Abstract
Background
Although environmental lead exposure is associated with significant deficits in cognition, executive functions, social behaviors, and motor abilities, the neuroanatomical basis for these impairments remains poorly understood. In this study, we examined the relationship between childhood lead exposure and adult brain volume using magnetic resonance imaging (MRI). We also explored how volume changes correlate with historic neuropsychological assessments.
Methods and Findings
Volumetric analyses of whole brain MRI data revealed significant decreases in brain volume associated with childhood blood lead concentrations. Using conservative, minimum contiguous cluster size and statistical criteria (700 voxels, unadjusted p < 0.001), approximately 1.2% of the total gray matter was significantly and inversely associated with mean childhood blood lead concentration. The most affected regions included frontal gray matter, specifically the anterior cingulate cortex (ACC). Areas of lead-associated gray matter volume loss were much larger and more significant in men than women. We found that fine motor factor scores positively correlated with gray matter volume in the cerebellar hemispheres; adding blood lead concentrations as a variable to the model attenuated this correlation.
Conclusions
Childhood lead exposure is associated with region-specific reductions in adult gray matter volume. Affected regions include the portions of the prefrontal cortex and ACC responsible for executive functions, mood regulation, and decision-making. These neuroanatomical findings were more pronounced for males, suggesting that lead-related atrophic changes have a disparate impact across sexes. This analysis suggests that adverse cognitive and behavioral outcomes may be related to lead's effect on brain development producing persistent alterations in structure. Using a simple model, we found that blood lead concentration mediates brain volume and fine motor function.
Using magnetic resonance imaging to assess brain volumes, Kim Cecil and colleagues find that inner-city children with higher blood lead levels showed regions of decreased gray matter as adults.
Editors' Summary
Background.
Lead is a highly toxic metal that is present throughout the environment because of various human activities. In particular, for many years, large amounts of lead were used in paint, in solder for water pipes, in gasoline, and in ceramic glazes. But, as the harmful health effects of lead have become clear, its use in these and other products has been gradually phased out. Breathing air, drinking water, or eating food that contains lead can damage almost every organ in the human body. The organ that is most sensitive to lead exposure is the brain, and children's brains are particularly vulnerable because they are still developing. Children who swallow large amounts of lead can develop widespread brain damage that causes convulsions and sometimes death. Children who are repeatedly exposed to low to moderate amounts of lead (e.g., through accidentally swallowing residues of old lead paint or contaminated soil) can develop learning or behavioral problems.
Why Was This Study Done?
Lead exposure has been linked with various types of brain damage. These include problems with thinking (cognition); difficulties with organizing actions, decisions, and behaviors (executive functions); abnormal social behavior (including aggression); and difficulties in coordinating fine movements, such as picking up small objects (fine motor control). However, we know little about how lead damages the brain in this way and little about which brain regions are affected by exposure to low to moderate levels of lead during childhood. In this study, the researchers wanted to test the possibility that childhood lead exposure might lead to shrinking (“volume loss”) parts of the brain, particularly the parts that are crucial to cognition and behavior. They therefore studied the relationship between childhood lead exposure and adult brain volume. They also explored whether there is a relationship between brain volume and measures of brain functioning, such as fine motor control, memory, and learning assessed during adolescence.
What Did the Researchers Do and Find?
Between 1979 and 1984, the researchers recruited babies born in poor areas of Cincinnati, where there were many old, lead-contaminated houses, into the Cincinnati Lead Study. They measured their blood lead levels regularly from birth until they were 78 months old and calculated each child's average blood lead level over this period. They then used brain scans (known as magnetic resonance imaging, or MRI) to measure the brain volumes of the participants when they were 19–24 years old. The researchers found that exposure to lead as a child was linked with brain volume loss in adulthood, particularly in men. There was a “dose-response” effect—in other words, the greatest brain volume loss was seen in participants with the greatest lead exposure in childhood. The brain volume loss was most noticeable in a part of the brain called the prefrontal cortex—especially a region called the “anterior cingulate cortex.” When they examined the relationship between brain volume and measures of brain functioning, they found a link between brain volume and fine motor control, but not with the other measures.
What Do These Findings Mean?
These findings indicate that childhood lead exposure is associated with brain volume loss in adults, in specific regions of the brain. These brain regions are responsible for executive functions, regulating behavior, and fine motor control. Lead exposure has a larger effect on brain volumes in men than in women, which might help to explain the higher incidence of antisocial behaviors among men than women. Overall, these findings may explain why children and adults who have a history of lead exposure have behavioral and other problems, and support ongoing efforts to reduce childhood lead exposure in the US and other countries.
Additional Information.
Please access these Web sites via the online version of this summary at http://dx.doi.org/10.1371/journal.pmed.0050112.
A PLoS Medicine Perspective article by David Bellinger further discusses this study and a related paper on child exposure to lead and criminal arrests in adulthood
Toxtown, an interactive site from the US National Library of Medicine, provides information on environmental health concerns including exposure to lead (in English and Spanish)
The US Environmental Protection Agency provides information on lead in paint, dust, and soil and on protecting children from lead poisoning (in English and Spanish)
Medline Plus and the US National Library of Medicine Specialized Information Services provide lists of links to information on lead and human health (in English and Spanish)
The US Centers for Disease Control and Prevention provides information about its Childhood Lead Poisoning Prevention Program
The UK Health Protection Agency also provides information about lead and its health hazards
Introduction
Lead is widely recognized as a potent neurotoxicant, yet debate continues as to what levels of exposure result in irreversible brain injury. Evidence of lead “poisoning” is typically observed only at blood lead concentrations greater than 40 μg/dl (1.93 μmol/l) [1]. Encephalopathy is typically associated with blood lead concentrations greater than 100 μg/dl (4.83 μmol/l), but may occur at blood lead concentrations as low as 70 μg/dl (3.38 μmol/l), manifesting with focal lesions in the basal ganglia, thalami, cerebellum, cortical gray matter, and subcortical white matter [2–5]. Clinical neuroimaging studies in children with low to moderate (5 to 40 μg/dl [0.24 to 1.93 μmol/l]) blood lead concentrations tend to have few specific findings characteristic of lead exposure. However, such blood lead levels increase the individual likelihood of impaired cognition and executive function, impulsiveness, aggression, and delinquent behavior [6–11].
