Abstract
The primary objective of this study was to determine whether cadmium (Cd) exposures alter reproduction in fathead minnows (Pimephales promelas). Pairs of sexually mature minnows were exposed to waterborne Cd at 0, 12.5, 25, or 50 μg/L for 21 days. During this exposure period, the reproductive success of pairs was assessed. Following the exposure, gonadosomatic index (GSI), male secondary sexual characteristics, male plasma 11-ketotestosterone (11-KT) concentrations, and female plasma estradiol (E2) concentrations were determined. Results of this study show that Cd exposures impair reproduction in fathead minnows. Pairs exposed to 50 μg/L experienced a significant decline in spawning frequency and fecundity relative to unexposed minnows. Cd exposures also caused alterations in male secondary sexual characteristics, as males exposed to 25 μg/L had significantly fewer nuptial tubercles than controls. Furthermore, males exposed to 12.5 μg/L Cd experienced a significant increase in plasma 11-KT concentrations relative to controls. No alterations in GSI or female plasma E2 concentrations were observed.
Cadmium (Cd) is a common environmental pollutant that poses a significant threat to the health of aquatic ecosystems due to its persistent release through anthropogenic activities such as mining and smelting operations (Henson and Chedrese 2004). Cd concentrations in the water range from background levels of 1 μg/L to 400 μg/L in contaminated sites (Tilton et al. 2003). Like many heavy metals, Cd has a long half-life and can bioaccumulate over time in various organs including the liver, kidneys, testes, and ovaries (Henson and Chedrese 2004). The effects of Cd on the health of freshwater fish have been well studied especially with regard to survival (Benoit et al. 1976; Middaugh and Dean 1977; Hwang et al. 1995), calcium ionoregulation (Roch and Maly 1979; Wendelaar Bonga and Lock 1992), and skeletal development (Muramoto 1981; Cheng et al. 2000). However, fewer studies have investigated the effects of Cd exposure on the reproductive success of fishes despite evidence that Cd can act as a reproductive toxin (Signhal et al. 1985) and endocrine disruptor (Henson and Chedrese 2004).
Studies have shown that exposure to Cd damages gonads and impairs gametogenesis. The testes appear to be particularly sensitive to Cd exposures. Several studies have shown that Cd causes testicular apoptosis and necrosis (Singhal et al. 1985; Das 1988). Exposure to 10 mg/L Cd increases capsase 3 gene expression, an indicator of apoptosis, in the testes of black gobies (Gobius niger) (Migliarini et al. 2005). Male and female Asian cyprinids (Labeo bata) exposed to a range of Cd concentrations (1–15 mg/L) for four months experienced reductions in gonadosomatic index (GSI) relative to unexposed fish (Das 1988). In addition, the testes of exposed males lacked spermatids and spermatozoa, while the ovaries of exposed females lacked mature oocytes and contained a significantly higher proportion of atretic follicles relative to ovaries from unexposed females.
In addition to alterations in gametogenesis, exposure to Cd has been shown to alter steroidogenesis. For example, female Atlantic croaker (Micropogonias undulates) exposed to Cd have significantly higher plasma estradiol (E2) concentrations relative to unexposed croaker (Thomas 1989). Alterations in circulating sex steroids have also been documented for male and female Japanese medaka (Oryzias latipes) exposed to Cd (Foran et al. 2002; Tilton et al. 2003). In addition, Cd reduces the expression of rainbow trout E2 receptor α (long isoform) genes in the liver and increases the expression of salmon gonadotropin releasing hormone genes in the brain (Vetillard and Bailhache 2004).
The abnormalities in gonad and endocrine function caused by exposure to Cd could potentially impair reproductive success. With this in mind, the primary goal of this study was to determine the effects of Cd exposures on reproduction in fathead minnows. The first objective of this study was to determine whether or not Cd exposures impair reproductive success as measured by spawning frequency, fecundity, clutch size, fertilization success, hatching success, and offspring mortality. The second objective was to determine if Cd-induced alterations in reproductive success were correlated with morphological and physiological endpoints associated with reproduction including secondary sexual characteristics, GSI, male 11-ketotestosterone (11-KT) concentrations, and female E2 concentrations.
