Abstract
Diel studies of an Emiliania huxleyi bloom within a mesocosm revealed a highly dynamic associated viral community, changing on small times scales of hours.
Emiliania huxleyi is a globally important unicellular marine phytoplankton. It is known to form blooms that can extend over 100 000 km2 and is known to have a significant role in the carbon and sulphur cycles in the ocean, as well as having an impact on the climate (Fuhrman, 1999). It is now widely accepted that large double-stranded DNA viruses are instrumental in the collapse of E. huxleyi blooms, playing key roles in structuring E. huxleyi communities (Bratbak et al., 1996a; Schroeder et al., 2002). Characterization of these large E. huxleyi-specific viruses (EhVs) has revealed that they belong to the Phycodnaviridae family of algal viruses (Schroeder et al., 2002). The molecular dynamics and virus succession within E. huxleyi blooms have been previously studied in mesocosm experiments by Schroeder et al. (2003) and Martínez-Martínez et al. (2007), who found the presence of genetically rich, but stable E. huxleyi communities, with the same virus and host genotypes dominating in subsequent years.
General virus abundance has been found to be dynamic in some marine systems, on as small a temporal scale as 10 min intervals, with numbers fluctuating by a factor of up to 4 within this timescale (Bratbak et al., 1996b). Moreover, diversity in a particular group of bacteriophage has previously been shown to be changeable on scales of hours to days, due to rapidly switching host lysis targets in the surface waters (Parada et al., 2008). However, there have been no studies of algal virus community dynamics with a similarly high temporal resolution. The purpose of this study was to extend existing research (Schroeder et al., 2003; Martínez-Martínez et al., 2007), using EhV-specific and E. huxleyi-specific PCR primers, combined with denaturing gradient gel electrophoresis (DGGE) and sequence analysis to investigate E. huxleyi-EhV dynamics over diel cycles in a mesocosm experiment.
A mesocosm experiment was carried out at the Marine Biological Field Station adjacent to Raunefjorden, 20 km south of Bergen, Norway (60°22.1′N; 5°28.1′E), in June 2008. The experimental devices consisted of transparent polyethylene enclosures (11 m3; 90% light penetration of photosynthetically active radiation) mounted on floating frames, moored along the south side of a raft. Nutrients were added to obtain a ratio of nitrogen: phosphorous of 15:1 (1.5 µM NaNO3; 0.1 µM KH2PO4) as described by Jacquet et al. (Jacquet et al., 2002). Sample collection started 6 days after the start of the experiment (day 6), where 700 mL water samples were collected in triplicate and filtered through 0.45 µm pore-size PALL Gelman filters from a single enclosure over three diel cycles. Samples were taken at intervals of 4 h, starting at 0600 on 10 June 2008 (day 6), until 0600 on 11 June 2008 (day 7); additional samples, to give a total of 10 per diel cycle were taken on days 8/9 and 11/12 (12–13 June 2008 and 15–16 June 2008, respectively). Total genomic DNA was immediately extracted from replicate filters using the DNeasy blood and tissue kit (Qiagen) according to manufacturer's recommendations, with the exception of the proteinase K incubation, which was extended to 30 min, and the elution volume, which was 100 µL. Host and internal virus community structure were determined by the method described in Schroeder et al. (2003). PCR amplification was achieved using primers specific to the genes encoding the major capsid protein (MCP) in EhV and calcium binding protein (GPA) in E. huxleyi (Schroeder et al., 2003). PCR products from the different time points were each analysed by DGGE, to visualize the respective E. huxleyi and EhV community structure over the diel cycles. Individual bands, corresponding to putative genotypes were selected and excised for EhV and E. huxleyi and were subsequently purified according to Schroeder et al. (2003) and were sequenced by Geneservice (Cambridge). High-quality sequences were selected and aligned with those available in the GenBank database and any novel sequences generated were deposited under accession numbers FJ668535–FJ668537.
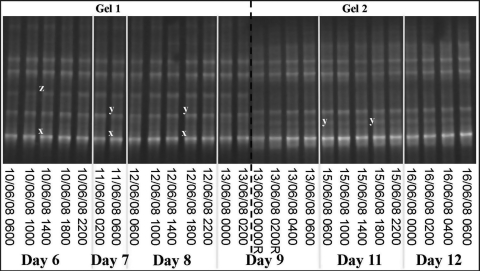
DGGE profiles were evaluated in conjunction with results from the analytical flow cytometry (AFC) counts of the mesocosm experiment for E. huxleyi and EhV, provided by W. H. Wilson and S. Kimmance (personal communication). AFC indicated typical dynamics of E. huxleyi growth in the mesocosm; days 6–7 were pre-bloom, days 8–9 were during the bloom and days 11–12 were within the bloom peak. Replicate DGGE gel images of amplified GPA from E. huxleyi showed a stable community structure, with at least five genotypes persisting at the same time throughout the three diel cycles, which extended over 7 days of the mesocosm experiment (Fig. 1). This is consistent with previous studies of E. huxleyi blooms in 2000 and 2003 (Martínez-Martínez et al., 2007). There was no change in genotypic composition in any of the samples analysed in this study, during the development of the bloom, observed up to day 12. At this point E. huxleyi cell numbers, counted by AFC, reached 1.7 × 105 cells mL−1. Sequencing of the excised bands from the E. huxleyi DGGE gel confirmed the presence of three genotypes previously detected in the 2000 and 2003 blooms, corresponding to sequences designated as OTU3 (band x), OTU5 (band z) and OTUf (band y) by Martínez-Martínez et al. (2007) (Fig. 1). Running multiple DGGE gels often results in minor variations in gel properties between gels. This can be seen in Fig. 1 where the positions of band z and band y are slightly lower in gel 2 compared with gel 1. Sequencing confirmed the identity of band y, hence we can infer that the band directly above is that of band z. These three genotypes identified were present throughout the duration of the bloom, once again highlighting the stability of the E. huxleyi community within the Norwegian Fjord.
