Abstract
Background:
Research on the translation of efficacious lifestyle change programs to prevent type 2 diabetes into community or clinical settings is needed.
Objectives:
To examine the reach, implementation, and efficacy of a 6-month lifestyle program implemented in primary care by nurse practitioners (NP) for adults at risk for type 2 diabetes.
Method:
The NP sites (n = 4) were randomized to an enhanced standard care program (1 NP and 1 nutrition session) or a lifestyle program (enhanced standard care and 6 NP sessions). These NPs recruited adults at-risk for diabetes from their practice (n = 58) with an acceptance rate of 70%.
Results:
The program reached a diverse, obese, moderately low-income sample. The NPs were able to successfully implement the protocols. The average length of the program was 9.3 months. Attendance was high (98%) and attrition low (12%). The NPs were able to adopt the educational, behavioral, and psychosocial strategies of the intervention easily. Motivational interviewing was more difficult for NPs. Mixed-model repeated measures analysis indicated significant trends or improvement in both groups for nutrition and exercise behavior. Participants of the lifestyle program demonstrated trends for better high density lipoprotein (HDL) as well as exercise behavior compared to enhanced standard care participants. Twenty-five percent of lifestyle participants met treatment goals of 5% weight loss compared to 11% of standard care participants.
Discussion:
A lifestyle program can be implemented in primary care by NPs, reach the targeted population, and be modestly successful. Further research is indicated.
Keywords: diabetes prevention, nurse practitioner, translation research
Type 2 diabetes (T2D) is emerging as a public health epidemic of the 21st century, with approximately 17 million persons affected in the United States. Ethnic minority persons have a disproportionate risk and are twice as likely as non-Hispanic White persons of similar age to develop T2D (Centers for Disease Control and Prevention [CDC], 2004), and T2D is the leading cause of blindness, renal failure, and nontraumatic amputation in adults in the US. In addition, T2D increases the risk of cardiovascular disease and stroke two- to four-fold (CDC, 2004). These complications often occur concomitantly and contribute to extensive disability, personal suffering, and significant societal costs. In the US, the economic costs associated with diabetes in 2007 were estimated to be $174 billion (American Diabetes Association, 2008). Therefore, the greatest opportunity in addressing the personal and societal burden of T2D is to prevent the progression of the disease.
Recent evidence demonstrates that individuals at risk for T2D can be identified, and T2D can be delayed, if not prevented, through lifestyle change programs. International trials have demonstrated a 31-58% reduction in the incidence of T2D for adults with impaired glucose tolerance (IGT) who participated in lifestyle change programs of weight reduction and physical activity compared to a control group (Pan et al., 1997; Tuomilehto et al., 2001). Most recently, the Diabetes Prevention Program (DPP), a large clinical trial in the US with an ethnically diverse sample of adults, provided evidence on the dramatic decrease in progression from IGT to T2D with a lifestyle change program (Knowler et al., 2002).
The DPP intervention protocol was based on behavioral science theories and included the following components: a provider-partnership model of care, education, behavioral support (i.e., goal setting, problem-solving), and motivational interviewing (Diabetes Prevention Research Group, 1999). Research supports that education, goal-setting, and problem-solving are effective in changing health-related behaviors (Foster, Makris, & Bailer, 2005; Nothwehr & Yang, 2007). Motivational interviewing is a collaborative counseling method for enhancing motivation to change by exploring and resolving ambivalence when individuals are having difficulty meeting mutually determined treatment goals (Rollnick, Miller, & Butler, 2007). Motivational interviewing has demonstrated efficacy in a wide range of health promotion interventions, including interventions to promote nutrition and physical activity (Resnicow et al., 2002; West, DiLillo, Bursac, Gore, & Greene, 2007). These components provided the conceptual framework for the lifestyle program protocol of this pilot study.
Results from lifestyle change trials emphasize the importance of lifestyle in the prevention of T2D. A strong correlation was seen between the ability to prevent T2D and the degree to which participants made the recommended lifestyle changes (Tuomilehto et al., 2001). Also encouraging from a translational perspective was that the lifestyle change goals that contributed to diabetes prevention in these studies were quite modest. Participants were counseled to lose 5-7% of body weight, reduce fat intake to < 30%, reduce saturated fat intake to < 10%, increase fiber intake to 15gm/1000kcal, and exercise for 30 minutes 5-7 days per week. Making these modest lifestyle changes also reduced the magnitude of cardiac risk factors of participants (e.g., hypertension; Tuomilehto et al., 2001). The challenge is how to provide research-based lifestyle change programs to at-risk populations that are aligned with current health care systems. The DPP was a proof of principle study demonstrating the ability to delay T2D with lifestyle change and therefore provided extreme measures to promote lifestyle change (e.g., frequent sessions, free sneakers) that are not translated easily into community or clinical settings.
Approaches to translate diabetes prevention programs into different settings have been investigated. A systematic review of community-based interventions to prevent or delay T2D (9 studies of adults) reported variable treatment models with very modest improvements in outcomes. The majority of studies in this review used one-group designs and were not based on the DPP, and few measured plasma glucose or insulin resistance (Satterfield et al., 2003). More recently, group-based lifestyle programs translating the DPP to the community have demonstrated preliminary efficacy in terms of participants meeting weight loss goals (Laatikainen et al., 2007; Seidel, Powell, Zgibor, Siminerio, & Piatt, 2008) and improving glucose tolerance and lipid profiles (Laatikainen et al., 2007) in one-group designs. Experimental research evaluating the translation of the DPP into community or clinical settings is indicated. The lessons learned from previous translational research that were applied in the development of the protocol for this study are highlighted in a table at the Editor's Website at http://www.nursing-research-editor.com
Primary care represents a setting to screen at-risk adults and implement interventions to prevent T2D. Primary care practitioners typically provide health care for a large percentage of the population and have the ability to follow-up with patients over time. In addition, many primary care practitioners have established relationships with patients, which may enhance the delivery and receptivity of the recommended lifestyle changes. However, lifestyle change counseling has been reported to be difficult to accomplish in many primary care settings. Providers report pessimism about the motivation of patients to change their lifestyles, skepticism about the efficacy of brief lifestyle change counseling, limited time to provide lifestyle change counseling, limited training on effective counseling techniques, and low reimbursement rates (Kristeller & Hoerr, 1997; Larme & Pugh, 1998).
