Abstract
Although fructose is commonly used as a sweetener, its effects on brain function are unclear. Using rat hippocampal slices, we found that fructose and mannose, like pyruvate, preserve ATP levels during 3-hours of glucose deprivation. Similarly, fructose and mannose restored synaptic potentials (EPSPs) depressed during glucose deprivation. However, restoration of synaptic responses was slow and only partial with fructose. EPSPs supported by mannose were inhibited by cytochalasin B (CCB), a glucose transport inhibitor, but were not inhibited by α-cyano-4-hydroxycinnamate (4-CIN), a monocarboxylate transport inhibitor, indicating that neurons use mannose via glucose transporters. In contrast, both CCB and 4-CIN depressed EPSPs supported by fructose, suggesting that fructose may be taken up by non-neuronal cells through CCB sensitive hexose transporters and metabolized to a monocarboxylate for subsequent use during neuronal respiration. Supporting this possibility, twenty minutes of oxygen deprivation in the presence of fructose resulted in functional and morphological deterioration whereas oxygen deprivation in the presence of glucose or mannose had minimal toxic effects. These results indicate that neuronal fructose utilization differs from glucose and mannose and likely involves release of monocarboxylates from glia.
Keywords: ATP, GLUT8, GLUT11, glycolysis, hexose, monocarboxylate
INTRODUCTION
Although glucose is the principal energy substrate in the mammalian central nervous system (CNS), there is evidence that other endogenous agents, including monocarboxylates and creatine (Nakashima et al., 2005), serve as energy substrates under certain conditions. In hippocampal slices, lactate (Schurr et al., 1988) and pyruvate (Izumi et al., 1994; 1997b) are examples of monocarboxylates that can sustain neuronal integrity in the absence of glucose.
Alternative energy substrates for glucose may not be limited to monocarboxylates, and hexoses other than glucose may also serve as CNS energy substrates. In the United States, it is estimated that per capita fructose consumption as corn syrup has increased to more than 40 g/day (Gaby, 2005). This dietary consumption raises questions about whether fructose can alter CNS energy metabolism (Funari et al., 2007). Prior studies indicate that fructose has memory-enhancing properties (Messier and White, 1987; Rodriguez et al., 1994) and can act as a neuroprotectant under some circumstances (Sapolsky, 1986). Moreover, high fructose intake may alter hypothalamic appetitive systems (Lindqvist et al., 2008).
These findings suggest that fructose has direct actions in the CNS, although whether fructose can be used as a brain energy substrate remains uncertain (Douard & Ferraris, 2008). Okada’s group initially reported that mannose and fructose partially preserve synaptic function in the absence of glucose in the guinea pig dentate gyrus (Saitoh et al., 1994). However, subsequent studies by the same group found that mannose and fructose fail to preserve synaptic transmission in the dentate gyrus and CA3 region, despite the fact that they sustain ATP levels (Kanatani et al, 1995; Wada et al., 1998). These studies raise important questions about whether the CNS has the ability to use fructose as an energy substrate.
In the present study, we examined whether fructose serves as an energy substrate in the CA1 region of rat hippocampal slices using cytochalasin B (CCB) to inhibit hexose transporters and α-cyano-4-hydroxycinnamate (4-CIN) to inhibit monocarboxylate transporters. In hippocampal slices, synaptic responses depress gradually following glucose removal or during administration of CCB. EPSPs sustained by glucose are not altered by 4-CIN but EPSPs sustained by monocarboxylates are rapidly depressed by 4-CIN, indicating that 4-CIN acts as an inhibitor of monocarboxylate transporters (Izumi et al., 1997a). The rapid decline of EPSPs following glucose deprivation in the presence of 4-CIN appears to result from block of monocarboxylate exchange between glia and neurons, suggesting that monocarboxylates released from glia fuel neurons when glucose use is limited (see also Allen et al., 2005; Sakurai et al., 2002; Cater et al., 2001). Because we previously observed that CCB suppresses glucose-supported EPSPs without affecting pyruvate-supported EPSPs at 50 μM, whereas 4-CIN suppresses pyruvate-mediated EPSPs without affecting glucose-supported EPSPs at 200 μM (Izumi et al., 1997a), we used 50 μM CCB and 200 μM 4-CIN to determine whether they affect mannose- and fructose-supported EPSPs. Using CCB and 4-CIN, we show that fructose supports neuronal function through release of monocarboxylates, likely produced in glia, rather than through a direct mechanism.
EXPERIMENTAL PROCEDURES
Hippocampal Slice Preparation
Slices were prepared from the septal half of the hippocampus using standard techniques. Postnatal day (PND) 30–34 albino rats were anesthetized with halothane and decapitated (Zorumski et al., 1996). Hippocampi were rapidly dissected and placed in artificial cerebrospinal fluid (ACSF) containing (in mM): 124 NaCl, 5 KCl, 2 MgSO4, 2 CaCl2, 1.25 NaH2PO4, 22 NaHCO3, 10 glucose, bubbled with 95% O2-5% CO2 at 4–6 °C, and sliced transversely into 500 μm slices using a WPI vibroslicer. Slices were then placed in an incubation chamber containing gassed ACSF for 2 hr at 30°C. At the time of study, slices were transferred one at a time to a submersion-recording chamber, and experiments were done at 30°C. Fructose, mannose and pyruvate were used with adjusted pH and a reduction in NaCl to maintain osmolality.
Synaptic Physiology
Extracellular recordings were obtained from the apical dendritic region for analysis of population EPSPs or the pyramidal cell body layer of CA1 for study of population spikes using 2 M NaCl glass electrodes with resistances of 5–10 M. Data were recorded and analyzed using an IBM-based computer system. The amplitude of population spikes and slopes of extracellularly recorded EPSPs were measured off-line using computer programs written in Axobasic (MDS Inc. Toronto, Canada). Analysis of EPSPs was confined to stimuli that evoked only small population spikes that did not distort the waveform. Evoked synaptic responses were elicited with 0.2 msec constant current pulses through a bipolar electrode (Rhodes Medical Instruments, Woodland Hill, CA) placed in the Schaffer collateral-commissural pathway. Synaptic responses in CA1 were monitored by applying single stimuli to the Schaffer collateral pathway every 60 sec at an intensity sufficient to elicit a 50%–60% maximal EPSP slope. This stimulation frequency yields stable synaptic responses and avoids alterations in synaptic function that can occur with higher frequencies of test pulses.