Fig. 1.
A representative DGGE image of Emiliania huxleyi-amplified PCR fragments of the GPA gene from three diel cycles of the mesocosm experiment, Norway, 2008. Letters indicate bands excised and confirmed by sequencing. Identical letters indicate bands with identical sequences, where x is identical to OTU3, y is identical to OTUf and z is identical to OTU5. The sample set was run across two gels, where the letter R indicates samples run on both gels to account for any variations in the gel properties.
Emiliania huxleyi virus (EhV) AFC counts showed 3.7 × 105 EhV-like viruses mL−1 on day 6 of the experiment and the EhV infectious community structure was found to be temporally variable on this day (Fig. 2). No pronounced diel variability in EhV-like virus counts was detected by AFC during the sampled period. At least five bands, corresponding to viral genotypes, were present during day 6, occurring individually at 0600, then with up to three genotypes co-occurring at a single time point later that day, on replicate DGGE gels (Fig. 2). During day 8 of the experiment, EhV counts increased in abundance to 4.6 × 105 EhV-like viruses mL−1 and the EhV community structure continued to be changeable, until one virus genotype (band d) became dominant towards the end of the day and into day 9, coinciding with a further increase in virus abundance, as counted by AFC. This same virus genotype persisted throughout days 11 and 12 (Fig. 2), and EhV virus counts continued to increase to 2.5 × 106 EhV-like viruses mL−1 at the end of this study. Sequencing of the EhV DGGE bands confirmed that the bands at different migration positions corresponded to different EhV sequences, with the presence of at least six dominant EhV genotypes being verified (bands a–f). Bands at the same migration positions but at different time points were additionally confirmed as being identical sequences. During days 6/7 (pre-bloom) and days 8/9 (during the bloom), sequencing confirmed the presence of a virus sequence identical to that of an English Channel isolate, EhV86 (band c), a virus genotype not detected before in Norwegian waters. Band b (corresponding to Norwegian genotype, OTU2) was also confirmed in the pre-bloom and during the bloom stages; this strain was previously detected in the 2000 mesocosm in the pre-bloom stage (Martínez-Martínez et al., 2007). Three new EhV genotypes, with unique sequences, were identified in this mesocosm experiment in the pre-bloom and during the bloom stages, represented as bands a, e and f and deposited as OTUs 24, 25 and 26, respectively (accession numbers FJ668535–FJ668537) (Fig. 2). There was a clear fluctuation in the occurrence all of these EhV genotypes, in the samples collected during the early stages of the bloom. Band d, confirmed as identical to the genotype OTU19, became the most dominant virus genotype when the bloom was reaching a peak; from day 9 at 0400 h until the end of the study (Fig. 2). Interestingly, the OTU19 genotype was not the dominant EhV genotype during the 2000 and 2003 blooms, with two other genotypes (designated as OTUs 1 and 3) being reported to dominate the virus communities in these mesocosms (Martínez-Martínez et al., 2007).
Fig. 2.
A representative DGGE image of Emiliania huxleyi virus-amplified PCR fragments of the MCP gene from three diel cycles of the mesocosm experiment, Norway, 2008. Letters indicate bands excised and confirmed by sequencing. Identical letters indicate bands with identical sequences, where a, e and f are new sequences (deposited as OTUs 24, 25 and 26, respectively), b is identical to OTU2, c is identical to EhV86 and d is identical to OTU19. The sample set was run across two gels, where the letter R indicates samples run on both gels to account for any variations in the gel properties.