Nurse practitioners (NPs) represent an overlooked yet ideal health professional to implement lifestyle change counseling in primary care. There are currently over 85,000 certified registered NPs in the United States; the majority (77%) are certified in family or adult specialties (American Academy of Nurse Practitioners, 2002). They have been reported to be particularly cost-effective in preventive care due to their expertise in counseling, health education, and case management (Hummel & Pirzada, 1994). In providing care to adults with T2D, NPs were more likely to provide health education about nutrition, weight, and exercise compared to physicians (Lenz, Mundinger, Hopkins, Lin, & Smolowitz, 2002). In addition, many NPs provide health care for individuals who would be otherwise underserved (Fairbanks, Montoya, & Viens, 2001). Therefore, NPs are health professionals with access to adults at risk for T2D and the expertise to implement a diabetes prevention program.
Designing studies to test the translation of a research-based program (with established efficacy in clinical trials) into the healthcare setting requires consideration of broad processes and outcomes of care. The RE-AIM (Reach, Efficacy, Adoption, Implementation, Maintenance) model was the organizing framework of this study as it was developed for use in evaluating the effectiveness of health behavior programs in terms of public health significance (Glasgow, Vogt, & Boles, 1999). The major premise of the model is that public health impact of programs require more than efficacy. Programs must also reach a diverse sample, representative of the population at-risk. They must be appealing to health care providers and realistic to adopt in specific practice settings. Programs also must be able to be implemented as intended. Finally, programs must be maintained by both the individual and the clinical setting. These dimensions, involving both individual and organizational factors, interact to determine the overall population-based impact of a program.
The purpose of this pilot study was to test the translation of the DPP modified specifically for NPs to deliver in the context of primary care. Specific aims of the study were: (a) to modify the DPP collaboratively with NPs for implementation in primary care; (b) to evaluate the reach, implementation, and preliminary efficacy of a 6-month Lifestyle Program provided in primary care by NPs for adults at risk for diabetes on clinical (weight change, waist circumference, insulin resistance, lipids), behavioral (nutrition, exercise), psychosocial (depressive symptoms), and participant satisfaction outcomes compared to enhanced standard care; and (c) to evaluate the effects of 5% weight loss on clinical, behavioral, and psychosocial outcomes.
Methods
Design
A mixed-method design was used to modify a lifestyle change program for primary care and to evaluate the processes and outcomes associated with implementing the program in NP practices. The study had two distinct phases: Phase I was an interpretive and participatory method with the purpose of modifying the intervention protocol for easier implementation in the NP practices. Phase II was a prospective clinical trial pilot study with cluster randomization and repeated measures to evaluate the reach, implementation, and preliminary efficacy of the modified lifestyle program.
Sample
A convenience sample of 4 NP primary care practice sites were recruited from a regional practice-based research network for NPs in New England through a mailed invitation (22% response rate). Nonrespondent NPs declined due to ongoing research, having patients not speaking English, or lack of time. The network had 68 members; 80% were certified as family NPs (56%) or adult NPs (24%) providing care for ethnically and racially diverse adults. A cluster randomization procedure using a computerized table of random numbers randomized 4 sites: 2 sites into the lifestyle change program and 2 sites into an enhanced standard care program. Each site had a different distribution of NPs working with study participants (site 1 and 3 had 2 NPs for the duration of the study, site 2 had one NP, and site 4 had 2 NPs, with the second NP replacing the first one due to illness).
Procedure
The NPs recruited a convenience sample of 58 adults at risk for T2D from their practices (31 treatment and 27 control group participants). The sample size for this pilot study was determined by a power analysis, recruiting 20% of what would be necessary for a clinical trial testing the intervention. Inclusion criteria were: (a) age 21 years or older; (b) medically stable and safe to exercise; (c) at risk for IGT, metabolic syndrome, or T2D; and (d) able to speak English. Potential participants were considered at-risk if they were overweight or obese (BMI ≥ 25kg/m2) and were age 65 years or older. Adults younger than 65 years and overweight or obese also were considered at-risk if they had any other risk factor for T2D (family history of T2D, history of gestational diabetes or giving birth to a baby ≥ 9 pounds, of an ethnic group at high risk for T2D, hypertension, or lipid abnormalities of high tryglicerides and low-density lipoproteins and low high-density lipoproteins). Exclusion criteria included: current participation in a commercial diet program or treatment of IGT with metformin. Institutional review board approval was obtained from all institutional review boards associated with the study.
Interventions
Enhanced standard care
After informed consent and baseline data collection, all participants (regardless of group assignment) received written information about diabetes prevention, a 20- to 30-minute individual session with their NP on the importance of a healthy lifestyle for the prevention of T2D, and a 45-minute individual session with a nutritionist hired for the study. The goals of the standard care approach were similar to the DPP and represented the current treatment recommendation for individuals at risk for T2D. Specifically, participants were encouraged to follow a healthy diet (limit calories, fat, and processed foods); to lose 5-7% of their initial weight through diet and exercise; and to increase their exercise gradually with a goal of at least 30 minutes of exercise (e.g., walking) 5 days per week. Training for NPs at the sites randomized to enhanced standard care only and study nutritionists consisted of a 2-hour education session reviewing the study protocols. Monthly meetings were conducted to discuss any implementation questions.
Lifestyle change program
The lifestyle change program for this pilot study was based on the protocol for the DPP (Diabetes Prevention Research Group, 1999). The goals for this program were identical to enhanced standard care, yet the approach was more intensive and based on behavioral science evidence which recognizes the difficulty inherent in diet and exercise lifestyle change. The lifestyle change program for this study provided: (a) culturally relevant education on nutrition, exercise, and T2D prevention; (b) behavioral support in collaboratively identifying lifestyle change goals and problem-solving barriers to change; and (c) motivational interviewing when participants were unable to achieve lifestyle goals. These components were identical to those utilized in the DPP. Training for NPs at the sites randomized to the lifestyle program consisted of training on the enhanced standard care protocol, self-study (reading and a 45-minute DVD on motivational interviewing), two 2-hour workshops on motivational interviewing (before study, at 3 months), a 2-hour education session reviewing the lifestyle program protocols, and monthly meetings with the primary investigator. Consultation with an expert on motivational interviewing was available throughout the study. Study nutritionists provided nutrition sessions at all sites and were blinded to the site assignment.
