Abstract
Objectives:
Cardiovascular responses to stressors are regulated by sympathetic activity, increased in black Americans, and associated with future cardiovascular morbidity. Our aim was to determine whether two functional variants in genes regulating sympathetic activity, a deletion in the α2C-adrenergic receptor (ADRA2C del322-325) and a G-protein β3 subunit variant (GNB3 G825T), affect cardiovascular responses to physiologic stressors and contribute to their ethnic differences.
Methods:
We measured heart rate and blood pressure responses to a cold pressor test (CPT) in 79 healthy subjects (40 blacks, 39 whites), aged 25.7 ± 5.3 years, and determined genotypes for the ADRA2C and GNB3 variants. We examined the response variables (increase in heart rate and blood pressure) in multiple linear regression analyses adjusting first for baseline measures, ethnicity, and other covariates, and then additionally for genotypes.
Results:
Black subjects had a greater heart rate response to CPT than whites (mean difference, 9.9 bpm; 95% confidence interval (CI), 4.1 to 15.6; P=0.001). Both the ADRA2C del/del (15.8 bpm; 95% CI, 8.0 to 23.7; P<0.001) and GNB3 T/T genotypes (6.8 bpm; 95% CI, 0.9 to 12.7; P=0.026) were associated with greater heart rate response. After adjusting for genotypes, the ethnic difference was abrogated (1.3 bpm; 95% CI, −5.4 to 8.0; P=0.70), suggesting that the genetic variants contributed substantially to ethnic differences.
Conclusions:
Variation in genes that regulate sympathetic activity affects hemodynamic stress responses and contributes to their ethnic differences. This study elucidates how genetic factors may in part explain ethnic differences in cardiovascular regulation.
Keywords: Cardiovascular physiology; Stress; Ethnic groups; Genetics; Receptors, Adrenergic, alpha-2; G-protein beta3 subunit
Introduction
The sympathetic nervous system regulates many physiologic responses, particularly those related to stress, and enhanced cardiovascular responses to physiologic and psychological stressors in young, healthy subjects predict long-term adverse cardiovascular outcomes [1]. Sympathetic activity is also increased in cardiovascular diseases such as heart failure, hypertension, arrhythmias, and sudden death, often before clinical manifestations of disease [2]. Thus, altered regulation of sympathetic activity plays a role in the pathogenesis of cardiovascular disease [2, 3].
A major regulator of sympathetic activity is the α2-adrenergic receptor (α2-AR) [4]. Since sympathetic activity is highly heritable [5, 6], and since many diseases associated with increased sympathetic activity have a genetic component, interest has focused on the specific role of α2-AR receptor subtypes (α2A, α2B and α2C) and their genetic variations in the regulation of sympathetic response [7, 8].
The α2C-AR on the presynaptic nerve terminal is part of a negative feedback system that inhibits further release of norepinephrine. This regulatory function is particularly important when sympathetic activity is increased [9]. Presynaptic α2C-ARs may also inhibit secretion of epinephrine from the adrenal gland [10], and postsynaptic receptors induce vasoconstriction [11]. Genetic variation in the α2C-AR may contribute to interindividual differences in autonomic response.
A common 12 base pair deletion in the coding region of the α2C-AR gene (ADRA2C) results in the deletion of four amino acids (del322–325) that results in markedly decreased agonist-mediated responses in vitro [12]. Thus, this less functional variant would be expected to result in increased sympathetic activity in vivo and increased risk for diseases associated with sympathetic activation. Indeed, one study reported an increased risk of systolic heart failure in African-Americans homozygous for the ADRA2C del322–325 variant [13], although a large bi-ethnic population study did not find an association between the deletion variant and imaging markers of systolic dysfunction [14]. The effect of the del322-325 variant on stress responses has not been studied.
Adrenergic receptors couple to heterotrimeric guanine-nucleotide-binding proteins (G proteins) to mediate intracellular signal transduction. A C825T polymorphism in the gene encoding the G protein β3-subunit (GNB3) results in enhanced G protein signalling in vitro [15]. Thus, genetic variation in GNB3 would be expected to contribute to variability in sympathetic responses to stress [16], but this has not been examined.
Although cardiovascular responses to physiologic and mental stressors have often been noted to be greater in African-Americans than Caucasians, the reasons for this enhanced response are not known [17]. Because the ADRA2C del322–325 and the GNB3 C825T variants are much more common in black Americans than whites (approximately 10-fold and 2.5-fold, respectively), we hypothesized that this could contribute to ethnic differences in cardiovascular stress responses. To test this hypothesis, we evaluated the role of the ADRA2C del322–325 and GNB3 C825T variants in sympathetic responses to the cold pressor test, a stimulus that results in a profound increase in sympathetic activity [18], and the contribution of these genetic variants to ethnic differences in responses.
Methods
Subjects
The Institutional Review Board of Vanderbilt University Medical Center approved the study protocol, and subjects gave written informed consent. Eighty subjects were studied: 72 (39 white and 33 black subjects) recruited by advertisement and from a volunteer database [19], and 8 black subjects with known ADRA2C del genotype. Four subjects homozygous for the deletion allele (Del/Del) and 4 homozygous for the insertion allele (Ins/Ins) were recruited to increase the number of individuals with these less common genotypes. All subjects also contributed data to a previous study [20].
African-American and Caucasian American subjects were eligible to participate if they were unrelated residents of middle Tennessee, between 18-45 years of age, and had no clinically significant abnormality based on medical history, physical examination, and routine laboratory testing. Family history of hypertension was determined by self-report. Subjects reported their ethnicity and that of their parents and grandparents using check-boxes to choose among “Caucasian”, “African-American”, “Hispanic”, “Chinese”, “Japanese”, and “other” (the latter to be specified). Multiple choices were permitted. A subject was assigned to an ethnic group when both parents and at least 3 of 4 grandparents were of the same ethnicity. Body mass index (kg/m2) was calculated as weight divided by height2 (kg/m2). Subjects were free of medications and dietary supplements for at least 2 weeks and received an alcohol- and caffeine-free diet (providing daily 150 mmol of sodium, 70 mmol of potassium, and 600 mmol of calcium) for 4 days prior to the study.
