Abstract
Objective
The aim of this study was to identify whether the rapid membrane-associated pathway of the glucocorticoid receptor (GR) is active in erythroid cells and plays any role in determining the reversible inhibition on erythroid maturation exerted by GR.
Materials and Methods
First we determined the biological effects (inhibition of apoptosis and induction of β-globin expression) induced in primary erythroblasts by EPO and the GR agonist dexamethasone, alone and in combination. Next, by biochemical analysis, we determined the association between GR and the erythropoietin receptor in proerythroblasts generated in vitro from 10 normal adult donors. These studies also analyzed the levels of STAT-5 phosphorylation induced when the cells were stimulated with dexamethasone alone or in combination with erythropoietin.
Results
Dexamethasone antagonized the β-globin mRNA increases but not the inhibition of apoptosis induced by EPO in primary cells. Dexamethasone also antagonized the ability of erythropoietin to induce STAT-5 phosphorylation in these cells. In fact, erythropoietin and dexamethasone alone, but not in combination, induced phosphorylation and nuclear translocation of STAT-5. The inhibition likely occurred through an interaction between the two receptors because GR became associated with the erythropoietin receptor and STAT-5 in cells stimulated with erythropoietin and dexamethasone.
Conclusion
These data suggest that glucocorticoids inhibit erythroid maturation not only through a transcriptional mechanism but also through a rapid membrane-associated pathway that interferes with the erythropoietin receptor signalling.
Keywords: Human Erythropoiesis, Primary Proerythroblasts, Erythropoietin Receptor, Glucocorticoid Receptor, STAT-5
INTRODUCTION
Extensive clinical studies have established a direct correlation between numbers of red cells present in the blood and concentrations of erythropoietin (EPO), a hormone mainly produced by the kidney, present in the sera (1,2). The correlation is established through the interaction of EPO with a specific receptor, EPO-R, present on the surface of the erythroid cells being developed in the marrow (3). In the circulation of normal individuals, variability has been described both in red cell number (normal ranges of hemoglobin in blood are 12–16 gr/L) and EPO concentration (4–26 U/mL) (4). Under steady state conditions, however, the two parameters are not correlated and other factors, such as sex, age, and possibly yet to be identified genetic determinants, appear to play a more important role than EPO in tuning the number of red cells in the blood (4). Earlier studies have identified nuclear receptor binding compounds, such as dexamethasone (5), estradiol (6) and thyroid hormone (7,8) as synergizing with EPO in inducing generation of erythroid bursts in bone marrow or blood mononuclear cells in culture. The fact, however, that the culture assay used in these early studies did not contain purified progenitor cells prevented from clarifying whether stimulation of these nuclear receptors affected erythroid maturation directly or indirectly, by promoting growth factor (GF) release by the accessory cells.
The issue of a cell autonomous effect of nuclear receptors on erythropoiesis has been recently addressed by genetic studies in vertebrates. In the mouse, the glucocorticoid receptor (GR) directly controls the speed of the erythroid recovery following stress (9,10). In fact, although the hematocrit (Hct) of mice genetically engineered to either lack GR or to carry a GR allele encoding a dimerization-deficient protein is apparently within normal ranges, the mice recover poorly from hemolytic anemia induced by phenylhydrazine (9). Indirect evidence that GR might be a key player in controlling erythropoiesis also in humans, is provided by the observation that polycythemia is the first manifestation of Cushing’s disease, a syndrome associated with chronic stimulation of GR (11). It is generally accepted that glucocorticoids enter cells by passive transfer and interact with GR within the cytoplasm (12,13). Binding of glucocorticoids to their receptor has been shown to induce receptor dimerization, STAT-5-phosphorylation and formation of GR/STAT-5 complexes. These complexes, migrate to the nucleus, where they activate/repress the expression of target genes, by binding to specific consensus sequences (12,13). In the case of erythroid cells, the target genes represent a subset of those controlled by EPO and stem cell factor (SCF), a growth factor exerting an important function at early stages of differentiation (14,15). Recent evidence, however, indicates that in addition to its transcriptional activity, GR can activate a rapid membrane-associated signaling in several cell systems (16). The possibility that such a rapid pathway might be active in erythroid cells has not been investigated to date.
The ability of glucocorticoids to modulate erythroid differentiation has been exploited by using liquid cultures that permit massive amplification of primary murine and human erythroid cells (14). Greater numbers (108–10) of proerythroblasts are generated under human erythroid mass amplification (HEMA) culture conditions, a refinement of this liquid system (17). Such large numbers of cells gave us the opportunity to clarify whether GR is present on the membranes and physically interacts with EPO-R in erythroid cells. The data presented here indicate that, in human erythroblasts, growth-factor stimulation induces association of GR on the membranes. In addition, in these erythroid cells, GR forms a complex with EPO-R and antagonizes its ability to phosphorylate STAT-5. Therefore, glucocorticoids interfere with EPO signaling not only through a transcriptional mechanism but also by directly interfering with EPO-R signalling possibly through a rapid membrane-associated pathway. We suggest that genetic heterogeneity in the glucocorticoid-receptor gene contributes, at least in part, to the variability in red cell numbers observed in the circulation of normal individuals under steady-state conditions.
MATERIALS AND METHODS
Human Subjects
Peripheral blood was collected from 15 normal adult donors at the transfusion center of “La Sapienza” University (Rome, Italy) according to guidelines established by Institutional Ethics Committee.
Culture of Human Proerythroblasts
Light density blood cells were separated by standard centrifugation over Ficoll-Hypaque (Amersham Pharmacia Biotec, Uppsala, Sweden) and cultured at a concentration of 106 cells/mL for 10–11 days in Iscove’s Modified Dulbecco’s Medium (IMDM; Invitrogen, Carlsbad, CA, USA) containing fetal bovine serum (FBS, 20% v/v, Hyclone, Logan, UT), bovine serum albumin (BSA, 4% wg/vol) SCF (10 ng/mL, Amgen, Thousand Oaks, CA, USA), EPO (3 U/mL, Epoetina alfa, Dompè Biotec, Milan, Italy), IL-3 (1 ng/mL, Bouty, Milan, Italy) and DXM (10−6M, Sigma, St. Louis, MO, USA) (HEMA conditions) (17). For growth factor-deprivation experiments, cells were incubate with IMDM supplemented with FBS (10% v/v).
Phenotypical analysis
Cells were spun onto coverslip (Shandon, Astmoor, England), fixed (4% paraformaldehyde/Phosphate-buffered saline, Invitrogen), saturated/permeabilized for 30 min with NET gel (150mM NaCl, 5mM EDTA, 50mM Tris-HCl pH 7.4, 0.05% NP-40, 0.25% Carrageenan Lambda gelatine, 0.02% NaN3) and were stained either with May-Grünwald-Giemsa or with an antibody specific for STAT-5 (sc-835), (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and then probed with the secondary FITC-conjugated antibody (Invitrogen, Carlsbad, CA) diluted 1:400 in NET gel. Nuclei were counterstained with DAPI (Sigma). Negative controls were represented by samples incubated with the secondary antibody only. For flow cytometric analysis, cells were suspended in Ca++ and Mg++-free phosphate-buffered saline, supplemented with 1% BSA, 2mM EDTA and 0.01% NaN3, labelled with propidium iodide (5μg/mL, Sigma) and either phycoerythrin (PE)-conjugated CD36 and fluorescein isothiocyanate (FITC)-conjugated CD235a (anti-glycophorin A), or appropriate isotype controls (all from Immunotech, Beckmann-Coulter, Milan, Italy) or FITC-Annexin V (PharMingen, San Diego, CA) and analyzed with a FACS Aria (Becton Dickinson, San Josè, CA).
Purification of Human CD4+ T cells
CD4+ T cells were purified from light density blood cells of normal volunteers, by negative selection using magnetic beads (Miltenyi Biotech, Auburn, CA, USA) coated with mAbs directed against CD8, CD19, CD16, CD56 and CD11b as described by the manifacture. Immune-depleted cells were >96% CD3+ as determined by FACS analysis.
Western Blot Assay
Cells were dissolved in lysis buffer (20mM Hepes pH 7.4, 50mM NaCl, 10mM EDTA pH 8.0, 2mM EGTA, 0.5% NP-40. 0.5mM DTT, 10mM NaMo, 10mM NaVO3, 100mM NaF, 50mM β-glycerophosphate, 100μg/ml leupeptin, 0.5mM PMSF). Proteins (30 μg) were separated on 10% SDS-PAGE and transferred to a nitrocellulose membrane. Blots were first incubated with anti-STAT-5, -EPO-R (1:200, C-20 sc-695 and M-20 sc-697), -GR, -HSP-90α/β and -Actin (sc-1616) antibodies (all 1:200 and from Santa Cruz Biotechnology Inc.), and then with anti-rabbit, -mouse or -goat horseradish peroxidase-coupled secondary antibodies (Calbiochem, San Diego, CA, USA) as appropriate. Immune complexes were identified using the enhanced chemiluminescence system (Amersham Biosciences). The immunoblotting bands were quantified by using the Quantity One 1D-Analysis Software (Bio-Rad).