Materials and Methods
Fish Maintenance
All fish used in these experiments were produced by the University of Nebraska at Omaha fathead minnow colony according to the protocols outlined in Peake et al. (2004).
Throughout the experiment, minnows were maintained in aerated, dechlorinated, heated (26°C) tap water and were kept under a photoperiod of 16:8 h light:dark. Static renewal of water in the aquaria occurred daily. Renewal water had the following water quality (mean ± SE): pH, 8.0 ± 0.04; conductivity, 636 ± 6 μS; hardness (expressed as mg/L CaCO3), 169 ± 1; alkalinity (expressed as mg/L CaCO3), 94 ± 2 and Ca, 39 ± 1 mg/L.
21-Day Breeding Study
Pairs of fathead minnows were exposed to 0, 12.5, 25, or 50 μg/L Cd for 21 days. Each exposure group initially consisted of 20 pairs of minnows. Some mispairing and mortality occurred reducing the number of pairs at the end of the 21-day exposure to 18, 15, 12, and 4 in the 0, 12.5, 25, and 50 μg/L groups, respectively. During the exposure period, the reproductive success of each pair was assessed using a modified version of the 21-day breeding assay (Ankley et al. 2001). Pairs were maintained in 40-L tanks heated to ∼26°C. Each tank contained two pairs separated by a plastic divider. Spawning events were recorded daily. Spawning frequency was calculated as the percentage of pairs to spawn per day. Spawning substrates with adhered eggs were removed from each tank every morning and placed in a 1-L beaker containing clean laboratory water maintained in a warm water bath at 26°C. Clutch size, the number of eggs on each substrate, was evaluated so that fecundity could be determined. Fecundity was measured as the number of eggs laid per day per female. The number of fertilized eggs was determined by counting the number of eggs with eye spots at 2 to 3 days post spawn. Fertilization success was measured as the percent of eggs fertilized in each clutch. When larvae began to hatch (∼4 days post spawn), they were transferred to 2-L beakers. Hatching success was measured as the percent of fertilized eggs to hatch. Mortality of hatched larvae was monitored for and offspring mortality was calculated as the percent of hatched larvae to survive to 48 h.
At the termination of the 21-day breeding study, minnows were sacrificed for plasma collection, assessment of male secondary sexual characteristics, and determination of GSI. Fish were anesthetized, weighed, and bled. To collect blood, the caudal peduncle was severed and blood was collected with heparinized micropipettes, which were then centrifuged to separate the plasma. Plasma was aspirated into a heparinized microcentrifuge tube and stored on ice until processing was complete. Plasma samples were then placed in the −80°C freezer. Secondary sexual characteristics were assessed by counting the number of nuptial tubercles and measuring the intraocular distance and head width of each male. Because head width and intraocular distance are positively correlated, an intraocular distance index was calculated as the intraocular distance/head width. Intraocular distance is a measurement that has been successfully used as a male secondary sexual characteristic in fathead minnows (Orlando et al. 2004). Both male and female gonads were removed and weighed for determination of GSI.
Plasma Sex Steroid Quantification
Male plasma samples were analyzed for 11-KT using a commercially available fathead minnow 11-KT enzyme-linked immunosorbent assay (EIA) kit (Cayman Chemical, Ann Arbor, MI) following the manufacturer's protocol. Similarly, female plasma samples were analyzed for E2 using a commercially available EIA kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's protocol. Sample concentrations were measured using a BioTek ELx800 automated microplate reader managed by the computer software KC Junior version 1.6.
Cd Analysis
During the experiments, a water exchange (>30% of total volume) was conducted daily. A stock solution of CdCl2 was used to manually dose all of the experimental tanks. Water samples (125 ml) were collected daily from each aquarium. Each water sample was acidified with nitric acid to a pH of <2.0 and stored at 4°C. Actual Cd concentrations were determined by flame or graphite furnace atomic absorption spectrometry according to previously established methods (US EPA 1983). The actual Cd concentrations during the exposures averaged 39.7 μg/L (79.4% of nominal) in the 50-μg/L exposure aquaria, 24.3 μg/L (97.2% of nominal) in the 25-μg/L exposure aquaria, 8.6 μg/L (79.4% of nominal) in the 12.5-μg/L exposure aquaria, and were below the detectable limit of 0.0025 μg/L in the 0-μg/L aquarium.