The NPs who worked at the lifestyle program sites participated in the modification process of the lifestyle protocol which was aimed at maintaining key elements of the DPP while simultaneously enhancing the ability to implement the protocol in their practice settings. The changes made to the DPP protocol for this study were: a reduction of the number of in-person sessions from 16 over 6 months to 6 in-person sessions and 5 phone sessions over 6 months; and the revision of some content to be provided for participants to complete at home. The reduction in time was based on the NP's consideration of cost and time constraints of primary care. In-person sessions were structured to be provided in a 20-minute office visit. All educational content of the DPP was provided; however, content was abbreviated and the nutritional content was revised slightly. The lifestyle protocol used in this study is provided in Table 1. Over the 6 months of the intervention, participants were to receive approximately 3 hours of in-person NP support and 1 hour of NP phone support in contrast to 12-16 hours of individual sessions in the DPP core curriculum.
Table 1.
Lifestyle Protocol
| Session | Topic |
|---|---|
| NP session 1A | Welcome to the Lifestyle Balance Program. Highlighted study goals: 7% weight loss and 150 minutes of weekly physical activity. |
| NP session 1B | Healthy Eating. Emphasized the importance of a regular meal pattern and eating slowly. Used the Food Guide Pyramid as a model for healthy eating and compared personal eating patterns to these recommendations. Recommended specific low-fat, low-calorie substitutes at each level of the food pyramid. |
| Take-home | Healthy Eating Part II. More information on low-fat, healthy eating using culturally relevant recipes and information. |
| NP session 2 | Get Started Being Active. Discussed physical activity and importance to weight loss. Helped participants learn to set incremental exercise goals. |
| Take-home | Get Started Being Active Part II. More information on the benefits of exercise and how to exercise safely. |
| NP session 3 | Tip the Calorie Balance. Discussed the fundamental principle of energy balance and what it takes to lose 1-2 pounds per week. |
| Take-home | Take Charge of What's Around You. Introduced the principle of stimulus control and ways to identify cues in the home environment that lead to unhealthy food and activity choices. |
| NP session 4A | Talk Back to Negative Thoughts. Practiced identifying common patterns of self-defeating, negative thoughts and ways to counter these thoughts with positive statements. |
| NP session 4B | The Slippery Slope of Lifestyle Change. Stressed that slips are normal and learning to recover quickly is the key to success. |
| Take-home | Four Keys to Healthy Eating Out. Introduced four basic skills for managing eating away from home: anticipating and planning ahead, positive assertion, stimulus control, and making healthy food choices. |
| NP session 5 | You Can Manage Stress. Highlighted the importance of coping with stress, including stress caused by the Lifestyle Program. |
| Take-home | Make Social Cues Work for You. Presented strategies for managing problem social cues, e.g., being pressured to overeat, and helped participants learn social cues to promote healthy behaviors. |
| NP session 6 | Ways to Stay Motivated. Enhanced motivation to maintain behavior change by reviewing participants' personal reasons for joining the Lifestyle Program and by recognizing personal successes thus far |
| Take-home | Ways to Stay Motivated Part II. |
Notes. NP = Nurse Practitioner
Outcome Measures
Data were collected at the individual (participant) and organizational (NP and site) levels at scheduled time points throughout the study to evaluate the reach, implementation, and preliminary efficacy of the lifestyle program. All data were collected by trained research assistants blinded to group assignment with the exception of glucose tolerance, insulin resistance, and lipids, which were collected by experienced lab personnel at each site and sent to one laboratory for analysis.
Reach
Recruitment rates were documented for each NP practice. Demographic and clinical data (e.g., age, gender, SES, ethnicity, health history) were collected using a standard form.
Implementation
Participant measures of implementation consisted of attendance, attrition, and a satisfaction survey. The satisfaction survey was a 7-item summated scale modified from the Diabetes Treatment Satisfaction Survey (Bradley, 1994) to evaluate a diabetes prevention program. Adequate internal consistency has been reported with the original scale (alpha = .82) and was demonstrated with the modified scale in this study (alpha = .86).
Organizational measures of implementation consisted of NP and nutrition session documentation forms which were created with components of each session itemized. The percent of protocol implementation was calculated by dividing the number of protocol items by the number of protocol items completed per session. The NPs were interviewed also at 3 and 6 months to address issues of implementation.
Efficacy
Efficacy data were collected on clinical outcomes (weight loss, waist circumference, insulin resistance, and lipid profiles), behavioral outcomes (nutrition and exercise), psychosocial outcomes (depressive symptoms), and participant satisfaction. All data were collected at baseline, 3 months, and 6 months with the exception of laboratory data which were collected at baseline and 6 months, and the satisfaction survey which was collected at 6 months. Efficacy data collection measures and times were based on the DPP study and modified for the short duration of this pilot study.
Weight loss was the primary outcome and was calculated as percent weight loss from baseline to 6 months. Insulin resistance (IR) was an additional primary clinical outcome. After an 8-hour fast, subjects had fasting insulin and glucose levels drawn, ingested a standard glucose load (75 gm), and had insulin and glucose drawn at 120 minutes. The IR was assessed using the homeostasis model assessment (HOMA) which has been shown to be a good approximation (r = .35, p < .05) of more complex tests (metabolic clearance rate for glucose [fasting insulin (u/IU/ml) × fasting glucose (mmol/l)/22.5]; Wallace, Levy, & Matthews, 2004). Glucose at 120 minutes was used to assess glucose tolerance (GTT). Waist circumference and lipid profiles were secondary clinical outcomes. Waist circumference was measured by positioning a tape measure snugly midway between the upper hip bone and the uppermost border of the iliac crest. In very overweight participants, the tape was placed at the level of the umbilicus (Klein et al., 2007). Lipid profiles (low density lipoprotein [LDL], HDL, total cholesterol, total triglycerides) were determined using fasting venous blood.
Diet and exercise health promoting behaviors were measured with the exercise and nutrition subscales of the Health Promoting Lifestyle Profile II (8 and 9 items, respectively) which has items constructed on a 4-point Likert scale and measure patterns of diet and exercise behavior (Walker, Sechrist, & Pender, 1987). This instrument has been used in diverse samples and demonstrates adequate internal consistency (r = 0.70 to 0.90 for subscales; Jefferson, Melkus, & Spollett, 2000). The alpha coefficients for the exercise and nutrition subscales in this study were .86 and .76, respectively. Data on approximate minutes per week of exercise were collected also.