Immunoprecipitation (IP) Assay
Whole cell extracts (30–50 μg) were incubated with either a polyclonal anti-EPO-R (18) or anti-STAT-5, anti-GR or anti-HSP-90α/β antibodies overnight at 4°C under rotation in lysis buffer (see above). Extracts were then incubated with Ultralink Immobilized Protein A/G sepharose (Pierce Biotech, Rockford, IL, USA) for 2 h at room temperature. Immunocomplexes were dissociated from the beads by boiling for 5′ in loading buffer, separated on SDS-PAGE under reducing conditions, transferred to nitrocellulose membranes, and analyzed by Western Blot with anti-STAT-5pY (1:1000, cat. 9351S, Cell Signaling, Lake Placid, NY, USA), -HSP-90α/β and -GR antibodies.
Cell Fractionation
Cytosol and membrane fractions were prepared by sequential lysis with buffers of increasing stringency, followed by ultracentrifugation, as described (19). Briefly, cells were lysed in cold lysis buffer A (20mM Tris-HCl pH 7.5, 1mM EDTA pH 8.0, 1mM EGTA, 2mM DTT, 1mM NaVO3, 1mM NaF, 50mM β-glycerophosphate, 100μg/ml leupeptin, 0.5mM PMSF), broken with 20 strokes of a Dounce homogenizer and centrifuged for 50′ at 45,000 rpm at 4°C with a Beckman Ultra Centrifuge TL100 (Beckman Coulter, Fullertone, CA, USA). Supernatants, containing the cytosol fraction, were kept on ice while pellets were suspended in cold lysis buffer B (1% Triton X-100, 20mM Tris-HCl pH 7.5, imM EDTA pH 8.0, 1mM EGTA, 2mM DTT, 1mM NaVO3, 1mM NaF, 50mM β-glycerophosphate, 100μg/ml leupeptin, 0.5mM PMSF), left on ice for 15′, vortexed 2–3 times, and centrifuged for 50′ at 55,000 rpm at 4 °C. The supernatants of this second centrifugation step contained the membrane fractions. Both the cytosol and membrane fractions were precipitated with 10 vol of cold acetone, kept overnight at −20 °C and centrifuged for 30′ at 10,000 rpm at 4°C. Pellets were suspended in 10mM Tris-HCl pH=7.5 to be analyzed by IP and western blot. Cytosol and membrane proteins (50 μg) were separately analyzed by IP as described above. As a control for fraction purity, blots were probed with anti-β-tubulin (1:200, sc-23949) and anti-spectrin α1 (1:200, sc-15371) (Santa Cruz Biotechnology) antibodies.
RNA isolation and semiquantitative and quantitative RT-PCR analysis
Total RNA was isolated from 106 cells using TRIZOL (Invitrogen). For semi-quantitative determinations, RNA (1μg) was reverse transcribed and amplified adapting to the human genes PCR conditions previously described for the corresponding murine genes (20). Conditions for quantitative RT-PCR determination of β-globin expression have been described (21). Levels of β-globin mRNA were expressed in arbitrary units, using hGAPDH as calibrator, according to the following algorithm: ΔCt=[Ctβ-globin − CtGAPDH], where Ct is the threshold cycle, and presented as 2−ΔCt.
Statistical Analysis
Statistical analysis was obtained by paired t-test or by analysis of variance, as appropriate, using the Origin 6.1 software for Windows (Microcal Software Inc, Northampton, MA, USA).
RESULTS
Human proerythroblasts are sensitive to GF-starvation and activate the maturation program within 24 h of exposure to EPO
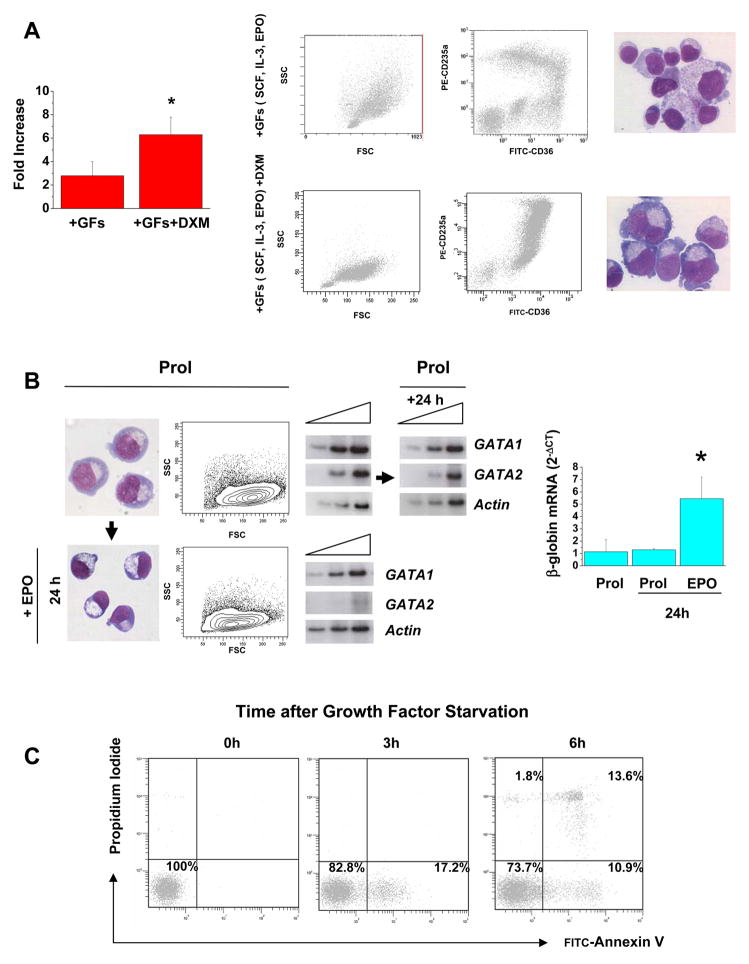
As expected (22), light density cells from the blood of normal adult donors cultured in the presence of SCF, EPO and IL-3 (GFs) generated, after 11 days of culture, cells of multiple lineages. However, only 20–50% of these cells had the antigenic profile of erythroid cells (CD235apos) (Figure 1A). The same cells cultured under HEMA conditions (GFs plus DXM) generated by day 11 many more cells (FI: 2.8±1.2 vs 6.2±1.6 in cultures without and with DXM, respectively, p<0.001), the majority of which were erythroid, as indicated by the few (<5%) cells that failed to express CD235a (Figure 1A). In addition, although these cells did not contain significant numbers of erythroid progenitors (< 0.1% of these cells generate BFU-E- or CFU-E- derived colonies in semisolid media) they remained immature, as indicated by their pro-erythroblast morphology and by the paucity of the cells expressing a mature CD36lowCD235apos profile (Figure 1A). In contrast to the constant number of cells generated in replicated HEMA cultures for each single donor (17), high variability was observed among the cells generated by different normal individuals (Figure 1A).
Figure 1. Glucocorticoids sustain massive generation of proerythroblasts in culture by preventing their maturation in response to EPO.
(A) Fold Increase, flow cytometry analyses (forward/size side scatter and CD36/CD236a expression) and May-Grunwald Giemsa staining of cells obtained after 11 days in cultures of light density blood cells of normal adult volunteers stimulated either with multi-lineage GFs (SCF, IL-3, and EPO) or with GFs and DXM in combination (HEMA conditions), as indicated. Fold increase is calculated with respect to the number of cells seeded at day 0 and is presented as mean (±SD) of 10 separate experiments, each one with an individual donor. The difference in FI observed between cultures stimulated with GFs and those stimulated with GFs + DXM is statistically significant (<0.001) by paired t-test. By flow cytometry, cultures stimulated with GFs contain both erythroid (CD235apos) and non-erythroid (neutrophils, CD235adim, and lymphocytes, CD235aneg) cells. By contrast, the majority (>90–95%) of the cells in cultures stimulated with GFs and DXM were erythroid (CD235apos). Furthermore, both by flow cytometry (lack of CD235ahighCD36dim) and morphological criteria, erythroid cells obtained in the presence of GFs and DXM remained immature. Cell morphology is presented at 40× original magnification.
(B) May-Grunwald-Giemsa staining, forward size scatter (FSC) analyses and semiquantitative RT-PCR analyses for the expression of GATA1, GATA2 and Actin (the triangle on the top indicates amplifications levels after 25, 27 and 30 cycles), and quantitative RT-PCR analysis for β-globin in proerythroblasts obtained after 11 days of HEMA culture incubated for 24 h either under HEMA conditions (Prol) or in medium containing EPO alone. The levels of β-globin expression are presented as mean (±SD) of 5 separate experiments (*, p<0.01 by paired t test). Original magnification 40X.