Resting measures
Studies were performed on the morning (8:00-10:00 am) of study day 5 in a temperature-controlled room (22°C) in the Vanderbilt University Clinical Research Center after an overnight fast. A 20 G intravenous cannula was inserted into an antecubital arm vein for blood sampling. After 30 minutes, during which subjects rested comfortably supine, blood pressure and heart rate were measured at the left brachial artery by a semi-automated device (Dinamap MPS; GE Medical Systems, Waukesha, WI, USA), with a cuff size appropriate for the arm circumference [21]. To allow habituation, readings were repeated 3-4 times, and the last 2 recordings, obtained one minute apart after 30 minutes of rest, were averaged. Then, a venous blood sample (15 mL) was drawn from the uncuffed arm for determination of plasma catecholamine concentrations and for DNA analysis.
Cold pressor test
All preparations for the cold pressor test were performed only after the resting measures had been obtained in order to minimize confounding through anticipation. With the subject remaining supine, the left foot was fully immersed up to the ankle in a tub filled with ice water (4°C) for 2 minutes. Two subsequent readings of blood pressure and heart rate were taken with the semi-automated device, starting at approximately 15 and 45 seconds after foot immersion. At 1 minute, an additional blood sample (10 mL) was taken for determination of plasma catecholamine concentrations.
Genotyping
DNA for all 79 subjects that completed the study was available for genotyping. After amplification of DNA fragments by polymerase chain reaction (PCR), the ADRA2C del322-325 variant was genotyped by DNA fragment analysis and confirmed by direct sequencing of 10 randomly selected samples. DNA fragment analysis was performed as described previously [22]. In short, a fluorescently labeled forward primer (5′-6-FAM-AGACGGACGAGAGCAGCGCA-3′) and a reverse primer (5′-AGGCCTCGCGGCAGATGCCGTACA-3′) were used to amplify DNA fragments by PCR. Amplicons were denatured at 95°C for 5 min, and fragment analysis performed on an ABI 3730 Genetic Analyzer and its Genotyper V.1.0.1 software.
Genotyping for the GNB3 C825T polymorphism (rs5443) was performed by allelic discrimination with TaqMan 5′-nuclease assays [23] on an ABI 7900 HT real-time PCR system (Applied Biosystems, Foster City, CA) using validated TaqMan probes. Genotypes were generated using a 95% quality value threshold. To ensure quality control, we included samples of known genotype with each genotyping run. Additionally, in a subset of 8 subjects, genotyping by PCR followed by detection of restriction fragment length polymorphisms, as previously described [24], gave concordant results. Genotyping success rate was 100% for the ADRA2C Del322-325 and 98.8% for the GNB3 C825T variant, yielding genotypes in 79 and 78 subjects, respectively.
Plasma catecholamine determination
Blood was collected into cooled heparinized tubes which were immediately placed on ice until centrifuged at 4°C for 10 minutes at 3,000 rpm. Plasma was harvested and stored in tubes containing 40 μL of reduced glutathione (6%) at −20°C until assayed. Norepinephrine and epinephrine concentrations were measured by high-performance liquid chromatography using electrochemical detection with dihydroxybenzylamine as internal standard [25].
Statistical analysis
Data are expressed as mean and standard deviation or 95% confidence interval (CI). Analyses were performed using the highest heart rate and blood pressure measurement obtained during the cold pressor test. Comparisons of demographic data and outcomes in the two ethnic groups and among genotypes were performed by Chi-square test, independent t-test, and one-way analysis of variance, or Mann Whitney U and Kruskal-Wallis test if the normality assumption was not met. Allele distribution was tested for deviations from Hardy-Weinberg equilibrium with the use of a chi-square test with 1 degree of freedom. Previous studies [13, 26] reported a recessive mode of inheritance for the functional effects of the ADRA2C deletion, and we therefore grouped homozygous and heterozygous insertion carriers and compared them to deletion homozygotes. In preliminary analyses we confirmed that, for both genes, heterozygous and homozygous carriers of the “wild-type” alleles (ADRA2C Ins322-325 and GNB3 C825, respectively) did not differ significantly in their responses, whereas genotypic differences existed between homozygous carriers of variant alleles and the two other genotype groups. Multiple linear regression models were used to assess effects of ADRA2C del322-325 and GNB3 C825T genotypes on systolic and diastolic blood pressure, heart rate, and plasma catecholamine concentrations at rest and on the change in these parameters during cold pressor testing, both before and after adjustment for potential confounding factors. These included ethnicity, sex, body mass index (BMI), family history of hypertension, age, and the resting measurement of the response variable. Since preselecting some subjects to enrich homozygous genotypes could have affected our results, we also performed analyses after excluding them. All tests were two-tailed, and a P-value of <0.05 was considered statistically significant. Analyses were performed with the statistical software SPSS (SPSS v.14.0, SPSS Inc., Chicago, IL, USA) and STATA 9.1 (StataCorp, College Station, TX, USA).
Results
Subject characteristics
Eighty subjects (39 whites and 41 blacks) were studied. One black subject, pre-selected for ADRA2C del/del genotype, could not complete the cold pressor test and was excluded from analysis. The demographic characteristics for the remaining 79 subjects are shown in Table 1. Blacks had a higher body mass index than whites (mean difference, 2.3 kg/m2; 95% CI, 0.6 to 4.0; P=0.009).
Table 1.
