The bony conformity of the glenoid and humeral head articular surfaces provides some of the stability of the shoulder. Frequently, patients with recurrent dislocations have bony deficits in one or both of these surfaces. Glenohumeral instability with substantial glenoid defects or engaging Hill–Sachs lesions poses a difficult challenge for orthopedic surgeons.
Burkhart and colleagues1 report a 67% recurrence rate using only soft-tissue repair in patients with recurrent dislocations and glenoid bony defects. Treatment options for glenoid bony defects vary from soft-tissue repair only if the defect is small to bone grafting2 and Bristow–Laterjet coracoid transfers if the defect is large.3,4 Small or nonengaging Hill–Sachs lesions are usually left alone while addressing the Bankart lesion, but they must be addressed when the Hill–Sachs lesion engages.1 Treatments include osteochondral grafting,5 infraspinatus transfer,6 humeral head plasty7 and derotational osteotomies.8 Ignoring a large deficit may lead to failure of the soft tissue Bankart repair.
We present the cases of 2 patients whose shoulders required interventions for both the humeral head and the glenoid to remain stable. We reconstructed the glenoid using a Latarjet procedure, and we treated the Hill–Sachs lesion with focal arthroplasty using the HemiCAP implant (Arthrosurface), a novel approach to the problem. At 1 year follow-up, neither patient had experienced a recurrence.
Case 1
An active 35-year-old, right-handed man presented with recurrent right shoulder instability despite having had a previous surgical stabilization procedure. Two years before presentation he had undergone an open Bankart repair for anterior instability, but within 1 year of the operation, he had dislocated the same shoulder while reaching back during a rock climbing venture. He displayed apprehension in the abducted external rotated position, but the results of range of motion, laxity indices, rotator cuff, posterior apprehension and neurovascular examinations were normal. Radiographs and magnetic resonance images (MRI) revealed a small Hill–Sachs lesion and a small bony and soft tissue Bankart lesion, so we concluded that failure was related to technical error or reinjury. Therefore, we decided to proceed with an arthroscopic revision of the Bankart repair. We confirmed that the glenoid loss and Hill–Sachs defects were small during the diagnostic arthroscopy, and we performed an isolated soft tissue Bankart repair.
The patient’s postoperative course and rehabilitation were uneventful, but 9 months later the patient dislocated his shoulder once more, this time while throwing a Frisbee.
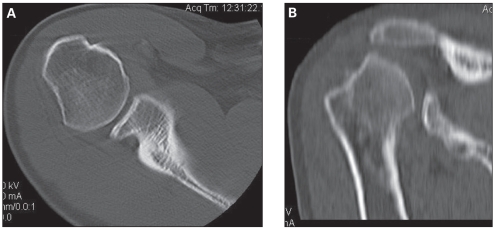
His range of motion was 160° of forward elevation, 60° of external rotation in adduction and T9 internal rotation. He had a markedly positive anterior apprehension test. We ordered repeat radiographs and a computed tomography (CT) scan. These investigations demonstrated increased bony loss with a moderate Hill–Sachs lesion 4 cm in diameter involving an estimated 40% of the humeral head and a substantial glenoid bony defect involving an estimated 30% of its surface (Fig. 1).
Fig. 1.
(A) Axial computed tomography (CT) scan evaluation demonstrates moderately sized Hill–Sachs and bony Bankart lesions. (B) Coronal reconstruction of the CT scan reveals the Hill–Sachs lesion.
We outlined the surgical options for the patient, including reconstruction of one or both of these defects. For the humerus, we included the HemiCAP implant as a novel option that only had short follow-up but held promise. The patient agreed to have the implant inserted if needed, and he agreed to a potential Latarjet procedure.
Under general anesthesia, his shoulder proved to be grossly unstable as it could easily be dislocated anteriorly and would even lock after dislocation. Using an anterior deltopectoral approach, we exposed the glenohumeral joint. The glenoid defect was large and we visually estimated it to comprise 33% of the total glenoid surface. The Hill–Sachs defect was large and we estimated it to involve 40% of the humeral head, confirming the findings from the CT scan. We therefore resurfaced the Hill–Sachs defect using the HemiCAP implant. A 25-mm implant with a 5 × 5–mm offset provided the best fit. Once we resurfaced the defect, we retested shoulder stability. Anterior instability was still present within a functional range of motion without any engagement of the now reconstructed humeral head defect. We performed a Latarjet coracoid transfer to address the anterior instability caused by the glenoid bone loss.
