Abstract
Stable isotope labeling by amino acids in cell culture (SILAC) is a powerful quantitative proteomics platform for comprehensive characterization of complex biological systems. However, the potential of SILAC-based approaches has not been fully utilized in human embryonic stem cell (hESC) research mainly because of the complex nature of hESC culture conditions. Here we describe complete SILAC labeling of hESCs with fully preserved pluripotency, self-renewal capabilities, and overall proteome status that was quantitatively analyzed to a depth of 1556 proteins and 527 phosphorylation events. SILAC-labeled hESCs appear to be perfectly suitable for functional studies, and we exploited a SILAC-based proteomics strategy for discovery of hESC-specific surface markers. We determined and quantitatively compared the membrane proteomes of the self-renewing versus differentiating cells of two distinct human embryonic stem cell lines. Of the 811 identified membrane proteins, six displayed significantly higher expression levels in the undifferentiated state compared with differentiating cells. This group includes the established marker CD133/Prominin-1 as well as novel candidates for hESC surface markers: Glypican-4, Neuroligin-4, ErbB2, receptor-type tyrosine-protein phosphatase ζ (PTPRZ), and Glycoprotein M6B. Our study also revealed 17 potential markers of hESC differentiation as their corresponding protein expression levels displayed a dramatic increase in differentiated embryonic stem cell populations.
Human embryonic stem cells (hESCs)1 are stem cells derived from the blastocyst inner cell mass. They are pluripotent; thus they are able to differentiate into any human cell type. The self-renewal capacity and pluripotency make hESCs an ideal system to study the processes of cell development and differentiation. Moreover hESC research is highly relevant for regenerative medicine, which aims at replacing or restoring tissue damaged by disease or injury through transplantation of functional hESCs (1,2). However, factors responsible for maintaining the undifferentiated and pluripotent nature of hESCs are still largely unknown. Before hESCs can be used for transplantation into the human body, reliable and reproducible protocols for differentiating them into specific cell types are needed. To create such protocols we need to develop a thorough understanding of the mechanisms maintaining the undifferentiated pluripotent nature of hESCs and those guiding their differentiation into specific lineages.
A number of factors involved in the maintenance of pluripotency have been described over the last few years (3). It has also been demonstrated that overexpression of some of these factors in somatic cells is sufficient to turn them into pluripotent stem cells very similar to hESCs (4–8). However, it is apparent that the processes occurring during such transformation are extremely complex. A large number of factors and pathways are involved in maintaining the pluripotent state and regulating self-renewal and differentiation. The process of specific hESC differentiation into distinct cell types is even less understood. Most current attempts to directionally differentiate hESCs are based on sequential application of empirically selected growth factors and consequent selection for markers expressed in the target cell types (9). A more systematic approach is needed to improve our understanding of the pathways that control the conversion of precursors into specific cell types, progressing toward the goal of reproducing these processes in vitro for the generation of functional cells and tissues for transplantation.
Comprehensive quantitative analysis of the hESC proteome would mean an important advance in understanding the nature of “stemness,” pluripotency, and differentiation. Several studies targeting various aspects of the hESC proteome have already been reported (for reviews, see Refs. 10 and 11). The task, however, is so enormous that further detailed analysis and novel strategies are necessary and will be of high interest and importance. In this regard, MS-based quantitative proteomics and in particular stable isotope labeling by amino acids in cell culture (SILAC) may greatly facilitate the process of defining the mechanisms of hESC self-renewal and differentiation. With SILAC, the entire proteome of a given cell population is metabolically labeled by heavy, non-radioactive isotopic variants of amino acids, thus making it distinguishable by MS analysis (12). Thereafter two or more distinctly SILAC-labeled cell populations can be mixed and analyzed in one MS experiment that allows accurate quantitation of proteins from the different cellular states (13). This versatile strategy has been demonstrated to be very useful for comprehensive characterization of complex biological phenomena (14–21) including in-depth comparison of signaling pathways to identify control points determining cell fate of adult mesenchymal stem cells (22).
Here we report a procedure for complete SILAC labeling of human ES cells. We show that these SILAC-encoded hESCs have preserved self-renewing undifferentiated status as well as pluripotent capabilities based on analysis of known markers. In addition, we further compared the overall proteomes and phosphoproteomes of SILAC-labeled hESCs and equivalent cells grown under conventional culture conditions. We next compared the membrane proteomes of undifferentiated and differentiated hESCs in a quantitative manner. Our analysis identified 811 membrane proteins, which to our knowledge is the largest data set of ES cell membrane proteome. This study also revealed 23 membrane proteins with large changes in their expression levels during the differentiation. Six of those cell surface molecules displayed more than 3-fold higher levels in the self-renewing cells, whereas the remaining 17 were identified as more abundant in the differentiated population. These may be useful as specific hESC markers for the corresponding ES cell state and help to shed light on the mechanisms for self-renewal and differentiation.
EXPERIMENTAL PROCEDURES
Cell Culture—
All three hESC lines used in this study, Odense-3, KMEB1, and HUES9, were maintained on Matrigel (BD Biosciences) in mouse embryonic fibroblast (MEF)-conditioned medium essentially as recommended by Geron Corp. (on-line “Protocols for Maintenance of Human Embryonic Stem Cells in Feeder Free Conditions”). Briefly the cell culture plastic surface was coated using 1 ml of Matrigel (prediluted to ∼0.3 mg/ml)/10 cm2. The MEF-conditioned medium (CM) was prepared as follows: hESC medium consisting of Knockout DMEM, 15% Knockout serum replacement, 1% penicillin/streptomycin, 1% non-essential amino acids, 1% Glutamax (all from Invitrogen), 0.5% human serum albumin, 0.1 mm β-mercaptoethanol (both from Sigma), and 10 ng/ml FGF2 (Invitrogen) was conditioned for 24 h on irradiated MEFs plated at a density of 56,000 cells/cm2 (0.3 ml of medium/cm2 of MEFs) and frozen at −20 °C. Immediately before use, medium was thawed out, sterile filtered, and supplemented with 10 ng/ml FGF2. For in vitro hESC differentiation regarding membrane marker determination, cells were induced to differentiate spontaneously by replacing the CM with non-conditioned medium not containing FGF2 as well (regular DMEM SILAC labeling medium supplemented with normal lysine (Lys0) and arginine (Arg0)). Cells were cultured in this medium for 2 days for the proteomics experiments or 4 days for quantitative PCR analyses. In both cases, 50 nm phorbol 12-myristate 13-acetate was further supplemented for the last 24 h of differentiation. Further details including cell differentiation, lysis, and fractionation are available in the supplemental methods.
Preparation of SILAC Labeling Medium—
CM was dialyzed three times against PBS using a membrane with a 3500-Da cutoff and once against DMEM containing 1% penicillin/streptomycin, 1% non-essential amino acids, and 1% Glutamax but lacking arginine and lysine. Heavy SILAC amino acids Arg6 (l-[U-13C6]arginine, Cambridge Isotope Laboratories) and Lys4 (l-[2H4]lysine, Sigma-Isotec) were supplemented to the medium at various concentrations as indicated in the text. Medium was then sterile filtered, and FGF2 (10 ng/ml) was added immediately before use. To achieve complete SILAC incorporation, hESCs were grown in the labeling medium for five passages prior to further manipulation of the cells.