(C) Flow cytometry analysis for Annexin V and propidium iodide staining of proerythroblasts generated in 11 days of HEMA cultures (GFs + DXM) and GF-starved for up to 6 h. Significant numbers (17%) of Annexin Vpos cells became detectable within 3 h of GF-starvation and ~25% of them became Annexin V and/or propidium iodide positive by 6 h.
To clarify whether the apparent block exerted by DXM on erythroid maturation was reversible, proerythroblasts obtained in HEMA were induced to mature with EPO for 24 h. EPO reduced the size of the cells and suppressed GATA-2 expression (23) while inducing GATA-1 and β-globin expression by 6 fold within 24 h. Morphology and expression profiling of the control proerythroblasts maintained in HEMA culture for the same period of time did not change (Figure 1B).
Primary proerythroblasts generated in HEMA culture were sensitive to apoptosis induced by GF-starvation. While the majority (>98%) of proerythroblasts generated in HEMA culture were Annexinneg, a significant proportion (17%) of them became Annexin Vpos within 3 h of GF-starvation. By 6 h, Annexin Vpos (11%) and Annexin VposPropidium Iodidepos (13%) cells became detectable and less than 50% of the cells was trypan blueneg by 8 h (Figure 1C).
DXM antagonizes the effects of EPO on maturation, but not those on survival, of human pro-erythroblasts
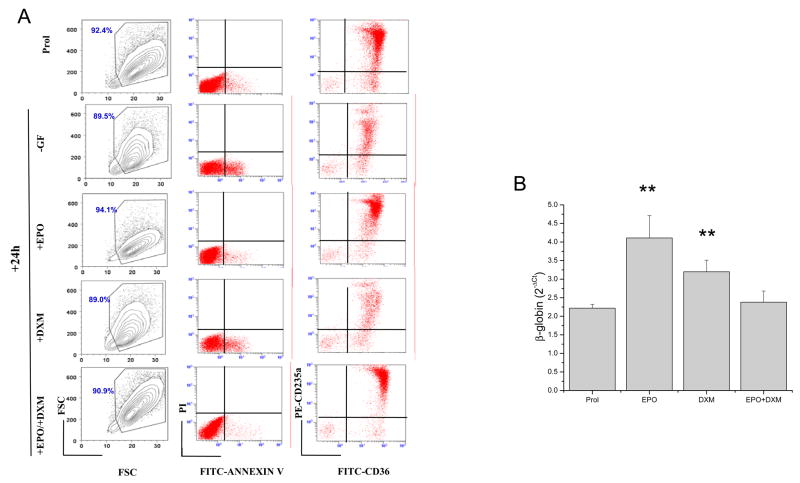
As shown in Figure 1, proerythroblasts generated ex vivo under HEMA conditions respond to EPO by repressing apoptosis and increasing β-globin expression. In order to identify which of these functions are inhibited by DXM, we compared size, viability and levels of β-globin expressed by proerythroblasts exposed for 24 h to EPO and DXM, either alone or in combination (Figure 2).
Figure 2. DXM antagonizes maturation (β-globin m-RNA induction), but not survival (prevention of apoptosis) signaling of EPO on human proerythroblasts.
A) Forward and size side scatter analysis, Annexin V/Propidium Iodide and CD36/CD235a staining of pro-erythroblasts obtained after 11 days in HEMA cultures (prol) and incubated for 24 h either without GFs or with EPO (3 U/mL), DMX (10−6 M) or the combination of both, as indicated. Equivalent numbers (106) of proerythroblasts were incubated in 1 mL of each culture condition. To highlight eventual cell loss due to cell death, the numbers of events analyzed in each sample correspond to equivalent culture volumes. Results are representative of those obtained in 3 separate experiments, each with a different donor.
B) Levels of β-globin expressed by adult erythroblasts obtained in HEMA culture and exposed for 24 h to EPO or DXM, alone or in combination. EPO and DXM alone but not in combination, significantly upregulated the levels of β-globin expressed by these cells. Results are presented as mean (±SD) of three separate experiments (p<0.01 by paired t-test).
The effects of 24 h incubation with medium without GFs or medium supplemented with EPO, DXM or the combination of both on induction of apoptosis was evaluated by flow cytometry (Figure 2B). At day 11 of HEMA culture, very few proerythroblasts (>95% CD235apos) were in apoptosis (<5% Annex Vpos). After 24 h of GF-starvation, the cells became low in number (<20% of the input, compare also the number of events in the FSC/SSC plots of Figure 2B), and >17% of them were Annexin Vpos. The cells that had died during GF-starvation were the more mature CD235ahigh cells (Figure 2B). Cells kept for 24 h in DXM alone were similar in number, frequency of Annexin Vpos and maturation state to those obtained in the absence of GFs. On the other hand, the cells in cultures containing EPO remained constant in number, with few Annexin Vpos cells (< 4%) (Figure 2B). In contrast, the cells in cultures containing both EPO and DXM were mostly alive (< 4% Annexin Vpos). Therefore DXM does not interfere with the anti-apoptotic effects of EPO on primary proerythroblasts.
As expected the level of β-globin mRNA expressed in ex vivo generated erythroblasts exposed for 24 h to EPO, increased by 2-fold (Figure 2C). The levels of β-globin expressed by these cells also increased upon exposure to DXM. Since the proerythroblasts that survived 24 h with DXM alone were the most immature, it is unlikely that the increases in β-globin induced by DXM were due to cell selection. By contrast β-globin mRNA levels did not increase when the proerythroblasts were exposed to EPO and DXM in combination. These results suggest that both EPO and DXM, when used alone, activate a signaling pathway, possibly represented by STAT5, that induces β-globin expression in primary proerythroblasts but that this pathway is not activated when the two factors are used in combination.
The protein content of primary human erythroblasts is not affected by GF-starvation or by other manipulations necessary for signaling studies
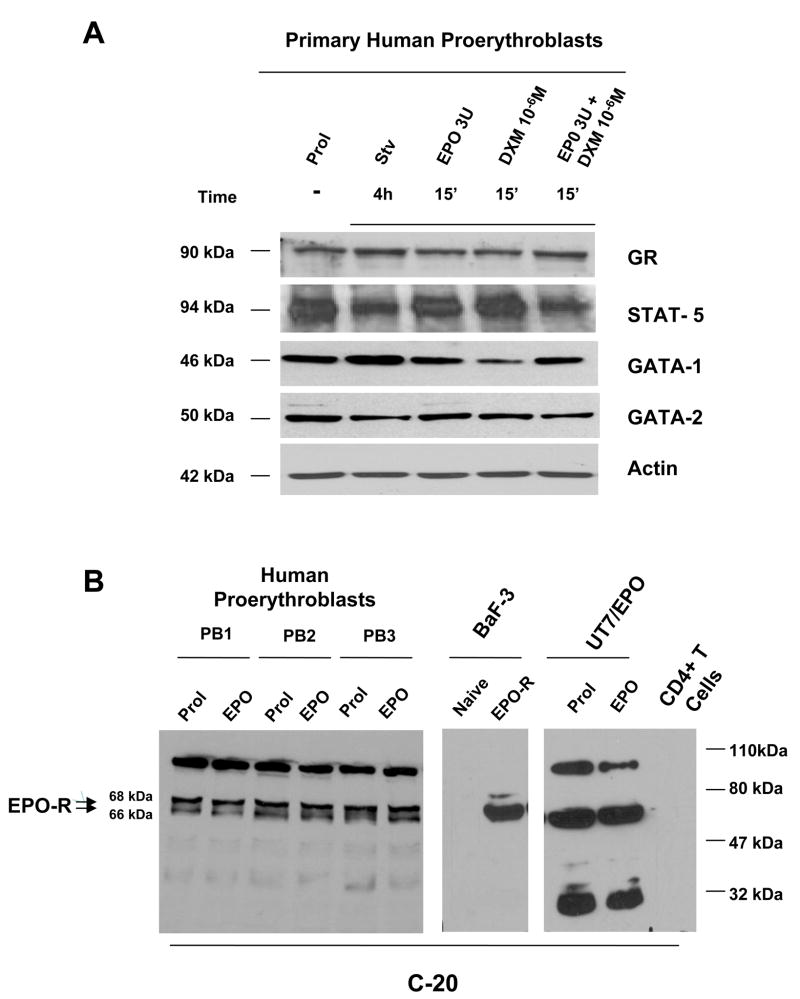
The use of primary cells for signaling studies is often discouraged by their low number and intrinsically high GF-sensitivity. The massive generation of human proerythroblasts in HEMA culture overcomes number limitations (21). Furthermore, the GF-starvation studies presented in Figures 1C identified a time window (3–4 h) during which significant numbers of cells survived the GF-withdrawal treatment necessary to bring the EPO-R and GR-signaling pathways to their resting configuration. In primary cells, however, protein turnover can be rapid (protein half-lives can be as low as 1 h) and GF-dependent. Therefore, 4 h of GF-starvation, although had not induced apoptosis, might bias the interpretation of signaling studies by altering the amount of proteins expressed by the cells. As shown in Figure 3, WB experiments ensured that the protein milieu in human proerythroblasts remained constant during the manipulations necessary for signaling studies. As additional control, proteins whose stability is known to be either EPO-sensitive (GATA-1) or -insensitive (GATA-2) (24–26) were included in the analysis.
