Abstract
Compromises between speed and accuracy are seemingly inevitable in decision-making when accuracy depends on time-consuming information gathering. In collective decision-making, such compromises are especially likely because information is shared to determine corporate policy. This political process will also take time. Speed–accuracy trade-offs occur among house-hunting rock ants, Temnothorax albipennis. A key aspect of their decision-making is quorum sensing in a potential new nest. Finding a sufficient number of nest-mates, i.e. a quorum threshold (QT), in a potential nest site indicates that many ants find it suitable. Quorum sensing collates information. However, the QT is also used as a switch, from recruitment of nest-mates to their new home by slow tandem running, to recruitment by carrying, which is three times faster. Although tandem running is slow, it effectively enables one successful ant to lead and teach another the route between the nests. Tandem running creates positive feedback; more and more ants are shown the way, as tandem followers become, in turn, tandem leaders. The resulting corps of trained ants can then quickly carry their nest-mates; but carried ants do not learn the route. Therefore, the QT seems to set both the amount of information gathered and the speed of the emigration. Low QTs might cause more errors and a slower emigration—the worst possible outcome. This possible paradox of quick decisions leading to slow implementation might be resolved if the ants could deploy another positive-feedback recruitment process when they have used a low QT. Reverse tandem runs occur after carrying has begun and lead ants back from the new nest to the old one. Here we show experimentally that reverse tandem runs can bring lost scouts into an active role in emigrations and can help to maintain high-speed emigrations. Thus, in rock ants, although quick decision-making and rapid implementation of choices are initially in opposition, a third recruitment method can restore rapid implementation after a snap decision. This work reveals a principle of widespread importance: the dynamics of collective decision-making (i.e. the politics) and the dynamics of policy implementation are sometimes intertwined, and only by analysing the mechanisms of both can we understand certain forms of adaptive organization.
Keywords: context-dependent behaviour, collective behaviour, group movement, recruitment, emigration
1. Introduction
From first-hand experience, and from first principles, one can deduce a mutual antagonism between speed and accuracy in many decisions. Accuracy may require extra information, but gathering, processing and sharing information take time. Compromises between speed and accuracy have been demonstrated in decision-making by humans (e.g. Edwards 1965; Vitevitch 2002), monkeys (e.g. Roitman & Shadlen 2002) and bees (Chittka et al. 2003). They are also a characteristic of ‘anytime algorithms’ in computing. These provide an early answer when interrupted but at the cost of accuracy (Dean & Boddy 1988).
Franks et al. (2002, 2003a), respectively, first predicted and then experimentally demonstrated speed–accuracy trade-offs in collective decision-making in house-hunting ants. For ants choosing new homes, faster decisions are more error-prone (Marshall et al. 2006; Pratt & Sumpter 2006). However, these earlier studies do not tell the full story. Indeed, they leave a central issue unresolved.
Paradoxically, in house-hunting ants, faster decisions could lead to slower emigrations, and nothing would be gained.
The reason for this potential paradox is that house-hunting ants use quorum sensing in such a way that two key processes are inseparable. Essentially, quorum sensing collates individual decisions into collective ones (i.e. corporate policy is determined politically); but quorum sensing may also limit the number of ants that know how to get into the new nest and can take an active role in expediting the emigration. Thus if the ants use a low quorum threshold (QT) in an emergency, the decision may be faster, and less well informed, and the emigration may be slower. Therefore, by making a quicker decision, the ants might get the worst of all possible worlds—a poor choice and a slow emigration, in which vulnerable members of the colony remain exposed for longer. Thus, because the QT sets a limit on both information gathering and training, the use of a low threshold in an emergency could turn a crisis into a chronic refugee problem. To understand this more fully, we will need to explain the natural history of this decision-making system.
When a colony needs to find a new home, certain active scouts leave their vulnerable nest-mates and start to search for something suitable. The majority of a colony's workers, however, remain at the old nest guarding the brood. Such workers take a passive role in the emigration but have a crucial role in colony maintenance. When active scouts strike lucky, they assess many nest variables (Mallon & Franks 2000; Mugford et al. 2001; Franks et al. 2003b, 2005, 2006a,b, 2007a), and, all else being equal, they recruit nest-mates more quickly to a high-quality nest than to a low-quality one (Mallon et al. 2001). Hesitation over poor nests will favour selection of better ones. Initial recruitment is typically by forward tandem running (Pratt et al. 2002). In this process, an ant that has found a suitable new nest, and knows an efficient route to it, leads a single nest-mate worker, i.e. an active scout that is still at the old nest, from the old nest forwards to the new one. Such tandem running is slow—but the ant following the tandem leader can learn the route to the new nest and later can either lead tandem runs itself or carry nest-mates to the new nest. Tandem running trains naive ants, who train others, thereby creating a positive-feedback information cascade. Moreover, such tandem running was the first case in animal behaviour to be shown to meet all the criteria of a strict definition of teaching (Franks & Richardson 2006). More recently, Richardson et al. (2007) have even shown that the teaching ant, i.e. the tandem leader, engages in three forms of evaluation, namely (i) the amount she has already invested, (ii) the quality of the goal and (iii) the rate of progress of the tandem. Forward tandem runs (FTRs), i.e. those from the old nest to the new one, continue until tandem leaders encounter a certain number of nest-mates in the new nest—the QT. The size of the QT varies with circumstances. In benign conditions in the laboratory, it is typically between approximately 10 and 20 workers (Pratt et al. 2002), whereas, the average colony has approximately 110 workers in total (Franks et al. 2006a). In harsh conditions, QTs are lower (Franks et al. 2003a). Moreover, if the ants have no need to move at all because their existing nest is fully functional but they have encountered something much better, they use a very high QT (Dornhaus et al. 2004). Moderate to large QTs are sufficiently high that it is unlikely that one ant alone would have created that quorum by leading very large numbers of nest-mates to the new nest in successive tandem runs (Pratt et al. 2002). Therefore, high QT's imply that many ants consider the new nest suitable (Franks et al. 2002). In this way, quorum sensing serves as a method of collating the separate evaluations of many individual ants (Pratt 2005). Quorum sensing is a wonderful device in collective decision-making.