Membrane Preparation—
Membrane preparation from Odense-3 and HUES9 cells was performed essentially as described previously (23). Cells were rinsed with PBS and harvested by incubation in prewarmed cell dissociation buffer (Invitrogen), vigorous pipetting, and centrifugation. Cell pellets were resuspended in sucrose buffer (255 mm sucrose, 20 mm HEPES, pH 7.4, and 1 mm EDTA) freshly supplemented with protease inhibitor mixture (Roche Applied Science), transferred to a prechilled Dounce homogenizer, and disrupted with a tight pestle. Cell lysates were clarified by centrifugation at 20,800 rcf for 10 min and transferred to ultracentrifuge tubes, and the membranes were pelleted by centrifugation at 245,000 rcf for 2 h. The membrane pellet was resuspended in ice-cold 100 mm Na2CO3 and incubated on ice for 30 min with occasional vortexing, and membranes were pelleted again by centrifugation at 245,000 rcf for 30 min. The membrane pellet was further rinsed once with 500 mm Na2CO3 and once with 50 mm Na2CO3.
Sample Preparation and MS Acquisition—
In-gel trypsin digestion and subsequent desalting and concentration of the samples for MS analysis were carried out as described previously (24,25). Enrichment of phosphopeptides and in-solution protein digestion were performed essentially as described previously (19) with minor modifications. Detailed protocols are available in the supplemental methods. All samples were analyzed by on-line C18 reverse-phase nanoscale LC-MS/MS. Analysis of the samples for determination of the proteome and phosphoproteome status of SILAC-labeled hESCs was performed on a 7-tesla Finnigan LTQ-FT Ultra (Thermo Fisher Scientific) instrument equipped with a nanoelectrospray ion source (Proxeon Biosystems) and connected to an Agilent 1100 nanoflow system (Agilent Technologies).
Proteins from membrane preparations were analyzed on an Agilent 1200 nanoflow system (Agilent Technologies) and an LTQ-Orbitrap XL (Thermo Fisher Scientific) equipped with a nanoelectrospray ion source (Proxeon Biosystems). Membrane digest from each ES cell line was divided into four equal parts (4 μg each). Each sample was then analyzed with one of the following precursor scan mass ranges: m/z 300–2000, m/z 300–600, m/z 550–850, or m/z 800–1800. Other parameters were essentially as described previously (26). Detailed acquisition methods for all experiments are provided in the supplemental methods.
MS Data Analysis—
For the experiments regarding proteome and phosphoproteome status of SILAC-labeled hESCs, MS2 peaks were centroided and merged into a single peak list file using the open source DTASupercharge software (SourceForge, Inc.) and searched with Mascot v2.2 (Matrix Science) against the human IPI protein database v3.31. Precursor mass tolerance was set at 15 ppm and 0.6 Da for MS2 fragments. For the samples enriched for phosphopeptides precursor tolerance was restricted to 5 ppm. Identified proteins were quantitated and validated using MSQuant software (SourceForge, Inc.). Criteria for validation of protein hits were as follows: individual peptide score above 25, protein score above 60, and at least two unique peptides per protein resulting in a false-positive rate (27) below 0.7%. Phosphopeptide hits were also stringently validated, and a Mascot score threshold of 25 was set to obtain a false-positive rate below 0.8%.
Data analysis for experiments regarding hESC membrane markers was performed as described above with the following exceptions. Precursor mass tolerance was restricted to 5 ppm, and the criteria for protein identification were peptide score above 21 and at least two unique peptides per protein. If, however, a protein was identified with only one peptide in one of the cell lines it was included if it was identified with at least two peptides in the other cell line. Further details are available in the supplemental methods. ProteinCenter software (Proxeon Biosystems) was used to extract membrane proteins based on Gene Ontology assignments (28). FASTA sequences from these proteins were submitted to the Phobius resource (29) for prediction of transmembrane domains and signal peptides in the sequence.
Additional Methods—
A detailed description of hESC differentiation, subcellular fractionation, and immunocytochemistry including antibody information; reverse transcription and real time quantitative PCR procedures including primer sequences; fluorescence-activated cell scanning; MS acquisition methods; the phosphopeptide enrichment protocol; and post-translational modification scoring are available in the supplemental methods. The data have also been deposited into the “Human Proteinpedia” (30).
RESULTS
SILAC-labeled hESCs Maintain Undifferentiated Self-renewal Status—
SILAC labeling of hESCs is not a trivial task because of the specific conditions required for maintaining hESCs in culture. ES cells are commonly grown on a feeder layer of MEFs to preserve the undifferentiated pluripotent state (31). The feeder cells, however, represent a substantial source of unlabeled amino acids that ultimately results in a low SILAC labeling efficiency of the ES cells, thereby considerably compromising the accuracy of quantitation (32). Moreover the presence of MEFs in the hESC culture leads to a substantial contamination of the sample with mouse proteins. Because the mouse and human proteomes share significant homology with a large number of the proteolytic peptides indistinguishable between the two species, such co-cultures may result in false assessment of MEF proteins to the hESC proteome and most likely dramatic quantitation errors in peptides common to both species.
We have therefore decided to use a previously described alternative approach that allows culturing human ES cells in a feeder-free environment. In this system, hESCs are grown on plastic dishes coated with Matrigel in CM collected from separate MEF cultures (33,34). Nevertheless such CM also contains a significant amount of unlabeled amino acids consequently leading to incomplete SILAC labeling. To remove those, we first dialyzed the CM against PBS and then against SILAC medium lacking arginine and lysine. Subsequently Lys4 (4 Da heavier from normal l-lysine) and Arg6 (6 Da heavier from normal l-arginine) were added to the dialyzed CM to create SILAC labeling medium for hESCs (for details see the supplemental methods).
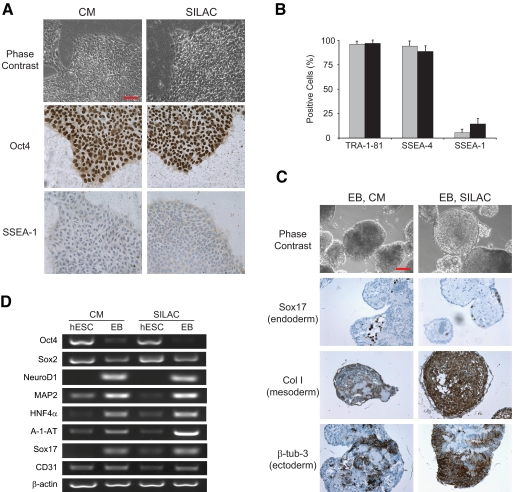
It was not immediately clear whether the dialyzed CM would maintain the undifferentiated growth of hESCs. It is possible that some factors released by MEFs into the CM and essential for hESC growth are lost during the dialysis. Thus, we maintained two parallel cultures of hESCs, one in normal CM and another in SILAC medium prepared as described above. After five doublings that are usually necessary for complete incorporation of labeled amino acids (12), the hESCs remained undifferentiated as demonstrated by morphology, positive staining for self-renewal marker Oct4, and negative staining for differentiation marker SSEA-1 (Fig. 1A). The self-renewal state was further confirmed by expression analysis of several self-renewal and differentiation markers by fluorescence-activated cell scanning (Fig. 1B). To ensure that the capacity of SILAC medium to support undifferentiated growth of hESCs was not cell line-specific, we repeated the experiments with three independent cell lines: Odense-3, KMEB1 (35), and HUES9 (36). The expression levels of all markers tested, namely SSEA-1, Oct4, SSEA-4, TRA-1-81, Nanog, Sox2, and Cripto, were comparable between the cells maintained in normal CM and those cultured in SILAC medium (supplemental Fig. 1).