Psychosocial data were collected on depressive symptoms as measured by the Center for Epidemiologic Studies-Depression Scale (CES-D), a widely used scale (Radloff, 1977). The CES-D consists of 20 items that address depressed mood, guilt or worthlessness, helplessness or hopelessness, psychomotor retardation, loss of appetite, and sleep disturbance. Each item is rated on a scale of 0 to 3 in terms of frequency during the past week. The total score may range from 0 to 60, with a score of 16 or more indicating impairment. High internal consistency, acceptable test-retest reliability, and good construct validity have been demonstrated (Posner, Stewart, Marin, & Perez-Stable, 2001). The alpha coefficient was .93 for the CES-D in this sample.
Data Analysis
Data were entered into databases (Microsoft Access or Excel) via an automated Teleform system. Mean substitution was employed for missing data of individual items on instruments (up to 15%). If more than 15% of items were missing (rare), the subscale or scale was coded as missing data. Descriptive statistics were calculated using frequency distributions and appropriate summary statistics for central tendency and variability (using SAS). The two groups were compared on major variables to make certain that the cluster random assignment equally distributed the sample. Variables unequally distributed were controlled for in subsequent analyses.
Reach and implementation were analyzed with descriptive statistics and content analysis of NP interviews and process notes. The hypothesis that at-risk adults who received the lifestyle change program would demonstrate better clinical, behavioral, and psychosocial outcomes than at-risk adults who received an enhanced standard care control program was tested using an intent to treat repeated measures mixed modeling procedure (PROC MIXED, SAS). Each model had fixed effects (group assignment, income, race, month, and month by group interaction with age as a covariate) and random effects (intercept and site for dependent variables with two evaluation time points; intercept, site, and month for dependent variables with three evaluation points). The main effect of time was estimated, which described the average monthly change in outcomes across both groups; and treatment by time interaction, which compared the rates of monthly change between treatment and control groups. Because it was possible that significant main effects or interaction effects would not be found due to the sample size, identification of trends of significance (e.g., p < .20) and effect sizes were examined. Participant satisfaction was analyzed with t-test analysis.
Results
Reach
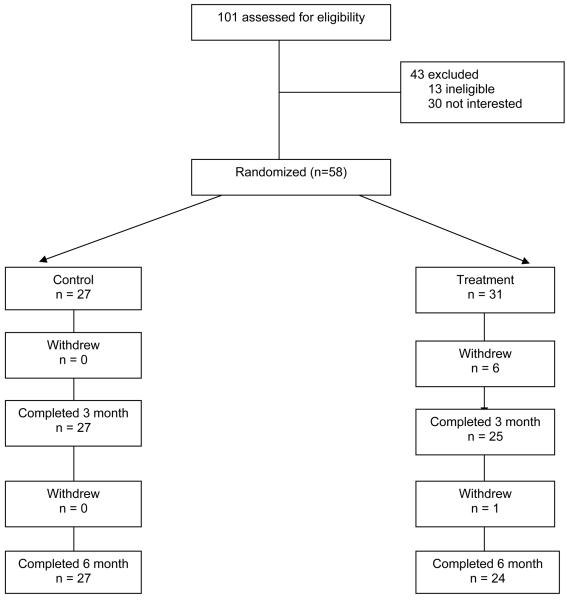
Fifty-eight participants were enrolled April-August 2006 and the study ended in September 2007. There was a 70% response rate to in-person recruitment at NP practices and a 12% attrition rate at 6 months (Figure 1). Those who did not complete the study were younger, had higher BMI, and lower low-density lipoproteins (p < .05). Reasons for attrition included: competing life demands such as medical issues (n = 4), move or lost to follow-up (n = 2), or preference for alternate treatment (n = 1).
Figure 1.
Consort Table
The sample represented diverse (45% White), primarily female (92%), obese, moderately-low income adults at risk for T2DM (Table 2). Thirty-three percent of the sample reported elevated depressive symptoms. There were no statistically significant differences between groups at baseline on clinical, behavioral, or psychosocial variables. There were more Black participants in the treatment group in contrast to more Hispanic participants in the control group (p = .01). More participants in the treatment group had moderately-low income compared to low income in the control group (p = .01).
Table 2.
Characteristics of Participants at Baseline by Treatment Group
| Characteristic | Treatment (n = 31) |
Control (n = 27) |
p |
|---|---|---|---|
| Age, years | |||
| Mean (±SD) | 48.2 (12.4) | 43.2 (13.2) | .1415* |
| Sex | |||
| Male, n (%) | 3 (50.0) | 3 (50.0) | 1.0000# |
| Female, n (%) | 28 (53.8) | 24 (46.2) | |
| Race | |||
| White, n (%) | 15 (57.7) | 11 (42.3) | .0116# |
| Black, n (%) | 14 (70.0) | 6 (30.0) | |
| Hispanic, n (%) | 2 (16.7) | 10 (83.3) | |
| Income | |||
| < $19,999, n (%) | 1 (9.1) | 10 (90.9) | .0176# |
| $20,000 - $39,999, n (%) | 12 (70.6) | 5 (29.4) | |
| $40,000 - $59,999, n (%) | 7 (58.3) | 5 (41.7) | |
| $60,000 - $99,999, n (%) | 7 (70.0) | 3 (30.0) | |
| $100,000 and greater, n (%) | 4 (50.0) | 4 (50.0) | |
| Clinical Variables | |||
| HOMA** | 5.6 (3.1) | 5.7 (5.4) | .5942* |
| Glucose120 (mg/dl) | 119.1 (40.9) | 109.8 (36.1) | .3670* |
| LDL (mg/dl) | 123.0 (38.0) | 109.7 (33.4) | .1164* |
| HDL (mg/dl) | 48.6 (13.1) | 43.7 (11.0) | .1295* |
| BMI (kg/m2) | 40.0 (9.0) | 37.4 (7.0) | .2262* |
| Waist (inches) | 45.3 (7.8) | 42.6 (6.9) | .1720* |
| Behavioral Variables | |||
| Physical Activity | 1.8 (0.5) | 1.9 (0.6) | .7747* |
| Minutes of Exercise/Week | 139.7 (191.3) | 129.5 (139.7) | .8162* |
| < 150 minutes, n (%) | 22 (57.9) | 16 (42.1) | .4130# |
| Nutrition (HPLP range 0-4) | 2.4 (0.6) | 2.4 (0.5) | .6695* |
| CES-D (range 0 – 60) | 12.1(10.7) | 14.9 (13.6) | .3890* |
| ≥ 16 total score, n (%) | 10 (52.6) | 9 (47.4) | 1.0000# |
Notes.