Once a QT has been satisfied, the active ants switch from tandem running to carrying ‘passive’ nest-mates: either other adults (brood-care workers and the queen) or brood (eggs, larvae or pupae). Carrying a nest-mate is three times quicker than leading a tandem run. However, transported ants do not learn the route, as they are being carried along and, thus, have not been trained to take an active role in the emigration. Moreover, because the QT sets a limit to tandem running and may limit the ants that know the route between the old and new nests, it sets a limit on the speed of the emigration. Low QTs mean faster and potentially less well-informed decisions and potentially slower emigrations, and, thus, greater exposure and risk to colony members. Hence, lowering the QT in an emergency would seem to make things worse. The notion of ‘any port in a storm’ implies a quick decision and rapid implementation to minimize risk. However, unless the ants have an additional mechanism to restore emigration speed, their use of low QTs in harsh conditions would appear to be maladaptive.
Reverse tandem runs (RTRs) might resolve this paradox. RTRs are the third form of recruitment exhibited during emigrations and occur from the new nest back to the old one. First, as described earlier, the ants begin with FTRs; second, they switch to carrying nest-mates; and then finally, as carrying continues, they typically begin RTRs. Earlier work by Pratt et al. (2002) has shown that RTRs occur sporadically during the transport phase; 99 per cent of reverse tandem leaders also recruited from the old nest; 84 per cent of RTRs ended with the leader picking up a nest-mate (adult or brood) at the old nest; 73 per cent of RTR followers were also, at some stage in the emigration, active recruiters from the old nest; and 53 per cent of RTR followers had previously engaged in such recruitment before following an RTR. Therefore, RTRs may, in part, serve to reactivate recruiters and it seems likely that all participants in FTRs and RTRs, both leaders and followers, are from the active group of workers.
Here, we report, for the first time, the results of new experiments to examine the role of reverse tandem runs and to determine whether they can resolve the paradox of faster decisions potentially causing slower emigrations.
There has been extensive mathematical modelling of the house-hunting algorithms of Temnothorax albipennis (Pratt et al. 2002, 2005; Marshall et al. 2006; Planqué et al. 2006, 2007; Pratt & Sumpter 2006). The most recent of these models by Planqué et al. (2007) focused on the possible role of RTRs and predicted that they might serve to restore the speed of emigrations when the ants have used a low QT and have made rather few FTRs.
Accordingly, here we test two alternative hypotheses for the function of RTRs. Hypothesis 1 is the simplest explanation for RTRs, namely that RTRs compensate for a disruption of FTRs. Thus, if FTRs have been unsuccessful for whatever reason, the ants may compensate by leading more RTRs.
Hypothesis 2 postulates that RTRs are related to scout dispersal (e.g. in emergencies) and the associated rarity of candidates to follow FTRs in the vicinity of the old nest. In an extreme emergency, scouts may disperse far and wide from the old nest in search of any port in a storm. Hence, when one does find a suitable new nest, it may later find few, if any, active ants at the old nest to recruit with an FTR. Therefore, such a pioneering scout might do the next best thing and begin recruiting by transporting a passive nest-mate to the new nest. Meanwhile, other scouts may have finally stumbled upon the same new nest by circuitous routes and may be available to be led in RTRs. These slow scouts may be partly disoriented by the time-consuming and haphazard path they have taken. It might then benefit the colony and their own inclusive fitness if they are shown, through following an RTR, a quicker path between the old and new nests so that they can help expedite the emigration by carrying nest-mates to the new nest.
2. Material and methods
Twenty queen-right colonies of T. albipennis were collected in October 2005 from south Dorset in the United Kingdom. Colonies were housed in artificial nests consisting of a cardboard perimeter sandwiched between two microscope slides 75 mm×50 mm, forming nesting cavities with internal dimensions 49 mm×30 mm×1.5 mm with an entrance tunnel 2 mm wide and 5 mm long. The artificial nests were located in large, square Petri dishes 22 cm×22 cm×2.2 cm, which acted as foraging arenas. Fluon-coated walls prevented the ants from escaping and a closed lid preserved humidity in the dish. Except during experiments, colonies had access to Drosophila, honey solution and water ad libitum. Throughout the study, colonies were kept on a low-vibration bench. Worker populations ranged from 49 to 311 and brood populations from 78 to 287 (for information on colony sizes, see Franks et al. 2006a). There were no significant differences in the median sizes of colonies used in the two treatments and two controls described below (Kruskal–Wallis test H=0.35, d.f.=3, p=0.951).
(a) Experiment 1: do reverse tandem runs compensate for a disruption of FTRs?
A new Petri dish was placed abutting the one containing the colony (figure 1). The new Petri dish contained a new nest site that was identical to the original one except that it had a removable cardboard cover to make it dark and hence more attractive than the original one (Franks et al. 2003b) to encourage an emigration (Franks et al. 2003b). The two nests, the old and the new one, were thus placed 33.5 cm apart, at opposite ends of the old and new dishes (figure 1). Two digital cameras (Nikon, Coolpix 889) were mounted on stands 18 cm above each nest. Each camera's field of view was adjusted to encompass the entire nest interior and the cardboard perimeters. Colonies were allowed at least 48 hours to settle into this environment before the experiment was performed so that they could learn landmarks (Pratt et al. 2001; McLeman et al. 2002) that remained throughout the experimental period.
