Abstract
Recent reports of selective disruption of stimulus control by drug administration and other disruptive operations in temporal discrimination procedures may be interpreted as a disruption of attention to the temporal sample stimuli. This experiment assessed the effects of nicotine, a compound that has been widely shown to increase measures of attention, on temporal discrimination performance. Pigeons responded under a psychophysical choice procedure in which responses to one key color were correct after presentation of four shorter sample durations and responses to another key color were correct after presentation of four longer sample durations. Performance under nicotine was characterized by using a model that differentiates the effects of nicotine on stimulus control, bias, and sensitivity of temporal discrimination. Nicotine selectively decreased the measure of stimulus control, but did not systematically affect the measures of timing. Mecamylamine (1.0 mg/kg) failed to antagonize the disruptive effects of nicotine. These results suggest that disruption of temporal discrimination performance in this preparation may not have been dependent on the specific pharmacology of nicotine and underscore the importance of quantitative separation of the effects of various manipulations on stimulus control from effects on timing.
Keywords: attention, nicotine, pigeon, stimulus control, temporal bisection, temporal discrimination, timing
Introduction
Considerable research interest has focused on the processes underlying accurate interval timing. The most influential theoretical account is scalar expectancy theory (SET; e.g. Gibbon, 1977). According to SET, accurate timing results from the output of a complex internal system composed of clock, memory, and decision stages. When timing begins, a switch is closed that allows pulses from a pacemaker to be gated into an accumulator. When an important or salient event occurs (e.g. delivery of food to a hungry animal), the number of pulses in the accumulator is transferred to reference memory. During the decision stage, the current value in the accumulator is compared with a value in reference memory. If the relative difference between the current value and the compared value is less than some threshold, the organism makes a response. Evidence from a large number of experiments using a wide variety of temporal discrimination procedures supports this model.
In terms of the neurophysiological underpinnings of timing, one popular account is the pacemaker—accumulator model (e.g., Buhusi and Meck, 2005). According to this account, dopamine (DA) determines the clock process of SET. Changes in the level of DA produce changes in the speed of the pace maker, thereby changing the rate at which pulses are emitted. Increases in DA level are predicted to increase the speed of the pacemaker, resulting in more pulses accumulated by the end of the to-be-timed interval and overestimation of time. In temporal bisection procedures, in which subjects categorize the duration of a presented sample as either short or long by selecting a corresponding choice option, this result is indicated by a leftward shift of the psychophysical function relating the proportion of responses to the ‘long’ response option to the duration of the sample. Conversely, decreases in the level of DA decrease the speed of the pacemaker, and produce underestimation of time, evidenced by a rightward shift of the psychophysical function. Figure 1 presents illustrative psychophysical functions showing accurate timing under control conditions, as well as overestimation and underestimation of time.
Fig. 1.
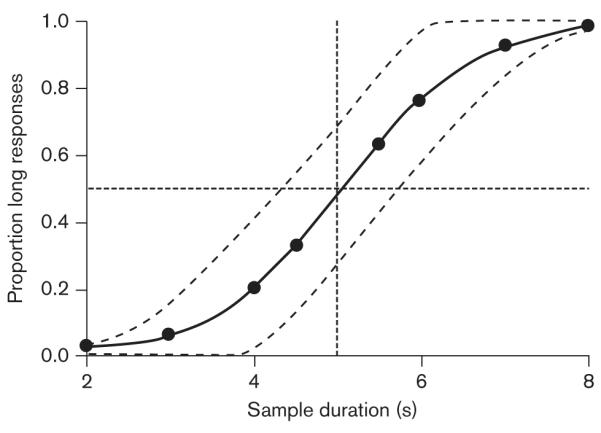
Hypothetical data showing the proportion of responses to the choice option corresponding to a long sample duration as a function of sample duration. Presented are psychophysical functions illustrating accurate temporal discrimination (solid line), overestimation (leftward shift), and underestimation (rightward shift) of the passage of time. The functions are constructed assuming a short/long category boundary of 5 s.
Although considerable evidence has supported the pacemaker—accumulator model (for review, see Meck, 1996), there are a number of results that are discrepant with model predictions (e.g. Chiang et al., 2000; Odum and Schaal, 2000; McClure et al., 2005; Ward and Odum, 2005; Odum and Ward, 2007). A growing number of studies report that DA agonist administration flattens the psychophysical function with no systematic lateral shifts, indicating a general disruption of temporal discrimination not accompanied by over or underestimation of time (for review, see Staddon and Higa, 2006).
There are a number of ways to theoretically characterize changes in psychophysical functions. A method described by Blough (1996) has been shown to have utility in interpreting the effects of drugs and other manipulations on temporal discrimination performance. Blough discussed three aspects of discrimination that affect performance: overall stimulus control, bias, and sensitivity. He suggested that measures of these factors could be obtained by fitting a cumulative normal function that estimates the upper and lower asymptotes, mean, and standard deviation (SD) of the obtained psychophysical function. This theoretical approach is illustrated in Fig. 2. The difference between the upper and lower asymptotes of the function (range) measures overall stimulus control, and indicates the degree to which choice responses are under the functional control of the presented sample stimuli. The mean of the function measures bias, and indicates whether choice responding is biased toward one response option over the other. The SD measures sensitivity or the precision of timing. Further-more, it is possible to separate quantitatively the effects of various manipulations on the range of the function from changes in the mean and SD by applying an asymptote correction that assumes perfect stimulus control (e.g. Heinemann et al., 1969) to the obtained psychophysical data before fitting the cumulative normal function.
Fig. 2.