Fig. 1.
SILAC-labeled hESCs maintain self-renewal capabilities and pluripotency. A, phase-contrast images of Odense-3 hESCs cultured in normal CM or SILAC medium (top panels) and immunostaining for Oct4 self-renewal and SSEA-1 differentiation markers (middle and bottom panels, respectively). Scale bar, 100 μm. B, quantitative assessment derived from fluorescence-activated cell scanning analyses for SSEA-4 and TRA-1-81 self-renewal and SSEA-1 differentiation markers. Gray and black bars represent CM and SILAC culture conditions, respectively. Error bars represent S.D. (n = 3). C, embryoid body in vitro differentiation assay of Odense-3 hESCs grown in CM or SILAC medium showing immunostaining for markers of all three germ layers. Scale bar, 100 μm. D, reverse transcription-PCR analysis of EB and undifferentiated hESCs for self-renewal (Oct4 and Sox2), ectoderm- (NeuroD1 and MAP2), endoderm- (HNF4α, A-1-AT, and Sox17) and mesoderm (CD31)-specific markers.
SILAC-labeled hESCs Remain Pluripotent—
We next examined whether the labeling procedure affects the pluripotency of hESCs. After maintaining hESCs in the conventional CM or SILAC medium for five passages, their differentiation capabilities were tested in an in vitro embryoid body (EB) formation assay. Cells from both conditions readily formed EBs, which were kept in culture for 20 days and subsequently subjected to immunocytochemistry and RT-PCR analysis. The results clearly demonstrated that SILAC labeling also preserved the pluripotency of the hESCs as we observed markers specific for all three germ layers, ectoderm, mesoderm, and endoderm (Fig. 1, C and D).
Efficiency of Labeling—
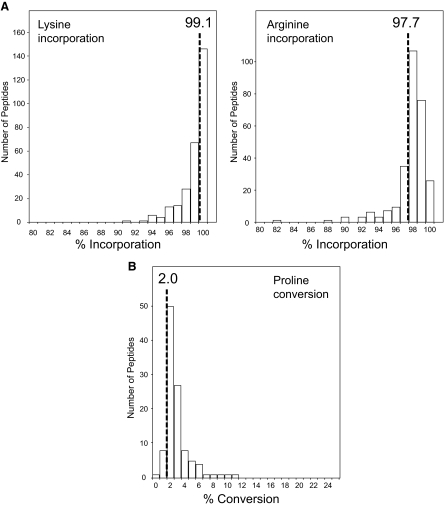
Having established that stemness of hESCs was unaffected by SILAC labeling, we next examined the incorporation efficiency of the labeled amino acids. As shown in Fig. 2A, hESCs were essentially fully labeled both on lysine and arginine residues after culture for five doublings in SILAC medium containing 146 mg/liter Lys4 and 28 mg/liter Arg6. The exact efficiency of labeling was estimated to be 99.1% for lysine and 97.7% for arginine (Fig. 2A and supplemental Table 1). Given atom purity of the stable isotope-labeled amino acids currently available, this represents most likely the maximal achievable labeling efficiency. In some mammalian cells, including hESCs, arginine can be metabolically converted to proline, consequently compromising the accuracy of quantitation (13, 37–39). Hence we performed a series of experiments using different amounts of Arg6 and observed only negligible levels of proline conversion when the arginine concentration in the labeling medium was in a range of 20–40 mg/liter (Fig. 2B, supplemental Fig. 2, and supplemental Table 2).
Fig. 2.
SILAC labeling efficiency in human ES cells. A, overall incorporation efficiencies evaluated by assessment of SILAC ratios between unlabeled and labeled versions of 280 lysine-containing peptides (left panel) and 270 arginine-containing peptides (right panel), respectively. B, degree of arginine to proline metabolic conversion in SILAC-labeled hESCs. Arg6 was supplied in the labeling medium at a concentration 21 mg/liter (see supplemental Fig. 2 for other arginine dilutions). The extent of conversion was estimated by quantitative assessment of 107 unique proline-containing peptides.
Proteome and Phosphoproteome Status of Undifferentiated SILAC-labeled hESCs—
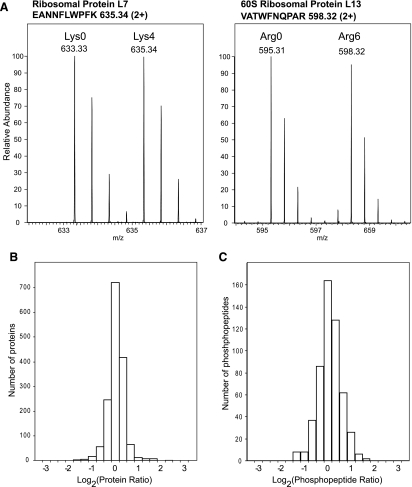
We next conducted two global MS-based studies targeting both protein expression and protein phosphorylation levels to quantitatively compare the proteomes of SILAC-labeled hESCs and the unlabeled cells grown in normal CM. These experiments were set to survey in an unbiased manner whether the labeling procedure had introduced any substantial changes in the proteome or phosphoproteome of the SILAC-labeled human ES cells. We mixed equal amounts of Lys4/Arg6-labeled hESCs and corresponding unlabeled cells grown in conventional CM. To reduce sample complexity, the mixed cells were subjected to subcellular fractionation and subsequent separation by one-dimensional SDS-gel electrophoresis. The entire gel lanes were sliced into individual gel blocks, in-gel digested with trypsin, and subjected to high accuracy LC-MS/MS analyses using an LTQ-FTICR mass spectrometer. We applied stringent criteria for protein identification including a requirement for two fully tryptic peptides in the correct SILAC state. The combined analysis of all gel slices yielded 1556 distinct hESC proteins with more than 99% certainty of identification as estimated by reverse database searching (27). We compared the relative abundances of these proteins between the two hESC populations using the ratios of intensities of the corresponding SILAC peptide pairs (Fig. 3A and supplemental Table 3). Protein ratios clustered tightly around the expected 1:1 value indicating that there were no major changes in the SILAC-labeled hESC proteome compared with the CM hESC proteome regarding protein expression levels (Fig. 3B). The average standard deviation for the entire data set was 13.9%, which compares well to the quantitation accuracy commonly observed in large scale SILAC analysis (16,22).
Fig. 3.
Quantitative comparison of the proteome and phosphoproteome of SILAC-labeled hESCs and corresponding unlabeled cells grown in conventional CM. A, examples from lysine- (left panel) and arginine (right panel)-containing SILAC peptide pairs demonstrating equal expression levels of the corresponding proteins as observed by MS. Shown is the distribution of the ratios of the identified 1556 hESC proteins (B) and 527 unique phosphorylated peptides (C) presented on a log scale.