Converging lines of evidence suggest that these cognitive, motor, and behavioral changes result from exposure of the developing central nervous system (CNS) to lead [12–16]. Although the underlying mechanisms of neurotoxicity are complex, lead appears to alter neurotransmitter release, leading to excitotoxicity and ultimately apoptotic changes (reviewed in [15,17,18]).
Studies of adult organolead manufacturing workers have demonstrated that the past cumulative lead dose (estimated from lead concentration in tibia) was associated with cognitive test scores and measures of composite and regional brain volumes. For example, Stewart and colleagues report longitudinal declines in function and stronger associations for decline in study participants with the apolipoprotein E 4 allele [19,20]. Analyses of brain images obtained with magnetic resonance imaging (MRI) indicate that smaller cingulate gyrus, insula, frontal gray matter, total gray matter, parietal white matter, and total brain volumes are associated with past cumulative lead doses decades after last occupational exposure [21]. These findings converge with a recent study showing that larger regional and composite brain volumes were associated with better cognitive function [22].
Despite the well-established link between lead exposure and cognitive deficits, few studies have directly examined the association between early childhood lead exposure and subsequent neurostructural features in adulthood. The purpose of this study was to investigate the effects of documented low to moderate childhood lead exposure, specifically mean childhood blood lead concentrations less than 40 μg/dl (1.93 μmol/l), on adult brain volume. We hypothesized that adults with higher childhood blood lead concentrations would demonstrate evidence of volume changes in neuroanatomical regions regulating cognitive and behavioral domains previously shown to be impaired with lead exposure. We also explored how volume changes correlate with factors derived from a comprehensive neuropsychological battery acquired during adolescence.
Methods
Participants
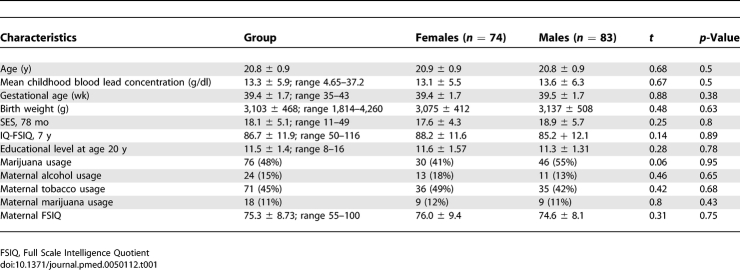
The individuals recruited for this investigation were participants in the Cincinnati Lead Study (CLS), an urban, inner-city cohort with detailed prenatal and postnatal histories of low to moderate lead exposure and behavioral outcomes monitored over 25 y. The CLS, a birth cohort recruited between 1979 and 1984, enrolled pregnant women who lived in neighborhoods with historically high rates of childhood lead poisoning. Exclusion criteria (see Text S1) for the mothers and infants were defined at study entry. This process netted newborns who were followed up quarterly through 5 y of age, semiannually from 5 to 6.5 y of age, again at age 10 y and between the ages of 15 and 17 y. A total of 157 CLS participants between the ages of 19 and 24 y provided informed consent and participated in this imaging study. A summary of their demographic features is presented in Table 1.
Table 1.
Characteristics of the Children and of their Mothers in the Cincinnati Lead Study (n = 157) with Comparison by Sex
Imaging Analysis Approach
We acquired whole-brain, three-dimensional, high-resolution 1.5 Tesla MRI data (see Text S1 for the detailed imaging protocol) to assess global and regional changes in brain tissue (gray matter, white matter, and cerebrospinal fluid [CSF]) volume for comparison with the mean of childhood blood lead concentrations (measured in μg/dl) collected between 3 and 78 mo of life using voxel-based morphometry (VBM) [23]. VBM involves normalizing individual structural MRI scans to a study-specific template to allow voxel-by-voxel comparisons between individuals. This approach allows for advanced statistical analysis throughout the brain and does not rely on the a priori designation of structures of interest or manual tracing or segmentation of brain structures. Consequently, VBM is well suited to our investigational study of the effects of lead exposure on brain volume.
Blood lead concentrations (μg/dl) were measured in this cohort every 3 mo from birth for the first 5 y of life and every 6 mo from 5 to 6.5 y. To represent lead dose, the mean of the 23 childhood blood lead assessments for each participant was employed for our analyses. While peak and annual composite measures of quarterly assessments were available, we regarded the arithmetic mean childhood blood lead concentration as best representing individual cumulative lead exposure for the participants. The mean childhood blood lead concentrations have been previously reported and used consistently in the publications regarding the CLS cohort [9,24]. When no blood lead data were obtained from a given time point, the missing values were imputed from a weighted average of a within-individual regression of blood lead on age and the cohort mean at each age. This imputation was performed to avoid simply excluding those participants who may have one or only a few missing blood lead values in the context of an otherwise data-rich exposure history. Analyses demonstrated that there were no significant differences in magnitude, direction, or statistical significance of blood lead regression coefficients when observed and imputed datasets were compared with observed-only datasets.