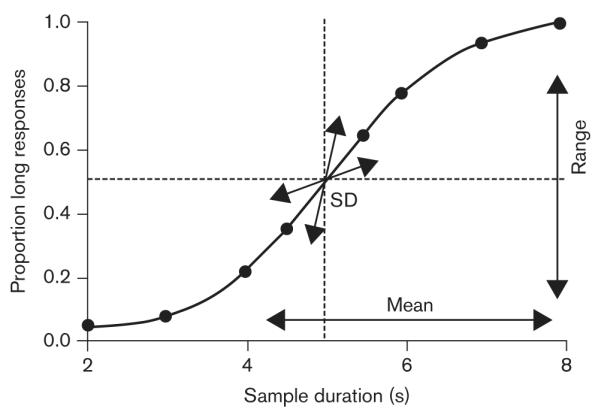
Theoretical approach to the characterization of psychophysical functions used in this experiment (and described by Blough, 1996), showing a model that provides estimates of overall stimulus control (range), bias (mean), and sensitivity (SD) of discrimination performance (after McClure et al., 2005). See text for details.
Using this theoretical framework, several recent studies have demonstrated that administration of both morphine and d-amphetamine produce selective effects on the range of the function (McClure et al., 2005; Ward and Odum, 2005; Odum and Ward, 2007). Specifically, administration of these drugs decreased the range, whereas the estimates of the mean and SD were not systematically affected. These results indicate that the drugs produced performance decrements through decreases in stimulus control, rather than through specific effects on timing.
Some authors have suggested that decreases in stimulus control after administration of drugs and other disruptors are mediated by attentional processes (Heinemann et al., 1969; Santi et al., 1995; Blough, 1996). This interpretation may be supported by reports of similar effects of drugs and other manipulations on the form of the psychophysical function. For example, Sutton and Roberts (2002) reported that illuminating a distracter light during sample presentation flattened the psychophysical function, indicating a general disruption of temporal discrimination, similar to the results from the drug studies discussed above. In addition, Ward and Odum (2007) recently quantified disruption by a similar manipulation according to the method proposed by Blough and found selective decreases in the range of the psychophysical function. Therefore, the administration of drugs and implementation of an experimental manipulation that was specifically designed to disrupt attention to temporal samples have both been shown to produce selective decreases in the measure of stimulus control. These results provide empirical support for the interpretation that decreases in stimulus control result from decreases in attention to temporal samples.
One way to examine the effects of various manipulations on attention to temporal samples is to disrupt temporal discrimination performance. The experiments described earlier have taken this approach. Another way is to examine temporal discrimination performance under some manipulation or condition that is predicted to increase attention to temporal samples. Nicotine (and nicotinic agonists) has been reported to increase measures of attention in both human and nonhuman experimental preparations (for reviews, see Levin and Simon, 1998; Levin et al., 2006). For example, nicotine improves choice accuracy and decreases omission errors and response latencies in the five-choice serial reaction time task in rats (e.g. Mirza and Stolerman, 1998; Stolerman et al., 2000; Bizarro and Stolerman, 2003), a procedure that measures sustained attention. In addition, nicotine improves performance on a sustained-attention task in pigeons (Lemmonds et al., 2002; Lemmonds and Wenger, 2003). Finally, nicotine has been shown to improve performance in an operant signal-detection procedure in rats (e.g. Rezvani et al., 2002). Thus, a large body of literature indicates that nicotine can improve attentional performance.
Few studies have assessed the effects of nicotine on temporal discrimination performance (Hinton and Meck, 1996; Levin et al., 1996, 1998; Bizot, 1997; Meck and Williams, 1997; Popke et al., 2000). Furthermore, there have been no studies that have explicitly characterized the effects of nicotine on ‘attention’ during temporal discrimination procedures. Thus, the effects of nicotine on attention during temporal discrimination procedures are not clear.
Given the recent interest in the potential role of attention in mediating disruption of temporal discrimination by drugs and other manipulations, together with the paucity of studies on the effects of nicotine on performance during temporal discrimination procedures, theoretical characterization of the effects of nicotine on temporal discrimination performance would be useful. This experiment used a psychophysical choice procedure in which pigeons categorized presented sample durations (houselight illumination) as either short or long by responding to a side key lit a corresponding color. Temporal discrimination performance under baseline, nicotine, and mecamylamine plus nicotine was characterized using a theoretical framework that can quantitatively differentiate between the effects of nicotine on stimulus control (attention), bias, and sensitivity of temporal discrimination.
Methods
Subjects
Four drug-naive White Carneau pigeons, with a history of exposure to a variety of experimental procedures, served as subjects. Pigeons were maintained at 80% ± 15 g of their free-feeding weight by postsession feeding as needed. Between sessions, pigeons were individually housed with free access to water in a temperature-controlled colony under a 12 : 12 h light : dark cycle.
Apparatus
Four Lehigh Valley Electronics sound-attenuating chambers were used. Chambers were constructed of painted metal with aluminum front panels. The chambers measured 30 cm across, 35 cm deep, and 35 cm high. Each front panel had three translucent plastic keys that could be lit from behind with red, white, and blue light and required a force of at least 0.10 N to record a response. Keys were 2.5 cm in diameter and 24 cm from the floor. A lamp (28 V, 1.1 W) that was mounted 4.5 cm above the center key served as a houselight. A rectangular opening that was 8.5 cm below the center key provided access to a solenoid-operated hopper filled with pelleted pigeon chow. During hopper presentations, the opening was lit with white light and the houselight and keylights were extinguished. Extraneous noise was masked by chamber ventilation fans and white noise. Contingencies were programmed and data were collected by a microcomputer using Med Associates interfacing and software (St Albans, Vermont, USA).
Procedure
Owing to the pigeons’ previous experimental history, no keypeck training was necessary, and the experimental procedure began immediately. Sessions were conducted 7 days a week at approximately the same time. We used a temporal bisection procedure during which the pigeons categorized the presented sample durations as being either of short or long duration by responding to a key that lit a corresponding color (see e.g. Stubbs, 1968). Trials began with the illumination of the center red key. This key served as a trial-ready stimulus to ensure that the pigeon was attending to the sample. A response to the center key darkened it and lit the houselight for the duration of the presented sample. After the sample presentation, the left and right keys were lit blue and white, each key color corresponding to either a short or a long sample duration. Durations shorter than 3 s were categorized as short, and durations longer than 3 s were categorized as long. The location of each color (left or right key) was randomly determined from trial to trial. A peck to the key that lit the color corresponding to the duration of the temporal sample (short or long) resulted in probabilistic 2.5 s access to food. A peck to the key that lit the other color produced a 2.5 s blackout. Key colors corresponding to short and long sample durations were counterbalanced across pigeons.