Demographic characteristics and resting cardiovascular measures.
| White subjects | Black Subjects | P-value | |
|---|---|---|---|
| n | 39 | 40 | |
| Female (%) | 17 (43.6%) | 19 (47.5%) | 0.73 |
| Age (years) | 26.0 ± 5.0 | 25.5 ± 5.6 | 0.65 |
| Height (m) | 1.72 ± 0.08 | 1.73 ± 0.11 | 0.69 |
| BMI (kg/m2) | 24.4 ± 3.5 | 26.6 ± 4.1 | 0.009 |
| Positive family history of hypertension [n (%)] |
15 (38.5%) | 20 (50%) | 0.30 |
| Heart rate (bpm) | 63.7 ± 10.1 | 64.2 ± 9.1 | 0.82 |
| Systolic blood pressure (mmHg) |
109.4 ± 8.9 | 114.8 ± 8.7 | 0.008 |
| Diastolic blood pressure (mmHg) |
64.8 ± 4.7 | 67.2 ± 7.8 | 0.10 |
| Plasma norepinephrine (pg/mL) |
249.8 ± 142.5 | 232.9 ± 131.6 | 0.59 |
| Plasma epinephrine (pg/mL) |
17.7 ± 20.0 | 24.5 ± 14.7 | 0.10 |
Continuous variables are represented as mean ± standard deviation. P-values are for ethnic comparisons (independent t-test / Chi-Square test).
Genetic analysis
Allele frequencies for the ADRA2C deletion were within the expected range and differed significantly among ethnic groups (3.8% for white and 46.3% for black subjects; P<0.001). The ADRA2C genotype distribution (Ins/Ins: 92.3% and 35.0%; Ins/Del: 7.7% and 37.5%; and Del/Del: 0.0% and 27.5% for whites and blacks, respectively) was consistent with Hardy-Weinberg equilibrium in whites (χ2=0.06, P=0.81) and blacks (χ2=2.42, P=0.12). For GNB3, frequencies of the T allele (39.5% and 85.0% for white and black subjects, respectively) and genotype distribution (CC: 39.5% and 2.5%; CT: 42.1% and 25.0%; TT: 18.4% and 72.5% for whites and blacks, respectively) were in the expected range, conformed with Hardy-Weinberg equilibrium (P=0.46 and P=0.74 for blacks and whites, respectively), and differed significantly between the two ethnic groups (P<0.001).
Resting cardiovascular measures
Resting cardiovascular measures in the two ethnic groups are shown in Table 1. Compared to whites, blacks had higher systolic blood pressure (mean difference, 5.4 mmHg; 95% CI, 1.5 to 9.4; P=0.008). In multiple linear regression models including sex, ethnicity, age, family history of hypertension, body mass index, and ADRA2C and GNB3 genotypes as covariates, higher resting systolic blood pressure was associated with male sex (P<0.001) and black ethnicity (P=0.017), and higher plasma norepinephrine concentrations were associated with female sex (P=0.015) and age (P=0.019). ADRA2C and GNB3 genotypes were not significantly associated with any of the dependent resting measures (systolic and diastolic blood pressure, heart rate, and catecholamine concentrations).
Responses to cold pressor test: Unadjusted analyses
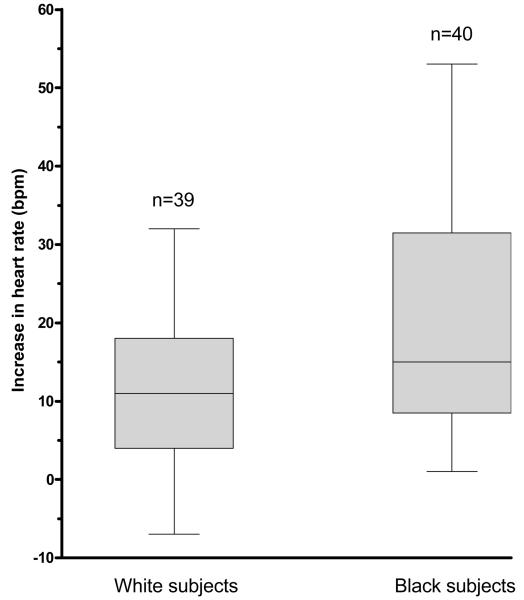
All cardiovascular measures and plasma catecholamine concentrations increased significantly during the cold pressor test (all P-values <0.002). Black subjects had a significantly greater unadjusted increase in heart rate than whites (mean difference, 8.4 bpm; 95% CI, 3.2 to 13.6; P=0.002; Table 2 and Figure 1a). There were no ethnic differences in the unadjusted rise in blood pressure or plasma catecholamine concentrations (Table 2).
Table 2.
Unadjusted response variables according to ethnicity and ADRA2C del322-325 and GNB3 genotypes
| Ethnicity | ADRA2C del322-325 genotype | GNB3 C/T genotype | |||||||
|---|---|---|---|---|---|---|---|---|---|
| White subjects |
Black Subjects |
P- value |
Ins/Ins or Ins/Del |
Del/Del | P- value |
CC or CT | TT | P- value |
|
| Δ Heart rate (bpm) |
11.6± 8.4 | 20.0 ± 14.0 | 0.002 | 13.4 ± 9.7 | 30.9 ± 15.8 | 0.004 | 12.1 ± 9.5 | 20.4 ± 13.8 | 0.003 |
| Δ Systolic BP (mmHg) |
18.2 ± 9.2 | 22.2 ± 15.2 | 0.16 | 18.8 ± 12.1 | 28.6 ± 13.3 | 0.016 | 18.1 ± 11.4 | 22.8 ± 13.8 | 0.10 |
| Δ Diastolic BP (mmHg) |
14.1 ± 7.3 | 15.3 ± 11.3 | 0.56 | 13.9 ± 9.2 | 19.7 ± 10.0 | 0.058 | 13.6 ± 8.8 | 15.8 ± 10.2 | 0.29 |
| Δ Plasma norepinephrine (pg/mL) |
100.5 ± 109.5 | 76.2 ± 80.6 | 0.28 | 91.5 ± 101.8 | 71.3 ± 55.8 | 0.35 | 97.8 ± 107.8 | 76.4 ± 81.9 | 0.35 |
| Δ Plasma epinephrine (pg/mL) |
6.7 ± 12.3 | 13.9 ± 18.0 | 0.13 | 8.9 ± 14.3 | 19.7 ± 21.9 | 0.22 | 9.8 ± 16.5 | 10.7 ± 14.9 | 0.74 |
P-values refer to the comparison between white and black subjects or the comparison between the genotype groups.
