), goat polyclonal human Ang-2 (1:160 dilution, R & D), mouse monoclonal human estrogen receptor α (NCL-ER6 F11, 1:40 dilution, Vector Laboratories, Inc., Burlingame, CA) or chicken polyclonal human estrogen receptor β (503, 1:1,000 dilution, supplied by Dr. Jan-Ake Gutafsson, Karolinska Institute, Sweden). Tissue was then incubated 1 h with biotinylated anti-goat, -mouse or chicken immunoglobulins (Vector Laboratories), immersed for 1 h in avidin-biotin-peroxidase complex (ABC Elite, Vector), developed using diaminobenzidine as the chromagen, and counterstained with Harris hematoxylin. Negative controls included omission of the primary antibody.
Statistics
Data were analyzed by unpaired Student's t test.
Results
Chronic Study
Serum estradiol and progesterone
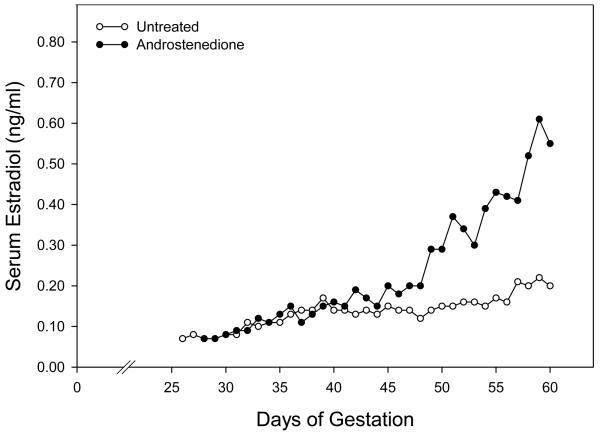
In untreated baboons, maternal peripheral serum estradiol concentrations increased gradually from approximately 0.10 ng/ml on day 25 to 0.20 ng/ml on day 60 of gestation (Fig. 1). Although serum estradiol levels in androstenedione-treated baboons were similar to those in untreated animals on days 25-40 (Fig. 1), estradiol increased thereafter reaching mean (± SE) levels on day 60 in the saphenous (0.51 ± 0.09 ng/ml, Fig. 1 and Table 1) and uterine (1.81 ± 0.31 ng/ml) veins that were approximately 3-fold greater (P<0.01) than in untreated animals (0.18 ± 0.03 and 0.57 ± 0.07 ng/ml, respectively). The increase in estrogen after day 40 apparently reflects the onset and progressive increase in expression of the P-450 aromatase by the placental trophoblast and thus capacity to aromatase androstenedione at this time in early baboon gestation (Albrecht and Pepe, unpublished data).
Fig. 1.
Maternal peripheral serum estradiol concentrations in baboons untreated or treated daily on days 25-59 of gestation (term is 184 days) with androstenedione (30 mg/day, sc). See Table 1 for details of animal treatment.
Table 1.
Maternal serum estradiol and progesterone levels in baboons
| Estradiol (ng/ml) |
Progesterone (ng/ml) |
||||
|---|---|---|---|---|---|
| Treatment | N | Saphenous | Uterine | Saphenous | Uterine |
| Untreated | 7 | 0.18 ± 0.03 | 0.59 ± 0.07 | 14.3 ± 2.3 | 38.8 ± 3.2 |
| Androstenedione | 7 | 0.51 ± 0.09* | 1.81 ± 0.31* | 16.9 ± 2.9 | 43.5 ± 7.2 |
Mean ± SE maternal saphenous and uterine vein serum estradiol and progesterone levels on day 60 of gestation in baboons untreated or treated daily on days 25-59 of gestation (term = 184 days) with androstenedione (30 mg/day, sc).
Values different (P<0.01) than in untreated baboons (Student's t test).
Serum progesterone levels on day 60 of gestation were similar in untreated and androstenedione-treated baboons in the maternal saphenous (14.3 ± 2.3 and 16.9 ± 2.9 ng/ml, respectively) and uterine (38.8 ± 3.2 and 43.5 ± 7.2 ng/ml) veins (Table 1).