In the postoperative period, we put the patient’s arm in a sling and performed protected passive range of motion (ROM) for the first 6 weeks. After 6 weeks, we allowed unrestricted passive ROM and began active ROM. We started active resisted ROM after 3 months and we allowed sports after 6 months.
At 1 year follow-up, the patient was free of pain and had excellent ROM without instability. His shoulder had 150° of forward elevation, 60° of external rotation, and T9 internal rotation, and he had a negative anterior apprehension test.
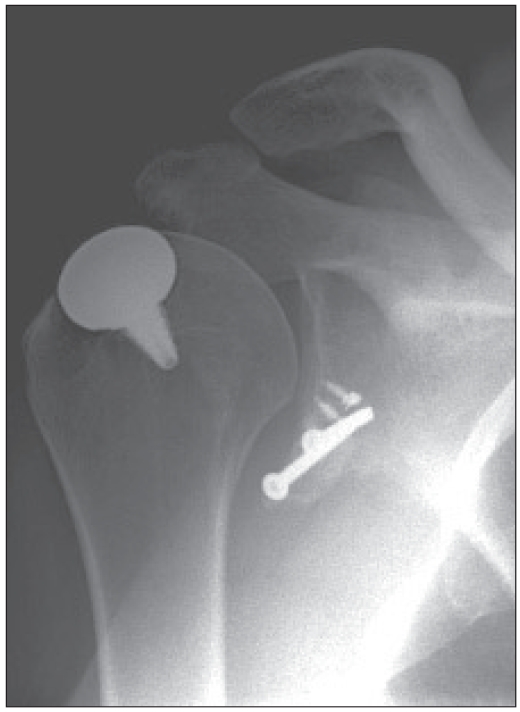
His score on the American Shoulder and Elbow Surgeons (ASES) shoulder scale,9 which is graded from 0 to 100 with a high score indicating good function, was 88 preoperatively and 92 postoperatively. His score on the Western Ontario Shoulder Instability Index (WOSI),10 which is graded from 0 to 2100 with a high score indicating poor function, improved from 1054 preoperatively to 306 postoperatively. The postoperative anteroposterior radiograph obtained at the latest follow-up is shown in Figure 2.
Fig. 2.
Postoperative radiograph showing the implant in proper position and the coracoid transfer bony block.
Case 2
A 25-year-old man was referred to us owing to right shoulder instability. His first dislocation occurred 4 years earlier when he fell out of bed. He received a sling support for 6 weeks. He subsequently experienced multiple recurrences, each during low-energy activities such as golf. Physiotherapy provided some relief, but persistent instability, pain and stiffness prompted him to seek medical help.
On examination he had 120° of forward elevation, 30° of external rotation in adduction and T12 internal rotation. He also had a markedly positive anterior apprehension test. Results of rotator cuff and neurovascular examinations were normal.
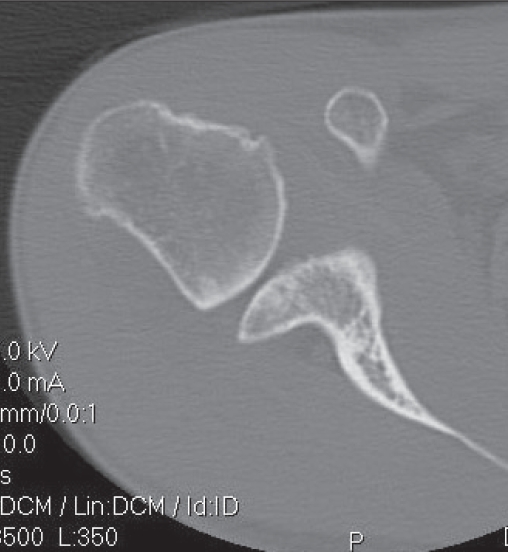
Radiographs revealed small Hill–Sachs and Bankart lesions and some moderate osteoarthritis. A CT scan revealed more extensive damage. We estimated the Hill–Sachs lesion to cover 33% of the humeral head and the bony glenoid loss to cover 33% of its total surface (Fig. 3).