Figure 1.
Figure 1a: Ethnic difference in heart rate response to cold pressor test. Boxes represent interquartile ranges, error bars the ranges for the unadjusted rise in heart rate during cold pressor testing. Black subjects had a higher unadjusted heart rate response than whites (P=0.002).
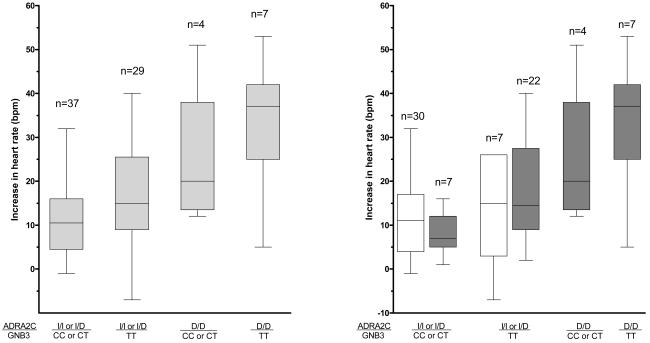
Figure 1b: The interaction between the ADRA2C and GNB3 genotypes and ethnicity on heart rate response during cold pressor test. Subjects were grouped by homozygosity for the variant alleles (D/D and TT for ADRA2C and GNB3, respectively). I=Insertion, D=Deletion. Boxes represent interquartile ranges, error bars the ranges. The left panel shows the unadjusted responses for the whole group, the right panel for whites (white bars) and blacks (grey bars) separately. There were no white subjects homozygous for the ADRA2C deletion. Heart rate response differed significantly among genotype groups (P<0.001).Within genotype groups with bi-ethnic representation, there was no ethnic difference (P>0.30).
Additionally, ADRA2C and GNB3 genotypes affected responses (Table 2, Figure 1b). Compared to carriers of the insertion allele (Ins/Ins and Ins/Del genotypes), subjects homozygous for the ADRA2C 322-325 deletion (Del/Del) had a greater increase in heart rate (P=0.004) and systolic blood pressure (P=0.016), and a trend to greater increase in diastolic blood pressure (P=0.058). Similarly, subjects homozygous for the GNB3 T variant (T/T) had a greater unadjusted increase in heart rate than carriers of the C allele (C/C and C/T genotypes) (P=0.003; Table 2, Figure 1b).
One black female subject, who was homozygous for the ADRA2C deletion and GNB3 T allele, had an extreme rise in plasma epinephrine concentrations during cold pressor testing (from 41 ng/mL at baseline to 347 ng/mL), representing an increase more that 18 times the interquartile range of the increase for all subjects (0-17 ng/mL). Increases in blood pressure, heart rate, and norepinephrine concentrations for this individual were between the 80th and 95th percentile of the range of the responses in the whole cohort. The marked epinephrine response to the cold pressor test in this individual was reproducible and thus not spurious. The analyses of epinephrine responses excluded this extreme outlier.
Heart rate responses: Adjusted analyses
Ethnicity
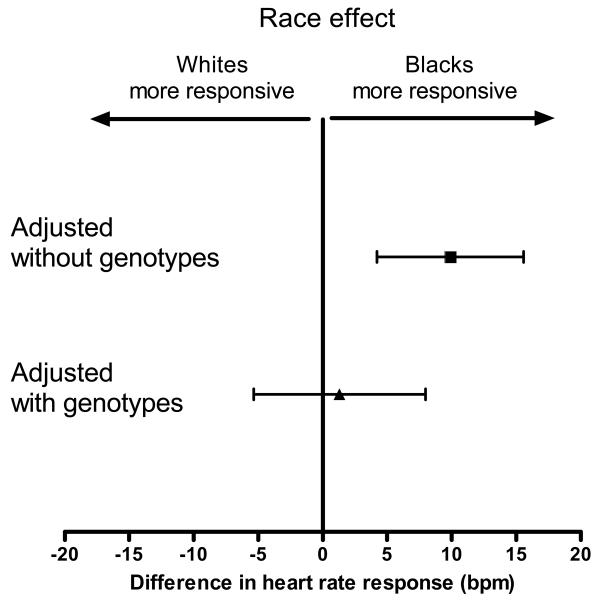
After adjustment for the resting heart rate, age, sex, BMI, and family history of hypertension, ethnicity remained strongly associated with the heart rate response to cold pressor testing (mean difference between blacks and whites, 9.9 bpm [95% CI, 4.1 to 15.6], P=0.001; Figure 2).
Figure 2. Effect of ethnicity on heart rate response before and after adjustment for ADRA2C del322-325 and GNB3 C825T genotypes.
The upper point estimate represents the ethnic difference after adjustment for all non-genetic covariates (P=0.001), the lower point estimate represent the ethnic difference after additional adjustment for ADRA2C and GNB3 genotypes. Error bars represent 95% confidence intervals. After adjustment for genotypes, the ethnic difference was abrogated (P=0.70).
Genotype
When genotypes were added to the model, ADRA2C (del/del) (P<0.001) and GNB3 (T/T) (P=0.026) were significantly associated with a greater increase in heart rate (Table 3). Testing for gene-gene interaction showed that the effects of both genes were largely independent of each other (P=0.84). A post-hoc sensitivity analysis restricted to black subjects only, in whom these genetic variants are much more common than in whites, yielded essentially equivalent results, although the association of GNB3 with the increase in heart rate was of borderline statistical significance (P=0.056). ADRA2C and GNB3 variants contributed substantially to the ethnic differences in heart rate response: After inclusion of genotypes in the multiple linear regression model, the strong association between ethnicity and heart rate response was abrogated (mean difference, 1.3 bpm [95% CI, −5.4 to 8.0 bpm]; P=0.70; Table 3; Figure 2). Moreover, there were no ethnic differences within subgroups with identical ADRA2C and GNB3 genotypes (P>0.30; Figure 1b). Excluding the 7 black subjects pre-selected for their ADRA2C genotype yielded essentially unchanged results.