Training
The baseline procedure included eight sample durations (2, 2.25, 2.5, 2.75, 3.25, 3.5, 3.75, and 4 s) that were selected randomly from trial to trial with the constraints that each sample duration appear once in each block of eight consecutive trials and that each sample duration be presented an equal number of times during the session. These sample durations were the result of several months of preliminary exploration with the general procedure, and were chosen because this range of sample durations produced enough errors that any improvement in accuracy (range increases) under nicotine would be apparent. Initially, only sample durations of 2 and 4 s were presented. If at any point during initial training, choice accuracy was low because of a pronounced color or side bias, the following correction procedure was implemented: a choice response to the key lit the color that did not correspond to the presented sample duration darkened both keys and initiated a 2.5 s blackout period, after which the entire trial was repeated with the same sample duration and comparison key colors in the same location. This process continued until a correct-choice response ended the trial in food. All four pigeons required correction at some point during initial training, but correction was not required for the remainder of the experiment.
Once accuracy with these sample duration endpoints (2 and 4 s) stabilized over the last five sessions, the next most extreme pair of sample durations was introduced (2.25 and 3.25 s). Once accuracy stabilized over the last five sessions with these four sample durations, the next most extreme pair of sample durations was introduced. This training schedule continued until all eight sample durations were included in the daily sessions.
Baseline
Once accuracy with all eight sample durations was stable by visual inspection over the last five sessions, the probability of reinforcement for the correct categorization of all sample durations was decreased from 1.0 to 0.4 over the course of 18–22 sessions. The reduction in probability of reinforcement was implemented to allow more trials per session. To allow drug absorption during test sessions, all sessions began with a 5-min chamber blackout. Trials were separated by a 10-s intertrial interval during which all chamber stimuli were extinguished. Daily sessions ended after 96 trials; therefore, each sample duration was presented 12 times per session.
Nicotine administration phase
Nicotine tests began when response accuracy was visually judged stable (no discernible trend or unusual variability; 40–60 sessions from the insertion of the final pair of intermediate samples, across pigeons). Nicotine hydrogen tartrate (Sigma St Louis, Missouri, USA) was dissolved in 0.9% saline and administered in a volume of 1.0 ml/kg of the 80% free-feeding body weight. Nicotine and vehicle were administered through intramuscular injections into the breast immediately before the pigeon was placed in the experimental chamber. To accustom the pigeons to the injection procedure, they were given several preliminary injections of saline. Results of these injections were excluded from the analyses. After the preliminary injections, nicotine and vehicle were given in the following order: 0.1, 0.3, 1.0, 0.03, 3.0 mg/kg, and saline. These doses were chosen because they produce a wide range of effects of nicotine (Lemmonds and Wenger, 2003). Nicotine test sessions were separated by at least three consecutive baseline sessions not preceded by an injection. The session immediately preceding a nicotine or vehicle session was designated as a control session. Dose—effect curves were determined with all doses before any dose was repeated. The effects of saline and each drug dose were determined three times for each pigeon.
Mecamylamine plus nicotine administration phase
Nicotine and mecamylamine plus nicotine tests began 20–22 sessions after the final saline determination for each pigeon. Mecamylamine (Sigma) was dissolved in 0.9% saline and administered in a volume of 1.0 ml/kg of the 80% free-feeding body weight. Mecamylamine, nicotine, and vehicle were administered through intramuscular injections as described above. A dose of 1.0 mg/kg nicotine was chosen for this phase based on the initial dose—effect curves showing that this dose produced a moderate disruption in the psychophysical function. A dose of 1.0 mg/kg mecamylamine was chosen because it has previously been shown to antagonize behavioral and physiological effects of similar doses of nicotine in pigeons (Chadman and Woods, 2004) and rats (e.g. Blondel et al., 2000). The effects of 1.0 mg/kg nicotine, 1.0 mg/kg nicotine plus 1.0 mg/kg mecamylamine, and saline were determined twice for each pigeon in the following order: 1.0 mg/kg mecamylamine plus 1.0 mg/kg nicotine, saline plus 1.0 mg/kg nicotine, and saline plus saline. Each injection was arranged in a way so that the initial injection (saline or mecamylamine) would precede the second injection (saline or nicotine) by 15 min. These dosing parameters have been used previously (e.g. Blondel et al., 2000).
Data analysis
Temporal-discrimination performance was assessed by recording the proportion of responses to the key color corresponding to the long sample duration after presentation of all sample durations. These data were averaged across all sessions of the same type (e.g. across control sessions, across sessions before which 0.1 mg/kg nicotine was administered). The proportion of long responses were plotted as a function of the presented sample duration and then further analyzed to obtain the range, mean, and SD of the function (Fig. 2).
The range of the function reflects the degree of overall stimulus control (Blough, 1996) and is equal to the value of the upper asymptote minus the value of the lower asymptote. A larger range indicates greater stimulus control, whereas decreases in the range indicate decreases in stimulus control. The mean is the time at which the proportion of responses to the long option is 0.5. This measure is the point of subjective equality and quantifies lateral shifts in the function. Decreases in the mean result from shifts of the function to the left (i.e. overestimation), whereas increases result from shifts to the right (i.e. underestimation; e.g., Meck, 1996). The SD is a measure of sensitivity to time, with smaller SD values indicating greater sensitivity to the differences between the short and long sample durations.