Placental and fetal body weights were not significantly different in untreated and androstenedione-treated baboons on day 60 of gestation (Table 2). However, the level of vascularization (i.e. number of blood vessels/mm2) of placental villous tissue determined by image analysis in our previous study (Albrecht et al., 2004) was increased by 50% in baboons in which estrogen levels were elevated by androstenedione treatment (Table 2).
Ang-1 and -2 mRNA
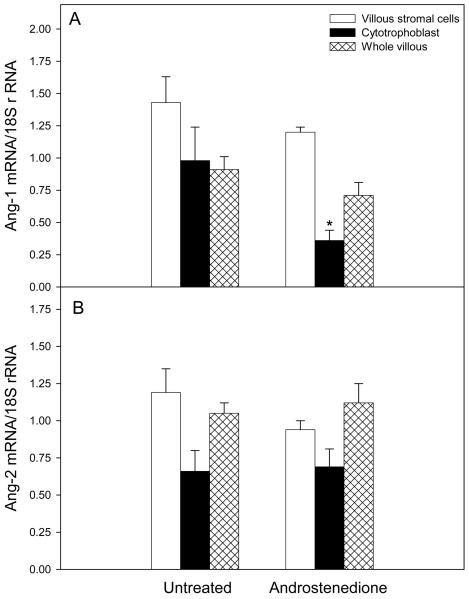
Placental cytotrophoblast Ang-1 mRNA level on day 60 of gestation in androstenedione-treated baboons (0.36 ± 0.08 relative to 18 S rRNA) was approximately one-third (P<0.05) of that in untreated animals (0.98 ± 0.26, Fig. 2A). However, Ang-1 mRNA levels in villous stromal cells and whole placental villous tissue were not significantly different in untreated (1.43 ± 0.20 and 0.91 ± 0.11, respectively) and androstenedione-treated (1.20 ± 0.04 and 0.72 ± 0.10) baboons.
Fig. 2.
Ang-1 (362-bp, panel A) and Ang-2 (535-bp, panel B) mRNA levels (relative to 18S rRNA) determined by RT-PCR in placental villous stromal and cytotrophoblast cells isolated by Percoll gradient centrifugation and whole villous tissue on day 60 of gestation from baboons untreated or treated daily on days 25-59 of gestation with androstenedione. Individual values are the means (± SE) of 7 baboons for each placental cell fraction and treatment group. *Significantly different (P<0.05, Student's t test) than in untreated animals.
In contrast to the decrease in cytotrophoblast Ang-1 mRNA expression, Ang-2 mRNA levels in villous cytotrophoblasts of untreated baboons (0.67 ± 0.14, Fig. 2B) were not significantly changed by androstenedione treatment (0.70 ± 0.12). Moreover, as shown for Ang-1, Ang-2 mRNA levels in isolated villous stromal cells and whole villous tissue were similar in untreated (1.19 ± 0.16 and 1.05 ± 0.07, respectively) and androstenedione treated (0.94 ± 0.06 and 1.12 and 0.13) baboons (Fig. 2B).
Acute study
Serum estradiol
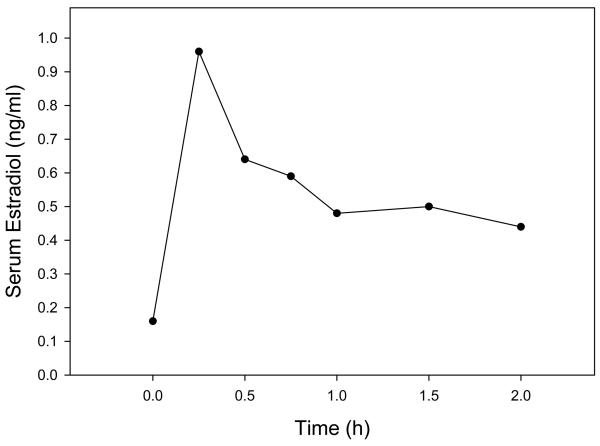
Maternal saphenous vein serum estradiol levels increased from 0.16 ± 0.01 ng/ml immediately before estradiol administration on day 54 of gestation (i.e. time 0, Fig. 3) to 0.96 ± 0.28 ng/ml 15 min after bolus iv injection of and sc implant of capsules containing estradiol. Serum estradiol concentrations then declined attaining a mean ± SE of 0.44 ± 0.03 ng/ml at 2 h, which was approximately 3-fold greater (P<0.01) than that in untreated baboons.