Table 3.
Adjusted responses of heart rate and systolic blood pressure to cold pressor testing
| Parameter | Coefficient | 95% confidence interval |
P-value | |
|---|---|---|---|---|
| Heart rate response |
Male sex | −0.8 | −6.2 to 4.5 | 0.75 |
| Black ethnicity | 1.3 | −5.4 to 8.0 | 0.70 | |
| Age | 0.1 | −0.4 to 0.5 | 0.83 | |
| Body Mass Index | −0.3 | −1.0 to 0.4 | 0.40 | |
| Positive FH of hypertension | −1.0 | −6.0 to 4.1 | 0.70 | |
| Baseline heart rate | −0.1 | −0.4 to 0.1 | 0.29 | |
| ADRA2C del/del genotype | 15.8 | 8.0 to 23.7 | <0.001 | |
| GNB3 T/T genotype | 6.8 | 0.9 to 12.7 | 0.026 | |
| Systolic blood pressure response |
Male sex | 2.7 | −4.5 to 10.0 | 0.45 |
| Black ethnicity | −0.7 | −8.8 to 7.5 | 0.87 | |
| Age | 0.5 | 0.0 to 1.1 | 0.065 | |
| Body Mass Index | −0.3 | −1.1 to 0.5 | 0.46 | |
| Positive FH of hypertension | 1.8 | −4.1 to 7.7 | 0.55 | |
| Baseline systolic blood pressure |
0 | −0.4 to 0.4 | 0.85 | |
| ADRA2C del/del genotype | 9.1 | 0.0 to 18.2 | 0.051 | |
| GNB3 T/T genotype | 5.7 | −1.2 to 12.7 | 0.10 |
Listed are covariates and multiple linear regression coefficients, their confidence intervals, and P-values for two response variables (changes from baseline in heart rate and systolic blood pressure). FH=family history; ADRA2C del/del genotype=homozygous ADRA2C 322-325 deletion genotype (coded as 1) compared to Ins/Ins and Ins/del genotype combined (coded as 0); GNB3 T/T genotype=homozygous GNB3 825 T/T genotype (coded as 1), compared to C/C and C/T combined (coded as 0). Sex and ethnicity were coded as 0/1 for female/male and white/black, respectively, so that the correlation coefficients reflect sex and ethnic differences, respectively.
Blood pressure responses: Adjusted analyses
Ethnicity
After adjustment for baseline blood pressure, sex, age, BMI, and family history of hypertension, the mean systolic blood pressure response was 5.5 mmHg (95% CI, −1.0 to 12.1) greater in blacks than whites (P=0.097)
Genotype
After adding ADRA2C and GNB3 genotypes to the model, the ADRA2C (del/del) genotype was associated with a greater systolic blood pressure response (P=0.051; Table 3). Additionally, the trend toward ethnic differences in systolic blood pressure response was no longer present (Table 3), suggesting that it was in part accounted for by ADRA2C genotype. For the diastolic blood pressure response, after adjustment for all covariates, there were no significant ethnic differences. Subjects with the ADRA2C del/del genotype increased diastolic blood pressure by a mean of 6.2 mmHg (95% CI, −0.8 to 13.2) more than subjects carrying the insertion allele (P=0.082).
Catecholamine responses: Adjusted analyses
After adjustment for all non-genetic covariates, black subjects had a greater increase in epinephrine (mean difference, 8.8 pg/mL [95% CI, 0.3 to 19.7]; P=0.044). After additional adjustment for ADRA2C and GNB3 genotypes, this ethnic difference was essentially unaffected (mean difference, 10.0 pg/mL; 95% CI, 1.0 to 16.6; P=0.027). After excluding the 7 pre-selected black subjects, the adjusted ethnic difference was attenuated (mean difference, 7.1 pg/mL; 95% CI, −2.5 to 16.8; P=0.145). Neither ADRA2C genotype (P=0.68) nor GNB3 genotype (P=0.34) were associated with the epinephrine response. Similarly, genotypes were not associated with norepinephrine response (P=0.77 and P=0.78 for ADRA2C and GNB3, respectively), and there were no ethnic differences (P=0.84).
Discussion
This study is the first to identify genetic variants that contribute to ethnic differences in cardiovascular stress response to the cold pressor test. Our main findings are that homozygosity for variants in ADRA2C and GNB3, key genes in the regulation of sympathetic signaling, is strongly associated with heart rate response to the cold pressor test, and contributes substantially to the ethnic differences observed. Moreover, there was a similar, but less pronounced ADRA2C genotype effect on blood pressure responses. Our findings suggest that these genetic variants contribute to enhanced cardiovascular stress responses in young, healthy subjects and raise the possibility that such variants may play a role in both the increased cardiovascular morbidity associated with such enhanced stress responses and with the interethnic differences seen in these responses and morbidity.
Cardiovascular responses to acute physical and mental stressors have been studied extensively, because they offer mechanistic and prognostic information beyond that provided by resting measures [27]. For example, enhanced blood pressure and heart rate responses to stress tests in young, healthy subjects were associated with subsequent higher resting heart rate, blood pressure, and hypertension [1, 28]. Thus, increased cardiovascular reactivity may not only define an intermediate phenotype predicting future cardiovascular disease but also reveal an underlying mechanism for the pathogenesis of adverse outcomes [29].
A classic experimental cardiovascular stressor is the cold pressor test; it elicits profound hemodynamic responses, greater than other physiological or psychological stress tests [1]. Most studies in bi-ethnic populations have found greater hemodynamic responses to the cold pressor test in young, healthy black subjects compared to whites [1, 18, 30-32]. Genetic contributions to these ethnic differences have not been elucidated. In our study, performed under controlled conditions including a standardized intake of salt, black subjects had a greater heart rate response, and a non-significant trend to higher systolic blood pressure response, in agreement with many previous studies [31, 32]. Furthermore, ethnic differences were largely accounted for by variants in ADRA2C and GNB3. Our study population was deliberately enriched with black subjects homozygous for either ADRA2C allele in order to better define the effect of this genetic variation on stress responses. Thus, given the contribution of this variant to cold pressor responses, our study was more likely to detect ethnic and genotypic differences than a study of randomly selected individuals. However, after excluding the preselected subjects from the analysis, similar findings were observed among the randomly selected individuals.