Figure 3. Exposure to GF-starvation and to EPO and DXM, alone and in combination, has modest effects on the EPO-R, GR, STAT-5, GATA-1 and GATA-2 content of human proerythroblasts.
A) Western blot analyses for GR, STAT-5, GATA-1 and GATA-2 of whole cell extracts from proerythroblasts obtained under HEMA (Prol) conditions, or GFs starved for 4 h (Stv) and then exposed for 15′ to EPO (3 U/mL) and DXM (10−6 M), alone or in combination as indicated. Equivalent amounts (30 μg) of protein were loaded in each lane. The source of each antibody is specified in Materials and Methods. The blots were reprobed with Actin as a loading control. The position of the bands of the expected molecular weight for each protein is indicated on the left.
B) Western blot analyses for EPO-R of whole cell extracts from proerythroblasts obtained in HEMA (Prol) culture from 3 different donors. The cells were also GF deprived for 4 h and then exposed for 15′ to EPO (3 U/mL). Naïve Ba/B3 cells and BaF3 cells transfected with the human EPO-R gene (29), UT7/EPO and CD4pos T cells were used as controls. The membrane was blotted with the C-20 antibody. Equivalent amounts (30 μg) of protein were loaded in each lane. The position of the molecular weight markers (in kDa) is presented on the right.
The different treatments did not change the intensities of the bands corresponding to the analyzed protein expressed by primary proerythroblasts, with the exception of an apparent reduction of STAT-5 in GF-starved cells and in cells stimulated with EPO and DXM in combination, and of a predicted (27) decrease in GATA1 content observed in cells treated with DXM alone (Figure 3A).
Recently it has been described that commercially available EPO-R antibodies may recognize other proteins, in addition to EPO-R (28). The EPO-R antibody to be used in our study was chosen on the basis of the results presented in Figure 3B. In these experiments, cell lysates of primary erythroblasts obtained from three separate donors and of naïve or human EPO-R-transduced BaF3 cells (29) were blotted in parallel with the antibody C-20, identified by Elliott et al. (28) as the most specific commercially available antibody. The human EPO-dependent erythroleukemic UT7/EPO cell line (30) and human adult CD4+ T cells were also included in the analysis as further positive and negative controls, respectively. The C-20 antibody recognized in primary erythroblasts three of the fours bands detected by Elliott et al., in various cell lines, with this same antibody. Two of these bands have the expected molecular weight (66 and 68 KDa) for EPO-R (18,31–33) and co-migrated with the EPO-R specific bands expressed by BaF3/EPO-R cells. The position of these bands on the gel corresponds to that of the EPO-R bands identified by Elliott et al (28). The levels of expression of EPO-R in primary proerythroblasts was low (<100-fold than in BaF3/EPO-R) and was not affected by stimulation with EPO (Figure 3B).
GF starvation and EPO or DXM alone induce HSP-90-independent association of GR with the cell membranes of human proerythroblasts
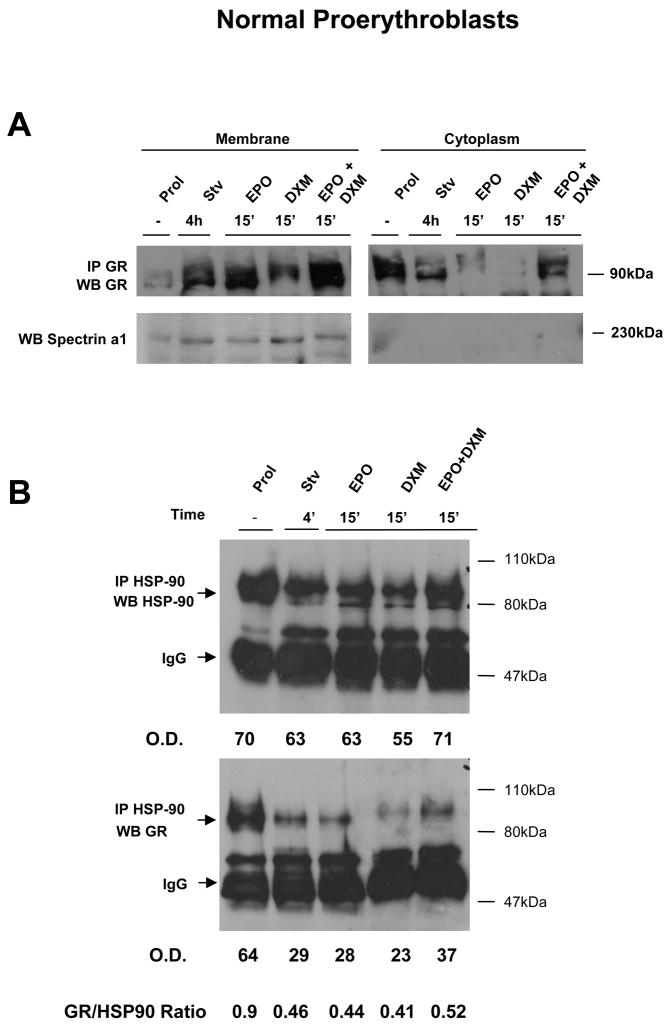
To characterize the molecular mechanism(s) underling the counteraction exerted by DXM the biological effects of EPO, we first determined the localization of GR within the human proerythroblasts (Figure 4). In these experiments, the membrane and cytosol fractions were prepared from proerythroblasts obtained from normal donors after 11 days of culture (Prol), GF starved for 4 h, and then treated either with EPO (3U/mL) and DXM (10−6M), alone or in combination, for 15′. The fractions were immunoprecipitated with anti-GR antibody and the immunoprecipitates analyzed by western blot with anti-GR. Since the cells analyzed were at a maturation stage in which spectrin becomes associated with the membranes, control blots were analysed with antibodies against spectrin α1, as a control for membrane-contamination of the cytosol fraction (Figure 3A).
Figure 4. GF starvation, and stimulation with EPO and DXM alone, induces HSP-90-independent translocation of GR from the cytosol to the cell membranes of normal proerythroblasts.
A) Localization of GR in normal proerythroblasts by cell fractionation experiments. Equivalent amount of proteins (30 μg) obtained from the membrane and the cytosol fraction of human proerythroblasts were immunoprecipitated with antibodies specific for GR, separated by SDS-PAGE and probed again by western blot with an antibody specific for GR. The cells were obtained either from HEMA culture (Prol) or GF starved for 4h (Stv) and then treated either with EPO, DXM or the combination of both, as indicated. The amount of spectrin α1 present in the membrane and cytosolic fractions was analyzed by western blot. The relative distribution of GR in the membrane and cytosol fraction, as corrected for the total amount of proteins obtained in the two fractions, of cells exposed to different stimuli is presented in Table 1.
B) Western blot analyses from HSP-90 and GR of whole cell extracts (50 μg) immunoprecipitated with an anti-HSP-90 antibody from human proerythroblasts obtained in HEMA (Prol), GF starved for 4 h (Stv), and then treated for 15′ with EPO (3 U/mL), DXM (10−6 M), or the combination of both, as indicated. The position of the molecular weight markers (in kDa) and of the bands of the expected size for HSP-90, GR and IgG are indicated. The relative intensity of the HSP-90 and GR bands observed in the different lanes was determined by spectrometry, expressed as optical density (OD) and used to calculate the ratio between the two bands.
Proerythroblasts from cultures contained low levels of membrane-associated GR (GRm/GRc ratio = 0.06) (Figure 4A and Table 1). GF starvation, however, induced a significant increase in the amount of GR present on the membranes of primary cells (GRm/GRc ratio = 0.39). Stimulation of the cells with EPO and DXM individually, but not in combination, further increased the amount of GR present on the membranes (GRm/GRc ratio = 1.4–2).
Table 1.
Relative distribution of GR in the membrane and cytosol fraction of human proerythroblasts stimulated with different GF-combinations.
| MEMBRANES | CYTOSOL | RATIOS | ||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | Proteins Recovered (μg) | Cells Analyzed (×106) | GR [O.D.] | Protein Recovered (μg) | Cells Analyzed (×106) | GR [O.D.] | ||
| Prol | 180 | 10 | 4.2 | 320 | 5.6 | 39.2 | 0.06 | |
| Stv | 140 | 13 | 21.3 | 320 | 5.6 | 23.3 | 0.39 | |
| EPO | 140 | 13 | 26.1 | 340 | 5.3 | 7.4 | 1.43 | |
| DXM | 90 | 20 | 16.8 | 300 | 6 | 2.4 | 2.1 | |
| EPO + DXM | 160 | 11 | 31.6 | 320 | 5.6 | 27.7 | 0.6 | |
where [GR] is the normalized O.D. value, and m and c indicate the membrane or cytoplasmic fraction.