Fig. 3.
Maternal peripheral serum estradiol levels on day 54 of gestation in baboons before and after a bolus iv injection of estradiol (1 μg/kg body weight) and sc implant of 3 silastic capsules containing estradiol at time 0 h. Values at each time point represent means of same 4 baboons.
Ang-1 and Ang-2 mRNA
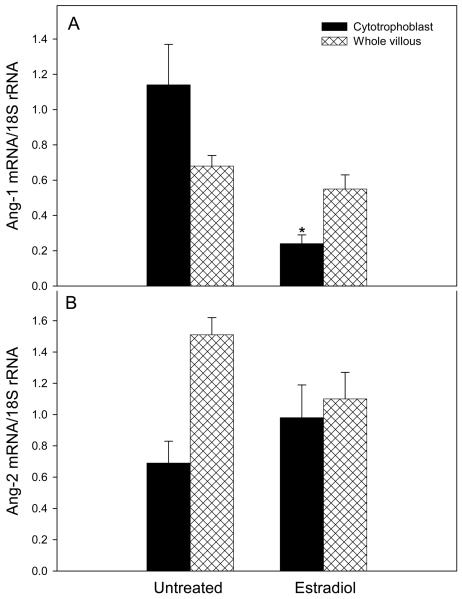
Within 2 h of acute administration of estradiol to animals on day 54 of gestation, placental villous cytotrophoblast Ang-1 mRNA was decreased to a level (0.24 ± 0.05) that was approximately one-fifth (P<0.05) of that in untreated/saline-treated (1.14 ± 0.23, Fig. 4A) animals. However, Ang-1 mRNA levels in whole placental villous tissue of estradiol-treated baboons (0.55 ± 0.08) was not significantly different from that in untreated/saline treated animals (0.68 ± 0.06).
Fig. 4.
Ang-1 (A) and Ang-2 (B) mRNA levels in placental villous cytotrophoblasts and whole villous tissue on day 54 of gestation in baboons untreated or treated for 2 h with saline vehicle (results combined, n=6) and 2 h after acute estradiol administration (n=4) as detailed in the legend of Fig. 3. *Value different (P<0.05, Student's t test) from that in untreated baboons.
Ang-2 mRNA levels in villous cytotrophoblasts and whole placental villous tissue of acutely estradiol-treated baboons (0.98 ± 0.21 and 1.10 ± 0.17, respectively, Fig. 4B) were not significantly different from values in untreated/saline-treated baboons (0.69 ± 0.14 and 1.51 ± 0.11, respectively).
Ang-1, Ang-2, and estrogen receptor α and β immunocytochemistry
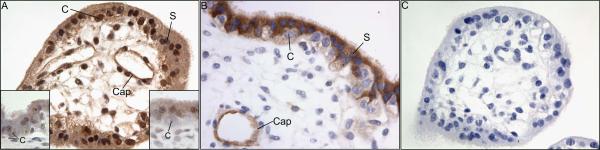
Ang-1 protein was abundantly expressed, as assessed by immunocytochemistry, in the cytotrophoblasts of the chorionic villi, as well as blood vessel walls (capillaries) located in the villous stroma on day 60 of baboon pregnancy (Fig. 5A). Immunostaining for Ang-1 also was observed within the syncytia and avascular cells in the villous stroma. Estrogen receptor α was also expressed within the nucleus of cytotrophoblasts of the baboon placenta (lower left corner of Fig. 5A), while immunostaining of estrogen receptor β appeared to be extremely light (lower right corner of Fig. 5A).
Fig. 5.
Ang-1 (A), estrogen receptor α (lower left corner of panel A), estrogen receptor β (lower right corner of panel A) and Ang-2 (B) immunocytochemistry on day 60 of gestation in untreated baboons. Panel C shows results for Ang-1 when primary antibody was omitted from the reaction. C, cytotrophoblast nucleus; S, syncytiotrophoblast nucleus; Cap, capillary.
Ang-2 protein was also localized in high level in the syncytiotrophoblast and in low level in cytotrophoblasts and vascular walls on day 60 of pregnancy in untreated baboons (Fig. 5B). Specificity of immunoreactivity was confirmed by the absence of Ang-1 (Fig. 5C) and Ang-2 (not shown) staining in the baboon placenta when primary antibodies were omitted from the reaction.