To obtain estimates of the mean and SD, we first normalized the functions (cf. Odum and Ward, 2007). If the functions were not normalized, and the asymptotes were to differ across drug doses (or determinations of a particular dose), the estimates of the mean and SD could be spuriously affected (for simulation and discussion, see McClure et al., 2005). Previous investigators have applied the asymptote correction to accommodate range decreases (decreases in stimulus control) that occur under disruption conditions (McClure et al., 2005; Ward and Odum, 2005, 2007; Odum and Ward, 2007). However, range increases (increases in stimulus control) as may occur under nicotine might also spuriously change the estimates of the mean and SD, particularly if these asymptote shifts were asymmetric. Therefore, normalizing the functions is also important here.
To normalize the functions, we used the following method. For the four shortest sample durations, we subtracted the proportion of long response at the 2 s sample duration from the obtained proportion of long response for each ‘short’ sample duration with the constraint that the result could not be less than zero. For the four longest sample durations, we added the difference between the proportion of long response at the 4 s sample duration and 1.0 to the obtained proportion of long response for each ‘long’ sample duration with the constraint that the result could not be more than 1.0. We used this method to normalize the functions, rather than the more common asymptote correction (e.g. Heinemann et al., 1969; Blough, 1996), because the functions were, in some cases, not strictly increasing as a function of sample duration at higher doses of nicotine and the more common correction in some cases magnified these irregularities. We have successfully used this method previously to characterize disruption by d-amphetamine (Odum and Ward, 2007). The normalized average psychophysical functions were fitted by a cumulative normal function with two free parameters (mean and SD). Fits were accomplished with the Microsoft (Redmond, Washington, USA) Excel spreadsheet program.
Statistical analyses
The effects of nicotine were assessed by means of repeated-measures analyses of variance (ANOVAs) conducted on the parameter estimates obtained during control and saline sessions, and from sessions under each dose of nicotine (or mecamylamine plus nicotine). Planned comparisons were conducted using Tukey’s test. As the latencies to respond to the trial-ready stimulus and choice response keys were not normally distributed, changes in latencies as a function of drug dose were compared using the nonparametric Friedman’s test. All statistical comparisons were conducted with a criterion significance level of P < 0.05.
Results
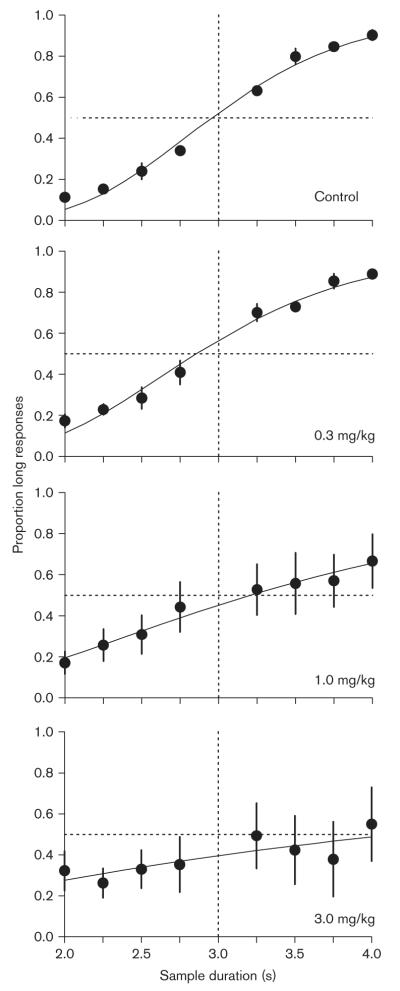
Figure 3 presents the mean proportion of responses to the long key color as a function of sample duration during control (top panel) and nicotine (lower panels) sessions. During control sessions the proportion of long responses was an increasing function of sample duration, indicating accurate temporal control. The cumulative normal function fit the control data well, accounting for 98.54% ( ± 0.64 SEM) of the variance across pigeons. Nicotine dose-dependently flattened the functions and increased the variability in the functions across determinations. At higher doses in particular, nicotine decreased the proportion of responses to the long key color after long sample durations (3–4 s), whereas the proportion of ‘long’ responses after short sample durations (2–3 s) was less affected.
Fig. 3.
Mean proportion of responses to the long key color as a function of sample duration during control sessions (top panel) and as a function of increasing nicotine dose (lower panels). The solid line through the data is the best fitting cumulative normal function (see text for details). Error bars indicate 1 standard error above and below the mean.
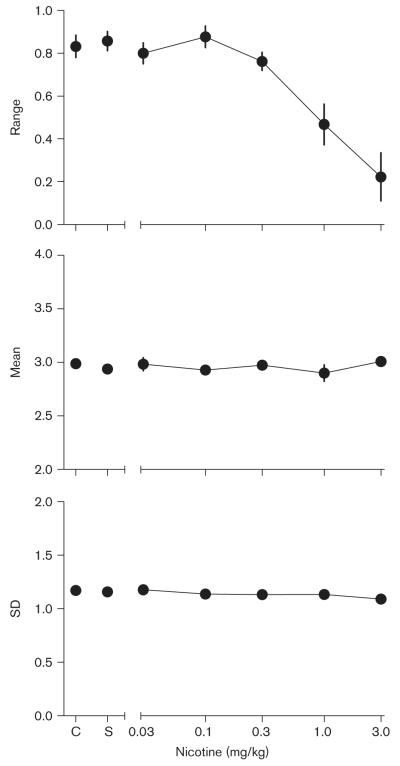
Figure 4 shows the effects of increasing doses of nicotine on the individual parameters of the psychophysical function. The top panel shows the effects of nicotine on the range of the function. The average range of the function during control conditions was 0.83 ( ± 0.05 SEM), indicating a relatively high level of overall stimulus control (but also indicating that decreasing the range of sample durations was effective in lowering accuracy). Saline administration had no systematic effects on the range of the function. Nicotine dose-dependently decreased the range of the function across birds, indicating a dose-dependent decrease of stimulus control. These results were confirmed by a repeated-measures ANOVA, which found a significant effect of nicotine dose [F(3,6)=14.49, P <0.01]. Planned Tukey’s multiple comparisons revealed that the range estimates at the 1.0 and 3.0 mg/kg doses of nicotine differed significantly from the range estimates at all other doses (including control and saline) but were not significantly different from one another.