In the second MS study, we first enriched phosphorylated peptides from the in-gel digested hESC samples prior to the MS analysis. As a result 527 unique phosphopeptides from 383 proteins were identified (supplemental Table 4). We assessed the distribution of the observed phosphorylation sites across Gene Ontology (GO) annotations (28) regarding protein function. Identified phosphopeptides spread well over various protein classes demonstrating that the analyses were not limited to high abundance cytoskeletal and housekeeping proteins (supplemental Fig. 3). Even categories of usually low abundance proteins like kinases, phosphatases, and transcription factors were well represented in the data set with 33, 41, and 39 members, respectively. Reanalyzing the data set for GO annotations based on cellular localization resulted in a similar picture of balanced protein distribution over different cellular compartments (supplemental Fig. 3). Importantly phosphopeptide ratios distributed closely around the 1:1 value indicating that the phosphorylation status was closely comparable between hESCs cultured in the normal CM or in the SILAC labeling medium (Fig. 3C). Thus, the SILAC-labeled hESCs should also be suitable for functional phosphoproteomics applications.
Membrane Proteomes of Undifferentiated and Differentiated hESCs—
Having ascertained the suitability of SILAC-labeled hESCs for quantitative proteomics studies, we applied this technology for the identification of hESC surface markers. Despite making the experiment technically more challenging, we chose to look at the early stage instead of terminal ES cell differentiation. The initial phases should reveal markers that are of higher functional relevance for hESC differentiation, thus further increasing the impact of the findings. For that purpose we cultured Odense-3 cells in medium containing normal lysine (Lys0) and arginine (Arg0), and we allowed the cells to undergo spontaneous differentiation for 2 days. In a parallel culture, another population of undifferentiated Odense-3 cells was labeled with Lys4 and Arg6. We then combined equal proportions of cells from both conditions and prepared one crude membrane extract. Proteins from the purified membranes were subsequently proteolytically digested in-solution and analyzed by high accuracy LC-MS/MS using an LTQ-Orbitrap mass spectrometer (supplemental Fig. 4). In addition, we performed the same experiment with HUES9 cells to ensure that the observed differential protein expression changes were not cell line-specific.
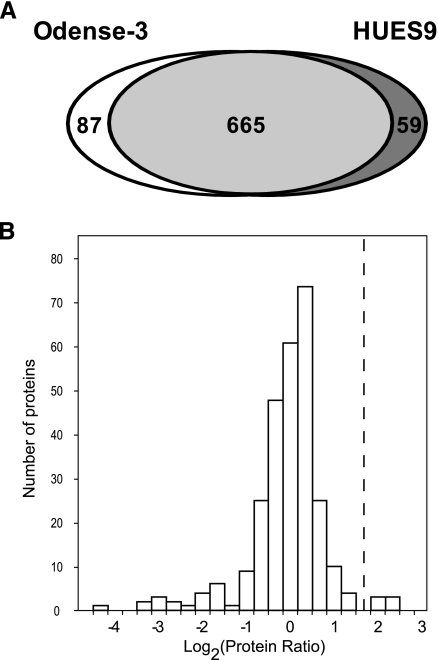
We again applied stringent criteria for identification including at least two unique peptides per protein and used the GO annotation for membrane assessment. As a result, 752 and 724 proteins were identified from Odense-3 and HUES9 cells, respectively, yielding in total 811 unique hESC membrane proteins (Fig. 4A and supplemental Table 5). This category includes true transmembrane as well as membrane-associated proteins. Therefore we performed further bioinformatics analyses, which revealed that 438 proteins of those possess at least one predicted transmembrane domain (29) (supplemental Table 5).
Fig. 4.
Quantitative determination of hESC membrane proteome. A, total number of membrane proteins identified from Odense-3 and HUES9 cell lines. B, distribution of protein ratios reflecting protein expression changes between differentiated and undifferentiated hESCs. Only membrane proteins identified in both Odense-3 and HUES9 cell lines with similar expression ratios (±50%) and at least one predicted transmembrane domain were included in the analysis. Ratios are averages of the values from the two cell lines and are presented on a log scale. The dashed line indicates the threshold (>3-fold change) set to discriminate potential hESC-specific markers from the remaining membrane proteins.
Distinguishing hESC Membrane Markers—
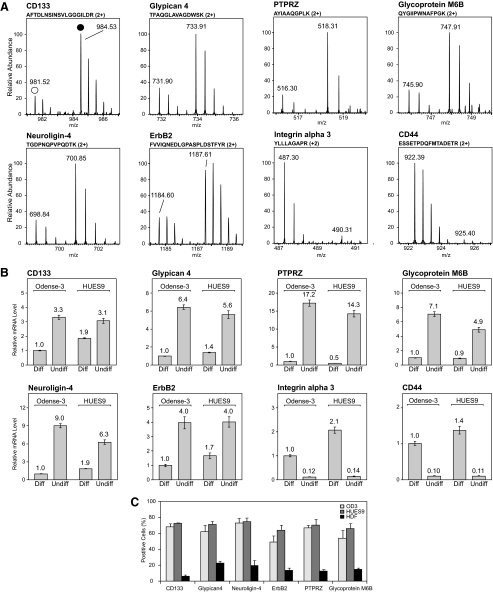
To identify candidate ES cell-specific surface markers from the large pool of membrane proteins we focused on transmembrane proteins that were expressed at significantly higher levels in the self-renewing undifferentiated precursors compared with differentiated cells. Our quantitative proteomics analyses revealed six such proteins from both ES cell lines displaying more than 3-fold higher expression in the undifferentiated state (Fig. 4B, Table I, and supplemental Table 7). All six candidates were found to be significantly up-regulated (p < 0.01) as determined by a statistical z-test commonly used in proteomics analyses because of its robustness toward outliers (32) (see the supplemental methods for details). Although CD133/Prominin-1 is a known ES cell marker (40), the remainder of the list includes functionally distinct novel candidates: a proteoglycan (Glypican-4), a type-B carboxylesterase (Neuroligin-4), a receptor tyrosine kinase (ErbB2), a receptor-type tyrosine-protein phosphatase (PTPRZ), and a multipass membrane proteolipid (Glycoprotein M6B) (Fig. 5A). For comparative purposes, we performed real time quantitative PCR analysis to examine the changes in mRNA levels of the corresponding proteins during hESC differentiation (Fig. 5B). Similar to the quantitative proteomics results, we observed a marked decrease in mRNA levels of the respective proteins in differentiated hESCs. We also analyzed the surface expression of the six potential self-renewal markers on hESCs and on human dermal fibroblasts by fluorescence-activated cell scanning (Fig. 5C). In hESCs all six proteins were expressed at a considerably higher level as compared with the somatic human dermal fibroblast cells. Taken together, our results indicate that these proteins are suitable candidates for specific hESC self-renewal surface markers that may be used in combination with already known markers for better phonotypical characterization of ES cells. Finally the expression of 17 proteins changed in the opposite direction during the differentiation, i.e. their levels were found to be more than 3 times lower in the undifferentiated hESCs (Fig. 5 and supplemental Table 6). This intriguing group contains both known differentiation markers and novel candidates that can be used to monitor the differentiation state of hESCs.