Discussion
The results of the present study show that placental villous cytotrophoblast Ang-1 expression was decreased by elevating the levels of estrogen chronically by androstenedione and acutely by estradiol administration in early baboon pregnancy. The estrogen-induced decrease in Ang-1 expression occurred specifically in placental cytotrophoblasts and not in cells isolated from the villous stroma. Moreover, the reduction in placental trophoblast angioregulatory factor expression was specific for Ang-1, whereas placental Ang-2 mRNA levels were unaltered and VEGF mRNA levels increased (Albrecht et al., 2004; Robb et al., 2004) by estrogen treatment of baboons. We recently reported that Ang-1 expression was decreased and Ang-2 expression was unchanged in both the placental cytotrophoblast and syncytiotrophoblast cell fractions coinciding with the elevation in estrogen of the second half of baboon pregnancy (Hildebrandt et al., 2001; Babischkin et al., 2007). Because syncytial tissue was not obtained in adequate amounts for analysis on day 60 in baboons of the present study, it is not known whether Ang-1 is also decreased in the syncytiotrophoblast by estrogen. Collectively, based on our present and recent findings we suggest that estrogen differentially regulates placental villous expression of VEGF, Ang-1 and Ang-2 in an angioregulatory and cell-specific manner during primate pregnancy.
Several observations suggest that the regulatory actions of estrogen on placental Ang-1 and VEGF formation are mediated by the estrogen receptor. Thus, estrogen receptor α and β were localized within the nuclei of placental cytotrophoblasts and the syncytiotrophoblast on day 60 of baboon gestation, as previously shown in the human trophoblast (Billiar et al., 1997; Bukovsky et al., 2003), providing a mechanism for the action of estrogen within the trophoblast. Moreover, estrogen receptor consensus sequences exist upstream of the promoter region of VEGF (Hyder and Stancel, 1999; Mueller et al., 2000). In addition, placental cytotrophoblast Ang-1 expression was decreased and VEGF expression increased (Robb et al., 2004) rapidly within 2 h of a bolus iv injection of estradiol to baboons, suggesting that the estrogen-induced effects on angioregulatory molecules do not involve cellular differentiation of the cytotrophoblast. However, whether the rapid effects of estrogen on placental Ang-1 and VEGF mRNA levels observed in baboons reflect transcriptional events remains to be determined. Moreover, angiopoietin protein levels need to be quantified in the placental cell isolates to determine whether translation of angiopoietin-1 mRNA into protein is also altered by estrogen during baboon pregnancy.
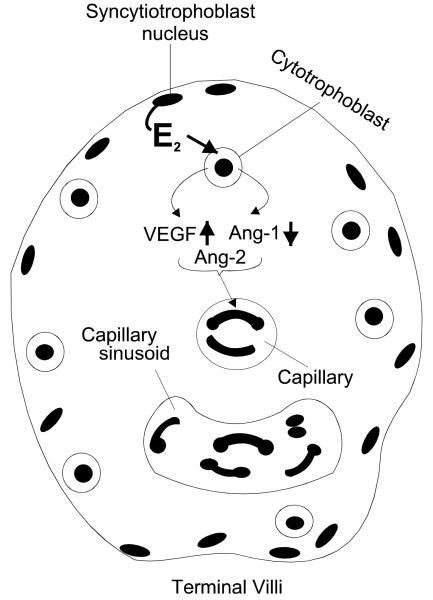
The physiological impact of the differential regulation of placental VEGF, Ang-1, and Ang-2 expression by estrogen also remains to be elucidated. However, it is likely that the estrogen-induced increase in VEGF, decrease in Ang-1 and maintenance of Ang-2 within the trophoblast are responsible, in part, for the unique pattern of neovascularization exhibited within the villous placenta during advancing primate pregnancy. VEGF has a pivotal role in stimulating vascular endothelial cell assembly into capillaries (Ferrara and Gerber, 2001), whereas Ang-1 stimulates vascular endothelial and smooth muscle cell cooption, thereby promoting arteriole-venule vessel formation, maturation and stabilization (Hanahan, 1997; Yancopoulos et al., 2000; Lobov et al., 2002; Visconti et al., 2002). Unopposed, Ang-2 causes vessel breakdown, but in the presence of VEGF Ang-2 appears to render endothelial cells responsive to VEGF (Maisonpierre et al., 1997; Yancopoulos et al., 2000; Visconti et al., 2002), causing angiogenesis and antagonizing the stabilizing effect of Ang-1 (Lobov et al., 2002; Asahara et al., 1998). Thus, the maturation of vessels into arterioles and venules within stem villi (Kaufmann et al., 2004; Charnock-Jones et al., 2004; Mayhew et al., 2004) in the first half of pregnancy when estrogen levels are low may be regulated by elevated trophoblast Ang-1 and presence of VEGF. In the second half of gestation, an extensive highly-coiled capillary sinusoidal network is formed and retained within the mature intermediate and terminal villi which comprise over half of the placental villous mass for maximal maternal-fetal exchange (Kaufmann et al., 2004; Charnock-Jones et al., 2004; Mayhew et al., 2004). We propose, as illustrated in Fig. 6, that in response to increasing levels of estrogen, the increased expression of VEGF, decreased expression of Ang-1 and sustained levels of Ang-2 by trophoblasts creates an environment for the development and retention of capillaries required for maximal blood exchange within the terminal villi.