Fig. 4.
Mean estimates of the individual parameters derived from fitting the cumulative normal function (see text for details) to the mean proportion — long data from each pigeon as a function of nicotine dose. The top panel shows estimates of the range, the middle panel displays estimates of the mean, and the bottom panel shows estimates of the SD. Other details as in Fig. 3.
The middle panel of Fig. 4 shows the average mean of the corrected psychophysical functions during control and saline sessions and as a function of nicotine dose. The average mean of the functions during control sessions was 3.00 s ( ± 0.03 SEM), indicating accurate estimation of the passage of time. Neither saline or nicotine administration had significant effects on the mean of the psychophysical function [F(3,6)=0.84, NS]. These results indicate that when changes in stimulus control are accounted for (by applying the correction to the psychophysical functions), nicotine had no systematic effect on the estimation of the passage of time (i.e. did not produce over or underestimation of time).
The bottom panel of Fig. 4 shows the average SD of the corrected psychophysical functions during control and saline sessions and across doses of nicotine. The average control SD for all pigeons was 1.17 ( ± 0.04 SEM). Neither saline or nicotine had any systematic effects on the SD of the function [F(3,6)=1.74, NS], indicating that when changes in overall stimulus control are taken into account, nicotine had no effect on the precision of temporal discrimination.
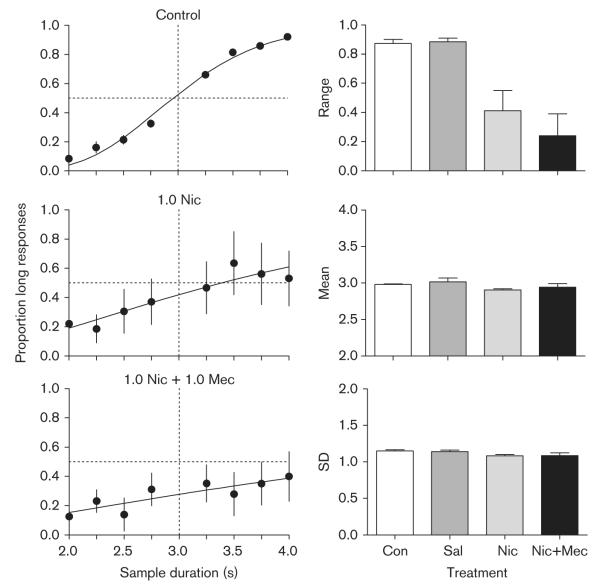
Figure 5 shows the results of the mecamylamine phase. The left panels show the proportion of responses to the long key color as a function of sample duration during control, nicotine, and mecamylamine plus nicotine sessions. The control data (top left panel) were well described by the cumulative normal function (Variance accounted for 98.51% ± 0.64 SEM) and are quite similar to those shown in Fig. 3. Administration of both 1.0 mg/kg nicotine (center left panel) and 1.0 mg/kg nicotine plus 1.0 mg/kg mecamylamine (bottom left panel) severely disrupted temporal discrimination performance, evidenced by a flattening of the psychophysical functions and increased variability across pigeons and determinations (similar to the two highest doses of nicotine in Fig. 3).
Fig. 5.
The left panel shows the mean proportion of responses to the long key color as a function of sample duration during control (top left panel), 1.0 mg/kg nicotine (center left panel), and 1.0 mg/kg mecamylamine plus 1.0 mg/kg nicotine (bottom left panel) sessions. The right panels show the estimates of the range (top right), mean (center right), and SD (bottom right) of the model fit to the psychophysical functions under control, saline, 1.0 mg/kg nicotine, and 1.0 mg/kg mecamylamine plus 1.0 mg/kg nicotine sessions. Other details as in Fig. 3.
The right panels of Fig. 5 show the individual parameter estimates of the psychophysical functions during control, saline, administration of 1.0 mg/kg nicotine, and the administration of 1.0 mg/kg mecamylamine plus 1.0 mg/kg nicotine. The top right panel shows the effects on the range of the function (overall stimulus control). During control sessions, the average range of the function was 0.87 ( ± 0.03 SEM). Saline had no effect on the range of the function. Both 1.0 mg/kg nicotine and 1.0 mg/kg mecamylamine plus nicotine decreased the range of the function. This effect was confirmed by a repeated measures ANOVA conducted on the range estimates from each treatment condition [F(3,3)=11.29, P < 0.05]. Planned comparisons indicated that the range estimates obtained under 1.0 mg/kg nicotine and 1.0 mg/kg mecamylamine plus 1.0 mg/kg nicotine differed significantly from those obtained under control and saline sessions, but did not differ from one another.
The center right and bottom right panels show the effects of nicotine and mecamylamine plus nicotine on the mean and SD, respectively, of the corrected psychophysical functions. The average mean and SD of the functions were 2.98 s ( ± 0.009 SEM) and 1.15 ( ± 0.02 SEM), respectively, similar to the estimates of these parameters from the nicotine phase. Administration of saline, nicotine, and mecamylamine plus nicotine did not change the estimates of the mean [F(3,3)=1.44, NS] or the SD [F(3,3)=2.54, NS]. Together, these results indicate that mecamylamine did not block the disruptive effects of nicotine on overall stimulus control, and neither nicotine or mecamylamine plus nicotine had systematic effects on the measures of timing, the mean and SD.
Table 1 shows median latencies to respond to the trial-ready and choice-response keys during both phases of the experiment. During the nicotine phase, latencies for both trial ready and choice responses during control sessions were around 1 s. Latencies under saline were similar to those under control. Nicotine increased the average median latency at the higher doses, but this effect was not significant on either trial-ready [Friedman statistic=10.77] or choice-response [Friedman statistic=7.36] latencies. During the mecamylamine phase, control and saline trial-ready and choice-response latencies were also around 1 s, similar to the results from the nicotine phase. Nicotine and mecamylamine plus nicotine increased the average median latency for trial-ready responding, whereas only mecamylamine plus nicotine increased the average choice response latency. These increases, however, were not significant on either trial-ready [Friedman statistic=2.10] or choice-response [Friedman statistic=5.00] latencies.