Cardiovascular responses to stress are heritable [33-36]. Therefore, genetic variation may contribute to interindividual and ethnic differences in cardiovascular reactivity. We studied two genes that encode for key regulators of sympathetic activity, the α2C-AR and G-protein β3-subunit. These genes have common variants (ADRA2C del322-325 and GNB3 C825T, respectively) with functional effects, and the variant alleles are much more common in black Americans than whites. Little is known about the effects of the del322-325 variant of ADRA2C.
Previously, we found no differences in resting plasma catecholamine concentrations, heart rate variability, and, as also reported in a large population study [37], no differences in resting blood pressure and heart rate among ADRA2C del322–325 genotypes [20]. In contrast, a study of 29 black subjects found significantly higher resting mean arterial pressure and catecholamine spillover in subjects homozygous for the ADRA2C deletion [26]. Since genetic variants affecting sympathetic activity may contribute differentially to resting cardiovascular measures and stress responses [36], the effect of the deletion variant may be more apparent in states of heightened adrenergic tone.
Concordant with this notion, in the present study subjects homozygous for the ADRA2C deletion had a 2-fold greater increase in heart rate, and a directionally similar systolic blood pressure response, but no differences in baseline measures. Importantly, these results remained significant after adjusting for a number of potential confounders. This finding is also concordant with findings in transgenic mice with targeted deletions of ADRA2C. At baseline, these animals do not have apparent abnormalities of adrenergic regulation, but in an experimental model of congestive heart failure they have higher plasma catecholamine concentrations, more severe disease, and a higher mortality [9].
The α2C-AR signals through G-proteins, and therefore genetic variation in the signalling pathway could amplify or mute the effects of ADRA2C variants. The C825T polymorphism of GNB3 is the most important functional variant shown to affect G protein signaling in vivo. Based on in vitro data, we expected the more active GNB3 genotype (TT) to be associated with greater α2C-AR activity and therefore attenuated stress responses. However, we observed the opposite: the TT genotype, independent of ADRA2C genotype, was associated with a significantly greater heart rate response. These findings are in line with another study in which the GNB3 TT genotype was associated with greater sympathetic activation upon orthostatic challenge [16]. The mechanisms for the pleiotropic effects associated with the GNB3 T allele variant are poorly understood [38], and may include the association of the β3-subunit with multiple other G-protein coupled receptors involved in cardiovascular control [39, 40], receptor cross-talk [41], or other direct effects of βγ dimers on cell function [42].
In our study, genotype effects on heart rate and blood pressure responses were not accompanied by corresponding differences in plasma catecholamines that would have explained the enhanced cardiovascular responsiveness in carriers of the more responsive genotypes. However, the antecubital venous plasma catecholamine concentration reflects the combined effect of systemic and local (forearm) release (spillover) and clearance. Thus, it is an indirect marker of sympathetic activity especially during dynamic changes in blood pressure and organ perfusion such as occur during the cold pressor test. Additionally, sympathetic responses are regulated in an organ-specific fashion [2, 43], and thus global measures of norepinephrine release may not provide information about cardiac release, for example. Organ-specific regional spill-over studies that adjust for clearance would be a more specific way to assess sympathetic tone in a given organ; such techniques, however, are unsuitable for larger studies due to their invasive nature and the use of radioactive tracers.
The rise in plasma epinephrine concentrations was 2-fold higher in subjects homozygous for the ADRA2C deletion variant, but this difference was not statistically significant in view of the high interindividual variability. Of note, in mice, the α2C-adrenergic receptor regulates epinephrine release from the adrenal gland, but there is conflicting information about the presence of α2C-adrenergic receptors in the human adrenal gland [44, 45]. Further studies of the effect of genetic variation in ADRA2C and GNB3 on organ-specific norpinephrine release and adrenal epinephrine secretion under conditions of stress will be of interest.
In conclusion, in this study, the ADRA2C del322-325 and GNB3 C825T variants were associated with enhanced hemodynamic responses to the cold pressor test, substantially accounting for the greater heart rate response in black subjects. Large longitudinal studies are necessary to examine whether these and other genetic variants are part of a common genetic basis linking stress test hyper-responsiveness in young adults to cardiovascular morbidity in later life.
Acknowledgements
The Vanderbilt DNA Resource Core and Vanderbilt University Center for Human Genetics Research provided technical assistance for this work.
Sources of Funding: This study was supported by US Public Health Service grants M01 RR-00095 from the National Center for Research Resources, P01 HL56693, GM31304, and a Pharmacogenetics Research Network Grant (U01 HL65962). Drs. Kurnik and Muszkat were recipients of a Merck Sharp & Dohme International Fellowship in Clinical Pharmacology.