Fig. 6.
Proposed role for estrogen (E2) in differentially regulating cytotrophoblast VEGF, Ang-1 and Ang-2 expression and consequently angiogenesis within placental terminal villi during primate pregnancy.
We believe that the regulatory effects of estrogen on placental VEGF and Ang-1 expression shown in our studies represent important new insight into the autocrine/paracrine roles which steroid hormones have on placental vascular development. Very little is known about the regulation of members of the angiopoietin family within the reproductive system, although estrogen was also shown to decrease Ang-1 expression in the lung, kidney and heart (Ye et al., 2002). Ang-2 is upregulated by chorionic gonadotropin in the rat testis (Haggstrom-Rudolfsson et al., 2003), while ACTH stimulated Ang-1 in the murine adult adrenal gland (Feraud et al., 2003) and Ang-2 in the human fetal adrenal in culture (Ishimoto et al., 2003). Moreover, hypoxia seems to have an important role in modulating Ang-2 formation in human placental villous explants (Zhang et al., 2001) and the action of estrogen on VEGF expression in the rodent uterus (Kazi et al., 2005). We suggest that the baboon provides an excellent nonhuman primate model to unravel in vivo the regulatory role that estrogen has on placental angiogenesis.
In summary, our present and recently published studies show that chronic and acute elevations in estrogen increased VEGF, decreased Ang-1, and had no effect on Ang-2 expression within placental cytotrophoblasts during early baboon pregnancy. Thus, estrogen differentially regulates placental villous expression of angioregulatory factors in a cell-specific manner. We propose that the estrogen-dependent differential regulation of VEGF, Ang-1, and Ang-2 is responsible for the unique pattern of neovascularization established within the villous placenta during primate pregnancy.
Table 2.
Placental and fetal body weights and placental blood vessel density in baboons.
| Treatment | N | Placental weight | Fetal body weight (g) |
No. blood vessels/mm2 (g) |
|---|---|---|---|---|
| Untreated | 7 | 31.7 ± 1.2 | 12.5 ± 0.5 | 493 ± 34 |
| Androstenedione | 7 | 25.1 ± 2.8 | 11.0 ± 1.3 | 743 ±27* |
Mean ± SE placental and fetal body weights and placental villous blood vessel density (as reported in our previous study, Albrecht et al., 2004) on day 60 of gestation in baboons untreated or treated daily on days 25-59 of gestation with androstenedione.
Values different (P<0.01) than in untreated baboons (Student's t test).
Acknowledgments
The secretarial assistance of Mrs. Wanda James with the manuscript is greatly appreciated.