ATP assay
ATP levels were determined by luminometry (Zylux, Maryville TN, USA) using an ATP assay kit (Calbiochem-Novabiochem, San Diego, CA, USA). The assay is based on the firefly luciferase-catalyzed oxidation of D-luciferin in the presence of ATP and oxygen, whereby the amount of ATP is quantified by the amount of light produced with a luminometer (Femtomaster FB12, Zylux Corp., Pfortzheim, Germany). Protein concentrations were determined by standard procedure (BioRad, Hercules CA, USA) (Bradford et al.,1976). In control hippocampal slices incubated with 10 mM glucose, ATP levels were 20.4 ± 2.6 nmole/mg protein (N=14). ATP concentrations from each whole slice were compared to matched controls from the same hippocampus incubated and measured simultaneously during each experiment.
Statistics
Differences between groups were analyzed using Student’s t-test and paired-pulse Student’s t-test (SigmaStat, Jandel Scientific Software, San Rafael, CA).
Materials
All chemicals, except for the ATP assay kit, were purchased from Sigma Chemical Co. (St. Louis, MO). Rats were purchased from Harlan Sprague Dawley Inc. (Indianapolis, IN).
RESULTS
Effects of Energy Substrates on ATP levels during Glucose Deprivation
Prior studies indicate that fructose and mannose preserve ATP levels in hippocampal slices (Saitoh et al., 1994, Kanatani et al, 1995, Wada et al., 1998). To confirm these observations, we initially compared the ability of fructose, mannose and pyruvate to preserve ATP levels in the absence of glucose. In hippocampal slices from PND 30 rats, ATP levels are depressed by > 60% during a three-hour period of glucose deprivation (Figure 1). This depression of ATP levels was largely prevented by administration of 10 mM pyruvate during the period of glucose deprivation (< 20% depression). Similarly, the reduction in ATP levels was effectively prevented by administration of either 10 mM mannose or 10 mM fructose (Figure 1), suggesting that these two hexoses can serve as energy sources during glucose deprivation in the hippocampus.
Figure 1.
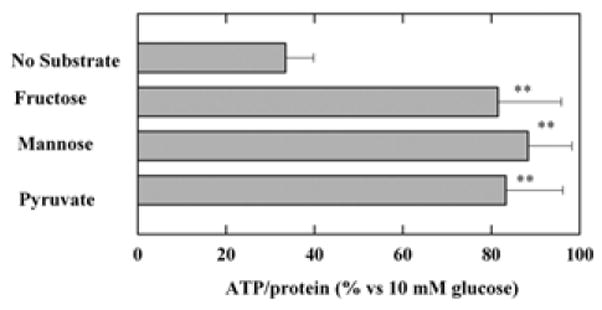
Effects of alternative energy substrates on ATP levels during glucose deprivation. ATP levels were measured relative to those observed following incubation of hippocampal slices with 10 mM glucose for three hours. Three-hour incubation of hippocampal slices in ACSF in the absence of exogenous energy substrates reduced ATP levels significantly compared to those in ACSF with 10 mM glucose (P<0.01 by Student t-test). In contrast, ATP levels were well preserved in the presence of 10 mM fructose, 10 mM mannose or 10 mM pyruvate. **<0.01 v.s. no substrates. Control ATP levels with 10 mM glucose was 19.5 ± 6.2 nmole/mg.
Effects of Energy Substrates on Synaptic Transmission during Glucose Deprivation
To determine whether the beneficial effects of mannose and fructose on hippocampal ATP levels result in improved synaptic function, we examined the effects of these energy substrates on CA1 transmission during glucose deprivation. In hippocampal slices, CA1 EPSPs were suppressed gradually during sixty minutes of glucose deprivation. In previous work, we found that release of endogenous monocarboxylates, likely from glia, helps to sustain synaptic responses following glucose removal and that inhibitors of monocarboxylate transport greatly speed the depression (Izumi et al., 1997a).
Following complete synaptic depression, EPSPs were restored almost completely within 20 min by administration of 10 mM mannose (EPSPs; 86 ± 2% of control baseline at 20 min, n=5, Figure 2). EPSPs sustained by 10 mM mannose were again gradually depressed by application of 50 μM CCB, a hexose transport inhibitor, with complete elimination of responses taking more than 40 min. The depressed EPSPs were restored by subsequent administration of 10 mM pyruvate in the presence of CCB (61 ± 20% 20 min after pyruvate administration). The restoration of EPSPs by pyruvate in the presence of CCB was blocked if 4-CIN was administered 10 min before pyruvate administration (EPSPs; 4 ± 2%, n=4, data not shown). In another set of experiments, EPSPs sustained by 10 mM mannose were not depressed by 200 μM 4-CIN, a monocarboxylate transport inhibitor (100 ± 8%, n=5, Figure 3), suggesting that the preservation of neuronal activity by mannose does not result from the uptake of mannose via 4-CIN-sensitive transporters or the release of endogenous monocarboxylates from glia.
Figure 2.
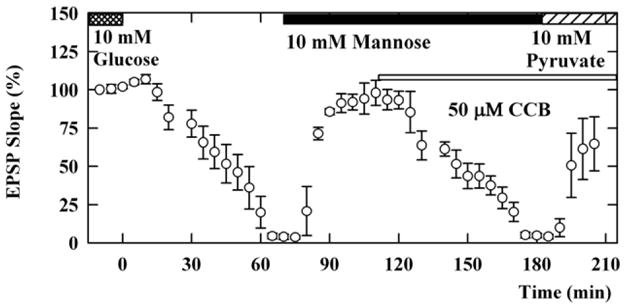
Effects of CCB on mannose-sustained EPSPs. Slices were initially incubated in ACSF containing 10 mM glucose (double hatched bar). Perfusion of glucose-free ACSF starting at time 0 gradually depressed EPSPs. After complete suppression of EPSPs, administration of 10 mM mannose (solid bar) almost fully restored EPSPs in the absence of glucose. Mannose-maintained EPSPs were again gradually depressed by 50 μM CCB (thin open bar). In the presence of CCB, the suppressed EPSPs were restored by administration of 10 mM pyruvate (striped bar).
Figure 3.