Statistical Analyses
Differences in reproductive endpoints between exposure groups were tested using one-way analysis of variance (ANOVA, Statveiw 5.0). When the assumption of homogeneity of variances was met, ANOVA was followed by Dunnett's tests. When the assumption was not satisfied, data were analyzed using the Welch ANOVA (JMP 5.0) followed by Kruskal-Wallis multiple comparison tests. The mortality of males and females was compared using chi-squared analysis corrected for continuity when appropriate. Statistical significance throughout the experiments was assumed at p ≤ 0.05.
Results
Reproductive Success
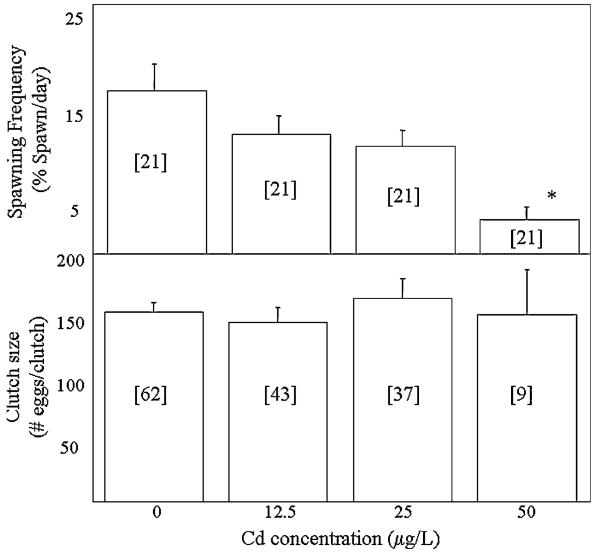
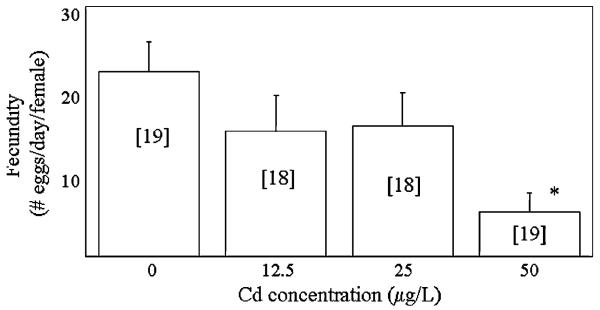
The average spawning frequency of minnows ranged from 3.5 to 16.8% of pairs spawning/days. There were significant differences in the spawning frequency between the groups (Welch ANOVA, p < 0.001, Figure 1). Specifically, the spawning frequency of pairs exposed to 50 μg/L Cd was significantly lower than that of pairs exposed to 0, 12.5, or 25 μg/L. Clutch size averaged between 144 and 163 eggs/clutch and no significant differences between groups were detected (Welch ANOVA, p = 0.83). The average fecundity of minnows ranged from 22.2 eggs/days in the unexposed group to 5.3 eggs/days in the 50-μg/L exposure group (Figure 2). Significant differences in the fecundity of groups were detected (Welch ANOVA, p < 0.01) with pairs exposed to 50 μg/L experiencing a significant reduction in fecundity relative to unexposed pairs.
Fig. 1.
Spawning frequency and clutch size of minnows exposed to 0, 12.5, 25 and 50 μg/L Cd. Numbers in brackets indicate sample size. *Significant difference from controls
Fig. 2.
Fecundity of minnows exposed to 0, 12.5, 25 and 50 μg/L Cd. Numbers in brackets indicate sample size. *Significant difference from controls
No significant differences in fertilization success, which averaged between 86% and 95% in each group, were detected (Welch ANOVA, p = 0.11; Table 1). Groups did not differ with regard to hatching success (ANOVA, p = 0.56), which averaged over 97% in each group. There were no significant differences in the offspring mortality among the groups (Welch ANOVA, p = 0.40).