The relative expression of GR between the membrane and cytoplasm fraction was then expressed as ratio between the normalized membrane and cytoplasmic values, as reported in the last line, according to the algorithm: Rm/Rc = [N]m/[N]c.
Since GR does not have a transmembrane spanning domain, association of GR with the cell membranes is thought to depend upon the interaction with the chaperone protein HSP-90, usually present on the membranes of many cell types (34). To identify whether the association of GR with the membranes of primary proerythroblasts was mediated by HSP-90, cell lysates from primary proerythroblasts incubated with the different GF combinations were immunoprecipitated with antibodies for HSP-90 and then analyzed by western blot with anti-HSP-90 (as a control) and anti-GR antibodies (Figure 4B). With the exception of a slightly higher (by 2-fold) levels of HSP-90 in cells from culture (Prol), equivalent amounts of HSP-90 were immunoprecipitated from all the other cell lysates investigated. Robust levels of GR were immunoprecipitated by the anti-HSP-90 in proerythroblasts obtained from HEMA cultures, a condition in GR was mainly localized in the cytosol (Figure 4A). Lower levels (by 3 fold) of GR were instead IP with the anti-HSP-90 antibody from GF starved cells, or from cells that had been treated with EPO or DXM, alone or in combinations (Figure 4B). Therefore, significant amounts of GR were associated with HSP-90 under Prol conditions, but not under GF-starvation and EPO and DXM stimulation when GR was preferentially localized on the cell membranes (Figure 4A,B).
In conclusion, GF starvation and stimulation with EPO and DXM promotes a HSP-90- independent association of GR to the cell membranes of human proerythroblasts.
DXM antagonizes EPO-induced phosphorylation and nuclear localization of STAT-5 in normal human proerythroblasts
The observation that GF-starvation induced human proerythroblasts to express GR on the cell membranes indicates that these cells might be induced to acquire the rapid membrane-associated GR signaling pathway. Since STAT-5 is immediately downstream of JAK2 of both EPO-R (35) and GR (10,36) signaling, it is possible that the membrane-associated GR might compete with EPO-R for STAT-5 signaling. To test this hypothesis, we analyzed the distribution within the cells and the phosphorylation status of STAT-5 in normal proerythroblasts obtained from HEMA culture (Prol) and after GF deprivation for 4 h (Stv) and then stimulated for various length of time either with EPO, DXM, or the combination of both.
Immunofluorescence studies with a STAT-5 antibody indicated a significant increase in STAT-5-immunostaining of the plasma membrane of normal proerythroblasts upon exposure to EPO and DXM alone, but not in combination (Figure 5A). In addition, EPO and DXM alone, but not in combination, significantly increased the nuclear localization of STAT-5 in these cells (Figure 5A and Table 2).
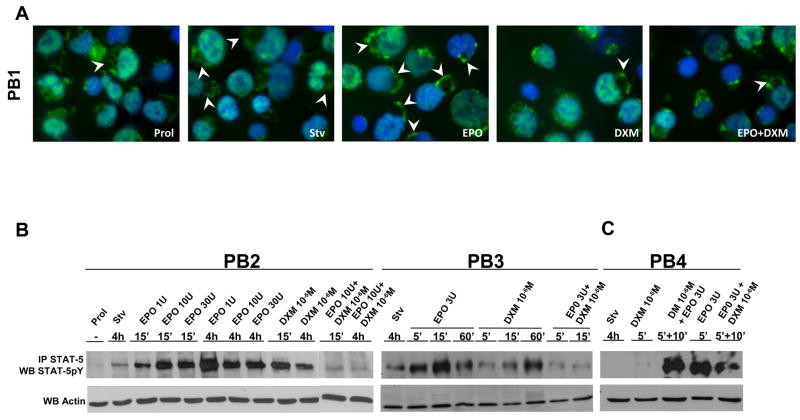
Figure 5. Levels of STAT-5 phosphorylation after exposure either to GF starvation, EPO and DXM, alone or in combination, of human proerythroblasts obtained from normal donors.
A) Immunofluorescence analyses with an anti-STAT-5 antibody of proerythroblasts obtained in HEMA culture (Prol) from a normal donor and treated as indicated in Figure 3B. Original magnification: 100×. Representative cells in which STAT-5 is localized on the plasma membrane are indicated by arrowheads. The microscopic images were acquired as described in the legend of Figure 3A. Quantification of the level of STAT-5 immunostaining in the nuclear area is presented in Table 1.
B) Levels of STAT-5-phosphorylation in proerythroblasts obtained in HEMA culture (Prol) from two normal donors (each panel a different donor), GF starved for 4 h (Stv) and then treated for 5′-4h either with EPO (1–30 U/mL), DXM (10−6 M) or the combination of both, as indicated. The specificity of the signal for the phosphorylated form of STAT-5 was increased by analyzing proteins that were immunoprecipitated from 50 μg of whole cell lysates with a STAT-5 antibody by western blot with an anti-STAT-5pY antibody. Equivalent amounts (10 μg) of pre-immunoprecipitated proteins were analyzed by western blot with an anti-Actin antibody, as quantitative control. Similar results were obtained in 10 additional experiments.
C) Levels of STAT-5-phosphorylation in proerythroblasts preincubated for 5′ either with DXM (10−6 M) or EPO (3 U/mL) and then exposed to EPO (3U/mL) or DXM (10−6 M) for 10′.
Table 2.
Intensity of the STAT-5 immunostaining (expressed as gray units) in the nuclear area of proerythroblasts derived in vitro from normal proerythroblasts stimulated with different GF-combinations.
| Nuclear STAT-5 immunostaining | |||
|---|---|---|---|
| Treatments | Minimum Intensity | Maximal Intensity | Average Intensity |
| Prol | 1392° | 2149° | 1764.7±123.2° |
| Stv | 1223* | 1914* | 1470.0±89.5* |
| EPO | 1344° | 2576*° | 1799.5±172.8° |
| DXM | 1439° | 2250° | 1833.1±130.6° |
| EPO + DXM | 1285* | 1865* | 1524.0±77.1* |
The intensity of the immunostaining was determined by analyzing thirty randomly selected nuclei per experimental point with the MetaMorph Imaging System (Molecular Devices, Sunnyvale, CA). The acquiring settings were maintained constant for all samples.
P values were calculated by Anova.
p< 0.001 with respect to the corresponding Prol cells
p< 0.01 with respect to the corresponding Stv cells
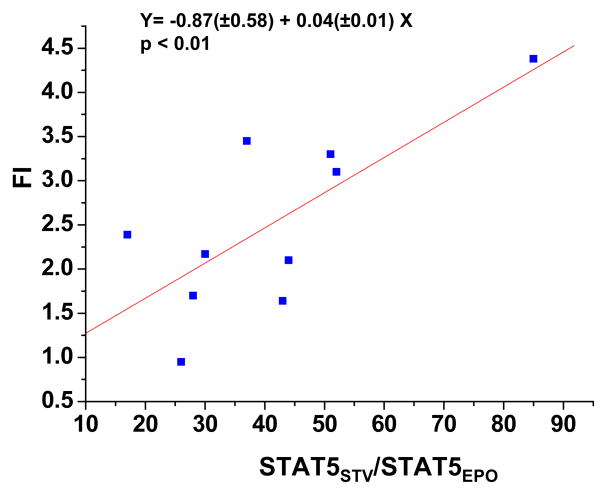
On the other hand, although comparable levels of total protein phosphorylation were detected in all the culture conditions, analyzed in this study (data not shown), STAT-5-phosphorylation was barely detectable in normal proerythroblasts generated in HEMA cultures (Figure 5B). GF deprivation alone induced some STAT-5-phosphorylation with a great level of donor-to-donor variability. A significant (p<0.01) linear correlation (Figure 6) existed between the levels of STAT-5 phosphorylation induced by GF-starvation (Figure 5) and the numbers of proerythroblasts, expressed as FI, generated under HEMA conditions observed in 10 independent experiments, each one with a separate donor (Figure 1). This correlation suggests that the two variabilities might be linked. EPO and DXM both induced a strong STAT-5-phosphorylation signal in normal proerythroblasts. In the case of EPO, the extent of the induction was concentration and time dependent. At the lower EPO concentrations tested (1 U/mL), maximal levels of STAT-5-phosphorylation were observed after 4 h of exposure, while at higher concentrations (3–30 U/mL), phosphorylation was maximal within 5′, started to decrease by 60′ and was still detectable after 4 h. Following DXM (10−6 M) exposure, STAT-5-phosphorylation became detectable after 15′ and was maximal after 60′. By contrast EPO and DXM in combination did not induce STAT-5-phosphorylation in normal proerythroblasts at any of the time points (5′, 15′ and 4 h) investigated (Figure 4B).
Figure 6. Correlation between the levels of STAT-5-phosphorylation induced by GF starvation and the number of proerythroblasts generated in HEMA cultures from different donors.