Table I.
hESC surface markers identified through SILAC-based proteomics
Surface markers were extracted from the total list of identified hESC membrane proteins based on criteria for membrane GO annotation, transmembrane domain prediction, and SILAC ratio. Values in parentheses indicate the corresponding S.D. undiff./diff., undifferentiated/differentiated; n/a, not applicable.
| Accession key | Protein name | Odense-3 ratio undiff./diff. | HUES9 ratio undiff./diff. | TMa | SPb |
|---|---|---|---|---|---|
| IPI00012540.1 | CD133/Prominin-1 | 4.9 (±0.7) | 4.2 (n/a) | 5 | Yes |
| IPI00232571.1 | Glypican-4 | 3.9 (±0.4) | 3.4 (±0.3) | 1 | Yes |
| IPI00171705.1 | Neuroligin-4 | 5.0 (±0.7) | 4.2 (±0.2) | 1 | Yes |
| IPI00300384.3 | ErbB2 | 3.7 (±0.3) | 3.0 (±0.4) | 1 | Yes |
| IPI00187158.2 | Glycoprotein M6B | 4.5 (±0.6) | 3.8 (n/a) | 4 | No |
| IPI00216283.1 | PTPRZ | 4.5 (±0.6) | 4.1 (±0.4) | 1 | Yes |
Number of predicted transmembrane domains.
Predicted signal peptide in the protein sequence.
Fig. 5.
Protein and mRNA levels of hESC membrane markers. A, quantitative proteomics assessment: examples of SILAC peptide pairs for the corresponding proteins from differentiated (Diff.) (hollow circle) and undifferentiated (Undiff.) (solid circle) Odense-3 cells. B, mRNA levels of the selected proteins in undifferentiated and differentiated Odense-3 and HUES9 cells evaluated by real time quantitative PCR. Target gene expression levels were normalized to expression of actin; the normalized values for differentiated Odense-3 cells were then set at 1 in all panels. Error bars represent S.D. (n = 3). C, quantitative assessment on the surface expression of the six potential hESC self-renewal markers by fluorescence-activated cell scanning (see also supplemental Fig. 5). Two human ES cell lines (Odense-3 (OD3) and HUES9) were compared with somatic cells (human dermal fibroblasts (HDF)). Error bars represent S.E.M. Experiments were performed in three biological replicas.
DISCUSSION
SILAC-labeled hESCs in our study maintained an undifferentiated self-renewing state and pluripotency and were suitable for functional studies using quantitative proteomics. We applied this technology to compare the membrane proteomes of undifferentiated and differentiated hESCs to identify ES cell-specific surface markers. Our quantitative proteomics strategy distinguished six surface markers specific for undifferentiated hESCs from the large pool of 811 identified membrane proteins. These include CD133/Prominin-1, Glypican-4, Neuroligin-4, ErbB2, PTPRZ, and Glycoprotein M6B.
CD133/Prominin-1 is known to be expressed in several adult stem cell types such as hematopoietic and neural stem cells (41,42). Interestingly in mouse embryonic stem cells, CD133 is thought to be a marker of early differentiation (43) and may be yet another marker emphasizing dissimilarities of mouse and human ES cells (44). Glypican-4 is a cell surface proteoglycan bearing heparin sulfates. It has been reported to bind FGF2 (45) and may thereby be involved in the process of FGF2-dependent self-renewal regulation as FGF2 is known to play an essential role in hESC self-renewal (46). Neuroligin-4 is a single pass transmembrane protein putatively involved in synapse formation. In hESCs, it may be involved in both cell-cell and cell-extracellular matrix (ECM) contact formation as hESCs depend highly on the presence of ECM and poorly tolerate dissociation into single cell suspension (47).
ErbB2 is a member of the epidermal growth factor receptor family (48). It plays a major role in cell proliferation and survival (49) and is also involved in certain developmental processes (50). ErbB2 signaling has been shown to be necessary for hESC self-renewal as well (51). It appears that these properties of ErbB2 signaling are also utilized as a common mechanism to promote the self-renewal of cancer cells, and ErbB2 is often found to be amplified in various human cancers (48).
PTPRZ is a receptor-type protein-tyrosine phosphatase and encodes a single pass membrane protein. It appears to play a role in heparin-binding growth factor signaling and may be involved in neural development (52). A role in neuronal development has been suggested for Glycoprotein M6B as well (53); however, no exact biochemical function has been described for the protein. This is also the case for CD133, and our identification of these proteins as hESC-specific markers may suggest new biological roles.
In addition, we identified 17 proteins with increased expression during differentiation. Although some of them have been shown to play a role in certain development and differentiation processes, e.g. Ephrin B2 (54), others appear to participate in cell adhesion (CD166), ECM-cell signal transduction (Axl receptor tyrosine kinase and various integrins), and cell-cell interaction (CD44). Their identification as hESC differentiation markers allows additional insight into both specific protein function and the mechanisms of the differentiation process. Beyond the identified surface markers, our study also provides comprehensive quantitative profiles of the hESC membrane proteome. The quantitative comparison of undifferentiated and differentiated hESC membranes may provide further insights into ES cell maintenance and differentiation.
MS-based technology has only recently been applied to ES cell biology, and it has already shown enormous potential (11,32, 55–59). Alternative procedures for SILAC labeling of hESCs have recently been reported (37,39). The addition of an accurate quantitative dimension will ensure an accumulation of better knowledge about the factors responsible for the stemness of hESCs that could greatly facilitate many aspects of ES cell research (2,60). Although significant progress has been made recently in deciphering the nature of pluripotency, a more detailed and thorough understanding of the mechanisms of self-renewal and differentiation is necessary to generate safe protocols for obtaining differentiated cells and tissues that could be used in regenerative medicine (2). SILAC-based proteomics and phosphoproteomics could help to unveil signaling pathways involved in both the maintenance of the hESC pluripotent state and the conversion processes into specific cell lineages, thereby providing valuable clues toward the understanding of the mechanisms controlling hESC self-renewal and differentiation.
Supplementary Material
Acknowledgments
We thank all members from the Center for Experimental BioInformatics for useful discussions. We are grateful to Dr. Joern Dengjel (Center for Experimental BioInformatics) and Dr. Shao-En Ong (The Broad Institute of the Massachusetts Institute of Technology and Harvard) for the critical reading of the manuscript. HUES9 cells were a generous gift from Dr. Douglas Melton (Harvard University). The Centre of Inflammation and Metabolism is supported by a grant from the Danish National Research Foundation.
Footnotes
Published, MCP Papers in Press, January 17, 2009, DOI 10.1074/mcp.M800287-MCP200
The abbreviations used are: hESC, human embryonic stem cell; CM, conditioned medium; DMEM, Dulbecco's modified Eagle's medium; EB, embryoid body; ECM, extracellular matrix; ES, embryonic stem; FGF, fibroblast growth factor; GO, Gene Ontology; IPI, International Protein Index; LTQ, linear trap quadrupole; MEF, mouse embryonic fibroblast; PTPRZ, receptor-type tyrosine-protein phosphatase ζ; SILAC, stable isotope labeling by amino acids in cell culture; Arg6, l-[U-13C6]arginine; Lys4, l-[2H4]lysine; rcf, relative centrifugal force.
This work was supported by a grant from the Lundbeck Foundation.
The on-line version of this article (available at http://www.mcponline.org) contains supplemental material.