Table 1.
Median (25th and 75th percentile in parentheses) trial-ready and choice-response latencies (in seconds) during control, saline and drug sessions for both phases of the experiment
| Control | Saline | 0.03 (mg/kg) | 0.1 (mg/kg) | 0.3 (mg/kg) | 1.0 (mg/kg) | 3.0 (mg/kg) | |
|---|---|---|---|---|---|---|---|
| Nicotine phase | |||||||
| Trial ready | 1.05 (0.72/1.54) | 1.05 (0.73/1.55) | 0.99 (0.70/1.52) | 0.98 (0.66/1.40) | 1.00 (0.70/1.43) | 1.10 (0.70/1.79) | 1.25 (0.81/2.27) |
| Choice | 1.05 (0.71/1.56) | 0.99 (0.70/1.45) | 0.93 (0.65/1.38) | 1.00 (0.68/1.44) | 1.00 (0.68/1.42) | 1.13 (0.78/1.80) | 1.35 (0.86/2.54) |
| Mecamylamine phase | Nic | Nic+Mec | |||||
| Trial ready | 0.93 (0.60/1.40) | 1.00 (0.65/1.47) | 1.59 (0.98/2.25) | 1.50 (0.90/2.92) | — | — | — |
| Choice | 0.98 (0.70/1.43) | 1.00 (0.65/1.50) | 0.93 (0.65/1.51) | 1.63 (0.93/3.10) | — | — | — |
Mec, mecamylamine; Nic, nicotine.
Discussion
During control sessions, choice responding was under temporal control, evidenced by the proportion of responses to the long choice option increasing as a function of sample duration. The range of the function (difference between the upper and lower asymptotes) indicated that the level of overall stimulus control was relatively high, but, importantly, not so high as to preclude range increases under nicotine. In addition, the estimates of the mean and SD of the corrected psychophysical functions indicated accurate estimation of the passage of time under control conditions. These results are in accord with other recent reports (e.g. McClure et al., 2005; Ward and Odum 2005, 2006, 2007; Odum and Ward, 2007).
Nicotine administration flattened the psychophysical functions, indicating a general disruption of temporal discrimination. This result replicates those from other assessments of the effects of various drugs on temporal discrimination performance (for review, see Staddon and Higa, 2006). Analysis of the individual parameter estimates derived from fitting the cumulative normal functions showed that nicotine dose-dependently decreased the range of the functions, indicating a dose-dependent decrease in stimulus control. The measures of bias and sensitivity, the mean and SD, were not affected by nicotine. These results indicate that when the decrease in overall stimulus control is taken into account (by correcting the proportion-long response data before estimating the mean and SD), nicotine had no effect on the two measures of timing. Administration of mecamylamine 15 min before nicotine did not antagonize the nicotine-induced decreases in overall stimulus control (the range decreased under nicotine plus mecamylamine), and did not affect the mean and SD of the functions.
The selective disruption of stimulus control by nicotine in this study is consistent with a number of recent reports of the effects of drugs and other manipulations on temporal discrimination performance. For example, McClure et al. (2005) and Ward and Odum (2005) reported selective decreases in stimulus control in a temporal discrimination procedure similar to that used in this study after administration of d-amphetamine and morphine, respectively. In addition, Ward and Odum (2007) recently assessed the effects of several non-pharmacological disruptors (presession feeding, intertrial interval food deliveries, extinction, and distraction) on temporal discrimination performance and found that all manipulations disrupted the performance by selectively decreasing stimulus control, rather than by affecting timing (for similar results, see Ward and Odum, 2006). Therefore, a growing body of literature suggests that disruption of temporal discrimination performance in this procedure by drugs and other manipulations may be mediated by decreases in stimulus control that occur in the absence of systematic distortions in timing.
The general disruption produced by nicotine in this study is in contrast to the results reported by Bizot (1997). He reported that nicotine (0.25 and 0.5 mg/kg) produced overestimation of time in a temporal bisection procedure, evidenced by a decrease in the proportion of responses to the ‘short’ option after presentation of a 12-s intermediate sample duration. There are a number of differences across experiments that may have contributed to the different results. First, the results reported by Bizot (1997) were based on only one determination of the effects of each dose of nicotine, and may therefore warrant caution in interpretation. In addition, the experimental design in this study resulted in each of the eight sample durations being experienced 12 times per session, including during drug-test sessions, whereas the design used by Bizot resulted in the 12-s intermediate sample duration being experienced only once per session during probe sessions (eight total probe sessions across the study) and 10 times per session during drug-test sessions (one session per dose). Perhaps this procedural difference, among others, contributed to the differing results across experiments.
The results obtained here also differ from those reported with rats by Meck and colleagues. Using the peak interval (PI) procedure, a procedure in which subjects must estimate the duration of an ongoing interval, Hinton and Meck (1996) and Meck and Williams (1997) reported a horizontal leftward shift in the response function after the administration of nicotine, consistent with over-estimation of time. With regard to this result, there are numerous differences between this procedure and the PI procedure, and future research is necessary to determine the specific reasons for the obtained differences across experiments. Nevertheless, although the disruption produced by nicotine in this experiment differs from the results of a few studies, it is consistent with the results of many other studies that have assessed the effects of drugs from a wide variety of pharmacological classes on temporal discrimination performance (for review, see Staddon and Higa, 2006).