Footnotes
Conflicts of interest: None declared
References
- 1.Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, et al. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110:74–78. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- 2.Robertson D, Biaggioni I, Burnstock G, Low PA. Primer on the autonomic nervous system. 2nd edition Elsevier Academic Press; San Diego, CA: 2004. [Google Scholar]
- 3.O'Connor DT, Insel PA, Ziegler MG, Hook VY, Smith DW, Hamilton BA, et al. Heredity and the autonomic nervous system in human hypertension. Curr Hypertens Rep. 2000;2:16–22. doi: 10.1007/s11906-000-0053-8. [DOI] [PubMed] [Google Scholar]
- 4.Flordellis C, Manolis A, Scheinin M, Paris H. Clinical and pharmacological significance of alpha2-adrenoceptor polymorphisms in cardiovascular diseases. Int J Cardiol. 2004;97:367–372. doi: 10.1016/j.ijcard.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Williams PD, Puddey IB, Beilin LJ, Vandongen R. Genetic influences on plasma catecholamines in human twins. J Clin Endocrinol Metab. 1993;77:794–799. doi: 10.1210/jcem.77.3.8370701. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy BP, Rao F, Botiglieri T, Sharma S, Lillie EO, Ziegler MG, et al. Contributions of the sympathetic nervous system, glutathione, body mass and gender to blood pressure increase with normal aging: influence of heredity. J Hum Hypertens. 2005;19:951–969. doi: 10.1038/sj.jhh.1001912. [DOI] [PubMed] [Google Scholar]
- 7.Dao TT, Kailasam MT, Parmer RJ, Le HV, Le Verge R, Kennedy BP, et al. Expression of altered alpha2-adrenergic phenotypic traits in normotensive humans at genetic risk of hereditary (essential) hypertension. J Hypertens. 1998;16:779–792. doi: 10.1097/00004872-199816060-00009. [DOI] [PubMed] [Google Scholar]
- 8.Kirstein SL, Insel PA. Autonomic nervous system pharmacogenomics: a progress report. Pharmacol Rev. 2004;56:31–52. doi: 10.1124/pr.56.1.2. [DOI] [PubMed] [Google Scholar]
- 9.Brede M, Wiesmann F, Jahns R, Hadamek K, Arnolt C, Neubauer S, et al. Feedback inhibition of catecholamine release by two different alpha2-adrenoceptor subtypes prevents progression of heart failure. Circulation. 2002;106:2491–2496. doi: 10.1161/01.cir.0000036600.39600.66. [DOI] [PubMed] [Google Scholar]
- 10.Brede M, Nagy G, Philipp M, Sorensen JB, Lohse MJ, Hein L. Differential control of adrenal and sympathetic catecholamine release by alpha 2-adrenoceptor subtypes. Mol Endocrinol. 2003;17:1640–1646. doi: 10.1210/me.2003-0035. [DOI] [PubMed] [Google Scholar]
- 11.Chotani MA, Mitra S, Su BY, Flavahan S, Eid AH, Clark KR, et al. Regulation of alpha(2)-adrenoceptors in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004;286:H59–H67. doi: 10.1152/ajpheart.00268.2003. [DOI] [PubMed] [Google Scholar]
- 12.Small KM, Forbes SL, Rahman FF, Bridges KM, Liggett SB. A four amino acid deletion polymorphism in the third intracellular loop of the human alpha 2C-adrenergic receptor confers impaired coupling to multiple effectors. J Biol Chem. 2000;275:23059–23064. doi: 10.1074/jbc.M000796200. [DOI] [PubMed] [Google Scholar]
- 13.Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB. Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med. 2002;347:1135–1142. doi: 10.1056/NEJMoa020803. [DOI] [PubMed] [Google Scholar]
- 14.Canham RM, Das SR, Leonard D, Abdullah SM, Mehta SK, Chung AK, et al. Alpha2cDel322-325 and beta1Arg389 adrenergic polymorphisms are not associated with reduced left ventricular ejection fraction or increased left ventricular volume. J Am Coll Cardiol. 2007;49:274–276. doi: 10.1016/j.jacc.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Siffert W, Rosskopf D, Siffert G, Busch S, Moritz A, Erbel R, et al. Association of a human G-protein beta3 subunit variant with hypertension. Nat Genet. 1998;18:45–48. doi: 10.1038/ng0198-45. [DOI] [PubMed] [Google Scholar]
- 16.Matsunaga T, Nagasumi K, Yamamura T, Gu N, Nishikino M, Ueda Y, et al. Association of C825T polymorphism of G protein beta3 subunit with the autonomic nervous system in young healthy Japanese individuals. Am J Hypertens. 2005;18:523–529. doi: 10.1016/j.amjhyper.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Stein CM, Lang CC, Xie HG, Wood AJ. Hypertension in black people: study of specific genotypes and phenotypes will provide a greater understanding of interindividual and interethnic variability in blood pressure regulation than studies based on race. Pharmacogenetics. 2001;11:95–110. doi: 10.1097/00008571-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Calhoun DA, Mutinga ML, Collins AS, Wyss JM, Oparil S. Normotensive blacks have heightened sympathetic response to cold pressor test. Hypertension. 1993;22:801–805. doi: 10.1161/01.hyp.22.6.801. [DOI] [PubMed] [Google Scholar]
- 19.Harris PA, Lane L, Biaggioni I. Clinical research subject recruitment: the Volunteer for Vanderbilt Research Program. J Am Med Inform Assoc. 2005;12:608–613. doi: 10.1197/jamia.M1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurnik D, Muszkat M, Friedman EA, Sofowora GG, Diedrich A, Xie HG, et al. Effect of the alpha2C-adrenoreceptor deletion322-325 variant on sympathetic activity and cardiovascular measures in healthy subjects. J Hypertens. 2007;25:763–771. doi: 10.1097/HJH.0b013e328017f6e9. [DOI] [PubMed] [Google Scholar]
- 21.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 22.Belfer I, Buzas B, Hipp H, Phillips G, Taubman J, Lorincz I, et al. Haplotype-based analysis of alpha 2A, 2B, and 2C adrenergic receptor genes captures information on common functional loci at each gene. J Hum Genet. 2005;50:12–20. doi: 10.1007/s10038-004-0211-y. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ. SNP genotyping by the 5'-nuclease reaction. Methods Mol Biol. 2003;212:129–147. doi: 10.1385/1-59259-327-5:129. [DOI] [PubMed] [Google Scholar]