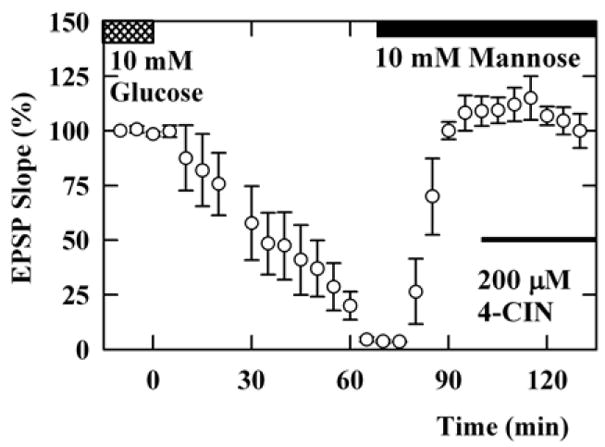
Effects of 4-CIN on mannose-sustained EPSPs. EPSPs supported by 10 mM glucose (double hatched bar) were gradually depressed by perfusion of ACSF without glucose. Administration of 10 mM mannose (solid bar) restored EPSPs. The mannose-supported EPSPs were not depressed by 200 μM 4-CIN (thin filled bar).
Similar to the effects of mannose, administration of 10 mM fructose also restored EPSPs during glucose deprivation. Unlike mannose, however, the recovery of EPSPs in the presence of fructose was slow and only partial (EPSP; 20 ± 9% 20 min after fructose perfusion, n=5, P<0.01, with gradual recovery to about 80% of control, Figure 4). EPSPs sustained by 10 mM fructose were diminished by CCB, suggesting that fructose requires a CCB-sensitive hexose transporter (EPSP; 22 ± 7% 20 min after CCB administration, Figure 4). However, the decay was faster than that observed with mannose (P<0.05 vs. mannose), suggesting a distinct mechanisms for fructose use or additional effects of CCB on fructose use.
Figure 4.
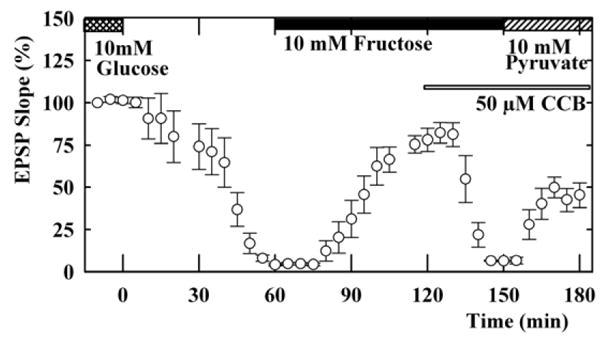
Effects of CCB on fructose-sustained EPSPs. Slices were initially incubated in ACSF containing 10 mM glucose (double hatched bar). Perfusion of glucose-free ACSF starting time 0 gradually depressed EPSPs. After complete suppression of EPSPs, administration of 10 mM fructose (solid bar) partially restored EPSPs in the absence of exogenous glucose. The fructose-sustained EPSPs were depressed by 50 μM CCB (thin open bar). In the presence of CCB, the suppressed EPSPs were restored by administration of 10 mM pyruvate (striped bar).
Because CCB, like other cytochalasins, also binds actin, we tested if fructose-sustained EPSPs are affected by cytochalasin D (CCD), an agent that more potently binds to actin but does not inhibit hexose tranporters (Cooper 1987). Because CCD depressed glucose-sustained EPSPs at 5 μM suggesting non-specific effects (Kim and Lisman, 1999, Chen, et al, 2004), we administered 1μM CCD, a concentration that binds actin but has no effect on glucose-supported synaptic responses. EPSPs restored by 10 mM fructose after glucose deprivation were not depressed by CCD (Figure 5), suggesting that effects on actin are unlikely to contribute to CCB-mediated inhibition of EPSPs sustained by fructose.
Figure 5.
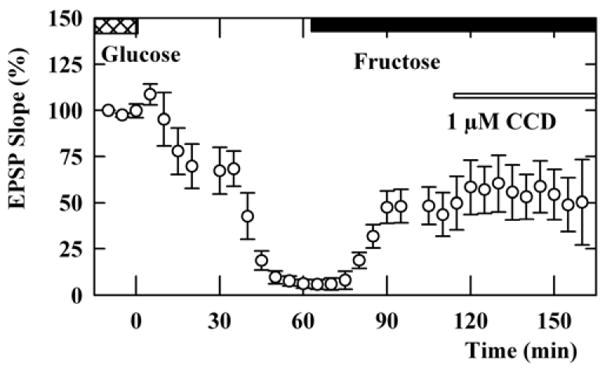
Effects of CCD on fructose-sustained EPSPs. Slices were initially incubated in ACSF containing 10 mM glucose (double hatched bar). Perfusion of glucose-free ACSF starting at time 0 gradually depressed EPSPs. After complete suppression of EPSPs, administration of 10 mM fructose (solid bar) partially restored EPSPs in the absence of exogenous glucose. The fructose-sustained EPSPs were not depressed by 1 μM CCD (thin open bar).
In another set of experiments, EPSPs sustained by fructose were also rapidly diminished by 4-CIN (EPSP; 5 ± 2% 20 min after 4-CIN administration, n=5, P<0.01 vs. mannose), and, in the presence of 4-CIN, the depressed EPSPs were restored by administration of 10 mM glucose (Figure 6, EPSP; 74 ± 2%). These observations suggest that fructose is either transported by both CCB- and 4-CIN-sensitive mechanisms or is indirectly utilized by neurons. The latter possibility further suggests that non-neuronal cells (glia) may be involved in the effects of fructose (Magistretti and Pellerin, 1996), nourishing neurons through the delivery of monocarboxylates such as pyruvate.
Figure 6.
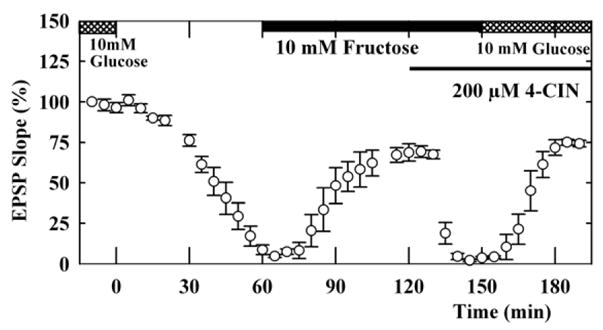
Effects of 4-CIN on fructose-sustained EPSPs. EPSPs sustained by 10 mM glucose (double hatched bar) were gradually depressed by perfusion of ACSF without glucose. Administration of 10 mM fructose (solid bar) restored EPSPs. In contrast to mannose-supported EPSPs, fructose-supported EPSPs were depressed by 200 μM 4-CIN (thin filled bar). In the presence of 4-CIN, replacement of glucose with fructose restored EPSPs.