Table 1.
Reproductive success of minnows exposed to 0, 12.5, 25, and 50 μg/L Cd
| Reproductive Parameter |
Exposure Concentration |
|||
|---|---|---|---|---|
| 0 μg/L | 12.5 μg/L | 25 μg/L | 50 μg/L | |
| Fertilization success (%) | 86 ± 3 (60) | 95 ± 1 (41) | 91 ± 3 (34) | 91 ± 3 (8) |
| Hatching success (%) | 98 ± 1 (55) | 100 ± .1 (41) | 97 ± 3 (34) | 98 ± 1 (8) |
| Offspring mortality (%) | 4 ± 2 (52) | 7 ± 3 (41) | 6 ± 2 (34) | 29 ± 16 (8) |
Data are expressed as mean ± standard error. Numbers in parentheses indicate sample size.
Secondary Sexual Characteristics and GSI
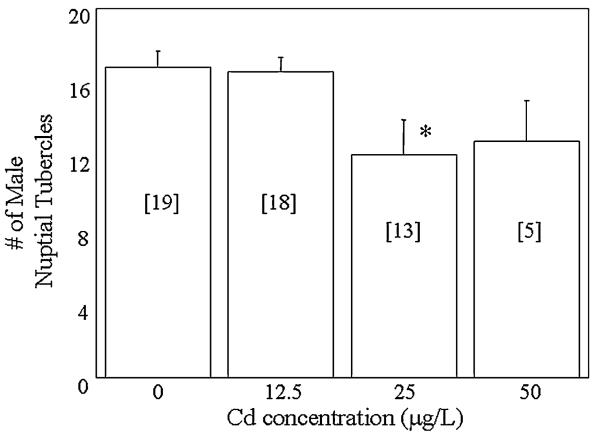
Males in the 0- and 12.5-μg/L exposure groups had an average of 17 breeding tubercles, while males in the 25- and 50-μg/L groups had an average of 12 breeding tubercles (Figure 3). Significant differences in the number of male breeding tubercles were detected between groups (ANOVA, p = 0.02). Specifically, males exposed to 25 μg/L Cd had significantly fewer breeding tubercles than unexposed males. The mean intraocular distance index ranged from 0.83 to 0.85 in each group. No significant differences in the intraocular distance index of males were detected (ANOVA, p = 0.50; Table 2). The average GSI of males ranged from 0.014 to 0.018 and no significant differences were detected between the groups (ANOVA, p = 0.40). Similarly, significant differences in female GSI, which averaged between 0.10 and 0.12 in each of the groups, were not detected (ANOVA, p = 0.44).
Fig. 3.
Number of breeding tubercles of males exposed to 0, 12.5, 25 and 50 μg/L Cd. Numbers in brackets indicate sample size. *Significant difference from controls
Table 2.
Male intraocular distance index and GSI of minnows exposed to 0, 12.5, 25, and 50 μg/L Cd
| Exposure Concentration |
||||
|---|---|---|---|---|
| Parameter | 0 μg/L | 12.5 μg/L | 25 μg/L | 50 μg/L |
| Intraocular distance index | 0.85 ± 0.01 (19) | 0.83 ± 0.01 (18) | 0.83 ± 0.02 (13) | 0.85 ± 0.02 (5) |
| Male GSI | 0.002 ± 0.002 (19) | 0.02 ± 0.001 (18) | 0.01 ± 0.001 (13) | 0.02 ± 0.002 (5) |
| Female GSI | 0.10 ± 0.01 (17) | 0.10 ± 0.01 (16) | 0.11 ± 0.01 (13) | 0.12 ± 0.03 (3) |
Data are expressed as mean ± standard error. Numbers in parentheses indicate sample size.