Fig. 3.
Axial computed tomography scan identifying large Hill–Sachs and bony Bankart lesions.
Despite some osteoarthritic changes and limited range of motion, the patient’s shoulder was severely unstable. We envisioned several surgical options; owing to the large nature of both the glenoid and the humeral head defects, a soft tissue repair alone seemed inadequate. We opted to perform a Latarjet procedure along with bone grafting and/or arthroplasty of the humeral head lesion with the patient’s agreement.
His shoulder dislocated readily under anesthesia and appeared grossly unstable. Using a deltopectoral approach, we visualized the glenohumeral joint and we estimated the glenoid defect to cover 50% of the glenoid. The Hill–Sachs lesion was also extensive, involving an estimated 40% of the head surface. Based on this finding, we proceeded with the head reconstruction using the HemiCAP implant. We used a 25-mm implant with a 4 × 4–mm offset.
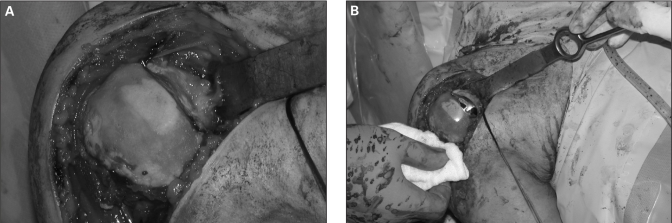
His shoulder was still unstable anteriorly after the HemiCAP reconstruction owing to the extensive glenoid bone loss, therefore we performed the Latarjet coracoid transfer also. The humeral head before and after insertion of the HemiCAP is shown in Figure 4. Postoperatively, the patient’s rehabilitation protocol mirrors the one used in the first patient’s case.
Fig. 4.
Intraoperative photographs displaying (A) the Hill–Sachs lesion and moderate osteoarthritis and (B) the HemiCAP implant in place.
At 1 year follow-up, the patient was doing well without recurrence and enjoyed 120° of forward elevation, 30° of external rotation in abduction and T12 internal rotation. His ASES score improved from 55 preoperatively to 78 postoperatively. His WOSI score improved from 1490 preoperatively to 561 postoperatively.
Discussion
Hill–Sachs and Bankart lesions are common findings after even a single dislocation.11 By ordering an MRI for every patient with an acute dislocation, Widjaja and colleagues12 found that 73% of patients with primary dislocated shoulders had Bankart lesions and 67% had Hill–Sachs lesions. The incidence and severity of these lesions increases in patients whose shoulders have dislocated multiple times. Yiannakopoulos and colleagues13 reviewed the arthroscopic findings of 23 patients with acutely dislocated shoulders and compared them with the findings in patients with shoulders that had dislocated multiple times (104 patients with an average of 8 dislocations) and found that Bankart/anterior labral periosteal sleeve avulsions occured in 78% of patients with acute dislocations and 97% of patients with chronic dislocations and that Hill–Sachs lesions occured in 65% of patients with acute dislocations and 93% of patients with chronic dislocations. They found that inverted pear glenoid occured in 15% of patients with chronic dislocations, but not in patients with acute dislocations.
Several studies have demonstrated the important role that glenoid bony defects can play in recurrence. Burkhart and colleagues1 found that a glenoid with an inverted pear shape led to a 67% failure rate of the soft tissue Bankart repair alone versus a 4% failure rate when no such defect was present. In a cadaver study, Itoi and colleagues14 found that a 21% loss of glenoid resulted in substantially lesser forces needed to dislocate the shoulder in abduction and internal rotation.
Surgical options to reconstruct the glenoid defects include glenoid osteochondral allografts, autogenous iliac crest grafts and Latarjet coracoid transfer.
Warner and colleagues2 reviewed the results of grafting the glenoid with iliac crest bone in 11 patients in whom the glenoid defect length exceeded the maximum anterior to posterior radius of the glenoid on 3-dimensional reconstruction CT scans. They found that at a mean follow-up of 33 months the mean ASES score improved from 65 to 94 and that the University of California, Los Angeles (UCLA) score improved from 18 to 33. There were no recurrence and the mean loss of range of motion was 7° of flexion, 14° of external rotation in abduction and 1 spinous process of internal rotation.