For our VBM analysis, we developed multiple regression models of volume for each tissue class (gray matter, white matter, and CSF) and mean childhood blood lead as the covariate of interest. A simple regression analysis identifies regions of interest (ROIs) where volume change is associated with lead dose. We analyzed the data for evidence of volume changes in both directions: gain and loss. Because of the numerous factors involved in brain development that could alter composite and regional brain volumes, we evaluated multiple variables for inclusion in the regression models. These variables have also been implicated as alternative and/or additive factors responsible for the cognitive and behavioral manifestations attributed to lead dose. Variables considered include participant age at time of imaging, current marijuana use (obtained from a urine drug screening collected at time of imaging), sex, birth weight, gestational age at birth, maternal IQ [25], maternal alcohol consumption during pregnancy, maternal marijuana use during pregnancy, maternal tobacco use during pregnancy, mean childhood Hollingshead socioeconomic status (SES) score [26], current SES score, and home environment (using the mean Home Observation for Measurement of the Environment score measured in early childhood [27]). Upon adding an individual putative confounder variable into the otherwise simple linear regression between volume and mean childhood blood lead concentration, the change of regression coefficient (beta) was calculated to evaluate the influence of the variable. This testing was conducted within the ROIs significantly correlated (unadjusted p ≤ 0.001; 700 voxel cluster threshold) with the mean childhood blood lead concentration. The variable was considered significant and retained for the subsequent final multivariate analysis if adding the variable caused greater than 20% of the pixels for the composite ROIs to have over a 10% change in the beta values. Two variables—age at time of imaging and birth weight—satisfied the criteria for inclusion and were included in the final multiple regression model. The effect of sex on lead-associated volumetric changes was assessed by testing for a sex-by-lead interaction in the whole cohort as well as in separate analyses for males and females. (Additional details are included in Text S1.)
Exploratory Analysis of Neuropsychological Outcome
The data collected from the CLS participants at the time of enrollment in the imaging study did not include neuropsychological measures. However, the factors for adolescent (approximately 15.6 y old) neuropsychological measures as described by Ris, and colleagues [28] were available for exploring the relationship of our structural data with functional outcomes. The overlap of the adolescent and adult waves of the CLS resulted in 120 participants having both brain imaging data (n = 157) and neuropsychological factor scores (n = 195). For these additional volumetric analyses, we took as the outcome measure the individual factor scores for the five categories: memory, learning/IQ, attention, visuoconstruction, and fine motor, and compared it with gray matter images covarying for sex. As with the primary analyses, regional increases and decreases in gray matter volume associated with respective factor scores were determined. Subsequently, the addition of 78-mo blood lead concentration as a covariate into the models would add negative volume associations to the respective model, effectively nulling common territories. As this represents an exploratory analysis, no variables other than sex were examined as possible covariates.
Ethical Oversight
The institutional review boards of the Cincinnati Children's Hospital Medical Center and the University of Cincinnati approved the study protocol. A Certificate of Confidentiality for the study was obtained from the National Institutes of Health.
Results
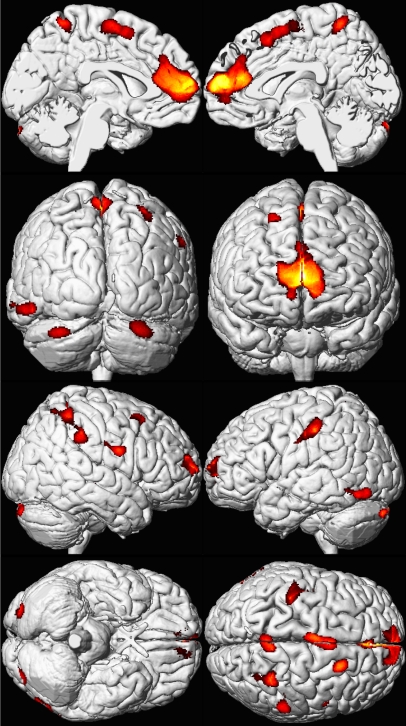
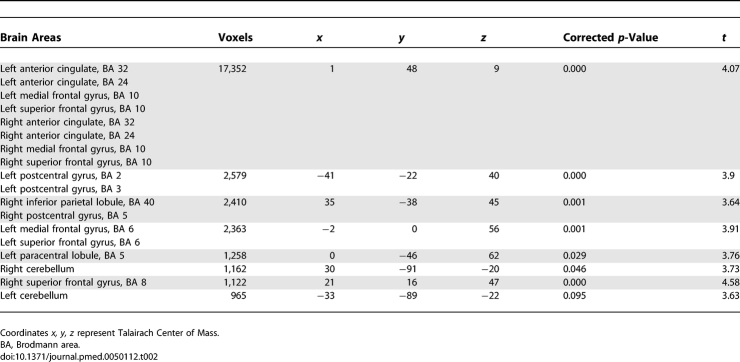
Higher mean childhood blood lead concentrations were associated with significant decrements in gray matter volume for several cortical regions (Figure 1; Table 2). The volumetric-based analysis revealed an inverse, linear dose-effect relationship between mean childhood blood lead concentration and brain volume in specific regions. (Figure 2). Prefrontal cortical areas of lead-related volumetric decline involved the medial and the superior frontal gyri comprising the ventrolateral prefrontal cortex (VLPFC) as well as the anterior cingulate cortex (ACC). Other areas of lead-related volume loss were located in postcentral gyri, the inferior parietal lobule, and the cerebellar hemispheres. Using conservative, minimum contiguous cluster size and statistical criteria (700 voxels, unadjusted p < 0.001), approximately 1.2% of the total gray matter was significantly and inversely associated with mean childhood blood lead concentration. No significant volume changes were observed within white matter or CSF volume.