Sex Steroid Concentrations
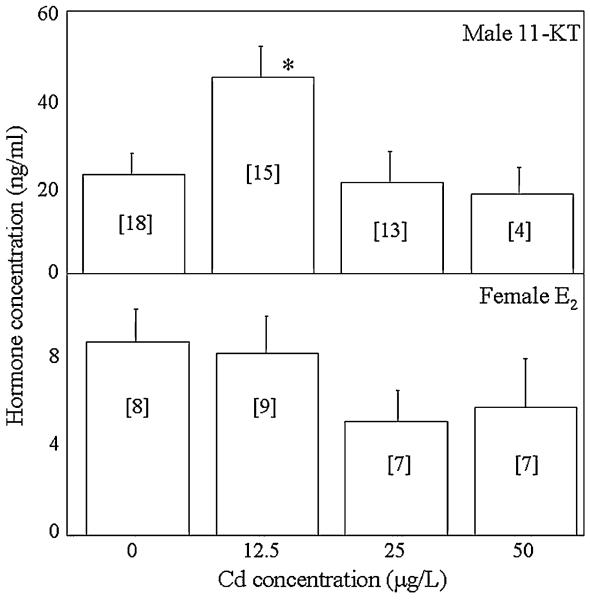
The average plasma 11-KT concentration of unexposed males was 22.5 ng/ml, while the average plasma E2 concentration of unexposed females was 8.8 ng/ml (Figure 4). Significant differences in male plasma 11-KT concentrations were detected (ANOVA, p = 0.02). Specifically, the average 11-KT concentration of males exposed to 12.5 μg/L was significantly greater than that of unexposed males. In contrast, no significant differences in female plasma E2 concentrations were detected (ANOVA, p = 0.38).
Fig. 4.
Plasma 11-KT concentrations of males (top) and E2 concentrations (bottom) of females exposed to 0, 12.5, 25, and 50 μg/L Cd. Numbers in brackets indicate sample size. *Significant difference from controls
Survival
Survival of males and females exposed to 0, 12.5, 25, and 50 μg/L Cd is shown in Table 3. A comparison of male and female survival revealed no differences between males and females at 0, 12.5, or 25 μg/L Cd (p = 1.0, 0.55, 0.09, respectively). However, at 50 μg/L, male minnows had significantly lower survival than females (p = 0.02).
Table 3.
Survival of male and female minnows exposed to 0, 12.5, 25, and 50 μg/L Cd
| Exposure Concentration |
||||
|---|---|---|---|---|
| Survival | 0 μg/L | 12.5 μg/L | 25 μg/L | 50 μg/L* |
| Male | 95% (20) | 89% (18) | 67% (18) | 26% (19) |
| Female | 95% (20) | 94% (18) | 94% (18) | 68% (19) |
Numbers in parentheses indicate sample size.
Significant difference between male and female mortality for a given exposure concentration.
Discussion
The primary objective of this study was to determine if exposure to Cd alters the reproductive success of fathead minnows. The second objective was to determine if Cd-induced alterations in reproductive success were correlated with morphological and physiological endpoints such as secondary sexual characteristics, GSI, female E2 concentrations, and male 11-KT concentrations. The results of this study clearly demonstrate that Cd exposures impair the reproductive success of fathead minnows as evidenced by a significant decline in spawning frequency. In no case was reproductive success associated with any of the morphological or physiological endpoints measured. The lack of correlation between fecundity and clutch size, fertilization success, hatching success, and GSI suggests that Cd does not reduce fecundity by altering gametogenesis or gonad condition. Furthermore, a lack of correlation between fecundity and circulating sex steroid concentrations suggests that Cd does not reduce fecundity by altering endocrine function. However, Cd-induced reductions in fecundity were correlated with spawning frequency, which is indicative of alterations in reproductive behavior.