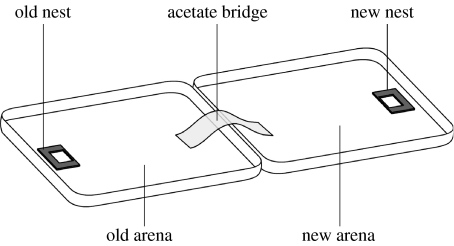
Figure 1.
Experimental arenas used to control dispersion of the ants. When the original nest has been destroyed, the second arena with the new nest site can be connected with an acetate bridge.
Emigrations were induced by removing the upper slide from the old nest to make it uninhabitable and an acetate bridge was introduced to link the two Petri dishes (figure 1). The bridge was made out of a rectangle of acetate 19×6 cm, which was transparent to deter colonies from nesting beneath its arch. The bridge was fixed to each dish by a strip of double-sided tape, such that it was flush to the dishes with no sticky surfaces exposed. To prevent the ants from escaping, the bridge did not touch the sides of the dishes. This design provided a clear division between the two Petri dish arenas. The new Petri dish was terra incognita for the ants. This design also created a substantial separation of the two nests and the relatively narrow bridge also served further to challenge the ants' navigational abilities (Pratt et al. 2001; McLeman et al. 2002). We favoured this design because it reduced the possibility of excited ants quickly finding the new nest by chance and should thereby have reduced the number of ants that had initially discovered the new nest. This should have increased the potential benefit of recruitment behaviours.
The colonies were allocated to give a similar distribution of colony sizes in the control (10 colonies) and treatment (10 colonies) groups. Colonies from the control group were allowed to emigrate undisturbed. By contrast, in the treatment group, we disrupted every FTR (that did not escape our attention). We wanted to do this without removing any ants or causing them to panic. Our procedure consisted of artificially stimulating the hind legs and abdomen of each tandem run leader with an eyelash mounted on a cocktail stick. We did this when a suitably large gap had naturally occurred between the leader and the follower. Such large gaps are common (Möglich 1978; Franks et al. 2002; Franks & Richardson 2006). Suitable stimulation with an eyelash is sufficient to encourage the leader to continue in the absence of its true follower (Möglich et al. 1974). We continued to tickle the leader with the eyelash, a few times per second, until the leader and the follower were approximately 2 cm apart so that they lost one another and the tandem run did not reform. FTRs were disrupted as soon as they were observed, i.e. almost always within a few centimetres of the old nest.
The numbers of successful forward and reverse tandem runs were recorded by two investigators until 20 min after the last brood item was moved into the new nest. We considered a successful tandem run to be the one that progressed to within a few millimetres of its target (i.e. the new or old nest). Some tandem runs aborted naturally during travel from one nest to the other either because the tandem was broken by a collision with nest-mates or because the follower became lost. Thus, we also recorded the number of partial tandem runs. Other components of the emigration were also recorded such as the time that elapsed between the opening of the old nest and the queen and last brood item being moved into the new nest. To record the numbers of ants in the old and new nests and hence the dynamics of the emigration, pictures of the old and new nests were taken immediately before and after the removal of the upper microscope slide of the original nest and every 10 min thereafter.
During each emigration, we observed a striking initial drop in worker numbers during the first 20 min of the emigration followed by a plateau phase (figure 2a, see also figure 3a for comparable data from experiment 2). This typical pattern was associated with the initial exodus of scouts from the old nest during the emigration. Thus, the number of scouts was estimated simply as the total number of ants in the colony minus the number of ants still in the old nest after 20 min.
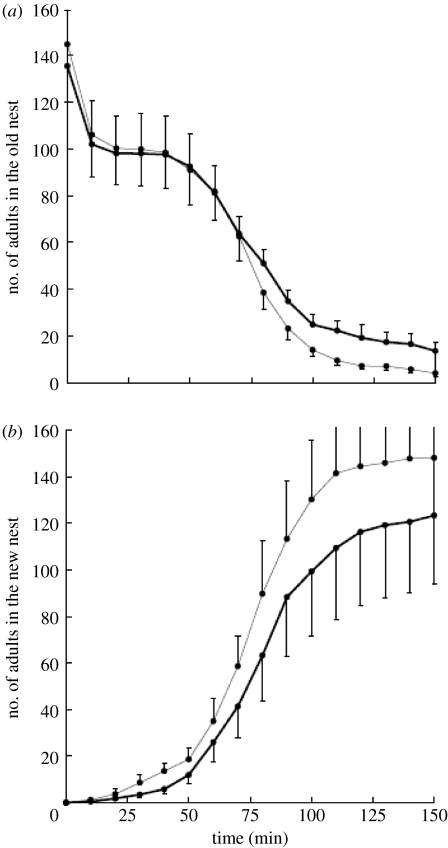
Figure 2.
Experiment 1. FTRs disrupted in the treatment but not in the control. Mean numbers of adults in the (a) old and (b) new nests for colonies from the control (grey line) or treatment (black line) group as a function of time since the old nest was destroyed. The error bars represent the standard error to the mean. FTRs expedite emigrations.