It has been recently reported that gap junctions and glio-neuronal interactions play a role in neuronal monocarboxylate use in the hippocampus (Rouach et al., 2008). Because we suspect that neuronal use of fructose is indirect through monocarboxylate transporters, we examined whether meclofenamic acid (MFA), a water-soluble gap junction inhibitor (Pan et al., 2007) alters fructose-sustained EPSPs. Fructose-sustained EPSPs after glucose deprivation were promptly depressed by 10μM MFA. Furthermore, EPSPs depressed in the presence of MFA were restored by administration of glucose (Figure 7, n=5), again supporting a distinct mechanism for fructose use.
Figure 7.
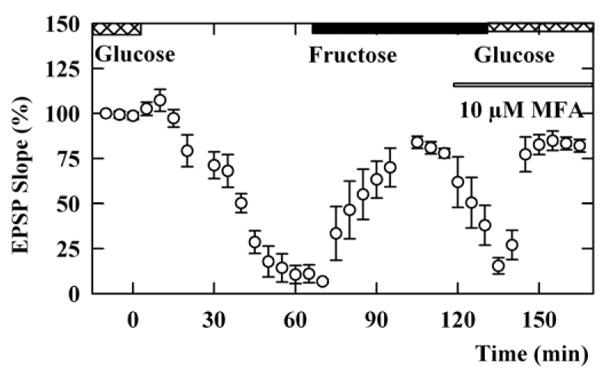
Effects of MFA on fructose-sustained EPSPs. EPSPs sustained by 10 mM glucose (double hatched bar) were depressed by perfusion of ACSF without glucose. Administration of 10 mM fructose (solid bar) restored EPSPs. The fructose-sustained EPSPs were depressed by 10 μM meclofenamic acid (MFA) (thin filled bar). In the presence of MFA, replacement of glucose with fructose restored EPSPs.
Effects of CCB and 4-CIN on ATP Levels Supported by Fructose
In slices from 5 animals, ATP levels were compared during 3 hour glucose deprivation with 10 mM fructose plus CCB or 4-CIN. Preservation of ATP levels by fructose is impaired by both CCB and 4-CIN, indicating that fructose is utilized through two distinct transporters (Fig. 8). In other experiments, 4-CIN did not depress ATP levels in the presence of 10 mM glucose (89 ± 11%, n=3). Figure 8 near here
Figure 8.
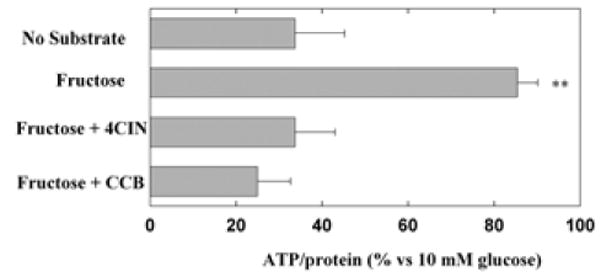
Effects of CCB and 4-CIN on ATP levels supported by fructose. ATP levels were measured relative to those observed following incubation of hippocampal slices with 10 mM glucose for three hours. Three-hour incubation of hippocampal slices in ACSF in the absence of exogenous energy substrates reduced ATP levels significantly compared to those in ACSF with 10 mM glucose (P<0.01 by paired Student t-test). ATP levels were preserved when 10 mM fructose was present during glucose deprivation (second bar, **<0.01 v.s. no fructose during glucose deprivation). ATP levels preserved in the presence of fructose were impaired by co-administration of either 4-CIN and CCB (third and fourth bars). ATP levels in the presence of either drug was significantly lower than those without these agents (P<0.01 v.s. fructose alone by paired Student t-test). Control ATP levels with 10 mM glucose were 20.6 ± 5.7 nmole/mg.
Effects of Energy Substrates on Hypoxia and Simulated Ischemia
If fructose helps to sustain neuronal function indirectly via metabolism in glia and subsequent release of endogenous monocarboxylates, then the use of fructose as an energy source should be restricted to conditions in which respiration remains intact because monocarboxylates require respiration for metabolic processing. To test this, we examined the effects of the energy substrates during hypoxia. Consistent with the requirement for respiration in the metabolic effects of monocarboxylates, EPSPs supported by 10 mM pyruvate were diminished by 20 min oxygen deprivation and did not recover following re-oxygenation (Figure 9, open triangles, n=4). In contrast, EPSPs supported by 10 mM glucose diminished during 20 min oxygen deprivation, but were completely restored by re-oxygenation in the presence of glucose (Izumi et al., 1998b; open circles, n=5). Similarly, in the presence of 10 mM mannose instead of glucose, suppression of EPSPs during oxygen deprivation was reversed by re-oxygenation (filled triangles, n=5). EPSPs supported by 10 mM fructose, however, were not restored during re-oxygenation (filled circles, n=5).
Figure 9.
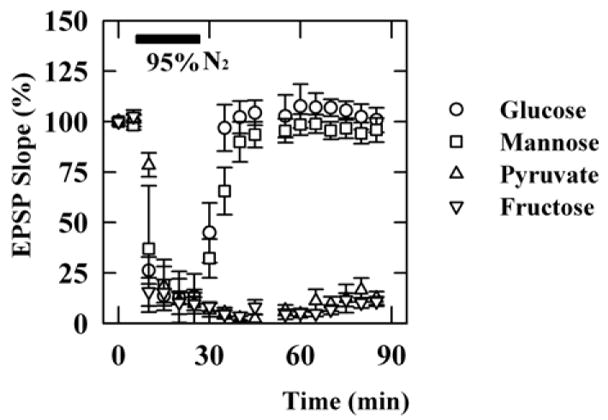
Effects of oxygen deprivation on EPSPs and ATP levels supported by various energy substrates. To induce hypoxia, oxygen (95% in normal ACSF) was replaced by nitrogen for 20 min (filled bar). A. EPSPs were complete suppressed during oxygen deprivation, but were restored when slices were re-oxygenated in the presence of 10 mM glucose (open circles) or 10 mM mannose (filled triangles). In contrast, EPSPs recorded in the presence of 10 mM pyruvate (open triangles) or 10 mM fructose (filled circles) did not recover after anoxia.