Reproductive Success
The results of this study indicate that Cd has an adverse effect on reproductive success. Minnows exposed to 50 μg/L Cd experienced a significant decline in fecundity relative to controls. Fecundity can be considered a function of spawning frequency and clutch size; therefore, alterations in fecundity can be credited to one or both of these variables. In the current study, the reduction in fecundity can be attributed to alterations in spawning frequency as minnows exposed to 50 μg/L experienced a significant decline in spawning frequency relative to controls, but maintained clutch sizes similar to those of controls. Other studies have also shown that changes in fecundity are linked to changes in spawning frequency rather than clutch size. For example, zebrafish (Danio rerio) exposed to E2 experience a reduction in spawning frequency and fecundity, but do not experience alterations in average clutch size (Brion et al. 2004). Also fathead minnows exposed to nonylphenol ethoxylate (Nichols et al. 2001) experience an increase in fecundity that is attributable to an increase in spawning frequency rather than changes in clutch size. In addition, fathead minnows exposed to 50 μg/L during female sexual differentiation (8–29 days old) also experienced a significant decline in spawning frequency relative to unexposed minnows; however, overall fecundity is maintained due to a significant increase in clutch size (Sellin and Kolok 2006). The impaired reproductive success of Cd-exposed minnows is primarily associated with a decrease in spawning frequency. It seems likely that this decline in spawning frequency is due to alterations in reproductive behavior rather than a direct alteration in gonad or endocrine function.
Secondary Sexual Characteristics and Reproductive Success
The male secondary sexual characteristics measured in this study did not correlate with the Cd-induced alterations in reproductive success. Minnows exposed to 50 μg/L Cd experienced a decline in fecundity; however, no significant differences in intraocular distance index or the number of nuptial tubercles were detected. In contrast, minnows exposed to 25 μg/L Cd had significantly fewer nuptial tubercles than controls, but did not experience a decline in reproductive success. These results indicate that secondary sexual characteristics are not predictive of Cd-induced reproductive impairment.
Gonad Health and Reproductive Success
While it is possible that the impaired reproductive success observed in this study is a result of altered gonad health, this seems unlikely given that clutch size, fertilization success, hatching success, and GSI were unaffected by Cd exposures. The average clutch size of minnows exposed to Cd did not differ from that of controls indicating that exposure to Cd did not affect the egg-producing capability of females. Fertilization and hatching success are thought to be an indicator of male and female gamete viability, respectively (Kime and Nash 1999). In the current study, exposure to Cd did not affect fertilization or hatching success indicating that gamete viability was not affected by Cd exposure. Other studies have yielded similar results. Specifically, Cd did not have adverse effects on the fertilization or hatching success of Japanese medaka exposed as adults (Foran et al. 2002; Tilton et al. 2003). In addition, the current study showed that exposure to Cd did not alter male or female GSI providing further evidence that gonad condition was not adversely affected. In contrast to this finding, other studies have shown that exposure to Cd reduces male GSI (Das 1988; Tilton et al. 2003). In the literature, there are conflicting reports on the effect of Cd on female GSI. Similar to the results of this study, Tilton et al. (2003) found that exposure to either 1, 5, or 10 μg/L Cd had no effect on the GSI of female medaka. However, Das (1988) showed that female Asian cyprinids experienced a significant reduction in GSI following exposure to 4–10 mg/L Cd, while Thomas (1989) found that exposure to 1 mg/L Cd significantly increased the GSI of female Atlantic croaker. It is likely that the conflicting results of these studies are related to differences in exposure dose, duration, and timing.
Endocrine Function and Reproductive Success
Several studies have shown that Cd can alter endocrine function. Atlantic croaker (Micropogonias undulates) exposed to 1 mg/L Cd have significantly higher plasma E2 concentrations than unexposed croaker (Thomas 1989). Female Japanese medaka exposed to 5 μg/L Cd experience a significant increase in circulating E2 levels relative to controls, while at 10 μg/L a reduction in E2 concentrations occurs (Tilton et al. 2003). Male medaka exposed to 1, 5, or 10 μg/L Cd do not experience alterations in plasma testosterone concentrations, but do experience a decline in gonadal testosterone release relative to controls (Tilton et al. 2003). In the present study, no alterations in the plasma E2 concentrations of females exposed to Cd were detected. In addition, males exposed to 25 and 50 μg/L Cd did not experience changes in 11-KT levels relative to controls. In contrast, male minnows exposed to 12.5 μg/L Cd experienced a significant increase in 11-KT concentrations. indicating that at this dose, Cd does affect endocrine function. However, no alterations in reproductive success were detected in fish exposed at 12.5 μg/L suggesting that 11-KT concentrations and reproductive success are not correlated with one another. Studies by Foran et al. (2002) and Tilton et al. (2003) also found that alterations in circulating sex steroid concentrations were not correlated with reproductive success. While the impaired reproductive success of Cd-exposed minnows may be related to alterations in endocrine function, this study does not provide evidence supporting this hypothesis. However, it should be noted that the results of this study do not rule out the possibility that Cd could impair reproduction by altering endocrine function at other points along the hypothalamus-pituitary-gonadal axis.