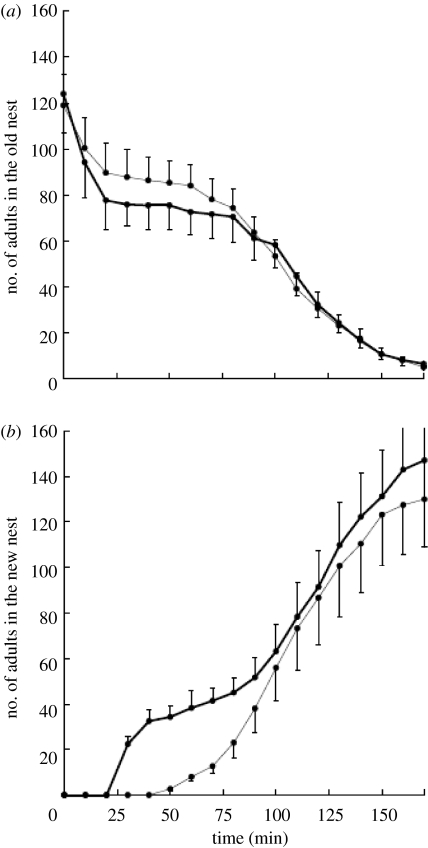
Figure 3.
Experiment 2. In the treatment, scouts were released in the arena with the new nest. In the control, scouts were released in the arena with the old nest. Mean numbers of individuals in the (a) old and (b) new nests for colonies from the control (grey line) or treatment (black line) group as a function of time since the old nest was destroyed. The error bars represent standard errors. In the treatment group, the ants used many reverse tandem runs. In the control group, the ants used many FTRs. RTRs can expedite emigrations.
(b) Experiment 2: is the abundance of reverse tandem runs influenced by the location of scouts and a reduction in the availability of potential tandem recruits at the old nest?
Several weeks after experiment 1 (so that the ants were not influenced by their prior experience—see Langridge et al. 2004), the same colonies were emigrated for a second time. Half of the treatment colonies in the first experiment became controls in the second, while half of the control colonies in the first experiment became treatments in the second. The experimental set-up was similar to experiment 1, with the addition of temporary arenas that served as corrals into which active scouts (as defined below) were imprisoned at the beginning of each emigration. Each temporary arena consisted of small (10 cm×10 cm×1.9 cm) Petri dishes, situated to one side of the large Petri dish that housed the original nest.
Colonies were induced to emigrate as before, however, the bridge was not introduced immediately. Colonies were allowed 5 min to relocate brood and settle after the nest had been opened. After this time and for a further 15 min period, ants scouting at least 2 cm from the nest perimeter were assumed to be active scouts and were aspirated from the old dish and isolated in the separate small Petri dish corral. Twenty minutes after starting the experiment, scouts belonging to control colonies were then gently transferred from the temporary arena into the centre of the old Petri dish, i.e. the one that housed the old nest, while those belonging to treatment colonies were gently deposited into the centre of the new Petri dish, i.e. the one housing the potential new nest site. Scouts in the treatments should have been much more disorientated than those in the controls. Colonies were subsequently left for 20 min, so transferred scouts could recover from the disturbance and investigate the particular large Petri dish to which they had been transferred. The bridge (figure 1) was then introduced and all subsequent emigration behaviour was recorded as in experiment 1.
(c) Statistical analysis
Effects of the treatment, colony size or number of scouts on the numbers of tandem runs were analysed using general linear model analyses of variance. Data on the dynamics of the emigration were analysed by means of Cox's (1972) proportional hazards models. Cox analysis is a standard technique for the analysis of censored survival data (Collett 1994; Dechaume-Moncharmont et al. 2003) and ecological and behavioural data (for example Dechaume-Moncharmont et al. 2005).
3. Results
(a) Experiment 1: do reverse tandem runs compensate for a disruption of FTRs?
(i) Number of tandem runs
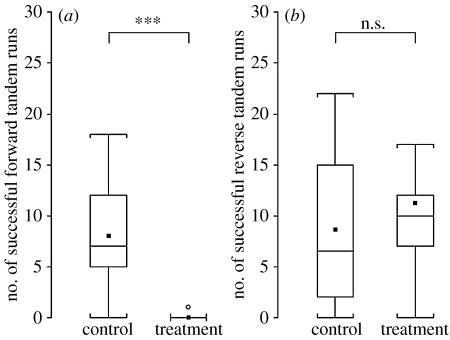
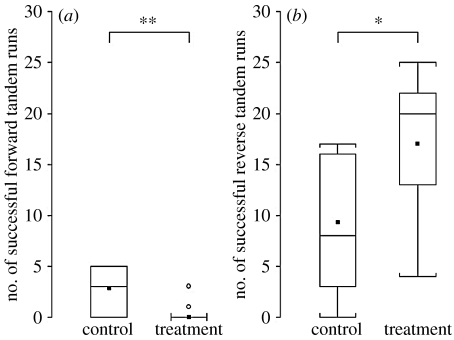
Disruption of FTRs during experiment 1 prevented almost every FTR from being successful. Thus, significantly more successful FTRs were recorded for the control colonies than for the treatment colonies (ANOVA: F1,18=19.47, p<0.001; figure 4a). However, there was no significant difference between the total numbers of FTRs (including partial and successful tandem runs) initiated by ants from control colonies or from treatment colonies before disruption by the experimenters (F1,18=3.02, p=0.100). This suggests that the ants in the treatment group neither tried harder to recruit by FTRs in response to low levels of success, nor did they give up on their attempts to lead FTRs. Furthermore, no significant difference was found between either the number of successful RTRs (F1,18=2.29, p=0.149; figure 4b) or the total number of RTRs initiated (F1,18=2.39, p=0.140) by control and treatment colonies. Colony size had no significant effect on the number of successful FTRs (F1,18=1.97, p=0.178); however, it did have a significant positive effect on the number of successful RTRs (F1,18=22.23, p<0.001). The number of scouts also had no significant effect on the number of successful FTRs (F1,16=2.05, p=0.173) or the number of successful RTRs (F1,16=0.44, p=0.517).