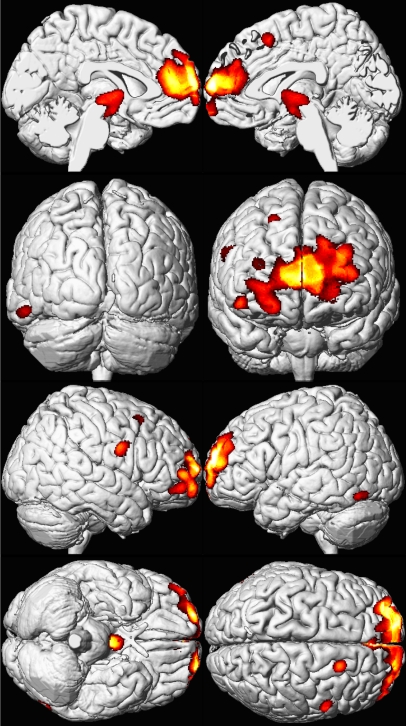
Figure 1. Regional Brain Volume Loss for the Cincinnati Lead Study Participants.
A composite representation of regions with significant volume loss for male and female CLS participants (n = 157) associated with mean childhood blood lead concentrations is shown with red and yellow clusters overlaid upon a standard brain template (seen at multiple angles; the first row presents views from the midline of the left and right hemispheres, respectively; the second row demonstrates views from the back and front of the cerebrum, respectively; the third row shows the lateral right and left hemispheres; and the fourth row shows views from below and above the cerebrum. Brain template source reference [51].
Table 2.
Coordinates of Significant Lead Associated Volume Loss for the CLS Participants
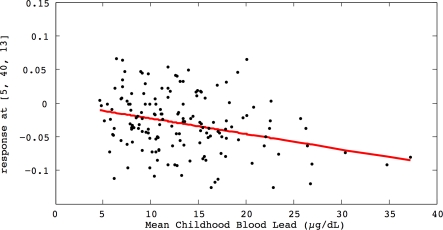
Figure 2. Regional Brain Volume Loss for the Cincinnati Lead Study Participants.
The relationship of individual brain volume with mean childhood blood lead concentrations within a medial frontal cluster is illustrated by this plot. The model is adjusted for age at time of scanning and birth weight, using a cluster threshold of 700 voxels and unadjusted p ≤ 0.001.
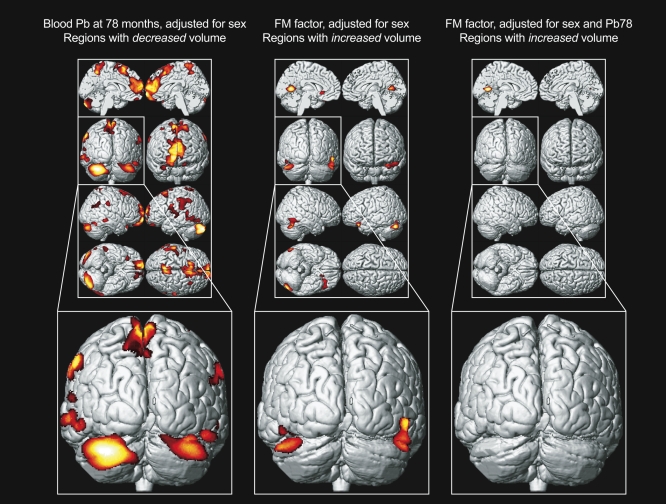
To explore the functional significance of these results, we compared neuropsychological factor scores and gray matter volume for the cohort. The analyses showed that the fine motor factor provided the strongest association, as it demonstrates a positive gray matter volume effect at a statistical threshold of p < 0.001 and minimum cluster of 700 voxels. The primary regions with this positive correlation included the cerebellar hemispheres bilaterally. These cerebellar regions correspond very well with the established, classical functional neuroanatomy implicated in the control of fine motor tasks (finger tapping and grooved peg board) comprising this factor. We found that the addition of the 78-mo blood lead concentration eliminated the association of fine motor function with cerebellar gray matter volume (Figure 3). Other factor scores (attention, visuoconstruction, memory, and learning/IQ) did not demonstrate such robust results with this simple overlap and elimination when adding lead into the sex-adjusted models.
Figure 3. Structure-Function Relationship.
Left, a composite representation of regions with significant volume loss for CLS participants associated with blood lead concentration at 78 mo of age and adjusted for sex is shown in red and yellow overlaid upon a standard brain template with the posterior coronal view highlighted below. Middle, analogous composite representation of regions with significant volume gain associated with fine motor factor scores and adjusted for sex. Right, analogous composite representation of regions with significant volume gain associated with fine motor factor scores, adjusted for sex and blood lead concentration at 78 mo of age. Brain template source reference [51].
In analyses stratified by sex, male participants demonstrated gray matter volume loss, primarily within the VLPFC and ACC (Figure 4). Again, using conservative minimum contiguous cluster size and statistical criteria (700 voxels, unadjusted p < 0.001), approximately 1.7% of the total gray matter volume loss of males was significantly and inversely associated with mean childhood blood lead concentration. In contrast, female participants demonstrated no significant gray matter volume loss in separate sex interaction models, with only a small parietal lobe foci represented (Figure 5). Notably, regions of gray matter volume loss in males were larger and more statistically significant than in the entire cohort, particularly within the frontal lobes. The mean childhood blood lead concentration did not significantly differ between the male (13.5 μg/dl [0.065 μmol/l], SD 6.3 μg/dl) and female participants (13.1 μg/dl [0.063 μmol/l], SD 5.5 μg/dl) (t = 0.67, p = 0.50). No demographic variables significantly differed between male and female participants.