Student's t-test;
Fisher's Exact test;
Log-Transformed for Analysis
HOMA = Homeostasis model assessment; LDL = low density lipoprotein; HDL = high density lipoprotein; BMI = body mass index; HPLP = Health promoting lifestyle profile; CES-D = Center for Epidemiologic Studies-Depression Scale
Implementation of the Intervention
Attendance for in-person sessions was high at 96%. Completion of phone calls for the lifestyle program was problematic with only a 37% success rate due to difficulty scheduling and making phone appointments (both providers and participants). Implementation of the lifestyle program took 9.3 months compared to the outlined protocol of 6.5 to 7 months due to NP illnesses, rescheduling of participant appointments, and the end of the year holidays. The protocols for in-person sessions were implemented with moderately good success. Implementation of the standard care NP protocol was 80%, implementation of the standard care nutrition protocol was 92%, and NP implementation of the lifestyle protocol was 76%. The NPs of the lifestyle program reported confidence in the ability to implement the educational and behavioral strategies of goal setting and problem-solving. All NPs reported that motivational interviewing was the most challenging aspect of the protocol to implement. The NPs reported difficulty in building motivation to change and in helping participants see that their behavior was inconsistent with personal values and goals; however, they worked consistently at improving their skills for the duration of the study. The NPs requested additional training and expert consultation throughout the course of the study. Protocol implementation increased over time. One factor that contributed to difficulty implementing the protocol was time as NPs were encouraged to complete sessions in 20 minutes to maintain their office schedule. The NPs reported that study participants often discussed psychosocial issues within the context of lifestyle change (e.g., stress of job), and this sometimes precluded the ability to complete all aspects of the protocol. In this situation, participants were encouraged to complete the session content at home using the standardized education handouts.
Preliminary Efficacy of the Intervention
Clinical outcomes
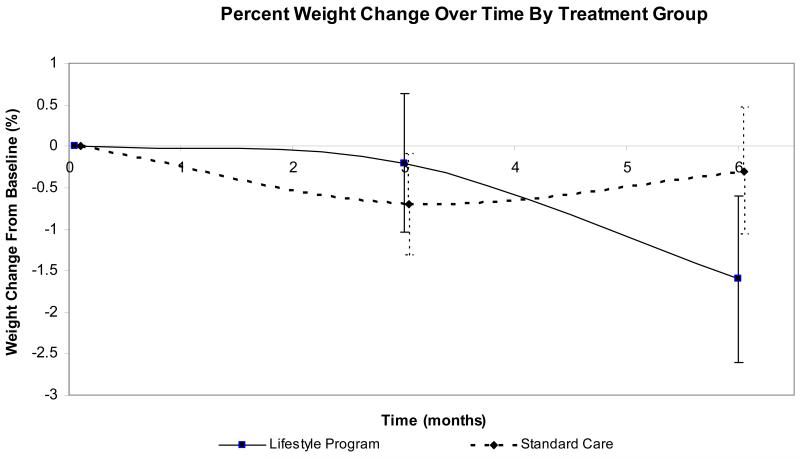
Results of the mixed model analyses for all outcomes are reported in Table 3. Participants in the lifestyle program demonstrated trends for greater percent weight loss (p = .08) and higher HDL levels (p = .21) over participants in the enhanced standard care program. Mean percent weight change from baseline between the two groups is shown in Figure 2. At 6 months, 25% of lifestyle participants achieved a weight loss goal of 5% compared to 11% of standard care participants. The HOMA levels demonstrated a trend to increase over time in both groups (p = .11). There were no significant differences or trends with respect to other clinical variables.
Table 3.
Estimates of Monthly Change in Outcome in Treatment and Control Groups
| Characteristic | Control group rate of change |
Treatment group rate of change |
pa | pb | Group effect size at 6 months |
|---|---|---|---|---|---|
| Clinical Variables | |||||
| Percent Change in Weight | 0.13 | −0.42 | .45 | .08 | 0.33 |
| HOMA** | 0.02 | 0.01 | .11 | .61 | 0.14 |
| Glucose 120 minutes (mg/dl) | 1.50 | 0.28 | .30 | .48 | 0.03 |
| Insulin 120 minutes (μU/ml) ** | 0.01 | −0.03 | .59 | .29 | 0.05 |
| LDL (mg/dl) | −0.14 | 0.07 | .94 | .87 | 0.28 |
| HDL (mg/dl) | 0.17 | 0.60 | .03 | .21 | 0.24 |
| BMI (kg/m2) | −0.02 | −0.03 | .55 | .97 | 0.11 |
| Waist (inches) | −0.03 | −0.19 | .33 | .35 | 0.28 |
| Behavioral Variables | |||||
| Physical Activity | 0.05 | 0.10 | <.0001 | .08 | 0.24 |
| Nutrition | 0.04 | 0.03 | .001 | .63 | 0.02 |
| CES-D (range 0-60) | −0.01 | −0.34 | .40 | .42 | 0.01 |
Notes.
Log-Transformed for Analysis
p-value for main effect of time (month) across both groups
p-value for interaction of treatment and time (month)
HOMA = Homeostasis model assessment; LDL = low density lipoprotein; HDL = high density lipoprotein; BMI = body mass index; CES-D = Center for Epidemiologic Studies-Depression Scale
Figure 2.
Weight Change From Baseline (%) by Group
Behavioral outcomes
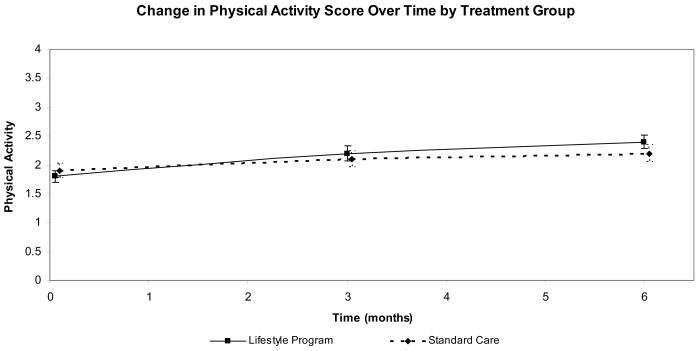
Participants in both groups demonstrated improvement over time in nutrition behavior (p = .001). Both groups also demonstrated a significant monthly increase in exercise behavior (p = .001), with lifestyle participants demonstrating trends toward greater improvement in exercise (p = .08; Figure 3). The percent of participants meeting the exercise goal of 150 minutes per week increased in the lifestyle group from 29% at baseline to 46% at 6 months and was relatively stable in the enhanced standard care group (39% to 40% at 6 months)
Figure 3.