Figure 4.
Experiment 1. Box-plots of the number of successful (a) forward and (b) reverse tandem runs in control and treatment groups (10 colonies in each group). Horizontal lines within boxes are medians, boxes show inter-quartile ranges and whiskers show entire range (excluding the outliers represented by circles). The squares indicate means. ***p<0.001.
(ii) Dynamics of colony emigration
We recorded the number of ants in both nests (figure 2). The treatment significantly decreased the probability of ants both leaving the old nest (Χ12=35.43, p<0.0001) and entering the new nest (Χ12=101.05, p<10−5). There was a significant positive effect of colony size on the probability of ants leaving the old nest (Χ12=33.05, p<0.0001) and entering the new one (Χ12=13.08, p<0.001).
(b) Experiment 2: is the abundance of reverse tandem runs influenced by the location of scouts and a reduction in the availability of potential tandem recruits at the old nest?
(i) Number of tandem runs
Successful FTRs were performed more often by control colonies compared with treatment colonies (F1,17=9.41, p=0.007; figure 5a). Successful RTRs were performed significantly more often by treatment colonies in experiment 2 (in which the scouts were placed in the arena containing the new nest), than by control colonies (in which the scouts were returned to the arena containing the old nest; F1,17=5.85, p=0.028; figure 5b).
Figure 5.
Experiment 2. Box-plots of the number of successful (a) forward and (b) reverse tandem runs in control and treatment groups (10 colonies in each group). Interpretation of box-plots and symbols as in the legend of figure 4. **p<0.01, *p<0.05.
There was no significant effect of the number of scouts corralled on the number of successful FTRs (F1,15=0.91, p=0.357) or successful RTRs (F1,15=2.73, p=0.121). Colony size had no significant effect on the number of successful FTRs (F1,17=1.69, p=0.212) and had a significant positive effect on the number of successful RTRs (F1,17=4.79, p=0.044).
(ii) Dynamics of colony emigration
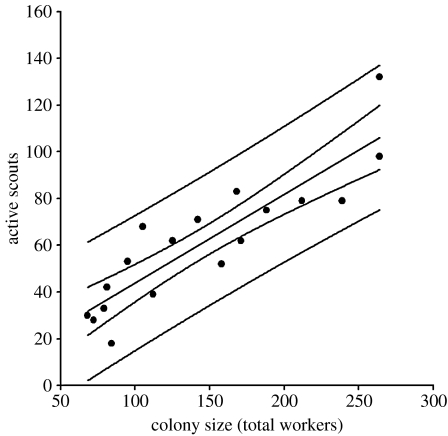
There was a significant positive relationship between colony size and the number of active ants that left the old nest in the period between 5 and 20 min after the old nest had been opened. The relationship is best described by active scouts=5.95+0.3795 (colony size) (r2=0.77, p<0.001).
These active ants were the ones collected and initially imprisoned. This procedure was identical in the treatment and control so the data were pooled. Active scouts, in colonies of all sizes, are approximately 40 per cent of a colony's total workforce (figure 6).
Figure 6.
Experiment 2. The relationship between colony size and the number of active ants that left the old nest in the period between 5 and 20 min after the old nest had been opened. The relationship is best described by active scouts=5.95+0.3795 (colony size) (r2=0.77, p<0.001). The central line is the fitted regression line; also shown are the 95% confidence limits for this line and for the dataset. One outlier has been removed.
The treatment significantly increased the probability of ants both leaving the old nest (Χ12=15.65, p<0.001, figure 3a) and entering the new nest (Χ12=4.96, p=0.026, figure 3b).
There was a significant positive effect of colony size on the probability of ants leaving the old nest (Χ12=21.08, p<0.001) but not on the probability of entering the new one (Χ12=1.65, p=0.19). The number of scouts had a significant effect on the probability of both leaving the old nest (Χ12=28.76, p<0.001) and entering the new one (Χ12=5.88, p=0.015). The number of ants in the new nest in experiment 2 (figure 3) shows different trends to those found in experiment 1 (figure 2). There was no increase at all in the number of ants in the new nest at the beginning of the emigration because the scouts were kept in the temporary corral. Moreover, as the ants from the treatment group were introduced into the new large Petri dish arena, they found the new nest more rapidly than the ants from the control group, which had been reintroduced to the original ‘old’ arena. Thus, the population in the new nest from the treatment group increased 20 min earlier than the corresponding populations in the control colonies. When this lag of 20 min was removed by transposing the data from the control emigrations forwards 20 min, the treatment was found to have no significant effect on the dynamics of new nest colonization (Χ12=1.64, p=0.20).
4. Discussion
The results reported here are the first systematic manipulation of the relative abundance of FTRs and reverse tandem runs (RTRs). Moreover, they not only explain why these house-hunting ants recruit in both directions but they also solve the prima facie paradox of quick decisions being of no value if they lead to slow implementation of choices.