Figure 4. Sex Influences Brain Volume Loss Associated with Lead Exposure (Males).
A composite representation of regions with significant volume loss for CLS participants associated with mean childhood blood lead concentrations is shown in red and yellow overlaid upon a standard brain template for males (n = 83). The model is adjusted for age at time of scanning and birth weight, using a cluster threshold of 700 voxels and unadjusted p ≤ 0.001. Views are the same as shown in Figure 1; brain template source reference [51].
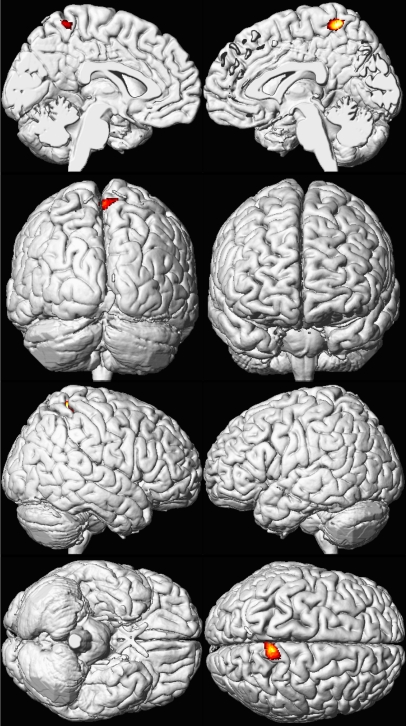
Figure 5. Sex Influences Brain Volume Loss Associated with Lead Exposure (Females).
A composite representation of regions with significant volume loss for CLS participants associated with mean childhood blood lead concentrations is shown in red and yellow overlaid upon a standard brain template for females (n = 74). The model is adjusted for age at time of scanning and birth weight, using a cluster threshold of 700 voxels and unadjusted p ≤ 0.001. Views are the same as shown in Figure 1; brain template source reference [51].
Discussion
Our study showed that higher mean childhood blood lead concentration is associated with region-specific reductions in adult gray matter volume. The findings suggest that childhood lead exposure is associated with volume loss in considerable portions of the prefrontal cortex, including the ACC and the VLPFC. The ACC, a component of the brain's limbic system positioned about the rostrum of the corpus callosum, processes cognitive and emotional information separately with distinguishable territories (reviewed in [29]). The functions attributed to the cognitive subdivision, located in the dorsal aspects of the ACC, include modulating attention and executive functions via sensory and/or response selection [29]. Additional attributed functions include anticipation of cognitively demanding tasks, error detection, monitoring completion, assessing potential conflicts, complex motor control, performing new behaviors, motivation and reward-based decision making [30–32]. The affective and emotional division, which is located ventrally, is associated with regulation of personal and social behavior, decision-making, and emotional responses. VLPFC has also been suggested as being similarly associated with mood regulation [33,34].
Volume loss in all of the aforementioned frontal brain regions, including both the cognitive and emotional territories of the ACC (Brodmann areas detailed in Table 2), is consistent with and potentially explanatory for cognitive and behavioral problems previously associated with lead exposure [8,12–14,28,35,36].These problems include general intellectual and executive functioning, antisocial behaviors, and attention deficit hyperactivity disorder (ADHD). Some studies report a structure–function relationship, which ultimately translates as brain volume change corresponding with altered function. In analyses of brain volumes and measures of cognitive function in adult organolead manufacturing workers, Schwartz and colleagues [22] showed that larger ROI volumes were associated with better cognitive function in five of six cognitive domains (visuoconstruction, processing speed, visual memory, executive functioning, and eye–hand coordination). Recent studies by Raine and colleagues suggest that deficits in cortical volume or activity found in select brain regions, such as prefrontal gray matter, may predispose a person to impulsive, aggressive, or violent behavior [37–39]. However, it is important to note that not all pathologies produce such straightforward correlations between structure and function, as some cognitive and behavioral functions are diffusely distributed such that the effects of other confounders (e.g., age) show greater influence on structural volumes [40].
Previously, Dietrich and colleagues reported the motor developmental status in 245 children of the CLS cohort at 6 y of age [9]. Following statistical adjustment for covariates, neonatal blood lead levels were associated with poorer performance on a measure of upper-limb speed and dexterity and the fine-motor composite. Postnatal blood lead levels remained significantly associated with poorer scores on measures of bilateral coordination, visual-motor control, upper-limb speed and dexterity, and the fine-motor composite. The strongest and most consistent relationships were observed with concurrent blood lead levels (mean 10.1 μg/dl [SD 5.6]). A 10 μg/dl increase in concurrent blood lead levels was associated with a 4.6 point (95% CI 2.1–7.1) decline in the fine motor composite score. The 78-mo postnatal blood lead levels were significantly associated with poorer fine-motor skills as indexed by covariate-adjusted factor scores derived from a factor analysis of a comprehensive neuropsychological battery conducted at 16 y of age [34]. The variables loading highly on the fine-motor component came from the grooved pegboard and finger tapping tasks. Following covariate pretesting and adjustment, the principal finding reported from the study was a significant main effect of 78-mo blood lead on the fine motor factor [34]. We now observe that the fine motor factor also provided the strongest association between brain structure and function in our exploratory analysis. The fine motor factor shows a positive brain volume association at the same statistical threshold (p < 0.001 and minimum cluster of 700 voxels) as our primary result. Upon the addition of 78-mo blood lead concentrations, we found that the association between gray matter volume in the cerebellum and the fine motor factor was eliminated. The regional concordance of the fine motor factor and lead volume effect in the cerebellum may reflect the fact that motor developmental outcomes are more sensitive indicators of lead's adverse effects on the CNS and are probably less confounded with social factors than cognitive, academic, and behavioral outcomes [9].