Exercise Change From Baseline (minutes per week) by Group
Psychosocial and satisfaction outcomes
While there was a decrease in depressive symptoms over time, this change was not significant. Participants of the lifestyle program were more satisfied with the program compared to standard care participants (t = 2.06; p = .048).
Effect of 5% Weight Loss on Clinical Outcomes
Mixed-model analysis comparing participants with 5% weight loss, regardless of group assignment, to participants who did not achieve 5% weight loss supported the beneficial effect of weight loss with respect to a decrease or a decreasing trend in HOMA (p = .10), GTT (p = .02), insulin at 120 minutes (p = .001), LDL (p = .10), triglyceride (p = .14), and cholesterol (p = .07; Table 4). Both groups improved their exercise (p = .001) and nutrition behaviors (p = .001), with a greater rate of increase in exercise among participants who achieved 5% weight loss (p = .01).
Table 4.
Estimates of Monthly Change in Outcomes in Group with 5% Weight Loss at 6 Months vs Group with <5% Weight Loss at 6 Months
| Characteristic | Rate of change in < 5% weight loss group |
Rate of change in 5% weight loss group |
pa | Group effect size at 6 months |
|---|---|---|---|---|
| Clinical variables | ||||
| HOMA** | ||||
| Glucose 120 minutes (mg/dl) | 0.02 | −0.03 | .10 | 0.45 |
| Insulin 120 minutes (μU/ml)** | 1.94 | −2.98 | .02 | 0.64 |
| LDL (mg/dl) | 0.02 | −0.14 | .0001 | 1.43 |
| HDL (mg/dl) | 0.34 | −2.38 | .10 | 0.50 |
| Triglyceride (mg/dl) ** | 0.45 | −0.02 | .30 | 0.44 |
| Cholesterol (mg/dl) | 0.003 | −0.03 | .14 | 0.59 |
| Waist (inches) | 0.78 | −3.22 | .07 | 0.59 |
| Behavioral variables | −0.07 | −0.24 | .38 | 0.24 |
| Physical activity | 0.05 | 0.15 | .01 | 0.87 |
| Nutrition (HPLP range 0-4) | 0.03 | 0.04 | .62 | 0.29 |
| CES-D (range 0 – 60) | −0.14 | −0.53 | .46 | 0.17 |
Notes.
Log-Transformed for Analysis
p-value for interaction of treatment and time (month)
HOMA = Homeostasis model assessment; LDL = low density lipoprotein; HDL = high density lipoprotein; BMI = body mass index; HPLP = Health promoting lifestyle profile; CES-D = Center for Epidemiologic Studies-Depression Scale
Discussion
Results of this study support the feasibility of implementing a diabetes prevention program by NPs in a primary care setting to adults at risk for T2D. Study participants were primarily female, of diverse race or ethnicity, obese, sedentary, of low to moderately-low income, and with elevated depressive symptoms, which is representative of adults at-risk for T2D. There were some differences with respect to race or ethnicity and income between groups which were due to the geographical location of NP practices. More importantly, the reach of the intervention to diverse racial and ethnic groups and to adults with low and moderately-low income needs to be highlighted as racial and ethnic differences in rates of obtaining routine preventive care have been documented (Kirk et al., 2005). While public health interventions are being advocated increasingly to reduce health disparities, continued attention to the implementation of interventions in community settings is indicated (Liburd & Vinicor, 2003). The NPs clearly reach this targeted population, providing culturally relevant care within indigenous community structures. The provision of culturally relevant care for diverse ethnic and racial groups appears critical to improved health outcomes in the prevention and management of chronic illnesses (Campbell et al., 2007; Whittemore, 2007).
Successful reach and implementation of study protocols are important results of this study. There was high attendance for in-person sessions and low attrition, critical elements to improving diabetes prevention efforts in vulnerable populations. Previous research implementing behavioral or psychological intervention research into primary care has demonstrated high attrition (30-40%) and considerable implementation issues (Zayas, McKee, & Jankowski, 2004). While there were some notable issues in the implementation of study protocols in this study (i.e., difficulty completing phone sessions, longer duration of program, frequent rescheduling of participant appointments), protocol implementation of in-person sessions was very good. The NPs were able to implement successfully an enhanced standard care and a lifestyle program aimed at T2D prevention within the context of primary care (i.e., 20-minute sessions) and without considerable training. Future dissemination of this program will be facilitated as NPs in both groups reported high levels of confidence in implementing the educational and behavioral strategies subsumed within the programs. Motivational interviewing was the only behavioral strategy that NPs in the lifestyle program expressed lack of confidence. The finding that NPs were able to implement many aspects of a lifestyle program easily is important, as lifestyle counseling has been reported to be difficult to implement by primary care physicians.
Preliminary efficacy results of the lifestyle program indicate modest improvements with respect to clinical and behavioral outcomes. Twenty-five percent of lifestyle participants achieved a 5% weight loss goal compared to 11% of participants in standard care. These results were obtained with a lifestyle program of much shorter duration than the DPP (4 hours vs. 12-16 hours), which is considerably less costly and potentially more acceptable to practitioners and participants. Weight loss of 5-7% in adults at risk for T2D improves glucose tolerance and has been shown consistently to reduce the risk for T2D (Colman et al., 1995; Rana, Li, Manson, & Hu, 2007). Modest weight loss was a strong predictor of T2D risk reduction in the DPP, with a 16% reduction in diabetes risk per kilogram of weight loss (Hamman et al., 2006).
Both lifestyle participants and standard care participants demonstrated improvements in diet and exercise behavior over time, additional behaviors shown to decrease T2D risk (Hamman et al., 2006). However, lifestyle participants demonstrated a trend toward greater improvements in exercise behavior. This finding is important as increasing exercise has been the primary behavior associated with risk reduction for type 2 diabetes, often in combination with weight loss. In the DPP, diabetes risk decreased as nutrition, exercise, and weight loss goals were met. Increased physical activity helped to sustain weight loss and for those participants not meeting the weight loss goal at 1 year, it helped to lower diabetes risk (Hammon et al., 2006). Exercise decreases glucose-stimulated insulin production, increases insulin sensitivity, and decreases abdominal adiposity, all risk factors for IGT and T2D (Bloem & Chang, 2008; Pratley et al., 2000). In a prospective study with adults at risk for T2D, a protective effect of exercise was observed, even in adults with high body mass index and glucose levels (Hu et al., 2004).