Although EPSPs were completely suppressed during oxygen deprivation regardless of the energy source, ATP levels after oxygen deprivation were surprisingly well preserved when slices were incubated with 10 mM glucose or 10 mM mannose (second and third bars in Figure 10, n=5). Twenty minutes of oxygen deprivation in the absence of any exogenous energy substrates (simulated ischemia) depressed ATP levels significantly (top bar in Figure 10). The same extent of depression was observed following anoxia in the presence of fructose (bottom bar in Figure 10). In another set of studies, anoxia in the presence of 10 mM pyruvate also significantly depressed ATP levels (22+5% of control, n=4). Figure 10 near here
Figure 10.
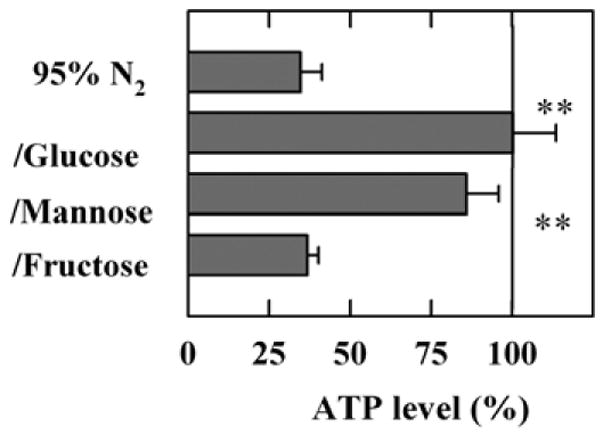
ATP levels in whole slices twenty minutes after oxygen deprivation. Oxygen deprivation depressed ATP levels in the absence of any exogenous energy substrate by > 60% (first bar, P < 0.01 v.s. control levels with glucose and oxygen). In the presence of 10 mM glucose or 10 mM mannose, ATP levels were well preserved relative to oxygenated slices in the presence of glucose. However, 10 mM fructose failed to maintain ATP levels during anoxia. **<0.01 v.s. simulated ischemia (top bar) by paired Student t-test). ATP levels in oxygenated controls slices were 21.0 ± 2.8 %.
DISCUSSION
There is considerable evidence that glucose is not the sole energy substrate used in the CNS. In hippocampal slices, the monocarboxylates, lactate (Schurr et al., 1988), pyruvate (Izumi et al., 1994) and β-hydroxybutyrate (Izumi et al., 1998a), sustain neuronal function during periods of glucose deprivation. In the present study, we provide evidence that hexoses other than glucose, specifically mannose and fructose, can support neuronal integrity in the hippocampus. Although plasma concentrations of mannose and fructose in healthy individuals are very low compared to glucose (Pitkänen, 1996), the concentrations of these hexoses in CSF are relatively higher (Kusmierz et al., 1989). While the physiological relevance of mannose as an energy substrate in the CNS is unclear, determining the possible roles of fructose in CNS energy metabolism is important because human fructose consumption is significant (Gaby, 2005) and it has been shown that fructose load affects brain function (Messier et al., 2007).
4-CIN is a useful tool for blocking monocarboxylate transporters selectively in hippocampal neurons, though at higher concentrations, 4-CIN also inhibits mitochondrial pyruvate uptake and oxidation in neurons and non-neuronal cells (Halestrap and Armston, 1984, McKenna et al., 2001,). Impairment of mitochondrial metabolism may suppress synaptic transmission (Tozzi et al., 2007). We previously observed that a high concentration of 4-CIN (2 mM) depresses glucose-supported EPSPs (Izumi et al., 1997a). Glucose-supported EPSPs are, however, not altered by 200 μM 4-CIN, suggesting that at 200 μM 4-CIN is more likely acting via effects on monocarboxylate transport than through inhibition of mitochondrial metabolism.
Similarly, CCB is a useful tool for blocking hexose transporters in hippocampal neurons. However, CCB, like other cytochalasins, also binds to actin. Among the various cytochalasins, CCD is the most potent in binding to actin, but does not inhibit hexose transporters (Cooper, 1987). Because it has been shown that CCD binds to actin and exhibits neuronal actions at 1 μM (Krucker et al., 2000, Wei et al., 2004) but at higher concentrations irreversibly inhibits glucose-sustained EPSPs and induces neurotoxicity (Kim and Lisman, 1999, Kim et al., 2002, Chen et al., 2004), we used 1 μM CCD for our studies. At 1 μM, CCD had no effect on fructose-sustained EPSPs. This observation suggests that actin-binding is unlikely to account for the depression of fructose-sustained EPSPs by CCB.
In the present study, we observed that 4-CIN does not block EPSPs supported by mannose, indicating that mannose is likely to be more directly utilized by neurons (Figure 11A). In contrast to mannose, fructose is transported by both 4-CIN and CCB-sensitive mechanisms. We hypothesize that fructose is first taken up by non-neuronal cells and then metabolized to a monocarboxylate for subsequent neuronal utilization (Figure 11B). Such a complex mechanism potentially underlying fructose utilization may be responsible for the only partial or less consistent ability of fructose to sustain EPSPs. In the CA3 region of guinea pig hippocampal slices, Okada’s group initially reported that EPSPs were sustained after switching energy substrates from glucose to fructose (Saitoh et al., 1994). However, the same group subsequently found that fructose failed to sustain EPSPs in spite of preserving ATP levels (Kanatani et al., 1995, Wada et al., 1998). Our results suggest that these inconsistencies may result from the apparent complex metabolic processing of fructose and factors that influence the integrity of glioneuronal interactions in slice preparations (Figure 11B).
Figure 11.