The largest area of significant volume loss associated with childhood lead exposure includes medial portions of the prefrontal cortex. Synaptic overproduction in the medial frontal lobe reaches a maximum between 3 and 4 y of age with synaptic pruning occurring in mid-to-late adolescence [41]. During the first 5 y of life, at least one of the quarterly blood lead assessments exceeded 10 μg/dl (0.48 μmol/l) for 99% of the cohort. The peak lead exposure occurred between 2 and 3 y of age for the participants. By adolescence, the mean blood lead concentrations for the cohort were 2.8 μg/dl (1.35 μmol/l) (SD 1.3 μg/dl). Given this critical time period in brain development and maturation with documented low to moderate level of lead exposure in the CLS cohort, the finding of reduced volume within the medial frontal lobe of the adult brains associated with increased childhood blood lead concentration is consistent with lead exposure impairing cortical development and maturation. Our findings also suggest that this structural change is permanent. Additional studies will be necessary to narrow the time points and possibly thresholds of irreversible injury, as our analyses reflect cumulative childhood lead dose. This finding is also consistent with experimental data indicating that low-level lead exposure causes significant reduction in neurite length in dopaminergic neurons [42]. However, the changes in volume detected by the VBM method could represent a variety of changes (i.e., neuronal size, reductions of dendritic arborization, or changes in neuropil) at the cellular level [43]. Experimental models with pathological analyses and possibly imaging studies could further complement the changes revealed by the findings of this VBM study. In addition, several studies using animal models suggest that lead exposure alters the formation and maintenance of myelin [15,44–46]. In our analyses, no changes in white matter volume were observed. However, while axonal and myelin volume are maintained, myelin organization may be altered as a result of lead exposure. Future studies with diffusion tensor imaging are needed to evaluate axonal and myelin integrity, since this method reveals quantitative information about the diffusion of water molecules about the myelin sheath of axons (see review [47]).
Evidence from behavioral studies in the CLS cohort, other epidemiological cohorts, and animal models indicate that males are more vulnerable to the effects of lead on executive function [28,36]. The higher incidence of ADHD and antisocial behaviors in males may be similarly consistent with greater susceptibility to childhood lead exposure. Froehlich and colleagues found that lead's effect on executive function arises from an interaction with the dopamine receptor D4 gene with exon III seven-repeat form (DRD4–7), with greater effect in males [36]. Braun and colleagues found that children with blood lead levels greater than 2 μg/dl (0.097 μmol/l), representing the top quintile of 4- to 15-y-old children in the United States, were four times more likely to have doctor-diagnosed ADHD [35]. Further studies are necessary to replicate our findings and provide clarification on the gene–environment interaction associated with greater vulnerability of males to lead exposure.
The significance and implications of the volume loss in the VLPFC and ACC in males are potentially profound. The increased sensitivity to lead in male participants has important sociological implications. Widely observed increases in rates of antisocial behaviors in young men no doubt are related to multiple cultural and biological factors, but negative behaviors in males may be exacerbated by the greater susceptibility of men to the toxic effects of lead exposure.
To our knowledge this is the first prospective study to directly examine the relationship between early exposure to lead and brain volume in adulthood. Lead dose as assessed by frequent serial blood lead determinations, assessment of a large number of potentially important covariate factors, and advanced imaging methods were unique aspects of this investigation. Furthermore, the sample was relatively homogenous with respect to sociodemographic variables such as SES and ethnicity, thus decreasing the extent to which strong confounding factors might generate spurious associations. In retrospect, a cumulative assessment of marijuana usage addressing the frequency and/or duration would have better characterized this potential confounder than urine screening for drug exposures at a single time point. Compared with VBM studies of psychiatric illnesses, we used a rigorous and conservative minimum contiguous cluster size and statistical criteria for significance (700 voxels, unadjusted p < 0.001) [33,48–50]. The VBM approach to quantifying brain volume eliminates bias from errors in the manual delineation of brain structures found with tracing studies. However, the VBM approach faces challenges in preprocessing of individual brains for group analyses; these limitations have been well described in the imaging literature (see Mechelli et al. [43]).
In summary, we found that early childhood lead exposure is associated with structural volume loss in the brain. The injury affects brain regions classically considered responsible for executive function, behavioral regulation, and fine motor control.We found that this volume loss persisted into adulthood. Our findings are consistent with and potentially explanatory for long-observed behavioral findings in children and adults with a history of lead exposure. Furthermore, decrements in brain volume associated with lead exposure were primarily present for male participants, independent of sex-related differences in blood lead concentrations and other demographic factors.
Supporting Information
(82 KB DOC)
Acknowledgments
The authors acknowledge the efforts of the MRI technologists Kendall J. O'Brien, BS, RT; and R. Scott Dunn, RT for providing imaging technical support. We are also grateful to members of the Cincinnati Lead Study Cohort and their families for their participation in this research.
Author contributions. KMC, CJB, KND, and BPL designed the experiments/the study. CJB, KND, JCE, and SW collected data or did experiments for the study. KMC, CJB, CMA, KND, MA, IE, and KJ analyzed the data. KMC, KND, and SW enrolled patients. KMC wrote the first draft of the paper. KMC, CJB, CMA, SW, RH, and BPL contributed to writing the paper. CJB designed part of the experiments/study and performed all the analysis. JCE reviewed the MRI studies. KMC, KND, and BPL participated in original conception of study, study design, and interpretation of results.