Footnotes
Supported by National Institutes of Health RO1 Research Grant HD13294
References
- Albrecht ED, Aberdeen GW, Pepe GJ. The role of estrogen in the maintenance of primate pregnancy. Am J Obstet Gynecol. 2000;182:432–438. doi: 10.1016/s0002-9378(00)70235-3. [DOI] [PubMed] [Google Scholar]
- Albrecht ED, Robb VA, Pepe GJ. Regulation of placental vascular endothelial growth/permeability factor expression and angiogenesis by estrogen during early baboon pregnancy. J Clin Endocrinol Metab. 2004;89:5803–5809. doi: 10.1210/jc.2004-0479. [DOI] [PubMed] [Google Scholar]
- Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, Yancopoulos GD, Isner JM. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233–240. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- Babischkin JS, Suresch DL, Pepe GJ, Albrecht ED. Differential expression of placental villous angiopoietin-1 and -2 during early, mid, and late baboon pregnancy. Placenta. 2007;28:212–218. doi: 10.1016/j.placenta.2006.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiar RB, Pepe GJ, Albrecht ED. Immunocytochemical identification of the oestrogen receptor in the nuclei of human placental syncytiotrophoblasts. Placenta. 1997;18:365–370. doi: 10.1016/s0143-4004(97)80071-9. [DOI] [PubMed] [Google Scholar]
- Brindle NPJ, Saharinen P, Alitalo K. Signaling and functions of angiopoietin-1 in vascular protection. Circul Res. 2006;98:1014–1023. doi: 10.1161/01.RES.0000218275.54089.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukovsky A, Caudle MR, Cekanova M, Fernando RI, Wimalasena J, Foster JS, Henley DC, Elder RF. Placental expression of estrogen receptor beta and its hormone binding variant–comparison with estrogen receptor alpha and a role for estrogen receptors in asymmetric division and differentiation of estrogen-dependent cells. Reprod Biol Endocrinol. 2003;1:36–56. doi: 10.1186/1477-7827-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolati B, Perkins J, Shams M, Rhaman M, Nijjar S, Qui Y, Kniss D, Dunk C, Yancopoulos G, Ahmed A. Angiopoietin-1 and angiopoietin-2 are differentially expressed during placental development and stimulate trophoblast proliferation, migration and release of nitric oxide. J Soc Gynecol Invest. 2000;7(Suppl)::158A. [Google Scholar]
- Charnock-Jones DS, Kaufmann P, Mayhew TM. Aspects of human fetoplacental vasculogenesis and angiogenesis. I. Molecular Regulation. Placenta. 2004;25:103–113. doi: 10.1016/j.placenta.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Clark DE, Smith SK, Sharkey AM, Charnock-Jones DS. Localization of VEGF and expression of its receptors flt and KDR in human placenta throughout pregnancy. Human Reprod. 1996;11:1090–1098. doi: 10.1093/oxfordjournals.humrep.a019303. [DOI] [PubMed] [Google Scholar]
- Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp Cell Res. 2006;312:630–641. doi: 10.1016/j.yexcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Feraud O, Mallet C, Vilgrain I. Expressional regulation of the angiopoietin-1 and -2 and the endothelial-specific receptor tyrosine kinase Tie2 in adrenal atrophy: a study of adrenocorticotropin-induced repair. Endocrinology. 2003;144:4607–4615. doi: 10.1210/en.2003-0099. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1999;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP. The role of vascular endothelial growth factor in angiogenesis. Acta Haematol. 2001;106:148–156. doi: 10.1159/000046610. [DOI] [PubMed] [Google Scholar]
- Goldman-Wohl DS, Ariel I, Greenfield C, Lavy Y, Yagel S. Tie-2 and angiopoietin-2 expression at the fetal-maternal interface: a receptor ligand model for vascular remodeling. Mol Human Reprod. 2000;6:81–87. doi: 10.1093/molehr/6.1.81. [DOI] [PubMed] [Google Scholar]
- Haggstrom-Rudolfsson S, Johansson A, Franck Lissbrant I, Wikstrom P, Bergh A. Localized expression of angiopoietin 1 and 2 may explain unique characteristics of the rat testicular microvasculature. Biol Reprod. 2003;69:1231–1237. doi: 10.1095/biolreprod.102.013375. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- Henson MC, Babischkin JS, Pepe GJ, Albrecht ED. Effect of the antiestrogen ethamoxytriphetol (MER-25) on placental low density liproprotein uptake and degradation in baboons. Endocrinology. 1988;122:2019–2026. doi: 10.1210/endo-122-5-2019. [DOI] [PubMed] [Google Scholar]