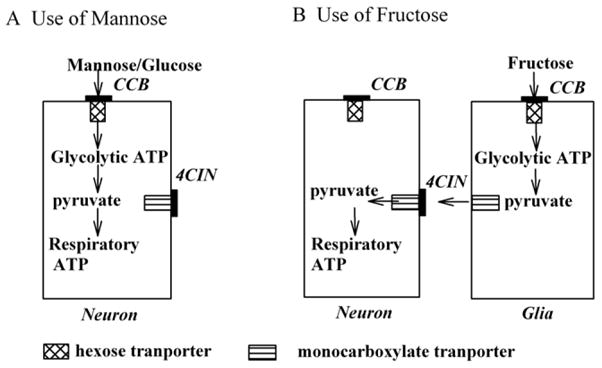
Hypothetical mechanisms for use of mannose and fructose by neurons. A. A scheme for the use of mannose by neurons is displayed. If mannose, like glucose, is directly used by neurons via a hexose transporter, CCB, but not 4CIN, inhibits its uptake. B. A scheme in which fructose indirectly fuels neurons via glia is shown. The block of monocarboxylate transporters by 4-CIN results in a failure of neurons to benefit from the presence of fructose, suggesting that monocarboxylates provide fuel to the neurons. In this scheme, CCB blocks fructose transport into non-neuronal cells.
Our results examining the effects of hexoses during oxygen deprivation argue against fructose being used directly by neurons as an energy substrate. Brief oxygen deprivation does not result in severe neuronal damage as long as hexoses are available for use by neurons, while deprivation of both oxygen and glucose results in severe neuronal damage even if pyruvate is present. This difference indicates that during anoxia glycolysis is important for preserving neuronal integrity.
Whereas glial glycolysis may be critical for glutamate metabolism and preventing excitotoxicity (Poitry et al., 2000), neuronal glycolysis may be crucial for maintaining synaptic function. This is because neurons lack glycogen and can preserve glycolytic energy metabolism only when hexoses are directly available. The failure of EPSPs to recover after oxygen deprivation in the presence of fructose indicates that fructose cannot be metabolized by neurons during oxygen deprivation. Even though it appears that fructose can be used by glia during anoxia, anoxia in the presence of fructose results in poor recovery of neuronal function. This suggests that neuronal glycolytic metabolism may be critical for preserving function during anoxia.
GLUT5, a specific fructose transporter, is expressed in the CNS (Shepherd et al., 1992; Maher et al., 1996; Payne et al., 1997) and insults that decrease the activity of the glucose transporters, GLUT1 and GLUT3, increase GLUT5 activity (Payne et al., 1997; Vannucci et al., 2003). This suggests that fructose transported through GLUT5 may be of particular importance under conditions where neuronal glucose uptake is impaired. However, GLUT5 is expressed only in microglia (Horikoshi, 2003). Furthermore, it has recently been reported that fructose loading improves memory tasks but does not increase GLUT5 expression in the brain (Messier et al., 2007), suggesting that the positive effects of fructose loading on cognitive function are independent of GLUT5 up-regulation. In the present study, the complete suppression of fructose-sustained EPSPs by CCB indicates that a hexose transporter sensitive to CCB is responsible for fructose utilization in the hippocampus. Additionally, CCB does not appear to inhibit GLUT5-mediated hexose uptake (Burant et al., 1992; Garriga et al., 2004). Taken together, these observations raise the possibility that fructose utilization in the CNS is mediated by a hexose transpoter distinct from GLUT5 and that other glucose transporters in CNS are involved in fructose uptake. Fructose is also transported by other Class II GLUTs such as GLUT9 and GLUT11 (Scheepers et al., 2005, Manolescu et al., 2007), both of which are expressed in brain (Doege et al., 2001; Wu et al., 2002; Augustin et al., 2004). In addition, GLUT8, also called X1, transports fructose (Ibberson et al., 2000) and is expressed in brain (Ibberson et al., 2002, Reagan et al., 2002, Widmer et al., 2005). Among these glucose transporters that promote fructose uptake, GLUT8 and GLUT11 are known to bind CCB (Ibberson et al. 2000, Wu et al., 2002), whereas GLUT9 is not sensitive to CCB (Doege et al., 2000, Augustin et al., 2004). Thus, GLUT8 and GLUT11 or an as yet unknown transporter may be involved in fructose uptake in the CNS. It should be also noted that these transporters may have high affinity for fructose which would allow them to scavenge the low concentrations of fructose that are likely found in the CSF.
In summary, our findings suggest that fructose is not directly utilized by neurons in the CA1 region of hippocampus. Different from glucose and mannose, fructose use appears to be complex with uptake and metabolism through a monocarboxylate transporter that is likely localized to non-neuronal cells. Although fructose utilization by neurons is indirect, fructose may still be a useful energy substrate for preserving neuronal activity when glucose transport is compromised, though we did not directly examine neuronal effects of lower concentrations of fructose that are more likely found in the CNS. Thus, further investigation is needed to determine the physiological role of fructose in the CNS.
Acknowledgments
This work was supported in part by National Institute of Health grants MH077791, AG18434 and AA12951, Alzheimer’s Disease Research Center of Washington University and the Bantly Foundation.