Although the nicotine-induced disruption of temporal discrimination performance observed in this study is consistent with the reported effects of other drugs and disruptors, it appears inconsistent with reports of enhanced cognitive function by nicotine in a variety of other experimental preparations (for review, see Levin et al., 2006). It should be noted, however, that improvements in cognitive performance are not always seen under nicotine. For example, Attaway et al. (1999) reported no effect of nicotine on acquisition of a serial pattern performance in rats. In addition, Levin and Torry (1996) reported that chronic administration of nicotine had no effect on radial arm maze performance in rats. Mirza and Bright (2001) found no enhancement of sustained attention under nicotine in the five-choice serial reaction time task in rats. Finally, Moragrega et al. (2003) reported that the acute administration of nicotine actually decreased performance in a water maze in mice. Thus, although many results indicate that nicotine enhances cognitive performance, it does not do so uniformly across studies, and such enhancement has been shown to vary with a variety of procedural and other factors, including the dose range and dosing regimen used, strain of animals, level of training before nicotine administration, among others (see Levin, 1992; e.g., Mirza and Stolerman, 1998; Levin and Simon, 1998, for reviews and discussion).
In this study, the failure of nicotine to enhance performance was not likely because of a ceiling effect on accuracy, as accuracy under baseline was well below maximal. In addition, the lack of a facilitative effect of nicotine was not likely because of the dose range used, as earlier experiments have shown a facilitative effect of nicotine on measures of sustained attention in pigeons using the dose range used here (Lemmonds et al., 2002; Lemmonds and Wenger, 2003). Therefore, improved performance under nicotine, had it occurred, would likely have been detected under the present experimental arrangement. Future research should attempt to more fully delineate and circumscribe the conditions (if any) under which nicotine enhances temporal discrimination performance within this procedure.
Regardless of the specific procedural or other aspects of this experiment which contributed to the results obtained here, this study is the first to characterize the effects of nicotine on the full psychophysical function in a temporal bisection procedure. The selective disruption of stimulus control observed here lends further support to the notion that disruption of temporal discrimination performance by drugs in these types of procedures may result from a general disruptive effect that is unrelated to the specific pharmacology of the drug (for discussion, see Odum and Schaal, 2000; Ward and Odum, 2006). The fact that the disruption produced by nicotine was not blocked by mecamylamine may provide support for the interpretation of the present results as being due to a general disruption of discrimination, rather than to a specific effect of nicotine. Conclusions based on this interpretation must be tempered, however, by the fact that only one dose of mecamylamine was administered in this study. Future studies should use a wider range of mecamylamine and nicotine combination doses.
In conclusion, the present results, together with those discussed previously, in which drugs and other disruptors produced selective decreases in stimulus control, indicate that assessing the effects of drugs and other disruptors on temporal discrimination performance using temporal bisection procedures may be problematic because such manipulations often severely decrease stimulus control. Decreased stimulus control leads to choice responses (the behavioral outcome from which the measure of timing in these procedures is derived) no longer being under the functional control of the presented sample durations. Thus, in these procedures, disrupting behavior in some fashion often compromises the very index of timing that is of interest under the disruption manipulation. Put another way, assessing the effects of some manipulation on timing per se in this procedure may not be possible because the measure of timing is distorted by decrements in other areas of discriminative performance that often occur under drugs and other manipulations. Other types of timing procedures which do not involve an explicit discrimination between two discrete duration categories (‘short’ and ‘long’), such as the PI procedure, may be more sensitive to the selective effects of various manipulations on timing (although there are challenges in interpreting ‘pure’ timing effects in other procedures as well; see Saulsgiver et al., 2006; Taylor et al., 2007, for considerations with analyzing data from the PI procedure). At the very least, the present results, when considered with reports of selective decreases in stimulus control following drugs and other disruptors, suggest that if the temporal-bisection procedure is used to characterize the disruptive effects of some manipulation on temporal-discrimination performance, the results should be analyzed using a theoretical framework that takes into account the almost certain decrease in stimulus control that occurs under such manipulations.
Acknowledgements
The authors thank Adam Kynaston for help in conducting this experiment. Portions of these data were presented at the 2008 Annual Meeting of the Association for Behavior Analysis in Chicago, Illinois. RDW is now at Columbia University. STB is now at the University of Nebraska.
References
- Attaway CM, Compton DM, Turner MD. The effects of nicotine on learning and memory: a neuropsychological assessment in young and senescent Fischer 344 rats. Physiol Behav. 1999;67:421–431. doi: 10.1016/s0031-9384(99)00081-5. [DOI] [PubMed] [Google Scholar]
- Bizarro L, Stolerman IP. Attentional effects of nicotine and amphetamine in rats at different levels of motivation. Psychopharmacology. 2003;170:271–277. doi: 10.1007/s00213-003-1543-6. [DOI] [PubMed] [Google Scholar]
- Bizot JC. Effects of psychoactive drugs on temporal discrimination in rats. Behav Pharmacol. 1997;8:293–308. doi: 10.1097/00008877-199708000-00003. [DOI] [PubMed] [Google Scholar]
- Blondel A, Sanger DJ, Moser PC. Characterisation of the effects of nicotine in the five-choice serial reaction time task in rats: antagonist studies. Psychopharmacology. 2000;149:293–305. doi: 10.1007/s002130000378. [DOI] [PubMed] [Google Scholar]