More than 50% of all Americans are not engaging in regular exercise or physical activity, and 25% report no leisure time physical activity (Zoeller, 2007). Participants in this study were not physically active at baseline, with only 34% reporting an exercise goal of 150 minutes per week. Thus, lifestyle programs that provide at-risk adults with strategies to initiate and maintain exercise safely are critical to T2D prevention. The increased intensity of the lifestyle program in this study appears to have enhanced participants' ability to engage in exercise.
Nutrition behaviors also play an important role in T2D risk reduction. Both groups in this study improved nutrition behaviors over time. Providing educational and behavioral strategies on nutrition and physical activity are foundational aspects to diabetes prevention programs. Meeting nutritional goals has been associated with weight loss maintenance which has been shown to improve diabetes risk factors (Avenell et al., 2004). Strategies to support weight loss as well as insulin sensitivity are important in T2D prevention. The beneficial effect of weight loss in adults at risk for diabetes was supported in this study as participants with a 5% weight loss demonstrated clinically significant improvements on important clinical outcomes of glucose, insulin resistance, and lipids as well as exercise behavior.
While this was a pilot study focused on the reach and implementation as well as preliminary efficacy, inadequate power precludes strong conclusions or clinical implications. Clearly, NPs represent a health care provider able to provide a diabetes prevention program within the context of primary care. The targeted population of diverse adults at risk for T2D was reached, NPs were able to implement the intervention components, and modest efficacy was achieved. Systems of care were complex and unique to each practice, and successful implementation required research support, primarily with scheduling of appointments.
Future research will be aimed at increasing the intensity of the intervention while considering the structure and processes of primary care. Eliminating phone sessions, providing additional in-person sessions, addressing elevated depressive symptoms, encouraging family participation at sessions, and developing a maintenance component to the program are revisions that are currently in progress. Previous research strongly supports increasing the intensity of behavioral interventions to increase the potential for weight reduction and maintenance (Norris et al., 2004). The challenge remains to identify the settings and sessions required. Typically, NPs provide ongoing follow-up care for adults at risk for T2D; thus, a maintenance component to this lifestyle program appears feasible. In addition, enhanced motivational interviewing training for NPs is indicated also. Strategies to simplify the implementation process for primary care office personnel are being explored also.
Conclusion
With the increasing prevalence of obesity and T2D risk, many adults would benefit from a preventive intervention. Yet, intensive lifestyle interventions are not implemented easily in the current health care system. Research is needed to evaluate less intensive interventions that take into consideration issues of reach and implementation as well as intervention efficacy. Results demonstrate a collaborative process of translating the DPP into the primary care setting, with NPs taking part in shaping and implementing the intervention protocol. While this was a small pilot study from one geographical area, with a relatively short program duration, preliminary results with respect to reach, implementation, and efficacy support further development and testing of this lifestyle program.
Acknowledgements
Supported by a grant from NIH/NIDDK R34DK070594. The clinical team was: Nanette Alexander, MSN, APRN; Alison Beale, RD; Diane Bussolini, RD; Elizabeth Magenheimer, MSN, APRN; Ulrike Muench, MSN, APRN; Karen Stemler, MS, APRN; Elizabeth Visone, APRN; Stephanie Wilborne, MSN, APRN; Stacie Zibel, MS, APRN. The research team was: Jo Cecille Demarest, MS, Project Director; Amy Triche, BA, Monika Haugstetter, BS, Felicia Lucas, BSN, Alyssa Roman, BA, Leah Swalley, BS, Research Assistants; Tony Ma, PhD, Data Manager;; Siobhan Thompson, MPH, Director of Research Administration Center for Self and Family Management of Vulnerable Populations; Judith Wylie-Rosett, EdD, RD, Consultant.
Contributor Information
Robin Whittemore, Yale School of Nursing New Haven, Connecticut.
Gail Melkus, Nursing Yale School of Nursing New Haven, Connecticut.
Julie Wagner, Division of Behavioral Sciences and Community Health University of Connecticut Health Center Farmington, Connecticut.
Veronika Northrup, Yale Center for Clinical Investigations New Haven, Connecticut.
James Dziura, Yale Center for Clinical Investigations New Haven, Connecticut.
Margaret Grey, Dean & Annie Goodrich Prof of Nursing Yale School of Nursing New Haven, Connecticut.
References
- American Academy of Nurse Practitioners Annual report 2002. 2002 Retrieved April 22, 2003, available from http://www.aanp.org.
- American Diabetes Association Economic costs of diabetes in the US in 2007. Diabetes Care. 2008;31(3):1–20. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al. Systemic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technology Assessment. 2004;8(21):1–182. doi: 10.3310/hta8210. [DOI] [PubMed] [Google Scholar]
- Bloem CJ, Chang AM. Short-term exercise improves beta-cell function and insulin resistance in older people with impaired glucose tolerance. The Journal of Clinical Endocrinology & Metabolism. 2008;93(2):387–392. doi: 10.1210/jc.2007-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley C. Diabetes treatment satisfaction questionnaire. In: Bradley C, editor. Handbook of psychology and diabetes. Harwood Academic Publishers; Australia: 1994. pp. 111–132. [Google Scholar]
- Campbell MK, Hudson MA, Resnicow K, Blakeney N, Paxton A, Baskin M. Church-based health promotion interventions: Evidence and lessons learned. Annual Review of Public Health. 2007;28:213–234. doi: 10.1146/annurev.publhealth.28.021406.144016. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . National diabetes fact sheet: General information and national estimates of diabetes in the United States, 2000. Author; Atlanta, GA: 2004. [Google Scholar]
- Colman E, Katzel LI, Rogus E, Coon P, Muller D, Goldberg AP. Weight loss reduces abdominal fat and improves insulin action in middle-aged and older men with impaired glucose tolerance. Metabolism. 1995;44(11):1502–1508. doi: 10.1016/0026-0495(95)90153-1. [DOI] [PubMed] [Google Scholar]