REFERENCES
- 1.Lerou, P. H., and Daley, G. Q. ( 2005) Therapeutic potential of embryonic stem cells. Blood Rev. 19, 321–331 [DOI] [PubMed] [Google Scholar]
- 2.Jaenisch, R., and Young, R. ( 2008) Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 132, 567–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohtsuka, S., and Dalton, S. ( 2008) Molecular and biological properties of pluripotent embryonic stem cells. Gene Ther. 15, 74–81 [DOI] [PubMed] [Google Scholar]
- 4.Hanna, J., Markoulaki, S., Schorderet, P., Carey, B. W., Beard, C., Wernig, M., Creyghton, M. P., Steine, E. J., Cassady, J. P., Foreman, R., Lengner, C. J., Dausman, J. A., and Jaenisch, R. ( 2008) Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell 133, 250–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakagawa, M., Koyanagi, M., Tanabe, K., Takahashi, K., Ichisaka, T., Aoi, T., Okita, K., Mochiduki, Y., Takizawa, N., and Yamanaka, S. ( 2008) Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 26, 101–106 [DOI] [PubMed] [Google Scholar]
- 6.Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., and Yamanaka, S. ( 2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 [DOI] [PubMed] [Google Scholar]
- 7.Takahashi, K., and Yamanaka, S. ( 2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 [DOI] [PubMed] [Google Scholar]
- 8.Yu, J., Vodyanik, M. A., Smuga-Otto, K., Antosiewicz-Bourget, J., Frane, J. L., Tian, S., Nie, J., Jonsdottir, G. A., Ruotti, V., Stewart, R., Slukvin, I. I., and Thomson, J. A. ( 2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 [DOI] [PubMed] [Google Scholar]
- 9.Jensen, J. ( 2007) Pathway decision-making strategies for generating pancreatic beta-cells: systems biology or hit and miss? Curr. Opin. Endocrinol. Diabetes Obes. 14, 277–282 [DOI] [PubMed] [Google Scholar]
- 10.Choi, M. Y., An, Y. J., Kim, S. H., Roh, S. H., Ju, H. K., Hong, S. S., Park, J. H., Cho, K. J., Choi, D. W., and Kwon, S. W. ( 2007) Mass spectrometry based proteomic analysis of human stem cells: a brief review. Exp. Mol. Med. 39, 690–695 [DOI] [PubMed] [Google Scholar]
- 11.Van Hoof, D., Mummery, C. L., Heck, A. J., and Krijgsveld, J. ( 2006) Embryonic stem cell proteomics. Expert Rev. Proteomics 3, 427–437 [DOI] [PubMed] [Google Scholar]
- 12.Ong, S. E., Blagoev, B., Kratchmarova, I., Kristensen, D. B., Steen, H., Pandey, A., and Mann, M. ( 2002) Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics 1, 376–386 [DOI] [PubMed] [Google Scholar]
- 13.Blagoev, B., and Mann, M. ( 2006) Quantitative proteomics to study mitogen-activated protein kinases. Methods 40, 243–250 [DOI] [PubMed] [Google Scholar]
- 14.Blagoev, B., Kratchmarova, I., Ong, S. E., Nielsen, M., Foster, L. J., and Mann, M. ( 2003) A proteomics strategy to elucidate functional protein-protein interactions applied to EGF signaling. Nat. Biotechnol. 21, 315–318 [DOI] [PubMed] [Google Scholar]
- 15.Ibarrola, N., Molina, H., Iwahori, A., and Pandey, A. ( 2004) A novel proteomic approach for specific identification of tyrosine kinase substrates using [13C]tyrosine. J. Biol. Chem. 279, 15805–15813 [DOI] [PubMed] [Google Scholar]
- 16.Blagoev, B., Ong, S. E., Kratchmarova, I., and Mann, M. ( 2004) Temporal analysis of phosphotyrosine-dependent signaling networks by quantitative proteomics. Nat. Biotechnol. 22, 1139–1145 [DOI] [PubMed] [Google Scholar]
- 17.Dengjel, J., Akimov, V., Olsen, J. V., Bunkenborg, J., Mann, M., Blagoev, B., and Andersen, J. S. ( 2007) Quantitative proteomic assessment of very early cellular signaling events. Nat. Biotechnol. 25, 566–568 [DOI] [PubMed] [Google Scholar]
- 18.Andersen, J. S., and Mann, M. ( 2006) Organellar proteomics: turning inventories into insights. EMBO Rep. 7, 874–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsen, J. V., Blagoev, B., Gnad, F., Macek, B., Kumar, C., Mortensen, P., and Mann, M. ( 2006) Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127, 635–648 [DOI] [PubMed] [Google Scholar]
- 20.Kruger, M., Kratchmarova, I., Blagoev, B., Tseng, Y. H., Kahn, C. R., and Mann, M. ( 2008) Dissection of the insulin signaling pathway via quantitative phosphoproteomics. Proc. Natl. Acad. Sci. U. S. A. 105, 2451–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishihama, Y., Sato, T., Tabata, T., Miyamoto, N., Sagane, K., Nagasu, T., and Oda, Y. ( 2005) Quantitative mouse brain proteomics using culture-derived isotope tags as internal standards. Nat. Biotechnol. 23, 617–621 [DOI] [PubMed] [Google Scholar]
- 22.Kratchmarova, I., Blagoev, B., Haack-Sorensen, M., Kassem, M., and Mann, M. ( 2005) Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science 308, 1472–1477 [DOI] [PubMed] [Google Scholar]
- 23.Foster, L. J., De Hoog, C. L., and Mann, M. ( 2003) Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc. Natl. Acad. Sci. U. S. A. 100, 5813–5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rappsilber, J., Ishihama, Y., and Mann, M. ( 2003) Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem. 75, 663–670 [DOI] [PubMed] [Google Scholar]
- 25.Shevchenko, A., Tomas, H., Havlis, J., Olsen, J. V., and Mann, M. ( 2006) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1, 2856–2860 [DOI] [PubMed] [Google Scholar]
- 26.Olsen, J. V., de Godoy, L. M., Li, G., Macek, B., Mortensen, P., Pesch, R., Makarov, A., Lange, O., Horning, S., and Mann, M. ( 2005) Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol. Cell. Proteomics 4, 2010–2021 [DOI] [PubMed] [Google Scholar]
- 27.Elias, J. E., Haas, W., Faherty, B. K., and Gygi, S. P. ( 2005) Comparative evaluation of mass spectrometry platforms used in large-scale proteomics investigations. Nat. Methods 2, 667–675 [DOI] [PubMed] [Google Scholar]