- Hildebrandt VA, Babischkin JS, Koos RD, Pepe GJ, Albrecht ED. Developmental regulation of vascular endothelial growth/permeability factor messenger ribonucleic acid levels in and vascularization of the villous placenta during baboon pregnancy. Endocrinology. 2001;142:2050–2057. doi: 10.1210/endo.142.5.8174. [DOI] [PubMed] [Google Scholar]
- Hyder SM, Stancel GM. Regulation of angiogenic growth factors in the female reproductive tract by estrogens and progestins. Mol Endocrinol. 1999;13:806–811. doi: 10.1210/mend.13.6.0308. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Dvorak HF. Angiogenesis: A dynamic balance of stimulators and inhibitors. Thromb Haemostasis. 1997;78:672–677. [PubMed] [Google Scholar]
- Ishimoto H, Ginzenger DG, Jaffe RB. Corticotropin regulates angiopoietin-1 and -2 RNA in the human fetal adrenal. J Soc Gynecol Invest. 2003;10:739. [Google Scholar]
- Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta. 2004;25:114–126. doi: 10.1016/j.placenta.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Kazi AA, Jones JM, Koos RD. Chromatin immunoprecipitation analysis of gene expression in the rat uterus in vivo: estrogen-induced recruitment of both estrogen receptor α and hypoxia-inducible factor 1 to the vascular endothelial growth factor promoter. Mol Endocrinol. 2005;19:2006–2019. doi: 10.1210/me.2004-0388. [DOI] [PubMed] [Google Scholar]
- Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., III Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta. 2004;25:127–139. doi: 10.1016/j.placenta.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Mueller MD, Vigne JL, Minchenko A, Lebovic DI, Leitman DC, Taylor RN. Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors alpha and beta. Proc Natl Acad Sci USA. 2000;97:10972–10977. doi: 10.1073/pnas.200377097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb VA, Pepe GJ, Albrecht ED. Acute temporal regulation of placental vascular endothelial growth/permeability factor expression in baboons by estrogen. Biol Reprod. 2004;71:1694–1698. doi: 10.1095/biolreprod.104.030882. [DOI] [PubMed] [Google Scholar]
- Sharkey AM, Charnock-Jones DS, Boocock CA, Brown KD, Smith SK. Expression of mRNA for vascular endothelial growth factor in human placenta. J Reprod Fertil. 1993;99:609–615. doi: 10.1530/jrf.0.0990609. [DOI] [PubMed] [Google Scholar]
- Torczynski RM, Fuke M, Bollon AP. Cloning and sequencing of a human 18S ribosomal RNA gene. DNA. 1985;4:283–291. doi: 10.1089/dna.1985.4.283. [DOI] [PubMed] [Google Scholar]
- Visconti RP, Richardson CD, Sato TN. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF) Proc Natl Acad Sci USA. 2002;99:8219–8224. doi: 10.1073/pnas.122109599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell BJ, Albrecht ED, Pepe GJ. Utilization of maternal and fetal androstenedione for placental estrogen production at mid and late baboon pregnancy. J Steroid Biochem Mol Biol. 1992;41:171–178. doi: 10.1016/0960-0760(92)90045-k. [DOI] [PubMed] [Google Scholar]
- Walsh SW. Regulation of progesterone and estrogen production during rhesus monkey pregnancy. In: Albrecht ED, Pepe GJ, editors. Perinatal Endocrinology; Research in Perinatal Medicine IV. Perinatology Press; Ithaca, NY: 1985. pp. 219–241. [Google Scholar]
- Wulff C, Wiegand M, Kreienberg R, Fraser HM. Angiogenesis during primate placentation in health and disease. Reproduction. 2003;126:569–577. doi: 10.1530/rep.0.1260569. [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Ye F, Florian M, Magder SA, Hussain SN. Regulation of angiopoietin and Tie-2 receptor in non-reproductive tissues by estrogen. Steroids. 2002;67:305–310. doi: 10.1016/s0039-128x(01)00163-5. [DOI] [PubMed] [Google Scholar]
- Zhang EG, Smith SK, Baker PN, Charnock-Jones DS. The regulation and localization of angiopoietin-1, -2, and their receptor Tie2 in normal and pathologic human placentae. Molecular Med. 2001;7:624–635. [PMC free article] [PubMed] [Google Scholar]