Abbreviations
- ACC
anterior cingulate cortex
- ADHD
attention deficit hyperactivity disorder
- CLS
Cincinnati Lead Study
- CSF
cerebrospinal fluid
- MRI
magnetic resonance imaging
- ROI
region of interest
- SES
Hollingshead socioeconomic scale
- VBM
voxel-based morphometry
- VLPFC
ventrolateral prefrontal cortex
Footnotes
Funding: This work was supported by grants from the National Institutes of Health, NIEHS P01 ES011261, NIEHS R01 ES015559 NIEHS R21 ES013524, NCI R01 CA112182, and the Environmental Protection Agency R82938901. These respective agencies had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing Interests: Two of the study's authors, BPL and RH, are on the editorial board of PLoS Medicine. BPL and KND sporadically serve as expert witnesses without personal financial gain.
References
- Staudinger KC, Roth VS. Occupational Lead Poisoning. American Family Physician. 1998;57:719–726. [PubMed] [Google Scholar]
- al Khayat A, Menon NS, Alidina MR. Acute lead encephalopathy in early infancy–clinical presentation and outcome. Ann Trop Paediatr. 1997;17:39–44. doi: 10.1080/02724936.1997.11747861. [DOI] [PubMed] [Google Scholar]
- Mani J, Chaudhary N, Kanjalkar M, Shah PU. Cerebellar ataxia due to lead encephalopathy in an adult. J Neurol Neurosurg Psychiatry. 1998;65:797. doi: 10.1136/jnnp.65.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atre AL, Shinde PR, Shinde SN, Wadia RS, Nanivadekar AA, et al. Pre- and posttreatment MR imaging findings in lead encephalopathy. AJNR Am J Neuroradiol. 2006;27:902–903. [PMC free article] [PubMed] [Google Scholar]
- Tuzun M, Tuzun D, Salan A, Hekimoglu B. Lead encephalopathy: CT and MR findings. J Comput Assist Tomogr. 2002;26:479–481. doi: 10.1097/00004728-200205000-00029. [DOI] [PubMed] [Google Scholar]
- Canfield RL, Henderson CR, Jr., Cory-Slechta DA, Cox C, Jusko TA, et al. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348:1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, et al. Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield RL, Gendle MH, Cory-Slechta DA. Impaired neuropsychological functioning in lead-exposed children. Dev Neuropsychol. 2004;26:513–540. doi: 10.1207/s15326942dn2601_8. [DOI] [PubMed] [Google Scholar]
- Dietrich KN, Berger OG, Succop PA, Hammond PB, Bornschein RL. The developmental consequences of low to moderate prenatal and postnatal lead exposure: intellectual attainment in the Cincinnati Lead Study Cohort following school entry. Neurotoxicol Teratol. 1993;15:37–44. doi: 10.1016/0892-0362(93)90043-n. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Stiles KM, Needleman HL. Low-level lead exposure, intelligence and academic achievement: a long-term follow-up study. Pediatrics. 1992;90:855–861. [PubMed] [Google Scholar]
- Needleman HL, Gatsonis CA. Low-level lead exposure and the IQ of children. A meta-analysis of modern studies. Jama. 1990;263:673–678. [PubMed] [Google Scholar]
- Needleman HL, Riess JA, Tobin MJ, Biesecker GE, Greenhouse JB. Bone lead levels and delinquent behavior. Jama. 1996;275:363–369. [PubMed] [Google Scholar]
- Stretesky PB, Lynch MJ. The relationship between lead exposure and homicide. Arch Pediatr Adolesc Med. 2001;155:579–582. doi: 10.1001/archpedi.155.5.579. [DOI] [PubMed] [Google Scholar]
- Stretesky PB, Lynch MJ. The relationship between lead and crime. J Health Soc Behav. 2004;45:214–229. doi: 10.1177/002214650404500207. [DOI] [PubMed] [Google Scholar]
- Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain. 2003;126:5–19. doi: 10.1093/brain/awg014. [DOI] [PubMed] [Google Scholar]
- Finkelstein Y, Markowitz ME, Rosen JF. Low-level lead-induced neurotoxicity in children: an update on central nervous system effects. Brain Res Brain Res Rev. 1998;27:168–176. doi: 10.1016/s0165-0173(98)00011-3. [DOI] [PubMed] [Google Scholar]
- Bressler JP, Goldstein GW. Mechanisms of lead neurotoxicity. Biochem Pharmacol. 1991;41:479–484. doi: 10.1016/0006-2952(91)90617-e. [DOI] [PubMed] [Google Scholar]
- Toscano CD, Guilarte TR. Lead neurotoxicity: from exposure to molecular effects. Brain Res Brain Res Rev. 2005;49:529–554. doi: 10.1016/j.brainresrev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Schwartz BS, Simon D, Bolla KI, Todd AC, et al. Neurobehavioral function and tibial and chelatable lead levels in 543 former organolead workers. Neurology. 1999;52:1610–1617. doi: 10.1212/wnl.52.8.1610. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Schwartz BS, Simon D, Kelsey K, Todd AC. ApoE genotype, past adult lead exposure, and neurobehavioral function. Environ Health Perspect. 2002;110:501–505. doi: 10.1289/ehp.02110501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart WF, Schwartz BS, Davatzikos C, Shen D, Liu D, et al. Past adult lead exposure is linked to neurodegeneration measured by brain MRI. Neurology. 2006;66:1476–1484. doi: 10.1212/01.wnl.0000216138.69777.15. [DOI] [PubMed] [Google Scholar]