The results of experiment 1 show that even a complete absence of successful FTRs did not lead to more RTRs. This refutes the hypothesis that RTRs directly compensate for too few successful FTRs. However, RTRs were significantly more abundant when the scouts were experimentally transferred (in experiment 2) to the vicinity of the new nest but did not ‘know’ how they got there (figure 5b). Accordingly, there were many more RTRs when the most active ants were more abundant near the new nest than at the old one (figure 5b). All of these results taken together strongly support the hypothesis that FTRs may occur when suitable recruits are available near the old nest and that RTRs may occur when suitable recruits are more available in the vicinity of the new nest. In both experiments, larger colonies produced significantly more RTRs. This is consistent with earlier results (Franks et al. 2006a). Larger colonies have more active workers (figure 6) so they may have a larger pool of potential RTR followers and leaders. In general, our observations suggest that tandem runs are initiated by tandem leaders rather than by lost scouts ‘asking for directions’. However, it obviously takes two to tandem and an FTR or RTR will only occur if both parties are willing and able actively to participate.
Overall, our results also strongly suggest that RTRs can rescue the fast dynamics of emigrations (figure 3), just as predicted by Planqué et al. (2007).
Our focus has been on how colonies maximize their emigration rates, as indicated by the gradients of the lines in figures 2 and 3, and hence how they minimize the average exposure of nest-mates to extranodal hazards. Note that total emigration times can be both highly variable and arguably not very meaningful if a colony takes several hours finally to retrieve the last brood item from the old nest.
In the light of these new results, we will now summarize our current understanding of this decision-making/choice implementation system.
In benign conditions, scouts slowly disperse from the old nest in search of the best available one. When they have found something suitable, they return to the old nest and can find many candidates among the active scout population that can be led in FTRs. Such recruitment builds a large quorum in a suitable nest. Hence, by the time the ants switch to recruitment by carrying, there is an abundance of active ants that have been taught an efficient emigration route between the old and new nests and can take an active role in carrying their more passive nest-mates quickly to the colony's new home. In such circumstances, we expect and find rather few reverse tandem runs. For example, the most benign conditions occur when the old nest remains intact and colonies are moving to improve. Under such circumstances, the ants use very high QTs, large numbers of FTRs and very few reverse tandem runs (Dornhaus et al. 2004).
By contrast, in harsh conditions many more scouts quickly leave the old nest (N. R. Franks 2003, personal observations; see also Pratt & Sumpter (2006) for a similar finding for a closely related species) dispersing rapidly far and wide looking, as it were, for any ‘port in a storm’, i.e. any moderately suitable new nest. When a scout finds something acceptable, and she returns to the old nest, very few scouts are available to be led in FTRs. Therefore, in an emergency, returning scouts do the next most useful task; they carry passive nest-mates (nest workers, the brood and even eventually the queen) to the new nest. (We define quorum sensing as complete when carrying begins. Therefore, by default, in emergency emigrations when the ants start carrying very soon, they have de facto typically used very small or non-existent QTs.) Carrying passive nest-mates continues but when a scout has deposited its load at the new nest, it attempts to lead RTRs from there (Pratt et al. 2002). This becomes increasingly easy as other scouts independently discover the new nest and become available to be led in an RTR. Why should these initially independent scouts be prepared to follow RTRs? One obvious explanation is that the longer they have taken to find the new nest the less direct will be the route they have taken to it from the old nest. Tandem runs teach effective and direct routes—but even these seem to get better through time (Pratt et al. 2005; Franks & Richardson 2006).
In an emergency emigration (i) FTRs can be rare, (ii) QTs appear to be low or non-existent, (iii) decision-making is quicker and more error-prone simply because it is less consensual and less well informed, (iv) carrying occurs sooner, and then (v) the ants may lead large numbers of reverse tandem runs to bring lost or disorientated scouts into an active role in the emigration. (For results supporting (i–iv), see Franks et al. 2003b, (v) applies to data reported in this paper.)
In the best of all possible (benign) worlds, FTRs might yield both more discerning collective decisions (because more individuals will contribute to nest evaluation) and faster emigrations if the positive feedback, associated with FTRs (which recruit further recruiters and so on), ‘kicks-in’ quickly. However, in harsh conditions, the ants use quick individual decisions and start emigrations directly by carrying because no active ant is available to be led in a FTR. Carrying is interspersed by reverse tandem runs (Planqué et al. 2006) that teach lost scouts to take an active role in the emigration (Richardson et al. 2007). Furthermore, any positive feedback associated with RTRs may also begin without delay (in the overall scheme of things, compared with FTR in benign conditions) because in harsh conditions latency periods associated with lengthy collective assessments will be minimized.
The emigration dynamics shown in figure 3a,b seem to imply that RTR's can lead to faster emigrations than FTR's. This, however, is almost certainly an artefact of scouts being transferred by hand to the new nest site. Nevertheless, these results do strongly suggest that reverse tandem runs can restore much of the speed of emigrations.
These interpretations also explain why some of our earlier results might appear anomalous. In certain of our experiments, in the laboratory under what we assume to be benign conditions, we sometimes observed more RTRs than FTRs and both may be very variable (Dornhaus et al. 2004; Franks et al. 2006a; E. A. Langridge 2000, personal communication; A. B. Sendova-Franks 1998, unpublished data). We now suspect that this is most often the case when, for technical reasons such as filming emigrations, the old nest and new one were very close together and in a small arena. This would have made the new nest quick and easy to discover by independent scouts. Hence a high quorum could be quickly met with very few FTRs. This interpretation is consistent with the data from experiment 2. In the treatment, the large numbers of scouts displaced by hand to the new arena found the new nest quickly (figure 3b) and this may have created an artificial quorum that helped to suppress FTRs (figure 5a; see also Pratt et al. 2002). In addition, if the new nest is extremely easy to find, many ants may find it so easily, and directly, that both FTRs and RTRs may be scarce because no ant needs to be taught the route (Franks & Richardson 2006; Richardson et al. 2007).