To compare the levels of STAT-5-phosphorylation in GF starved cells obtained from different donors, the intensities of the bands were normalized to those observed with cells stimulated for 15′ with 3 U/mL of EPO. The number of proerythroblasts obtained in HEMA was, instead, expressed as Fold Increase (FI). Each symbol represents a different donor. The equation of the linear relationship between the two values, indicated on the top, is statistically significant (p<.01).
To clarify the hierarchy of the interaction between EPO-R and GR under co-stimulation conditions, the levels of STAT-5-phosphorylation induced by 10′ of stimulation with EPO or DXM in cells that had been pre-incubated for 5′ with either DXM or EPO were measured (Figure 5C). Preincubation with DXM did not affect the ability of the cells to phosphorylate STAT-5 in response to EPO while the strong STAT-5-phosphorylation signal induced by incubating the cells for 5′ with EPO was quickly quenched by additional 10′ exposure to DXM.
These results indicate that GR exerts a dominant antagonistic effect on the ability of EPO-R to recruit to the cell membranes and to phosphorylate STAT-5.
Interaction of EPO-R, GR and STAT-5 in proerythroblasts stimulated with EPO and DXM, either alone or in combination
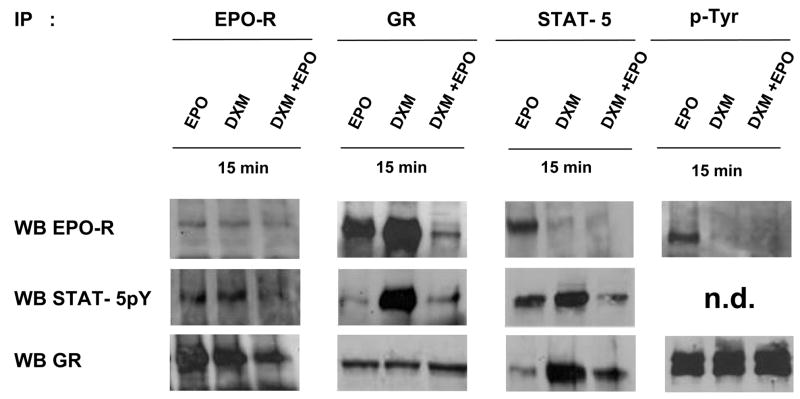
To understand why, when used in combination, both DXM and EPO failed to phosphorylate STAT-5, experiments were undertaken to identify which receptor STAT-5 was associated with, in cells exposed to different GF. Proerythroblasts obtained at day 11 of HEMA were GF-starved for 4 h and then treated either with EPO (3U/mL), DXM (10−6M) or the combination of both for 15′. Whole cell extracts were IP with antibodies specific for EPO-R (18), GR, STAT-5 and phosphotyrosine (p-Tyr) and the IP proteins were analyzed by WB with antibodies specific for EPO-R, GR, and STAT-5pY (Figure 7). As a quantitative control of the experiment, all the IP obtained with anti-EPO-R, by WB contained equivalent amount of EPO-R, while those obtained with anti-GR, by WB contained equivalent amounts of GR (Figure 7).
Figure 7. Interaction of GR, EPO-R and STAT-5pY in human proerythroblasts treated either with EPO and DXM alone, but not in combination.
Whole cell extracts (50 μg) were IP either with anti-EPO-R, -GR, -STAT-5 or -p-Tyr specific antibodies. The different IP were divided into aliquots that were independently separated by SDS-PAGE, transferred to nitrocellulose membranes, and blotted with antibodies that recognize EPO-R (C-20), STAT5pY and GR, as indicated. The WB with anti-STAT-5pY of IP obtained with anti-p-Tyr is not presented because of its high background (n.d.). Similar results were obtained in 3–5 additional experiments, each with a separate donor.
Surprisingly, IP obtained with anti-EPO-R from cells treated either with EPO or DXM, by WB not only were positive for GR, but contained more GR than IP obtained from cells being treated with the combination of both. The reciprocal was also true, IP obtained with anti-GR from cells exposed to EPO or DXM, by WB were positive for EPO-R, at levels much higher than those from cells being treated with EPO and DXM in combination (Figure 7). WB with anti-STAT-5pY of IP performed with anti-STAT-5 indicated once again that significant levels of STAT-5 phosphorylation were detectable primarily in cells incubated either with EPO or DXM, but not in cells stimulated with the two factors in combination. By WB, IP with anti-STAT-5 were positive for EPO-R in cells stimulated with EPO and positive for GR in those stimulated with DXM, respectively. These results indicate that in primary cells STAT-5pY had become associated either with EPO-R or with GR, depending on the stimulus to which the cells had been exposed. As a confirmation, WB with anti-STAT-5pY detected high levels of the protein in IP obtained with anti-GR only in cells exposed to DXM. By contrast, WB with anti-STAT-5pY in IP obtained with EPO-R detected the presence of the protein both in cells exposed to EPO, as expected, and in those exposed to DXM (Figure 7). As a last control, EPO-R was detected by WB, in IP with the p-Tyr antibodies only from cells exposed to EPO while GR was detected in IP from cells in all the conditions analyzed. Therefore, EPO-R was phosphorylated only in proerythroblasts stimulated with EPO while GR was constitutively phosphorylated in these cells.
DISCUSSION
It is here documented that human proerythroblasts remain immature as long as maintained in the presence of DXM but that, once exposed to EPO alone, mature within 24 h by decreasing GATA-2 and activating β-globin expression (Figure 1 and 2). The presence of DXM counteracted the maturation (β-globin induction) but not the anti-apoptotic effect induced by EPO on these cells (Figure 2 and Table 1). These results indicate that DXM allows massive generation of erythroid cells in culture because, in addition to its genomic effects, antagonizes the effects of EPO on maturation, retarding the cells at a stage in which they proliferate in response to GFs.
Biochemical studies indicated that GR is recruited to the cell membranes of primary erythroblasts after exposure to GR-deprivation. The association of GR to the membranes is further increased by exposing the cells to EPO and DXM alone, but not in combination (Figure 4 and Table 1). In addition, in these primary cells, both EPO and DXM alone induce association with the membranes and phosphorylation and migration of STAT-5 to the nucleus, while their combination is ineffective (Figure 5 and Table 2). A series of IPs, followed by WB, indicated that EPO-R and GR are present as a complex in normal human proerythroblasts stimulated either with EPO or DXM, that p-STAT-5 becomes part of this complex in association either with EPO-R or GR, depending on the stimulus to which the cells are exposed, and that the physical association between the two receptors is reduced when the cells are stimulated with EPO and DXM in combination (Figure 7). These results support the hypothesis that GR signalling in primary erythroblasts involves interaction with EPO-R. This hypothesis explains how GR may have access to JAK2, the first element of its signalling pathway in erythroid cells. In fact, since JAK2, as other members of the JAK family, is mainly localized on the cell membranes (37), the cytosolic form of GR has poor access to the first element of its signaling cascade unless it migrates to the membranes. The steric mechanism that underlies the inhibition of the EPO-R activity by GR can be explained by two, non mutually exclusive, models depending whether the physical interaction between the two receptors occurs within the cytoplasm and/or on the plasma membrane. The first model is supported by the observation that the majority of EPO-R is localized on the Golgi and endoplasmic reticulum where it is assembled into EPO-R/JAK2 complexes that are translocated to the plasma membrane (38). According to this model, association between GR and EPO-R inside the cell, by dislodging JAK2 from the complex, inhibits EPO signalling by preventing the migration of its receptor to the plasma membrane. In other words, GR would exert in the erythroid lineage the same function played by LNK in megakaryocytes. LNK, in fact, by binding to MPL, prevents its translocation to the cell surface and reduces the responsiveness of megakaryocytes to thrombopoietin, the MPL ligand (39–41). The second model is consistent with the canonical membrane-associated GR signalling described in other cell types (16) and is supported by the observation that DXM antagonized some (β-globin activation) but not all (anti-apoptotic) of the effects induced by EPO in human proerythroblasts (Figure 2), an indication that, at least some, EPO-R is present on the plasma membrane of DXM treated cells. In this model, steric interference between the two receptors when both of them are engaged by their respective ligands dissociates JAK2 from the complex, halting the ability of both receptors to phosphorylate STAT-5. Additional studies are necessary to formally identify the cellular localization of the interaction between EPO-R and GR in this primary erythroid cells.
Previous studies have described physical association of EPO-R with other receptors. The possibility exist that the specificity of the EPO-R functions are determined by the type of complex it forms in a cell. In mouse, EPO-R can become associated with c-KIT, the receptor for SCF, and stimulation with SCF of BaF3 cells ectopically engineered to express both c-KIT and EPO-R results in EPO-R activation in the absence of EPO (42). The observation that fetal, but not adult, BFU-E differentiate in cultures stimulated with SCF alone (43), and gain of function experiments with fetal EPO-Rnull cells (44), have suggested that c-KIT/EPO-R interactions might specifically support erythropoiesis in the fetal liver, when EPO production is poor. On the other hand, human EPO-R can also form a complex with the β chain common to the IL-3 and GM-CSF receptor (45). Although this complex does not have apparent hematopoietic functions (46), it has been implicated in the transduction of EPO-R signal in nonhematopoietic cells (47). This paper adds GR to the list of receptors that can form a complex with human EPO-R.