Hovelius and colleagues15 prospectively studied the results of 118 Bristow–Latarjet procedures. Of these 118 patients, 111 (94%) had not undergone previous shoulder surgeries. At 2-year follow-up, 1 patient (1%) had experienced a redislocation that did not require surgery, and 103 (87%) had excellent and 13 (11%) had good results on Rowe scoring. The average loss of external rotation in abduction was 8°, whereas the loss of internal rotation averaged 1.3 spinal levels. After 15 years, 1 patient (1%) required reoperation for instability, 4 patients (3%) experienced a recurrence and 12 (10%) experienced subluxation. The Rowe scoring was excellent in 90 (76%), good in 26 (22%), fair in 1 (1%) and poor in 1 (1%).
Humeral head defects can also lead to recurrence when engaging.1 Many treatment options exist for these osteochondral defects, including humeral head plasty, infraspinatus transfer, osteochondral grafting and rotation osteotomy. The evidence for each of these options, however, is largely anecdotal and based on small series or case reports.
In a case series of 18 patients, Miniaci and colleagues16 describe the result of reconstructing Hill–Sachs lesions with osteochondral humeral head allografts along with a capsular plication or Bankart repair. All patients had a failed previous repair and all had engaging Hill–Sachs lesions involving more than 25% of the articular margin. At an average follow-up of 50 months, there were no recurrences, and 16 (89%) had returned to work. Two patients (11%) had partial graft collapses, 3 (17%) experienced early osteoarthritis and 2 (11%) had their screws removed because of pain at the extremes of external rotation.
Weber and colleagues8 retrospectively reviewed the results of 180 rotational osteotomies and found a 5.7% redislocation rate. They found that 162 patients (90%) had a good to excellent result; 2 patients (1%) underwent reoperation because of dislocations, 6 (3%) because of delayed or nonunion and 1 (0.5%) because of over-rotation at the osteotomy site. A total of 107 plates (59%) were removed after 1–2 years.
We present in this paper a novel approach to deal with the problematic large engaging Hill–Sachs lesion using the HemiCAP9,17 partial humeral head resurfacing implant. The implant comprises a round cap-like articular surface component made of chrome-cobalt that secures via taper lock to a titanium-coated peg that is cemented into the humeral head subchondral bone. The system’s instrumentation and the implant’s various sizes and offsets allow the surgeon to reproduce the humeral head contour precisely with little bone resection. Fit is determined such that the implant sits flush with the articular margin of the defect without extending into the insertion of the rotator cuff while reproducing both the superior-inferior and the medial-lateral radii of curvatures of the defect. Despite the round implant sometimes only filling about three-quarters of the wedge shaped Hill–Sachs defect, engagement is prevented and incongruity is substantially reduced.
Davidson and colleagues,18 in a non–peer reviewed publication, prospectively examined 62 patients with a mean age of 60 years who underwent HemiCAP humeral resurfacing for shoulder pain. The indications were gleno-humeral arthritis in 45 patients (73%), avascular necrosis in 8 (13%), focal humeral head full thickness defect in 4 (6%), acromio-humeral arthritis in 4 (6%) and rheumatoid arthritis in 1 (2%). They excluded patients with instability. Thirteen patients (20%) had concomitant rotator cuff repairs, 10% had labral débridement or repair and 19% had subacromial decompression and distal clavicle excision. At a mean follow-up of 8 months, the mean Western Ontario Osteoarthritis Score improved from 247 preoperatively to 1234 postoperatively, the mean ASES score improved from 38.4 to 69.3, pain on a scale of 0 to 100 improved from 54 to 18. There was no evidence of loosening or migration on radiographic review.
To our knowledge, there are no studies to date on the HemiCAP implant used in the setting of instability. There are disadvantages to using an implant to reconstruct the humeral head. Specifically, there are no long-term studies on the HemiCAP implant at this point and as such the long-term survivorship rates are not known. As patients presenting with Hill–Sachs lesions tend to be young, this caveat may be relevant but some features of the HemiCAP implant in the shoulder may give it more longevity. First, the implant contains no polyethylene component, which is believed to be one of the major culprits in osteolysis. Second, failure of shoulder arthroplasty is linked more often to the glenoid component than to the humerus, upon which this implant lies. Third, the use of an implant that preserves bone stock makes eventual revision simpler.