- 28.Ashburner, M., Ball, C. A., Blake, J. A., Botstein, D., Butler, H., Cherry, J. M., Davis, A. P., Dolinski, K., Dwight, S. S., Eppig, J. T., Harris, M. A., Hill, D. P., Issel-Tarver, L., Kasarskis, A., Lewis, S., Matese, J. C., Richardson, J. E., Ringwald, M., Rubin, G. M., and Sherlock, G. ( 2000) Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25, 25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kall, L., Krogh, A., and Sonnhammer, E. L. ( 2007) Advantages of combined transmembrane topology and signal peptide prediction–the Phobius web server. Nucleic Acids Res. 35, W429–W432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathivanan, S., Ahmed, M., Ahn, N. G., Alexandre, H., Amanchy, R., Andrews, P. C., Bader, J. S., Balgley, B. M., Bantscheff, M., Bennett, K. L., Bjorling, E., Blagoev, B., Bose, R., Brahmachari, S. K., Burlingame, A. S., Bustelo, X. R., Cagney, G., Cantin, G. T., Cardasis, H. L., Celis, J. E., Chaerkady, R., Chu, F., Cole, P. A., Costello, C. E., Cotter, R. J., Crockett, D., DeLany, J. P., De Marzo, A. M., DeSouza, L. V., Deutsch, E. W., Dransfield, E., Drewes, G., Droit, A., Dunn, M. J., Elenitoba-Johnson, K., Ewing, R. M., Van Eyk, J., Faca, V., Falkner, J., Fang, X., Fenselau, C., Figeys, D., Gagne, P., Gelfi, C., Gevaert, K., Gimble, J. M., Gnad, F., Goel, R., Gromov, P., Hanash, S. M., Hancock, W. S., Harsha, H. C., Hart, G., Hays, F., He, F., Hebbar, P., Helsens, K., Hermeking, H., Hide, W., Hjerno, K., Hochstrasser, D. F., Hofmann, O., Horn, D. M., Hruban, R. H., Ibarrola, N., James, P., Jensen, O. N., Jensen, P. H., Jung, P., Kandasamy, K., Kheterpal, I., Kikuno, R. F., Korf, U., Korner, R., Kuster, B., Kwon, M. S., Lee, H. J., Lee, Y. J., Lefevre, M., Lehvaslaiho, M., Lescuyer, P., Levander, F., Lim, M. S., Lobke, C., Loo, J. A., Mann, M., Martens, L., Martinez-Heredia, J., McComb, M., McRedmond, J., Mehrle, A., Menon, R., Miller, C. A., Mischak, H., Mohan, S. S., Mohmood, R., Molina, H., Moran, M. F., Morgan, J. D., Moritz, R., Morzel, M., Muddiman, D. C., Nalli, A., Navarro, J. D., Neubert, T. A., Ohara, O., Oliva, R., Omenn, G. S., Oyama, M., Paik, Y. K., Pennington, K., Pepperkok, R., Periaswamy, B., Petricoin, E. F., Poirier, G. G., Prasad, T. S., Purvine, S. O., Rahiman, B. A., Ramachandran, P., Ramachandra, Y. L., Rice, R. H., Rick, J., Ronnholm, R. H., Salonen, J., Sanchez, J. C., Sayd, T., Seshi, B., Shankari, K., Sheng, S. J., Shetty, V., Shivakumar, K., Simpson, R. J., Sirdeshmukh, R., Siu, K. W., Smith, J. C., Smith, R. D., States, D. J., Sugano, S., Sullivan, M., Superti-Furga, G., Takatalo, M., Thongboonkerd, V., Trinidad, J. C., Uhlen, M., Vandekerckhove, J., Vasilescu, J., Veenstra, T. D., Vidal-Taboada, J. M., Vihinen, M., Wait, R., Wang, X., Wiemann, S., Wu, B., Xu, T., Yates, J. R., Zhong, J., Zhou, M., Zhu, Y., Zurbig, P., and Pandey, A. ( 2008) Human Proteinpedia enables sharing of human protein data. Nat. Biotechnol. 26, 164–167 [DOI] [PubMed] [Google Scholar]
- 31.Skottman, H., and Hovatta, O. ( 2006) Culture conditions for human embryonic stem cells. Reproduction 132, 691–698 [DOI] [PubMed] [Google Scholar]
- 32.Graumann, J., Hubner, N. C., Kim, J. B., Ko, K., Moser, M., Kumar, C., Cox, J., Scholer, H., and Mann, M. ( 2008) Stable isotope labeling by amino acids in cell culture (SILAC) and proteome quantitation of mouse embryonic stem cells to a depth of 5,111 proteins. Mol. Cell. Proteomics 7, 672–683 [DOI] [PubMed] [Google Scholar]
- 33.Xu, C., Inokuma, M. S., Denham, J., Golds, K., Kundu, P., Gold, J. D., and Carpenter, M. K. ( 2001) Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 19, 971–974 [DOI] [PubMed] [Google Scholar]
- 34.Fletcher, J. M., Ferrier, P. M., Gardner, J. O., Harkness, L., Dhanjal, S., Serhal, P., Harper, J., Delhanty, J., Brownstein, D. G., Prasad, Y. R., Lebkowski, J., Mandalam, R., Wilmut, I., and De Sousa, P. A. ( 2006) Variations in humanized and defined culture conditions supporting derivation of new human embryonic stem cell lines. Cloning Stem Cells 8, 319–334 [DOI] [PubMed] [Google Scholar]
- 35.Prokhorova, T. A., Harkness, L. M., Frandsen, U., Ditzel, N., Burns, J. S., Schroeder, H. D., and Kassem, M. ( 2009) Teratoma formation by human embryonic stem cells is site-dependent and enhanced by the presence of Matrigel. Stem Cells Dev. 18, 47–54 [DOI] [PubMed] [Google Scholar]
- 36.Cowan, C. A., Klimanskaya, I., McMahon, J., Atienza, J., Witmyer, J., Zucker, J. P., Wang, S., Morton, C. C., McMahon, A. P., Powers, D., and Melton, D. A. ( 2004) Derivation of embryonic stem-cell lines from human blastocysts. N. Engl. J. Med. 350, 1353–1356 [DOI] [PubMed] [Google Scholar]
- 37.Bendall, S. C., Hughes, C., Stewart, M. H., Doble, B., Bhatia, M., and Lajoie, G. A. ( 2008) Prevention of amino acid conversion in SILAC experiments with embryonic stem cells. Mol. Cell. Proteomics 7, 1587–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ong, S. E., Kratchmarova, I., and Mann, M. ( 2003) Properties of 13C-substituted arginine in stable isotope labeling by amino acids in cell culture (SILAC). J. Proteome Res. 2, 173–181 [DOI] [PubMed] [Google Scholar]
- 39.Van Hoof, D., Pinkse, M. W., Oostwaard, D. W., Mummery, C. L., Heck, A. J., and Krijgsveld, J. ( 2007) An experimental correction for arginine-to-proline conversion artifacts in SILAC-based quantitative proteomics. Nat. Methods 4, 677–678 [DOI] [PubMed] [Google Scholar]
- 40.Assou, S., Le Carrour, T., Tondeur, S., Strom, S., Gabelle, A., Marty, S., Nadal, L., Pantesco, V., Reme, T., Hugnot, J. P., Gasca, S., Hovatta, O., Hamamah, S., Klein, B., and De Vos, J. ( 2007) A meta-analysis of human embryonic stem cells transcriptome integrated into a web-based expression atlas. Stem Cells 25, 961–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizrak, D., Brittan, M., and Alison, M. R. ( 2008) CD133: molecule of the moment. J. Pathol. 214, 3–9 [DOI] [PubMed] [Google Scholar]