- Schwartz BS, Chen S, Caffo B, Stewart WF, Bolla KI, et al. Relations of brain volumes with cognitive function in males 45 years and older with past lead exposure. Neuroimage. 2007;37:633–641. doi: 10.1016/j.neuroimage.2007.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Dietrich KN, Succop PA, Berger OG, Hammond PB, Bornschein RL. Lead exposure and the cognitive development of urban preschool children: the Cincinnati Lead Study cohort at age 4 years. Neurotoxicol Teratol. 1991;13:203–211. doi: 10.1016/0892-0362(91)90012-l. [DOI] [PubMed] [Google Scholar]
- Silverstein AB. Two- and four-subtest short forms of the WAIS-R: a closer look at validity and reliability. J Clin Psychol. 1985;41:95–97. doi: 10.1002/1097-4679(198501)41:1<95::aid-jclp2270410116>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Cirino PT, Chin CE, Sevcik RA, Wolf M, Lovett M, et al. Measuring socioeconomic status: reliability and preliminary validity for different approaches. Assessment. 2002;9:145–155. doi: 10.1177/10791102009002005. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Caldwell BM. Home observation for measurement of the environment: a revision of the preschool scale. Am J Ment Defic. 1979;84:235–244. [PubMed] [Google Scholar]
- Ris MD, Dietrich KN, Succop PA, Berger OG, Bornschein RL. Early exposure to lead and neuropsychological outcome in adolescence. J Int Neuropsychol Soc. 2004;10:261–270. doi: 10.1017/S1355617704102154. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci. 2004;7:1370–1375. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- Davis KD, Taylor KS, Hutchison WD, Dostrovsky JO, McAndrews MP, et al. Human anterior cingulate cortex neurons encode cognitive and emotional demands. J Neurosci. 2005;25:8402–8406. doi: 10.1523/JNEUROSCI.2315-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler CM, DelBello MP, Jarvis K, Levine A, Adams J, et al. Voxel-based study of structural changes in first-episode patients with bipolar disorder. Biol Psychiatry. 2007;61:776–781. doi: 10.1016/j.biopsych.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environ Health Perspect. 2006;114:1904–1909. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, Lanphear BP, Dietrich KN, Cory-Slechta DA, Wang N, et al. Interactive Effects of a DRD4 Polymorphism, Lead, and Sex on Executive Functions in Children. Biol Psychiatry. 2007;62:243–249. doi: 10.1016/j.biopsych.2006.09.039. [DOI] [PubMed] [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Arch Gen Psychiatry. 2000;57:119–127. 128–119. doi: 10.1001/archpsyc.57.2.119. discussion. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Lencz T, Bihrle S, Lacasse L, et al. Prefrontal white matter in pathological liars. Br J Psychiatry. 2005;187:320–325. doi: 10.1192/bjp.187.4.320. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Lencz T, Bihrle S, LaCasse L, et al. Volume reduction in prefrontal gray matter in unsuccessful criminal psychopaths. Biol Psychiatry. 2005;57:1103–1108. doi: 10.1016/j.biopsych.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Visser PJ, van Boxtel MP, Jolles J. The relation between global and limbic brain volumes on MRI and cognitive performance in healthy individuals across the age range. Neurobiol Aging. 2000;21:569–576. doi: 10.1016/s0197-4580(00)00133-0. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Huang FN, Vemuri MC. Effects of low-level lead exposure on cell survival and neurite length in primary mesencephalic cultures. Neurotoxicol Teratol. 2003;25:555–559. doi: 10.1016/s0892-0362(03)00018-7. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-Based Morphometry of the Human Brain: Methods and Applications. Current Medical Imaging Reviews. 2005;1:105–113. [Google Scholar]
- Deng W, McKinnon RD, Poretz RD. Lead exposure delays the differentiation of oligodendroglial progenitors in vitro. Toxicol Appl Pharmacol. 2001;174:235–244. doi: 10.1006/taap.2001.9219. [DOI] [PubMed] [Google Scholar]
- Dabrowska-Bouta B, Sulkowski G, Bartosz G, Walski M, Rafalowska U. Chronic lead intoxication affects the myelin membrane status in the central nervous system of adult rats. J Mol Neurosci. 1999;13:127–139. doi: 10.1385/JMN:13:1-2:127. [DOI] [PubMed] [Google Scholar]
- Coria F, Monton F. Recovery of the early cellular changes induced by lead in rat peripheral nerves after withdrawal of the toxin. J Neuropathol Exp Neurol. 1988;47:282–290. doi: 10.1097/00005072-198805000-00007. [DOI] [PubMed] [Google Scholar]
- Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Adler CM, Levine AD, DelBello MP, Strakowski SM. Changes in gray matter volume in patients with bipolar disorder. Biol Psychiatry. 2005;58:151–157. doi: 10.1016/j.biopsych.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Wilke M, Kaufmann C, Grabner A, Putz B, Wetter TC, et al. Gray matter-changes and correlates of disease severity in schizophrenia: a statistical parametric mapping study. Neuroimage. 2001;13:814–824. doi: 10.1006/nimg.2001.0751. [DOI] [PubMed] [Google Scholar]
- Wilke M, Kowatch RA, DelBello MP, Mills NP, Holland SK. Voxel-based morphometry in adolescents with bipolar disorder: first results. Psychiatry Res. 2004;131:57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Brain atlases of normal and diseased populations. Int Rev Neurobiol. 2005;66:1–54. doi: 10.1016/S0074-7742(05)66001-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(82 KB DOC)