Resurfacing one side of a joint may lead to cartilage erosion on the other side in areas where the implant articulates, and this may lead to pain. This has proved to be a problem in the hip when only the femoral side is resurfaced. The shoulder, however, is not a weight-bearing joint. Furthermore, the area of metal to cartilage contact is much smaller as most of the humeral cartilage remains in place with the HemiCAP implant.
Advantages of using the HemiCAP implant over autogenous bone grafting include the absence of donor site morbidity and, possibly, a more accurate contouring and a shorter operative time.
Avoiding iliac crest bone graft donor site morbidity can be clinically beneficial. In a retrospective review of 66 anterior iliac crest bone graft procedures for the treatment of chronic osteomyelitis with a minimum 2-year follow-up, Ahlmann and colleagues19 found that 1 patient (2%) had pain for 6 or more months and that 3 patients (5%) had a sensory disturbance in the lateral femoral cutaneous nerve distribution for 6 or more months. In a prospective study involving 208 patients in 4 centres, Sasso and colleagues20 examined morbidity associated with anterior iliac crest bone grafting used in anterior lumbar interbody fusion and found that only 2 patients (1%) had no donor site pain at discharge. The number of patients increased to 35 patients (17%) at 6 weeks and 89 patients (43%) at 3 months, but 68 patients (33%) still reported some pain after 1 year. Each patient answered 2 questions about their pain on a visual analogue scale of 0 to 10. The first question asked about the intensity of pain with 0 indicating no pain and 10 indicating extreme pain, and the second question asked about the frequency of the pain with 0 indicating none of the time and 10 indicating all of the time. After combining the results of these questions, the mean pain score at the donor site was 12.8 out of 20 at discharge, 7.3 at 6 weeks, 3.8 at 3 months and 1.8 at 1 year follow-up.
Another advantage of the HemiCAP implant is the absence of disease transmission associated with allografts. The implant is more readily available than allografts and avoids problems associated with graft resorption and hardware prominence.
Our case series shows that the combination of large glenoid and humeral bony defects encountered with some patients who experience recurrent shoulder dislocations can be effectively treated with a good functional outcome. In one patient, the WOSI score improved from 1054 to 306, while in the other patient, the WOSI score improved from 1490 to 561. Although the experience with this focal arthroplasty technique is limited and the follow-up is relatively short, our results are encouraging. Hopefully our report can provide the impetus for further studies related to this clinical problem using this management option.
It is worth noting, however, that most patients with bony deficits on both the humeral head and the glenoid can suitably be treated by reconstructing only one of the deficits, but occasionally both defects may require intervention. To date, there are no validated preoperative guidelines for when both procedures are required; intra-operative assessment remains our best tool. When CT scans show that both the humeral head and the glenoid have more than 30% surface loss, the treating physician should be prepared to deal with both problems if instability persists intraoperatively despite fixing one defect. The treating physician may wish to refer to a subspecialist surgeon working at a tertiary care centre where such a dual intervention is best handled.
Footnotes
Competing interests: None declared.