- 42.Pfenninger, C. V., Roschupkina, T., Hertwig, F., Kottwitz, D., Englund, E., Bengzon, J., Jacobsen, S. E., and Nuber, U. A. ( 2007) CD133 is not present on neurogenic astrocytes in the adult subventricular zone, but on embryonic neural stem cells, ependymal cells, and glioblastoma cells. Cancer Res. 67, 5727–5736 [DOI] [PubMed] [Google Scholar]
- 43.Kania, G., Corbeil, D., Fuchs, J., Tarasov, K. V., Blyszczuk, P., Huttner, W. B., Boheler, K. R., and Wobus, A. M. ( 2005) Somatic stem cell marker prominin-1/CD133 is expressed in embryonic stem cell-derived progenitors. Stem Cells 23, 791–804 [DOI] [PubMed] [Google Scholar]
- 44.Rao, M. ( 2004) Conserved and divergent paths that regulate self-renewal in mouse and human embryonic stem cells. Dev. Biol. 275, 269–286 [DOI] [PubMed] [Google Scholar]
- 45.Hagihara, K., Watanabe, K., Chun, J., and Yamaguchi, Y. ( 2000) Glypican-4 is an FGF2-binding heparan sulfate proteoglycan expressed in neural precursor cells. Dev. Dyn. 219, 353–367 [DOI] [PubMed] [Google Scholar]
- 46.Levenstein, M. E., Ludwig, T. E., Xu, R. H., Llanas, R. A., VanDenHeuvel-Kramer, K., Manning, D., and Thomson, J. A. ( 2006) Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells 24, 568–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoffman, L. M., and Carpenter, M. K. ( 2005) Characterization and culture of human embryonic stem cells. Nat. Biotechnol. 23, 699–708 [DOI] [PubMed] [Google Scholar]
- 48.Citri, A., and Yarden, Y. ( 2006) EGF-ERBB signalling: towards the systems level. Nat. Rev. Mol. Cell Biol. 7, 505–516 [DOI] [PubMed] [Google Scholar]
- 49.Hynes, N. E. ( 1993) Amplification and overexpression of the erbB-2 gene in human tumors: its involvement in tumor development, significance as a prognostic factor, and potential as a target for cancer therapy. Semin. Cancer Biol. 4, 19–26 [PubMed] [Google Scholar]
- 50.Negro, A., Brar, B. K., and Lee, K. F. ( 2004) Essential roles of Her2/erbB2 in cardiac development and function. Recent Prog. Horm. Res. 59, 1–12 [DOI] [PubMed] [Google Scholar]
- 51.Wang, L., Schulz, T. C., Sherrer, E. S., Dauphin, D. S., Shin, S., Nelson, A. M., Ware, C. B., Zhan, M., Song, C. Z., Chen, X., Brimble, S. N., McLean, A., Galeano, M. J., Uhl, E. W., D'Amour, K. A., Chesnut, J. D., Rao, M. S., Blau, C. A., and Robins, A. J. ( 2007) Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood 110, 4111–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakaguchi, N., Muramatsu, H., Ichihara-Tanaka, K., Maeda, N., Noda, M., Yamamoto, T., Michikawa, M., Ikematsu, S., Sakuma, S., and Muramatsu, T. ( 2003) Receptor-type protein tyrosine phosphatase ζ as a component of the signaling receptor complex for midkine-dependent survival of embryonic neurons. Neurosci. Res. 45, 219–224 [DOI] [PubMed] [Google Scholar]
- 53.Olinsky, S., Loop, B. T., DeKosky, A., Ripepi, B., Weng, W., Cummins, J., Wenger, S. L., Yan, Y., Lagenaur, C., and Narayanan, V. ( 1996) Chromosomal mapping of the human M6 genes. Genomics 33, 532–536 [DOI] [PubMed] [Google Scholar]
- 54.Davy, A., and Soriano, P. ( 2007) Ephrin-B2 forward signaling regulates somite patterning and neural crest cell development. Dev. Biol. 304, 182–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baharvand, H., Fathi, A., van Hoof, D., and Salekdeh, G. H. ( 2007) Concise review: trends in stem cell proteomics. Stem Cells 25, 1888–1903 [DOI] [PubMed] [Google Scholar]
- 56.Van Hoof, D., Passier, R., Ward-Van Oostwaard, D., Pinkse, M. W., Heck, A. J., Mummery, C. L., and Krijgsveld, J. ( 2006) A quest for human and mouse embryonic stem cell-specific proteins. Mol. Cell. Proteomics 5, 1261–1273 [DOI] [PubMed] [Google Scholar]
- 57.Yocum, A. K., Gratsch, T. E., Leff, N., Strahler, J. R., Hunter, C. L., Walker, A. K., Michailidis, G., Omenn, G. S., O'Shea, K. S., and Andrews, P. C. ( 2008) Coupled global and targeted proteomics of human embryonic stem cells during induced differentiation. Mol. Cell. Proteomics 7, 750–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dormeyer, W., van Hoof, D., Braam, S. R., Heck, A. J., Mummery, C. L., and Krijgsveld, J. ( 2008) Plasma membrane proteomics of human embryonic stem cells and human embryonal carcinoma cells. J. Proteome Res. 7, 2936–2951 [DOI] [PubMed] [Google Scholar]
- 59.Nagano, K., Taoka, M., Yamauchi, Y., Itagaki, C., Shinkawa, T., Nunomura, K., Okamura, N., Takahashi, N., Izumi, T., and Isobe, T. ( 2005) Large-scale identification of proteins expressed in mouse embryonic stem cells. Proteomics 5, 1346–1361 [DOI] [PubMed] [Google Scholar]
- 60.Adewumi, O., Aflatoonian, B., Ahrlund-Richter, L., Amit, M., Andrews, P. W., Beighton, G., Bello, P. A., Benvenisty, N., Berry, L. S., Bevan, S., Blum, B., Brooking, J., Chen, K. G., Choo, A. B., Churchill, G. A., Corbel, M., Damjanov, I., Draper, J. S., Dvorak, P., Emanuelsson, K., Fleck, R. A., Ford, A., Gertow, K., Gertsenstein, M., Gokhale, P. J., Hamilton, R. S., Hampl, A., Healy, L. E., Hovatta, O., Hyllner, J., Imreh, M. P., Itskovitz-Eldor, J., Jackson, J., Johnson, J. L., Jones, M., Kee, K., King, B. L., Knowles, B. B., Lako, M., Lebrin, F., Mallon, B. S., Manning, D., Mayshar, Y., McKay, R. D., Michalska, A. E., Mikkola, M., Mileikovsky, M., Minger, S. L., Moore, H. D., Mummery, C. L., Nagy, A., Nakatsuji, N., O'Brien, C. M., Oh, S. K., Olsson, C., Otonkoski, T., Park, K. Y., Passier, R., Patel, H., Patel, M., Pedersen, R., Pera, M. F., Piekarczyk, M. S., Pera, R. A., Reubinoff, B. E., Robins, A. J., Rossant, J., Rugg-Gunn, P., Schulz, T. C., Semb, H., Sherrer, E. S., Siemen, H., Stacey, G. N., Stojkovic, M., Suemori, H., Szatkiewicz, J., Turetsky, T., Tuuri, T., van den Brink, S., Vintersten, K., Vuoristo, S., Ward, D., Weaver, T. A., Young, L. A., and Zhang, W. ( 2007) Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat. Biotechnol. 25, 803–816 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.