Therefore, in sum, we suggest that reverse tandem runs enable colonies to implement quick decisions rapidly. This interpretation is also compatible with certain RTRs also serving to reactivate scouts who had played an active recruitment role earlier in the emigration (Pratt et al. 2002).
These ants recruit in both directions when they emigrate, so that they can both decide quickly and emigrate quickly. They are thus very likely to have speed–accuracy trade-offs not just in the initial decision-making stages of their house hunting—but these should also feed forward into global compromises between accuracy of choice and speed of implementation.
Temnothorax albipennis also have an additional mechanism for expediting nest choice and emigrations in an emergency. They reconnoitre for potential nest sites before they have any need to emigrate and they remember poor ones with landmarks and mark them with pheromones. This presumably enables them to focus their search elsewhere for better nests if and when they become beset by the emergency of homelessness (Franks et al. 2007b).
Our results therefore support the view that colony-level emigration behaviour emerges from interactions among individuals following local, but sophisticated, behavioural rules (Camazine et al. 2001; Pratt et al. 2005). This indicates yet another example of a sophisticated strategy that T. albipennis has evolved in the process of efficiently finding, choosing and moving in to a new home.
Our study shows that decision-making may mean very little in isolation. It is not enough just to make a decision, either quickly or accurately. What is the value of a decision if it is not implemented well? Recall the scathing epitaph ‘He never said a foolish thing, nor ever did a wise one’.
Acknowledgments
N.R.F. wishes to thank the Biotechnology and Biological Sciences Research Council (BBSRC) for research grant (E19832), which supported F.-X.D.-M. We also gratefully acknowledge Elizabeth Langridge, Ana Sendova-Franks, Bob Planqué, Nathalie Stroeymeyt, Elva Robinson and other members of the Bristol ant lab for their helpful discussions.
Footnotes
One contribution of 11 to a Theme Issue ‘Group decision making in humans and animals’.
References
- Camazine S., Deneubourg J.-L., Franks N.R., Sneyd J., Theraulaz G., Bonabeau E. Princeton University Press; Princeton, NJ: 2001. Self-organization in biological systems. [Google Scholar]
- Chittka L., Dyer A.G., Bock F., Dornhaus A. Bees trade off foraging speed for accuracy. Nature. 2003;424:388. doi: 10.1038/424388a. doi:10.1038/424388a [DOI] [PubMed] [Google Scholar]
- Collett D. Chapman & Hall; London, UK: 1994. Modelling survival data in medical research. [Google Scholar]
- Cox D.R. Regression models and life tables (with discussion) J. R. Stat. Soc. B. 1972;34:187–220. [Google Scholar]
- Dean, T. L. & Boddy, M. 1988 An analysis of time-dependent planning. In Proc. 7th Nat. Conf. on Artificial Intelligence (eds T. M. Mitchell & R. G. Smith), pp. 49–54. Menlo Park, CA: AAAI Press.
- Dechaume-Moncharmont F.X., Decourtye A., Hennequet C., Pons O., Pham-Delègue M.-H. Statistical analysis of honeybee survival after chronic exposure to insecticides. Environ. Toxicol. Chem. 2003;22:3088–3094. doi: 10.1897/02-578. doi:10.1897/02-578 [DOI] [PubMed] [Google Scholar]
- Dechaume-Moncharmont F.X., Azzouz H., Pons O., Pham-Delègue M.H. Soybean proteinase inhibitor does not affect the foraging strategy of free flying honeybees. Apidologie. 2005;36:421–430. doi:10.1051/apido:2005031 [Google Scholar]
- Dornhaus A., Franks N.R., Hawkins R.M., Shere H.N.S. Ants move to improve—colonies of Leptothorax albipennis emigrate whenever they find a superior nest site. Anim. Behav. 2004;67:959–963. doi:10.1016/j.anbehav.2003.09.004 [Google Scholar]
- Edwards W. Optimal strategies for seeking information: models for statistics, choice reaction times, and human information processing. J. Math. Psychol. 1965;2:312–329. doi:10.1016/0022-2496(65)90007-6 [Google Scholar]
- Franks N.R., Richardson T. Teaching in tandem-running ants. Nature. 2006;439:153. doi: 10.1038/439153a. doi:10.1038/439153a [DOI] [PubMed] [Google Scholar]
- Franks N.R., Pratt S.C., Mallon E.B., Britton N.F., Sumpter D.J.T. Information flow, opinion polling and collective intelligence in house-hunting social insects. Phil. Trans. R. Soc. B. 2002;357:1567–1583. doi: 10.1098/rstb.2002.1066. doi:10.1098/rstb.2002.1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N.R., Dornhaus A., Fitzsimmons J.P., Stevens M. Speed versus accuracy in collective decision making. Proc. R. Soc. B. 2003a;270:2457–2463. doi: 10.1098/rspb.2003.2527. doi:10.1098/rspb.2003.2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N.R., Mallon E.B., Bray H.E., Hamilton M.J., Mischler T.C. Strategies for choosing among alternatives with different attributes: exemplified by house-hunting ants. Anim. Behav. 2003b;65:215–223. doi:10.1006/anbe.2002.2032 [Google Scholar]