The human GR gene is localized on the short arm of chromosome 5, a region frequently deleted in myelodysplastic syndrome and in acute myelogenous leukaemia (48). The human GR gene is highly polymorphic and its complex structure includes several alternative splicing and starting sites giving rise to an extreme large numbers of different GR isoforms found expressed in the human population (49,50). Such polymorphism is emerging as a leading cause for the heterogeneity of the response to glucocorticoids in the treatment of stress in humans (51–53). In this regard, it is interesting that GF-starvation induced some donor-dependent STAT-5 phosphorylation in human proerythroblasts (Figure 5). Since the GF-deprived media to which the cells were exposed contained some (10%) FBS, a source of glucocorticoids, we believe that the correlation between extent of STAT-5 phosphorylation induced by GF-starvation and numbers of erythroblasts generated in HEMA culture (Figure 7) reflects a correlation between affinity of the GR isoform for DXM and strength of its antagonistic effects on EPO-R signaling. According to this interpretation, polymorphism at the GR locus may represent one of the genetic factors that tune the number of red cells in the blood under conditions of steady-state hematopoiesis in humans. In fact, the size of the erythroid mass is determined both by the number of erythroid progenitors being recruited and by the number of cell divisions allowed at each stage of maturation. Given the fact that in vivo the number of proerythroblasts is higher than that of progenitor cells, the number of divisions that the former perform before terminal differentiation plays an important role in determining how many red cells will be produced by each progenitor cell. By retarding the EPO maturation signal, DXM would favor erythroblast proliferation and increase the cellular output of the erythropoietic process.
The hypothesis that GR polymorphism affects the Hct of normal individuals is in apparent contrast with the observation that the Hct of mice carrying the GRnull or a dimerization-defective GR defective mutation is apparently normal (9,10). However the statistical power of these studies is flawed by the small cohort of animals (5–6 mice per experimental group) analyzed. In the regard, using relatively low numbers of mice (14–18 per group), our laboratory was originally unable to detect statistically significant differences between the Hct expressed by mice carrying the hypomorphic Gata1low mutation (a mutation that reduces Gata1 expression in erythroid cells) and their wild-type littermates at 1 month of age (54). However, a recent reanalyzes of the data that included a larger cohort of animals (27 wild-type and 32 Gata1low littermates) revealed that although the Hct of Gata1low mice was within normal ranges, the average Hct of Gata1low mice was statistically lower than that of wild type mice (40.5±0.6, Min 34 − Max 45.4 vs 46.2±0.7, Min 37 − Max 56.6, respectively, p<0.001). This consideration strongly indicates that larger cohorts of GR deficient mice must be analyzed to be able to define the effects of these mutations on the Hct of mice under steady state condition.
In conclusion, we observed a membrane-associated interaction between GR and EPO which interfered with the ability of both receptors to phosphorylate STAT-5 and that may retard the ability of EPO-R to transduce a maturation signal for primary human proerythroblasts.
Acknowledgments
This study was supported by Ministero per la Ricerca Scientifica, grant no. RBNE0189JJ_003, RBNE015P72_003, and by Ministero per la Salute (ISS-NIH scientific cooperation) and the National Cancer Institute, grant no. P01-CA108671, USA.
Human recombinant SCF was provided by Amgen (Thousand Oaks, CA, USA; MTA no. 19982634-005). Catherine Lacombe and Patrick Majeux are gratefully thanked for the gift of the polyclonal anti-EPO-R antibody. Dr. John W. Adamson is gratefully acknowledged for the generous gift of his time for discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adamson JW, Eschbach JW. Erythropoietin for end-stage renal disease. N Engl J Med. 1998;339:625–627. doi: 10.1056/NEJM199808273390910. [DOI] [PubMed] [Google Scholar]
- 2.Ebert BL, Bunn HF. Regulation of the erythropoietin gene. Blood. 1999;94:1864–1877. [PubMed] [Google Scholar]
- 3.Constantinescu SN, Ghaffari S, Lodish HF. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends Endocrinol Metab. 1999;10:18–23. doi: 10.1016/s1043-2760(98)00101-5. [DOI] [PubMed] [Google Scholar]
- 4.Spivak JL. The biology and clinical applications of recombinant erythropoietin. Semin Oncol. 1998;25:7–11. [PubMed] [Google Scholar]
- 5.Golde DW, Bersch N, Cline MJ. Potentiation of erythropoiesis in vitro by dexamethasone. J Clin Invest. 1976;57:57–62. doi: 10.1172/JCI108269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singer JW, Adamson JW. Steroids and hematopoiesis. III. The response of granulocytic and erythroid colony-forming cells to steroids of different classes. Blood. 1976;48:855–864. [PubMed] [Google Scholar]
- 7.Golde DW, Bersch N, Chopra IJ, Cline MJ. Thyroid hormones stimulate erythropoiesis in vitro. Br J Haematol. 1977;37:173–177. doi: 10.1111/j.1365-2141.1977.tb06833.x. [DOI] [PubMed] [Google Scholar]
- 8.Daniak N, Hoffman R, Maffei LA, Forget BG. Potentiation of human erythropoiesis in vitro by thyroid hormone. Nature. 1978;272:260–262. doi: 10.1038/272260a0. [DOI] [PubMed] [Google Scholar]
- 9.Bauer A, Tronche F, Wessely O, et al. The glucocorticoid receptor is required for stress erythropoiesis. Genes Dev. 1999;13:2996–3002. doi: 10.1101/gad.13.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolznig H, Grebien F, Deiner EM, et al. Erythroid progenitor renewal versus differentiation: genetic evidence for cell autonomous, essential functions of EpoR, Stat5 and the GR. Oncogene. 2006;25:2890–2900. doi: 10.1038/sj.onc.1209308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gursoy A, Dogruk UA, Ayturk S, et al. Polycytemia as the first manifestation of Cushing’s disease. J Endocrinol Invest. 2006;29:742–744. doi: 10.1007/BF03344186. [DOI] [PubMed] [Google Scholar]
- 12.Vardimon L, Ben-Dror I, Oren A, Polak P. Cytoskeletal and cell contact control of the glucocorticoid pathway. Mol Cell Endocrinol. 2006;252:142–147. doi: 10.1016/j.mce.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Rogatsky I, Ivashkiv LB. Glucocorticoid modulation of cytokine signaling. Tissue Antigens. 2006;68:1–12. doi: 10.1111/j.1399-0039.2006.00599.x. [DOI] [PubMed] [Google Scholar]
- 14.von Lindern M, Zauner W, Mellitzer G, et al. The glucocorticoid receptor cooperates with the erythropoietin receptor and c-Kit to enhance and sustain proliferation of erythroid progenitors in vitro. Blood. 1999;94:550–559. [PubMed] [Google Scholar]
- 15.Kolbus A, Blazquez-Domingo M, Carotta S, et al. Cooperative signaling between cytokine receptors and the glucocorticoid receptor in the expansion of erythroid progenitors: molecular analysis by expression profiling. Blood. 2003;102:3136–3146. doi: 10.1182/blood-2003-03-0923. [DOI] [PubMed] [Google Scholar]
- 16.Tasker JG, Di S, Malcher-Lopes R. Minireview: rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology. 2006;147:5549–5556. doi: 10.1210/en.2006-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Migliaccio G, Di Pietro R, di Giacomo V, et al. In vitro mass production of human erythroid cells from the blood of normal donors and of thalassemic patients. Blood Cells Mol Dis. 2002;28:169–180. doi: 10.1006/bcmd.2002.0502. [DOI] [PubMed] [Google Scholar]
- 18.Walrafen P, Verdier F, Kadri Z, et al. Both proteasomes and lysosomes degrade the activated erythropoietin receptor. Blood. 2005;105:600–608. doi: 10.1182/blood-2004-03-1216. [DOI] [PubMed] [Google Scholar]
- 19.Fragale A, Tartaglia M, Bernardini S, et al. Decreased proliferation and altered differentiation in osteoblasts from genetically and clinically distinct craniosynostotic disorders. Am J Pathol. 1999;154:1465–1477. doi: 10.1016/S0002-9440(10)65401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Migliaccio AR, Rana RA, Sanchez M, et al. GATA-1 as a regulator of mast cell differentiation revealed by the phenotype of the GATA-1low mouse mutant. J Exp Med. 2003;197:281–296. doi: 10.1084/jem.20021149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Baldassarre A, Di Rico M, Di Noia A, et al. Protein kinase Calpha is differentially activated during neonatal and adult erythropoiesis and favors expression of a reporter gene under the control of the (A)gamma globin-promoter in cellular models of hemoglobin switching. J Cell Biochem. 2007;101:411–424. doi: 10.1002/jcb.21189. [DOI] [PubMed] [Google Scholar]