Abbreviations
- ATP
adenosine triphosphate
- EPSPs
excitatory postsynaptic potentials
- CCB
cytochalasin B
- 4-CIN
α-cyano-4-hydroxycinnamate
- CNS
central nervous system
- PND
Postnatal day
- ACSF
artificial cerebrospinal fluid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen NJ, Karadottir R, Attwell D. A preferential role for glycolysis in preventing the anoxic depolarization of rat hippocampal area CA1 pyramidal cells. J Neurosci. 2005;25:848–859. doi: 10.1523/JNEUROSCI.4157-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin R, Carayannopoulos MO, Dowd LO, Phay JE, Moley JF, Moley KH. Identification and characterization of human glucose transporter-like protein-9 (GLUT9): alternative splicing alters trafficking. J Biol Chem. 2004;16:16229–16236. doi: 10.1074/jbc.M312226200. [DOI] [PubMed] [Google Scholar]
- Bradford M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burant CF, Takeda J, Brot-Laroche E, Bell BI, Davidson NO. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem. 1992;25:14523–14526. [PubMed] [Google Scholar]
- Cater HL, Benham CD, Sundstrom LE. Neuroprotective role of monocarboxylate transport during glucose deprivation in slice cultures of rat hippocampus. J Physiol. 2001;531:459–466. doi: 10.1111/j.1469-7793.2001.0459i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bourne J, Pieribone VA, Fitzsimonds RM. The role of actin in the regulation of dendritic spine morphology and bidirectional synaptic plasticity. Neuroreport. 2004;15:829–832. doi: 10.1097/00001756-200404090-00018. [DOI] [PubMed] [Google Scholar]
- Cooper JA. Effects of Cytochalasin and Phalloidin on Actin. J Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doege H, Bocianski A, Joost HG, Schurmann A. Activity and genomic organization of human glucose transporter 9 (GLUT9), a novel member of the family of sugar-transport facilitators predominantly expressed in brain and leucocytes. Biochem J. 2000;350:771–776. [PMC free article] [PubMed] [Google Scholar]
- Doege H, Bocianski A, Scheepers A, Axer H, Eckel J, Joost HG, Schurmann A. Characterization of human glucose transporter (GLUT) 11 (encoded by SLC2A11), a novel sugar-transport facilitator specifically expressed in heart and skeletal muscle. Biochem J. 2001;359:443–449. doi: 10.1042/0264-6021:3590443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douard V, Ferraris RP. Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Endocrinol Metab. 2008;295:E227–237. doi: 10.1152/ajpendo.90245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funari VA, Crandall JE, Tolan DR. Fructose metabolism in the cerebellum. Cerebellum. 2007;6:130–140. doi: 10.1080/14734220601064759. [DOI] [PubMed] [Google Scholar]
- Gaby AR. Adverse effects of dietary fructose. Altern Med Rev. 2005;10:294–306. [PubMed] [Google Scholar]
- Garriga C, Barfull A, Planas JM. Kinetic characterization of apical D-fructose transport in chicken jejunum. J Membr Biol. 2004;197:71–76. doi: 10.1007/s00232-003-0640-0. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Armston AE. A re-evaluation of the role of mitochondrial pyruvate transport in the hormonal control of rat liver mitochondrial pyruvate metabolism. Biochem J. 1984;223:677–685. doi: 10.1042/bj2230677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi Y, Sasaki A, Taguchi N, Maeda M, Tsukagoshi H, Sato K, Yamaguchi H. Human GLUT5 immunolabeling is useful for evaluating microglial status in neuropathological study using paraffin sections. Acta Neuropathol. 2003;105:157–162. doi: 10.1007/s00401-002-0627-4. [DOI] [PubMed] [Google Scholar]
- Ibberson M, Riederer BM, Uldry M, Guhl B, Roth J, Thorens B. Immunolocalization of GLUTX1 in the testis and to specific brain areas and vasopressin-containing neurons. Endocrinology. 2002;143:276–284. doi: 10.1210/endo.143.1.8587. [DOI] [PubMed] [Google Scholar]
- Ibberson M, Uldry M, Thorens B. GLUTX1, a novel mammalian glucose transporter expressed in the central nervous system and insulin-sensitive tissues. J Biol Chem. 2000;275:4607–4612. doi: 10.1074/jbc.275.7.4607. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Benz AM, Zorumski CF, Olney JW. Effects of lactate and pyruvate on glucose deprivation in rat hippocampal slices. NeuroReport. 1994;5:617–620. doi: 10.1097/00001756-199401000-00021. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Benz AM, Clifford DB, Zorumski CF. Nitric oxide inhibitors attenuate ischemic degeneration in the CA1 region of rat hippocampal slices. Neurosci Lett. 1996;210:157–160. doi: 10.1016/0304-3940(96)12669-0. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Benz AM, Katsuki H, Zorumski CF. Endogenous monocarboxylates sustain hippocampal synaptic function and morphological integrity during energy deprivation. J Neurosci. 1997a;17:9448–9457. doi: 10.1523/JNEUROSCI.17-24-09448.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Katsuki H, Zorumski CF. Monocarboxylates (pyruvate and lactate) as alternative energy substrates for the induction of long-term potentiation in rat hippocampal slices. Neurosci Lett. 1997b;232:17–20. doi: 10.1016/s0304-3940(97)00567-3. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Ishii K, Katsuki H, Benz AM, Zorumski CF. beta-Hydroxybutyrate fuels synaptic function during development. Histological and physiological evidence in rat hippocampal slices. J Clin Invest. 1998a;101:1121–1132. doi: 10.1172/JCI1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Katsuki H, Benz AM, Zorumski CF. Oxygen deprivation produces delayed inhibition of long-term potentiation by activation of NMDA receptors and nitric oxide synthase. J Cereb Blood Flow Metab. 1998b;18:97–108. doi: 10.1097/00004647-199801000-00010. [DOI] [PubMed] [Google Scholar]
- Kanatani T, Mizuno K, Okada T. Effects of glycolytic metabolites on preservation of high energy phosphate level and synaptic transmission in the granule cells of guinea pig hippocampal slices. Experientia. 1995;51:213–216. doi: 10.1007/BF01931098. [DOI] [PubMed] [Google Scholar]
- Kim CH, Lisman JE. A role of actin filament in synaptic transmission and long-term potentiation. J Neurosci. 1999;11:4314–4324. doi: 10.1523/JNEUROSCI.19-11-04314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Mitsukawa K, Yamada MK, Nishiyama N, Matsuki N, Ikegaya Y. Cytoskeleton disruption causes apoptotic degeneration of dentate granule cells in hippocampal slice cultures. Neuropharmacology. 2002;42:1109–1118. doi: 10.1016/s0028-3908(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Krucker T, Siggins GR, Halpain S. Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proc Natl Acad Sci U S A. 2000;97:6856–6861. doi: 10.1073/pnas.100139797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmierz J, DeGeorge JJ, Sweeney D, May C, Rapoport SI. Quantitative analysis of polyols in human plasma and cerebrospinal fluid. J Chromatogr. 1989;497:39–48. doi: 10.1016/0378-4347(89)80003-9. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Baelemans A, Erlanson-Altertsson C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul Pept. 2008;150:26–32. doi: 10.1016/j.regpep.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Cellular bases of brain energy metabolism and their relevance to functional brain imaging: evidence for a prominent role of astrocytes. Cerebral Cortex. 1996;6:50–61. doi: 10.1093/cercor/6.1.50. [DOI] [PubMed] [Google Scholar]