References
- 1.Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill–Sachs lesion. Arthroscopy. 2000;16:677–94. doi: 10.1053/jars.2000.17715. [DOI] [PubMed] [Google Scholar]
- 2.Warner JJ, Gill TJ, O’Hollerhan JD, et al. Anatomical glenoid reconstruction for recurrent anterior glenohumeral instability with glenoid deficiency using an autogenous tricortical iliac crest bone graft. Am J Sports Med. 2006;34:205–12. doi: 10.1177/0363546505281798. [DOI] [PubMed] [Google Scholar]
- 3.Bigliani LU, Newton PM, Steinmann SP, et al. Glenoid rim lesions associated with recurrent anterior dislocation of the shoulder. Am J Sports Med. 1998;26:41–5. doi: 10.1177/03635465980260012301. [DOI] [PubMed] [Google Scholar]
- 4.Hovelius L, Akermark C, Albrektsson B, et al. Bristow-Latarjet procedure for recurrent anterior dislocation of the shoulder. year follow-up study on the results of 112 cases. A . Acta Orthop Scand. 1983;54:2–5. 284–90. doi: 10.3109/17453678308996571. [DOI] [PubMed] [Google Scholar]
- 5.Chapovsky F, Kelly JD., IV Osteochondral allograft transplantation for treatment of glenohumeral instability. Arthroscopy. 2005;21:1007. doi: 10.1016/j.arthro.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Connolly JF. Humeral head defects associated with shoulder dislocations—their diagnostic and surgical significance. Instr Course Lect. 1972;21:42–54. [Google Scholar]
- 7.Re P, Gallo RA, Richmond JC. Transhumeral head plasty for large Hill–Sachs lesions. Arthroscopy. 2006;22:798.e.1–4. doi: 10.1016/j.arthro.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 8.Weber BG, Simpson LA, Hardegger F. Rotational humeral osteotomy for recurrent anterior dislocation of the shoulder associated with a large Hill–Sachs lesion. J Bone Joint Surg Am. 1984;66:1443–50. [PubMed] [Google Scholar]
- 9.Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3:347–52. doi: 10.1016/S1058-2746(09)80019-0. [DOI] [PubMed] [Google Scholar]
- 10.Kirkley A, Griffin S, McLintock H, et al. The development and evaluation of a disease-specific quality of life measurement tool for shoulder instability. The Western Ontario Shoulder Instability Index (WOSI) Am J Sports Med. 1998;26:764–72. doi: 10.1177/03635465980260060501. [DOI] [PubMed] [Google Scholar]
- 11.Taylor DC, Arciero RA. Pathologic changes associated with shoulder dislocations. Arthroscopic and physical examination findings in first-time, traumatic anterior dislocations. Am J Sports Med. 1997;25:306–11. doi: 10.1177/036354659702500306. [DOI] [PubMed] [Google Scholar]
- 12.Widjaja AB, Tran A, Bailey M, et al. Correlation between Bankart and Hill–Sachs lesions in anterior shoulder dislocation. ANZ J Surg. 2006;76:436–8. doi: 10.1111/j.1445-2197.2006.03760.x. [DOI] [PubMed] [Google Scholar]
- 13.Yiannakopoulos CK, Mataragas E, Antonogiannakis E. A comparison of the spectrum of intra-articular lesions in acute and chronic anterior shoulder instability. Arthroscopy. 2007;23:985–90. doi: 10.1016/j.arthro.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Itoi E, Lee SB, Berglund LJ, et al. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82:35–46. doi: 10.2106/00004623-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Hovelius L, Sandström B, Sundgren K, et al. One hundred eighteen Bristow-Latarjet repairs for recurrent anterior dislocation of the shoulder prospectively followed for fifteen years: study I–clinical results. J Shoulder Elbow Surg. 2004;13:509–16. doi: 10.1016/j.jse.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Miniaci A, Gish MW. Management of anterior glenohumeral instability associated with large Hill–Sachs defects. Tech Shoulder Elbow Surg. 2004;5:170–5. [Google Scholar]
- 17.Kirker-Head CA, Van Sickle DC, Ek SW, et al. Safety of, and biological and functional response to, a novel metallic implant for the management of focal full-thickness cartilage defects: preliminary assessment in an animal model out to 1 year. J Orthop Res. 2006;24:1095–108. doi: 10.1002/jor.20120. [DOI] [PubMed] [Google Scholar]
- 18.Davidson PA, Lemak LJ, Uribe JW, et al. Anatomic humeral head resurfacing. [(accessed 2009 Apr. 15)];Review of clinical outcomes and case presentations. Available: www.arthrosurface.com/templates/Arthro_fourmangos/images/pdfs/pn0020_0100rev-b.pdf.
- 19.Ahlmann E, Patzakis M, Roidis N, et al. Comparison of anterior and posterior iliac crest bone grafts in terms of harvest-site morbidity and functional outcomes. J Bone Joint Surg Am. 2002;84-A:716–20. doi: 10.2106/00004623-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Sasso RC, LeHuec JC, Shaffrey C Spine Interbody Research Group. Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18(Suppl):S77–81. doi: 10.1097/01.bsd.0000112045.36255.83. [DOI] [PubMed] [Google Scholar]