- Diabetes Prevention Research Group Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22(4):623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks J, Montoya C, Viens DC. Factors influencing the recruitment and retention of nurse practitioners into rural, underserved, and culturally diverse areas. The American Journal for Nurse Practitioners. 2001;5:21–31. [Google Scholar]
- Foster GD, Makris AP, Bailer BA. Behavioral treatment of obesity. The American Journal of Clinical Nutrition. 2005;82(1 Suppl):230S–235S. doi: 10.1093/ajcn/82.1.230S. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. American Journal of Public Health. 1999;89(9):1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Lindstrom J, Valle TT, Eriksson JG, Jousilahti P, Silventoinen K, et al. Physical activity, body mass index, and risk of type 2 diabetes in patients with normal or impaired glucose regulation. Archives of Internal Medicine. 2004;164(8):892–896. doi: 10.1001/archinte.164.8.892. [DOI] [PubMed] [Google Scholar]
- Hummel J, Pirzada S. Estimating the cost of using non-physician providers in an HMO: Where would the savings begin? HMO Practice. 1994;8(4):162–164. [PubMed] [Google Scholar]
- Jefferson VW, Melkus GD, Spollett GR. Health promotion practices of young black women at risk for diabetes. The Diabetes Educator. 2000;26(2):295–302. doi: 10.1177/014572170002600210. [DOI] [PubMed] [Google Scholar]
- Kirk JK, Bell RA, Bertoni AG, Arcury TA, Quandt SA, Goff DC, Jr., et al. A qualitative review of studies of diabetes preventive care among minority patients in the United States, 1993-2003. The American Journal of Managed Care. 2005;11(6):349–360. [PubMed] [Google Scholar]
- Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, et al. Waist Circumference and Cardiometabolic Risk: A consensus statement from Shaping America's Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition and the American Diabetes Association. Obesity. 2007;15(5):1061–1067. doi: 10.1038/oby.2007.632. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England Journal of Medicine. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristeller JL, Hoerr RA. Physician attitudes toward managing obesity: Differences among six specialty groups. Preventive Medicine. 1997;26(4):542–549. doi: 10.1006/pmed.1997.0171. [DOI] [PubMed] [Google Scholar]
- Laatikainen T, Dunbar JA, Chapman A, Kilkkinen A, Vartiainen E, Heistaro S, et al. Prevention of type 2 diabetes by lifestyle intervention in an Australian primary health care setting: Greater Green Triangle (GGT) diabetes prevention project. BMC Public Health. 2007;7:249. doi: 10.1186/1471-2458-7-249. doi:10.1186/1471-2458-7-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larme AC, Pugh JA. Attitudes of primary care providers toward diabetes: Barriers to guideline implementation. Diabetes Care. 1998;21(9):1391–1396. doi: 10.2337/diacare.21.9.1391. [DOI] [PubMed] [Google Scholar]
- Lenz ER, Mundinger MO, Hopkins SC, Lin SX, Smolowitz JL. Diabetes care processes and outcomes in patients treated by nurse practitioners or physicians. The Diabetes Educator. 2002;28(4):590–598. doi: 10.1177/014572170202800413. [DOI] [PubMed] [Google Scholar]
- Liburd LC, Vinicor F. Rethinking diabetes prevention and control in racial and ethnic communities. Journal of Public Health Management Practice, Supplement. 2003:S74–S79. doi: 10.1097/00124784-200311001-00013. [DOI] [PubMed] [Google Scholar]
- Nothwehr F, Yang J. Goal setting frequency and the use of behavioral strategies related to diet and physical activity. Health Education Research. 2007;22(4):532–538. doi: 10.1093/her/cyl117. [DOI] [PubMed] [Google Scholar]
- Norris SL, Zhang X, Avenell A, Gregg E, Bowman B, Schmid CH, et al. Long-term effectiveness of weight-loss interventions in adults with pre-diabetes: a review. American Journal of Preventive Medicine. 2004;28(1):126–139. doi: 10.1016/j.amepre.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- Posner SF, Stewart AL, Marin G, Perez-Stable EJ. Factor variability of the Center for Epidemiological Studies Depression Scale (CES-D) among urban Latinos. Ethnicity & Health. 2001;6(2):137–144. doi: 10.1080/13557850120068469. [DOI] [PubMed] [Google Scholar]
- Pratley RE, Hagberg JM, Dengel DR, Rogus EM, Muller DC, Goldberg AP. Aerobic exercise training-induced reductions in abdominal fat and glucose-stimulated in responses in middle-aged and older men. Journal of American Geriatrics Society. 2000;48(9):1055–1061. doi: 10.1111/j.1532-5415.2000.tb04780.x. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for researching the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rana JS, Li TY, Manson JE, Hu FB. Adiposity compared with physical Inactivity and risk of type 2 diabetes in women. Diabetes Care. 2007;30(1):53–58. doi: 10.2337/dc06-1456. [DOI] [PubMed] [Google Scholar]
- Resnicow K, DiIorio C, Soet JE, Ernst D, Borrelli B, Hecht J. Motivational interviewing in health promotion: It sounds like something is changing. Health Psychology. 2002;21(5):444–451. [PubMed] [Google Scholar]
- Rollnick S, Miller WR, Butler CC. Motivational interviewing in health care. Guilford Press; New York: 2007. [Google Scholar]
- Satterfield DW, Volansky M, Caspersen CJ, Engelgau MM, Bowman BA, Gregg EW, et al. Community-based lifestyle interventions to prevent type 2 diabetes. Diabetes Care. 2003;26(9):2643–2652. doi: 10.2337/diacare.26.9.2643. [DOI] [PubMed] [Google Scholar]
- Seidel MC, Powell RO, Zgibor JC, Siminerio LM, Piatt GA. Translating the Diabetes Prevention Program into an urban medically underserved community: A nonrandomized prospective intervention study. Diabetes Care. 2008;31(4):684–689. doi: 10.2337/dc07-1869. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Illanne-Parrika P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. The New England Journal of Medicine. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Walker SN, Sechrist KR, Pender NJ. The Health-Promoting Lifestyle Profile: Development and psychometric characteristics. Nursing Research. 1987;36(2):76–81. [PubMed] [Google Scholar]
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- West DS, DiLillo V, Bursac Z, Gore SA, Greene PG. Motivational Interviewing improves weight loss in women with type 2 diabetes. Diabetes Care. 2007;30(5):1081–1087. doi: 10.2337/dc06-1966. [DOI] [PubMed] [Google Scholar]
- Whittemore R. Culturally competent interventions for Hispanic adults with type 2 diabetes: A systematic review. Journal of Transcultural Nursing. 2007;18(2):157–166. doi: 10.1177/1043659606298615. [DOI] [PubMed] [Google Scholar]
- Zayas LH, McKee MC, Jankowski KR. Adapting psychosocial intervention research to urban primary care environments: A case example. Annals of Family Medicine. 2004;2(5):504–508. doi: 10.1370/afm.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RF. Prescribing physical activity for cardiovascular and metabolic health. American Journal of Lifestyle Medicine. 2007;1(2):99–102. [Google Scholar]