Previous studies using DGGE have shown the appearance and disappearance of free EhV genotypes, present in the water column, prior to the development of a bloom (Schroeder et al., 2003). This has been attributed to EhV infection of the host, resulting in the disappearance of the virus genotypes from the water column, before their reappearance if infection was successful. This study focused mainly on internalized viruses, indicating successful infections, as DNA extractions were performed on material collected on 0.45 μm filters. Our data complement the results of previous studies, while uncovering a much greater variability of infectious EhVs over the course of a developing E. huxleyi bloom. Furthermore, four EhV genotypes were identified in this study that previously had not been detected in Norwegian waters; three of these being newly identified genotypes. No temporal variation of E. huxleyi genotypes was evident during the bloom yet a highly dynamic virus community was detected. A possible explanation for the high temporal variability of the EhV population is that the different virus strains detected have different timescales of infection. In laboratory studies, the virus strain, EhV86, has been found to preferentially infect E. huxleyi during the night, with the viruses being released during the day (unpublished data). If this is the case for other virus strains, it could explain some of the temporal variation observed, with the internal viruses detected at different times being related to their differing infection time points. Another possible explanation for the high variability of the EhV community during the early and pre-bloom stages is that propagation strategies may differ between EhV strains, with some potentially having a slower release of viruses or differing lengths of their infection cycles. This may mean that infection and release of viruses is not synchronous, thereby accounting for the variability detected. There are relatively little data available regarding the infection strategies of different EhV strains; however, it is apparent that they do have different host ranges (Allen et al., 2007). Some EhV genotypes are only distinguishable by a single base pair change in the MCP region amplified in this study, yet despite this relative similarity, they may have different host ranges, contributing to an extremely dynamic virus community. The success of one dominant virus strain in the latter stages of the bloom suggests that EhV genotypes are not phenotypically identical and that this dominant strain could have had a competitive advantage, such as a differing infection strategy or broader host range. Based on previous studies, the dominant virus genotype (band d/OTU19) was responsible for the demise of the bloom, since the EhVs that dominated during the bloom peak in 2000 and 2003 continued to be dominant in the post-bloom stage (Martínez-Martínez et al., 2007). Moreover, the change in the bloom-termination EhV genotype in 2008, when compared with 2000 and 2003, suggests a greater rate of EhV evolution when compared with a relatively stable host community. This observation is not unprecedented as it is well accepted that viral genomes evolve faster than their host genome counterparts (e.g. Drake et al., 1998).
The discovery of a highly temporally variable community structure in eukaryotic marine algal viruses has implications for future, and the interpretation of past, research. It is possible that studies with a broad temporal resolution, such as one sample per day, will not provide an accurate view of the aquatic virus community. A sample taken at the same time each day may over or under estimate the diversity in a given environment, especially if virus infection is related to diel cycles. This novel insight provided here, into viral community dynamics over a period of hours may help to further understand the ability of viruses to structure phytoplankton communities. Moreover, it may also complicate the assumption of a ‘steady state’ in virus induced mortality estimates.
FUNDING
D.C.S. is funded by NERC through the core strategic research programme Oceans2025 (R8-H12-52).
ACKNOWLEDGEMENTS
We thank all the participants in the mesocosm experiment in June 2008 at the Marine Biological Station, Nr Raunefjorden, Norway. In particular, we thank Willie H. Wilson and Susan Kimmance for kindly providing us with flow cytometry data.
REFERENCES
- Allen M., Martínez-Martínez J., Schroeder D.C., et al. Use of microarrays to assess viral diversity: from genotype to phenotype. Environ. Microbiol. 2007;9:971–982. doi: 10.1111/j.1462-2920.2006.01219.x. [DOI] [PubMed] [Google Scholar]
- Bratbak G., Wilson W.H., Heldal M. Viral control of Emiliania huxleyi blooms? J. Mar. Syst. 1996;a 9:75–81. [Google Scholar]
- Bratbak G., Heldal M., Thingstad T.F., et al. Dynamics of virus abundance in coastal seawater. FEMS Microbiol. Ecol. 1996;b 19:263–269. [Google Scholar]
- Drake J.W., Charlesworth B., Charlesworth D., et al. Rates of spontaneous mutation. Genetics. 1998;148:1667–1686. doi: 10.1093/genetics/148.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. Marine viruses and their biogeochemical and ecological effects. Nature. 1999;399:541–548. doi: 10.1038/21119. [DOI] [PubMed] [Google Scholar]
- Jacquet S., Heldal M., Iglesias-Rodriguez D., Larsen A., Wilson W.H., Bratbak G. Flow cytometric analysis of an Emiliania huxleyi bloom terminated by viral infection. Aquat. Microb. Ecol. 2002;27:111–124. [Google Scholar]
- Martínez-Martínez J., Schroeder D.C., Larsen A., et al. Molecular dynamics of Emiliania huxleyi and co-occurring viruses during two separate mesocosm studies. Appl. Environ. Microbiol. 2007;73:554–562. doi: 10.1128/AEM.00864-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada V., Baudoux A.-C., Sintes E., et al. Dynamics and diversity of newly produced virioplankton in the North Sea. ISME J. 2008;2:924–936. doi: 10.1038/ismej.2008.57. [DOI] [PubMed] [Google Scholar]
- Schroeder D.C., Oke J., Malin G., et al. Coccolithovirus (Phycodnaviridae): Characterisation of a new large dsDNA algal virus that infects Emiliana huxleyi. Arch. Virol. 2002;147:1685–1698. doi: 10.1007/s00705-002-0841-3. [DOI] [PubMed] [Google Scholar]
- Schroeder D.C., Oke J., Hall M.J., et al. Virus succession observed during an Emiliania huxleyi bloom. Appl. Environ. Microbiol. 2003;69:2484–2490. doi: 10.1128/AEM.69.5.2484-2490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]