- 24.Ohshiro Y, Ueda K, Wakasaki H, Takasu N, Nanjo K. Analysis of 825C/T polymorphism of G proteinbeta3 subunit in obese/diabetic Japanese. Biochem Biophys Res Commun. 2001;286:678–680. doi: 10.1006/bbrc.2001.5450. [DOI] [PubMed] [Google Scholar]
- 25.He HB, Deegan RJ, Wood M, Wood AJ. Optimization of high-performance liquid chromatographic assay for catecholamines. Determination of optimal mobile phase composition and elimination of species-dependent differences in extraction recovery of 3,4-dihydroxybenzylamine. J Chromatogr. 1992;574:213–218. [PubMed] [Google Scholar]
- 26.Neumeister A, Charney DS, Belfer I, Geraci M, Holmes C, Sharabi Y, et al. Sympathoneural and adrenomedullary functional effects of alpha2C-adrenoreceptor gene polymorphism in healthy humans. Pharmacogenet Genomics. 2005;15:143–149. doi: 10.1097/01213011-200503000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Menkes MS, Matthews KA, Krantz DS, Lundberg U, Mead LA, Qaqish B, et al. Cardiovascular reactivity to the cold pressor test as a predictor of hypertension. Hypertension. 1989;14:524–530. doi: 10.1161/01.hyp.14.5.524. [DOI] [PubMed] [Google Scholar]
- 29.Lillie EO, O'Connor DT. Early phenotypic changes in hypertension: a role for the autonomic nervous system and heredity. Hypertension. 2006;47:331–333. doi: 10.1161/01.HYP.0000203980.44717.aa. [DOI] [PubMed] [Google Scholar]
- 30.Light KC, Obrist PA, Sherwood A, James SA, Strogatz DS. Effects of race and marginally elevated blood pressure on responses to stress. Hypertension. 1987;10:555–563. doi: 10.1161/01.hyp.10.6.555. [DOI] [PubMed] [Google Scholar]
- 31.Arthur CM, Katkin ES, Mezzacappa ES. Cardiovascular reactivity to mental arithmetic and cold pressor in African Americans, Caribbean Americans, and white Americans. Ann Behav Med. 2004;27:31–37. doi: 10.1207/s15324796abm2701_5. [DOI] [PubMed] [Google Scholar]
- 32.Parmer RJ, Cervenka JH, Stone RA, O'Connor DT. Autonomic function in hypertension. Are there racial differences? Circulation. 1990;81:1305–1311. doi: 10.1161/01.cir.81.4.1305. [DOI] [PubMed] [Google Scholar]
- 33.Ditto B, France C. Carotid baroreflex sensitivity at rest and during psychological stress in offspring of hypertensives and non-twin sibling pairs. Psychosom Med. 1990;52:610–620. doi: 10.1097/00006842-199011000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Busjahn A, Faulhaber HD, Viken RJ, Rose RJ, Luft FC. Genetic influences on blood pressure with the cold-pressor test: a twin study. J Hypertens. 1996;14:1195–1199. doi: 10.1097/00004872-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 35.McCaffery JM, Pogue-Geile MF, Ferrell RE, Petro N, Manuck SB. Variability within alpha- and beta-adrenoreceptor genes as a predictor of cardiovascular function at rest and in response to mental challenge. J Hypertens. 2002;20:1105–1114. doi: 10.1097/00004872-200206000-00021. [DOI] [PubMed] [Google Scholar]
- 36.Choh AC, Czerwinski SA, Lee M, Demerath EW, Wilson AF, Towne B, et al. Quantitative genetic analysis of blood pressure response during the cold pressor test. Am J Hypertens. 2005;18:1211–1217. doi: 10.1016/j.amjhyper.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 37.Li JL, Canham RM, Vongpatanasin W, Leonard D, Auchus RJ, Victor RG. Do Allelic Variants in α2A and α2C Adrenergic Receptors Predispose to Hypertension in Blacks? Hypertension. 2006;47:1140–1146. doi: 10.1161/01.HYP.0000217972.80731.ef. [DOI] [PubMed] [Google Scholar]
- 38.Siffert W. Effects of the G protein beta 3-subunit gene C825T polymorphism: should hypotheses regarding the molecular mechanisms underlying enhanced G protein activation be revised? Focus on “A splice variant of the G protein beta 3-subunit implicated in disease states does not modulate ion channels”. Physiol Genomics. 2003;13:81–84. doi: 10.1152/physiolgenomics.00031.2003. [DOI] [PubMed] [Google Scholar]
- 39.Macrez-Lepretre N, Kalkbrenner F, Schultz G, Mironneau J. Distinct functions of Gq and G11 proteins in coupling alpha1-adrenoreceptors to Ca2+ release and Ca2+ entry in rat portal vein myocytes. J Biol Chem. 1997;272:5261–5268. doi: 10.1074/jbc.272.8.5261. [DOI] [PubMed] [Google Scholar]
- 40.Macrez N, Morel JL, Mironneau J. Specific galpha11beta3gamma5 protein involvement in endothelin receptor-induced phosphatidylinositol hydrolysis and Ca2+ release in rat portal vein myocytes. Mol Pharmacol. 1999;55:684–692. [PubMed] [Google Scholar]
- 41.Talaia C, Queiroz G, Pinheiro H, Moura D, Goncalves J. Involvement of G-protein betagamma subunits on the influence of inhibitory alpha2-autoreceptors on the angiotensin AT1-receptor modulation of noradrenaline release in the rat vas deferens. Neurochem Int. 2006;49:698–707. doi: 10.1016/j.neuint.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Tedford HW, Zamponi GW. Direct G protein modulation of Cav2 calcium channels. Pharmacol Rev. 2006;58:837–862. doi: 10.1124/pr.58.4.11. [DOI] [PubMed] [Google Scholar]
- 43.Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertens. 1999;17:719–734. doi: 10.1097/00004872-199917060-00001. [DOI] [PubMed] [Google Scholar]
- 44.Lymperopoulos A, Rengo G, Funakoshi H, Eckhart AD, Koch WJ. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nat Med. 2007;13:315–323. doi: 10.1038/nm1553. [DOI] [PubMed] [Google Scholar]
- 45.Berkowitz DE, Price DT, Bello EA, Page SO, Schwinn DA. Localization of messenger RNA for three distinct alpha 2-adrenergic receptor subtypes in human tissues. Evidence for species heterogeneity and implications for human pharmacology. Anesthesiology. 1994;81:1235–1244. doi: 10.1097/00000542-199411000-00018. [DOI] [PubMed] [Google Scholar]