- Blough DS. Error factors in pigeon discrimination and delayed matching. J Exp Psychol Anim Behav Process. 1996;22:118–131. [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Chadman KK, Woods JH. Cardiovascular effects of nicotine, chlorisondamine and mecamylamine in the pigeon. J Pharm Exp Ther. 2004;308:73–78. doi: 10.1124/jpet.103.057307. [DOI] [PubMed] [Google Scholar]
- Chiang TJ, Al-Ruwaitea ASA, Mobini S, Ho MY, Bradshaw CM, Szabadi E. The effect of d-amphetamine on performance on two operant timing schedules. Psychopharmacology. 2000;150:170–184. doi: 10.1007/s002130000422. [DOI] [PubMed] [Google Scholar]
- Gibbon J. Scalar expectancy theory and Weber’s law in animal timing. Psychol Rev. 1977;84:279–325. [Google Scholar]
- Heinemann EG, Avin E, Sullivan MA, Chase S. Analysis of stimulus generalization with a psychophysical method. J Exp Psychol. 1969;80:215–224. [Google Scholar]
- Hinton SC, Meck WH. Increasing the speed of an internal clock: the effects of nicotine on interval timing. Drug Dev Res. 1996;38:204–211. [Google Scholar]
- Lemmonds CA, Wenger GR. Effects of drugs of abuse and signal predictability in two models of sustained attention in pigeons. Behav Pharmacol. 2003;14:279–294. doi: 10.1097/01.fbp.0000080418.18561.93. [DOI] [PubMed] [Google Scholar]
- Lemmonds CA, Williams DK, Wenger GR. Effects of pentobarbital, d-amphetamine, and nicotine on two models of sustained attention in pigeons. Psychopharmacology. 2002;163:391–398. doi: 10.1007/s00213-002-1013-6. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotinic systems and cognitive function. Psychopharmacology. 1992;108:417–431. doi: 10.1007/BF02247415. [DOI] [PubMed] [Google Scholar]
- Levin ED, Simon BB. Nicotinic acetylcholine receptor involvement in cognitive function in animals. Psychopharmacology. 1998;138:217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- Levin ED, Torry D. Acute and chronic nicotine effects on working memory in aged rats. Psychopharmacology. 1996;123:88–97. doi: 10.1007/BF02246285. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Sparrow E, Hinton SC, Erhardt D, Meck WH, et al. Nicotine effects on adults with attention/deficit hyperactivity disorder. Psychopharmacology. 1996;123:55–63. doi: 10.1007/BF02246281. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, Hinton SC, Meck WH, Rose JE, March J. Transdermal nicotine effects on attention. Psychopharmacology. 1998;140:135–141. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- McClure EA, Saulsgiver KA, Wynne CDL. Effects of d-amphetamine on temporal discrimination in pigeons. Behav Pharmacol. 2005;16:193–208. doi: 10.1097/01.fbp.0000171773.69292.bd. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Brain Res Cogn Brain Res. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. NeuroReport. 1997;8:2831–2835. doi: 10.1097/00001756-199709080-00005. [DOI] [PubMed] [Google Scholar]
- Mirza NR, Bright JL. Nicotine-induced enhancements in the five-choice serial reaction time task in rats are strain-dependent. Psychopharmacology. 2001;154:8–12. doi: 10.1007/s002130000605. [DOI] [PubMed] [Google Scholar]
- Mirza NR, Stolerman IP. Nicotine enhances sustained attention in the rat under specific task conditions. Psychopharmacology. 1998;138:266–274. doi: 10.1007/s002130050671. [DOI] [PubMed] [Google Scholar]
- Moragrega I, Carrasco MC, Vicens P, Redolat R. Spatial learning in male mice with different levels of aggressiveness: effects of housing conditions and nicotine administration. Behav Brain Res. 2003;147:1–8. doi: 10.1016/s0166-4328(03)00112-8. [DOI] [PubMed] [Google Scholar]
- Odum AL, Schaal DW. The effects of morphine on fixed-interval patterning temporal discrimination. J Exp Anal Behav. 2000;74:229–243. doi: 10.1901/jeab.2000.74-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL, Ward RD. Characterizing the effects of d-amphetamine on temporal discrimination. Behav Process. 2007;175:156–166. doi: 10.1016/j.beproc.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Popke EJ, Mayorga AJ, Fogle CM, Paule MG. Effects of acute nicotine on several operant behaviors of rats. Pharmacol Biochem Behav. 2000;65:247–254. doi: 10.1016/s0091-3057(99)00205-1. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Bushnell PJ, Levin ED. Effects of nicotine and mecamylamine on choice accuracy in an operant visual signal detection task in female rats. Psychopharmacology. 2002;164:369–375. doi: 10.1007/s00213-002-1221-0. [DOI] [PubMed] [Google Scholar]
- Santi A, Weise L, Kuiper D. Amphetamine and memory for event duration in rats and pigeons: disruption of attention to temporal samples rather than changes in the speed of the internal clock. Psychobiology. 1995;23:224–232. [Google Scholar]
- Saulsgiver KA, McClure EA, Wynne CDL. Effects of D-amphetamine on the behavior of pigeons exposed to the peak procedure. Behav Process. 2006;71:268–285. doi: 10.1016/j.beproc.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Staddon JER, Higa JJ. Interval timing. Nat Rev Neurosci. 2006;7:1–2. [Google Scholar]
- Stolerman IP, Mirza NR, Hahn B, Shoaib M. Nicotine in an animal model of attention. Eur J Pharmacol. 2000;393:147–154. doi: 10.1016/s0014-2999(99)00886-9. [DOI] [PubMed] [Google Scholar]
- Stubbs DA. The discrimination of stimulus duration by pigeons. J Exp Anal Behav. 1968;11:223–238. doi: 10.1901/jeab.1968.11-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton JE, Roberts WA. The effect of nontemporal information processing on time estimation in pigeons. Learn Motiv. 2002;33:123–140. [Google Scholar]
- Taylor KM, Horvitz JC, Balsam PD. Amphetamine affects the start of responding in the peak interval-timing task. Behav Process. 2007;74:168–175. doi: 10.1016/j.beproc.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Ward RD, Odum AL. Effects of morphine on temporal discrimination and color matching: general disruption of stimulus control or selective effects on timing? J Exp Anal Behav. 2005;84:401–415. doi: 10.1901/jeab.2005.94-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Odum AL. Effects of prefeeding, intercomponent-interval food, and extinction on temporal discrimination and pacemaker rate. Behav Process. 2006;71:297–306. doi: 10.1016/j.beproc.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Ward RD, Odum AL. Disruption of temporal discrimination and the choose-short effect. Learn Behav. 2007;35:60–70. doi: 10.3758/bf03196075. [DOI] [PubMed] [Google Scholar]