- Maher F, Davies-Hill TM, Simpson IA. Substrate specificity and kinetic parameters of GLUT3 in rat cerebellar granule neurons. Biochem J. 1996;315:827–831. doi: 10.1042/bj3150827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolescu AR, Augustin R, Moley K, Cheeseman C. A highly conserved hydrophobic motif in the exofacial vestibule of fructose transporting SLC2A proteins acts as a critical determinant of their substrate selectivity. Mol Membr Biol. 2007;24:455–463. doi: 10.1080/09687680701298143. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Hopkins IB, Carey A. Alpha-cyano-4-hydroxycinnamate decreases both glucose and lactate metabolism in neurons and astrocytes: implications for lactate as an energy substrate for neurons. J Neurosci Res. 2001;66:747–754. doi: 10.1002/jnr.10084. [DOI] [PubMed] [Google Scholar]
- Messier C, White NM. Memory improvement by glucose, fructose, and two glucose analogs: a possible effect on peripheral glucose transport. Behav Neural Biol. 1987;48:104–127. doi: 10.1016/s0163-1047(87)90634-0. [DOI] [PubMed] [Google Scholar]
- Messier C, Whately K, Liang J, Du L, Puissant D. The effects of a high-fat, high-fructose, and combination diet on learning, weight, and glucose regulation in C57BL/6 mice. Behav Brain Res. 2007;178:139–145. doi: 10.1016/j.bbr.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Tomi M, Tachikawa M, Watanabe M, Terasaki T, Hosoya K. Evidence for creatine biosynthesis in Muller glia. Glia. 2005;52:47–52. doi: 10.1002/glia.20222. [DOI] [PubMed] [Google Scholar]
- Pan F, Mills SL, Massey SC. Screening of gap junction antagonists on dye coupling in the rabbit retina. Vis Neurosci. 2007;24:609–618. doi: 10.1017/S0952523807070472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J, Maher F, Simpson IA, Mattice LA, Davies P. GLUT5 glucose transporter expression in microglial cells. Glia. 1997;21:327–331. doi: 10.1002/(sici)1098-1136(199711)21:3<327::aid-glia7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pitkänen E. Mannose, mannitol, fructose and 1,5-anhydroglucitol concentrations measured by gas chromatography/mass spectrometry in blood plasma of diabetic patients. Clinica Chemica Acta. 1996;251:91–103. doi: 10.1016/0009-8981(96)06284-5. [DOI] [PubMed] [Google Scholar]
- Poitry S, Poitry-Yamate C, Ueberfeld J, MacLeish PR, Tsacopoulos M. Mechanisms of glutamate metabolic signaling in retinal glial (Müller) cells. J Neurosci. 2000;20:1809–1821. doi: 10.1523/JNEUROSCI.20-05-01809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan LP, Rosell DR, Alves SE, Hoskin EK, McCall AL, Charron MJ, McEwen BS. GLUT8 glucose transporter is localized to excitatory and inhibitory neurons in the rat hippocampus. Brain Res. 2002;932:129–134. doi: 10.1016/s0006-8993(02)02308-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez WA, Horne CA, Mondragon AN, Phelps DO. Comparable dose-response functions for the effects of glucose and fructose on memory. Behav Neural Biol. 1994;61:162–169. doi: 10.1016/s0163-1047(05)80070-6. [DOI] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Okada Y, Nabetani M. Effect of mannose, fructose and lactate on the preservation of synaptic potentials in hippocampal slices. Neurosci Lett. 1994;171:125–128. doi: 10.1016/0304-3940(94)90621-1. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Yang B, Takata T, Yokono K. Synaptic adaptation to repeated hypoglycemia depends on the utilization of monocarboxylates in guinea pig hippocampal slices. Diabetes. 2002;51:430–438. doi: 10.2337/diabetes.51.2.430. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoid toxicity in the hippocampus: reversal by supplementation with brain fuels. J Neurosci. 1986;6:2240–2244. doi: 10.1523/JNEUROSCI.06-08-02240.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheepers A, Schmidt S, Manolescu A, Cheeseman CI, Bell A, Zahn C, Joost HG, Schurmann A. Characterization of the human SLC2A11 (GLUT11) gene: alternative promoter usage, function, expression, and subcellular distribution of three isoforms, and lack of mouse orthologue. Mol Membr Biol. 2005;22:339–351. doi: 10.1080/09687860500166143. [DOI] [PubMed] [Google Scholar]
- Schurr A, West CA, Rigor BM. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science. 1988;240:1326–1328. doi: 10.1126/science.3375817. [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Gibbs EM, Wesslau C, Gould GW, Kahn BB. Human small intestine facilitative fructose/glucose transporter (GLUT5) is also present in insulin-responsive tissues and brain. Investigation of biochemical characteristics and translocation. Diabetes. 1992;41:1360–1365. doi: 10.2337/diab.41.10.1360. [DOI] [PubMed] [Google Scholar]
- Tozzi A, Costa C, Di Filippo M, Tantucci M, Siliquini S, Belcastro V, Parnetti L, Picconi B, Calabresi P. Memantine reduces neuronal dysfunctions triggered by in vitro ischemia and 3-nitropropionic acid. Exp Neurol. 2007;207:218–226. doi: 10.1016/j.expneurol.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Simpson IA. Developmental switch in brain nutrient transporter expression in the rat. Am J Physiol Endocrinol Metab. 2003;285:E1127–1134. doi: 10.1152/ajpendo.00187.2003. [DOI] [PubMed] [Google Scholar]
- Wada H, Okada Y, Uzuo T, Nakamura H. The effects of glucose, mannose, fructose and lactate on the preservation of neural activity in the hippocampal slices from the guinea pig. Brain Res. 1998;788:144–150. doi: 10.1016/s0006-8993(97)01532-1. [DOI] [PubMed] [Google Scholar]
- Wei J, Zhang M, Zhu Y, Wang JH. Ca(2+)-calmodulin signalling pathway up-regulates GABA synaptic transmission through cytoskeleton-mediated mechanisms. Neuroscience. 2004;127:637–647. doi: 10.1016/j.neuroscience.2004.05.056. [DOI] [PubMed] [Google Scholar]
- Widmer M, Uldry M, Thorens B. GLUT8 subcellular localization and absence of translocation to the plasma membrane in PC12 cells and hippocampal neurons. Endocrinology. 2005;146:5727–4736. doi: 10.1210/en.2005-0668. [DOI] [PubMed] [Google Scholar]
- Wu X, Li W, Sharma V, Godzik A, Freeze HH. Cloning and characterization of glucose transporter 11, a novel sugar transporter that is alternatively spliced in various tissues. Mol Genet Metab. 2002;76:37–45. doi: 10.1016/s1096-7192(02)00018-5. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Mennerick S, Izumi Y. Assessment of synaptic effects of nitric oxide in hippocampal neurons. Methods Neurosci. 1996;31:283–329. [Google Scholar]