- 22.Migliaccio G, Migliaccio AR, Druzin ML, et al. Long-term generation of colony-forming cells in liquid culture of CD34+ cord blood cells in the presence of recombinant human stem cell factor. Blood. 1992;79:2620–2627. [PubMed] [Google Scholar]
- 23.Leonard M, Brice M, Engel JD, Papayannopoulou T. Dynamics of GATA transcription factor expression during erythroid differentiation. Blood. 1993;82:1071–1079. [PubMed] [Google Scholar]
- 24.Ribeil JA, Zermati Y, Vandekerckhove J, et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. 2007;445:102–105. doi: 10.1038/nature05378. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez-Hernandez A, Ray P, Litos G, et al. Acetylation and MAPK phosphorylation cooperate to regulate the degradation of active GATA-1. EMBO J. 2006;25:3264–3274. doi: 10.1038/sj.emboj.7601228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghinassi B, Verrucci M, Jelicic K, Di Noia A, Migliaccio G, Migliaccio AR. Interleukin-3 and erythropoietin cooperate in the regulation of the expression of erythroid-specific transcription factors during erythroid differentiation. Exp Hematol. 2007;35:735–747. doi: 10.1016/j.exphem.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Chang T-J, Scher BM, Waxman S, Scher W. Inhibition of mouse GATA-1 function by glucocorticoid receptor: possible mechanism of steroid inhibition of erythroleukemia cell differentiation. Mol Endo. 1993;7:528–542. doi: 10.1210/mend.7.4.8502237. [DOI] [PubMed] [Google Scholar]
- 28.Elliott S, Busse L, Bass MB, et al. Anti-Epo receptor antibodies do not predict Epo receptor expression. Blood. 2006;107:1892–1895. doi: 10.1182/blood-2005-10-4066. [DOI] [PubMed] [Google Scholar]
- 29.Jones SS, D’Andrea AD, Haines LL, Wong GG. Human erythropoietin receptor: cloning, expression and biological characterization. Blood. 1990;76:31–35. [PubMed] [Google Scholar]
- 30.Komatsu N, Yamamoto M, Fujita H, et al. Establishment and characterization of an rythropoietin-dependent subline, UT-7/Epo, derived from human leukemia cell line, UT-7. Blood. 1993;82:456–464. [PubMed] [Google Scholar]
- 31.Longmore GD, Pharr PN, Lodish HF. A constitutively activated erythropoietin receptor stimulates proliferation and contributes to transformation of multipotent, committed nonerythroid and erythroid progenitor cells. Mol Cell Biol. 1994;14:2266–2277. doi: 10.1128/mcb.14.4.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu X, Gross AW, Lodish HF. Active conformation of the erythropoietin receptor: random and cysteine-scanning mutagenesis of the extracellular juxtamembrane and transmembrane domains. J Biol Chem. 2006;281:7002–7011. doi: 10.1074/jbc.M512638200. [DOI] [PubMed] [Google Scholar]
- 33.Watowich SS, Hilton DJ, Lodish HF. Activation and inhibition of erythropoietin receptor function: role of receptor dimerization. Mol Cell Biol. 1994;14:3535–3549. doi: 10.1128/mcb.14.6.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dittmar KD, Hutchison KA, Owens-Grillo JK, Pratt WT. Reconstitution of the steroid receptor/hsp90 heterocomplex assembly system of rabbit reticulocyte lysate. J Biol Chem. 1996;271:12833–12839. doi: 10.1074/jbc.271.22.12833. [DOI] [PubMed] [Google Scholar]
- 35.Witthuhn BA, Quelle FW, Silvennoinen O, et al. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74:227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- 36.Engblom D, Kornfeld JW, Schwake L, et al. Direct glucocorticoid receptor-Stat5 interaction in hepatocytes controls body size and maturation-related gene expression. Genes Dev. 2007;21(10):1157–1162. doi: 10.1101/gad.426007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Behrmann I, Smyczek T, Heinrich PC, et al. Janus kinase (Jak) subcellular localization revisited. J Biol Chem. 2004;279:35486–35493. doi: 10.1074/jbc.M404202200. [DOI] [PubMed] [Google Scholar]
- 38.Huang LJ-s, Constantinescu SN, Lodish HF. The N-terminal domain of Janus kinase 2 is required for Golgi processing and cell surface expression of erythropoitin receptor. Mol Cell. 2001;8:1327–1338. doi: 10.1016/s1097-2765(01)00401-4. [DOI] [PubMed] [Google Scholar]
- 39.Bersenev A, Wu C, Balcerek J, Tong W. Lnk controls mouse hematopoietic stem cell self-renewal and quiescence through direct interactions with JAK2. J Clin Invest. 2008;8:2832–2844. doi: 10.1172/JCI35808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tong W, Lodish HF. Lnk inhibits Tpo-mpl signaling and Tpo-mediated megakaryocytopoiesis. J Exp Med. 2004;5:569–580. doi: 10.1084/jem.20040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Velazquez L, Cheng AM, Fleming HE, et al. Cytokine signaling and hematopoietic homeostasis are disrupted in Lnk-deficient mice. J Exp Med. 2002;12:1599–1611. doi: 10.1084/jem.20011883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu H, Klingmuller U, Besmer P, Lodish HF. Interaction of the erythropoietin and stem cell-factor receptors. Nature. 1995;377:242–246. doi: 10.1038/377242a0. [DOI] [PubMed] [Google Scholar]
- 43.Kurata A, Mancini GC, Alespeiti G, Migliaccio AR, Migliaccio G. Stem cell factor induces proliferation and differentiation of fetal progenitor cells in the mouse. Br J Haematol. 1998;101:676–687. doi: 10.1046/j.1365-2141.1998.00775.x. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki N, Ohneda O, Takahashi S, et al. Erythroid-specific expression of the erythropoietin receptor rescued its null mutant mice from lethality. Blood. 2002;100:2279–2288. doi: 10.1182/blood-2002-01-0124. [DOI] [PubMed] [Google Scholar]
- 45.Jubinsky PT, Krijanovski OI, Nathan DG, Tavernier J, Sieff CA. The beta chain of the interleukin-3 receptor functionally associates with the erythropoietin receptor. Blood. 1997;90:1867–1873. [PubMed] [Google Scholar]
- 46.Scott CL, Robb L, Papaevangeliou B, Mansfield R, Nicola NA, Begley CG. Reassessment of interactions between hematopoietic receptors using common beta-chain and interleukin-3-specific receptor beta-chain-null cells: no evidence of functional interactions with receptors for erythropoietin, granulocyte colony-stimulating factor, or stem cell factor. Blood. 2000;96:1588–1590. [PubMed] [Google Scholar]
- 47.Mennini T, De Paola M, Bigini P, et al. Nonhematopoietic erythropoietin derivates prevent motoneuron degeneration in vitro and in vivo. Mol Med. 2006;12:153–160. doi: 10.2119/2006-00045.Mennini. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lezon-Geyda K, Najfeld V, Johnson EM. Deletions of PURA, at 5q31, and PURB, at 7p13, in myelodisplastic syndrome and progression to acute myeloid leukemia. Leukemia. 2001;15:954–962. doi: 10.1038/sj.leu.2402108. [DOI] [PubMed] [Google Scholar]
- 49.Lu NZ, Cidlowsky JA. Glucocorticoid receptor isoforms generate transcription specificity. Trends Cell Biol. 2006;16:301–307. doi: 10.1016/j.tcb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Van Rossum EF, Lamberts SW. Polymorphisms in the glucocorticoid receptor gene and their associations with metabolic parameters and body composition. Rec Prog Horm Res. 2004;59:333–357. doi: 10.1210/rp.59.1.333. [DOI] [PubMed] [Google Scholar]
- 51.Meijer OC. Understanding stress trough the genome. Stress. 2006;9:61–67. doi: 10.1080/10253890600799669. [DOI] [PubMed] [Google Scholar]
- 52.De Rijk R, De Kloet ER. Corticosteroid receptor genetic polymorphisms and stress responsivity. Endocrine. 2005;28:263–270. doi: 10.1385/ENDO:28:3:263. [DOI] [PubMed] [Google Scholar]
- 53.Van Rossum EF, Binder EB, Majer M, et al. Polymorphism of the glucocorticoid receptor gene and major depression. Biol Psychiatry. 2006;59:681–688. doi: 10.1016/j.biopsych.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 54.Vannucchi AM, Bianchi L, Cellai C, et al. Accentuated response to phenylhydrazine and erythropoietin in mice genetically impaired for their Gata-1 expression (GATA-1low mice) Blood. 2001;97:3040–3050. doi: 10.1182/blood.v97.10.3040. [DOI] [PubMed] [Google Scholar]