- Franks N.R., Hooper J.W., Webb C., Dornhaus A. Tomb evaders: house-hunting hygiene in ants. Biol. Lett. 2005;1:190–192. doi: 10.1098/rsbl.2005.0302. doi:10.1098/rspb.2005.0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N.R., Dornhaus A., Best C.S., Jones E.L. Decision-making by small & large house-hunting ant colonies: one size fits all. Anim. Behav. 2006a;72:611–616. doi:10.1016/j.anbehav.2005.11.019 [Google Scholar]
- Franks N.R., Dornhaus A., Metherell B.G., Nelson T.R., Lanfear S.A.J., Symes W.S. Not everything that counts can be counted: ants use multiple metrics for a single nest trait. Proc. R. Soc. B. 2006b;273:165–169. doi: 10.1098/rspb.2005.3312. doi:10.1098/rspb.2005.3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N.R., Dornhaus A., Hitchcock G., Guillem R., Hooper J., Webb C. Avoidance of conspecific colonies during nest choice by ants. Anim. Behav. 2007a;73:525–534. doi:10.1016/j.anbehav.2006.05.020 [Google Scholar]
- Franks N.R., Hooper J.W., Dornhaus A., Aukett P.J., Hayward A.L., Berghoff S.M. Reconnaissance and latent learning in ants. Proc. R. Soc. B. 2007b;274:1505–1509. doi: 10.1098/rspb.2007.0138. doi:10.1098/rspb.2007.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge E.A., Franks N.R., Sendova-Franks A.B. Improvement in collective performance with experience in ants. Behav. Ecol. Sociobiol. 2004;56:523–529. doi:10.1007/s00265-004-0824-3 [Google Scholar]
- Mallon E., Franks N.R. Ants estimate area using Buffon's needle. Proc. R. Soc. B. 2000;267:765–770. doi: 10.1098/rspb.2000.1069. doi:10.1098/rspb.2000.1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon E.B., Pratt S.C., Franks N.R. Individual and collective decision-making during nest site selection by the ant Leptothorax albipennis. Behav. Ecol. Sociobiol. 2001;50:352–359. doi:10.1007/s002650100377 [Google Scholar]
- Marshall J.A.R., Dornhaus A., Franks N.R., Kovacs T. Noise, cost and speed-accuracy trade-offs: decision-making in a decentralized system. J. R. Soc. Interface. 2006;3:243–254. doi: 10.1098/rsif.2005.0075. doi:10.1098/rsif.2005.0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeman M.A., Pratt S.C., Franks N.R. Navigation using visual landmarks by the ant Leptothorax albipennis. Insectes Soc. 2002;49:203–208. doi:10.1007/s00040-002-8302-2 [Google Scholar]
- Möglich M. Social organization of nest emigration in Leptothorax (Hym. Form.) Insectes Soc. 1978;25:205–225. doi:10.1007/BF02224742 [Google Scholar]
- Möglich M., Maschwitz U., Hölldobler B. Tandem calling: a new kind of signal in ant communication. Science. 1974;186:1046–1047. doi: 10.1126/science.186.4168.1046. doi:10.1126/science.186.4168.1046 [DOI] [PubMed] [Google Scholar]
- Mugford S.T., Mallon E.B., Franks N.R. The accuracy of Buffon's needle: a rule of thumb used by ants to estimate area. Behav. Ecol. 2001;12:655–658. doi:10.1093/beheco/12.6.655 [Google Scholar]
- Planqué R., Dornhaus A., Franks N.R., Kovacs T., Marshall J.A.R. Weighting waiting in collective decision making. Behav. Ecol. Sociobiol. 2006;61:347–356. doi:10.1007/s0265-006-0263-4 [Google Scholar]
- Planqué R., Dechaume-Moncharmont F.-X., Franks N.R., Kovacs T., Marshall J.A.R. Why do house-hunting ants recruit in both directions? Naturwissenschaften. 2007;94:911–918. doi: 10.1007/s00114-007-0273-8. doi:10.1007/s00114-007-0273-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt S. Quorum sensing by encounter rate in the ant Temnothorax albipennis. Behav. Ecol. 2005;16:488–496. doi:10.1093/beheco/ari020 [Google Scholar]
- Pratt S.C., Sumpter D.J.T. A tunable algorithm for collective decision-making. Proc. Natl Acad. Sci. USA. 2006;103:15 906–15 910. doi: 10.1073/pnas.0604801103. doi:10.1073/pnas.0604801103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt S.C., Brooks S.E., Franks N.R. The use of edges in visual navigation by the ant Leptothorax albipennis. Ethology. 2001;107:1125–1136. doi:10.1046/j.1439-0310.2001.00749.x [Google Scholar]
- Pratt S.C., Mallon E.B., Sumpter D.J.T., Franks N.R. Quorum sensing, recruitment, and collective decision-making during colony emigration by the ant Leptothorax albipennis. Behav. Ecol. Sociobiol. 2002;52:117–127. doi:10.1007/s00265-002-0487-x [Google Scholar]
- Pratt S.C., Sumpter D.J.T., Mallon E.B., Franks N.R. An agent-based model of collective nest choice by the ant Temnothorax albipennis. Anim. Behav. 2005;70:1023–1036. doi:10.1016/j.anbehav.2005.01.022 [Google Scholar]
- Richardson T.O., Sleeman P.A., McNamara J.M., Houston A.I., Franks N.R. Teaching with evaluation in ants. Curr. Biol. 2007;17:1520–1526. doi: 10.1016/j.cub.2007.08.032. doi:10.1016/j.cub.2007.08.032 [DOI] [PubMed] [Google Scholar]
- Roitman J.D., Shadlen M.N. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J. Neurosci. 2002;22:9475–9489. doi: 10.1523/JNEUROSCI.22-21-09475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitevitch M.S. Influence of onset density on spoken word recognition. J. Exp. Psychol. Hum. Percep. Perform. 2002;28:270–278. doi: 10.1037//0096-1523.28.2.270. doi:10.1037/0096-1523.28.2.270 [DOI] [PMC free article] [PubMed] [Google Scholar]