Behavior and Reproductive Success
An alternative explanation for the altered reproductive success of Cd-exposed minnows is that Cd impairs reproductive behaviors. In this study, overall fecundity was associated with spawning frequency. Specifically, minnows exposed to 50 μg/L Cd experienced a significant reduction in spawning frequency that led to a reduction in fecundity. Other studies investigating the consequences of chemical exposures on reproduction have hypothesized that reduced spawning frequency is caused by a disruption in mating behavior (Brion et al. 2004). It is plausible that exposure to Cd could alter reproductive behaviors as several studies have shown that Cd can affect the behaviors of fish. For example, pairs of rainbow trout (Oncorhynchus mykiss) exposed to sublethal concentrations of Cd engaged in a significantly lower number of aggressive attacks than pairs of unexposed trout (Sloman et al. 2003a). Furthermore, when a Cd-exposed and unexposed trout were paired, the Cd-exposed fish became subordinate. Exposure to Cd has also been shown to reduce the ability of rainbow trout to detect and respond to chemical alarm signals associated with predators (Scott et al. 2003). It has been hypothesized that these behavioral changes are related to alterations in olfaction (Sloman et al. 2003b). Disruption of olfaction could impair several olfaction-mediated behaviors including reproduction (Laberge and Hara 2001).
Survival
Though not central to the main objective of this study, it is interesting that males exposed to 50 μg/L Cd had a significantly lower survival than females exposed to the same concentration. A study by Hatakeyama and Yasuno (1987) also revealed sex-based differences in the survival of fish exposed to Cd. Specifically, male guppies (Poecilia reticulata) fed Cd-accumulated midge larvae for six months had a significantly higher survival than females. Sex differences in response to environmental exposures have recently been recognized as an emerging field of biological research especially with regard to heavy metals and endocrine-disrupting compounds (Keitt et al. 2004). It has been proposed that those investigating the outcomes of environmental exposures should include sex as a variable in all studies. The results of this study provide support for this suggestion by clearly demonstrating that gender influences the outcome of Cd exposures.
Acknowledgments
This study was partially supported by the University of Nebraska at Omaha (UNO) Department of Biology, the UNO Graduate College, and by the Nebraska Water Center. Additional support was given by Environmental Protection Agency Greater Research Opportunities Fellowship no. 91636301-0, NIH National Institute of Environmental Health Science, grant 1 R15 ES11788-01, and NIH grant 1 P20 RR16469 from the BRIN Program of the National Center for Research Resources.
References
- Ankley GT, Jensen KM, Kahl MD, Korte JJ, Makynen EA. Description and evaluation of a short-term reproduction test with the fathead minnow (Pimephales promelas) Environ Toxicol Chem. 2001;20:1276–1290. [PubMed] [Google Scholar]
- Benoit DA, Leonard EN, Christensen GM, Fiandt JT. Toxic effects of cadmium on three generations of brook trout (Salvelinus fontinalis) Trans Am Fish Soc. 1976;105:550–556. [Google Scholar]
- Brion F, Tyler CR, Palazzi X, Laillet B, Porcher JM, Garric J, Flammarion P. Impacts of 17B-estradiol, including environmentally relevant concentrations, on reproduction after exposure during embryo-larval-, juvenile- and adult-life stages in zebrafish (Danio rerio) Aquat Toxicol. 2004;68:193–217. doi: 10.1016/j.aquatox.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Cheng SH, Wai AWK, So CH, Wu RSS. Cellular and molecular basis of cadmium-induced deformities in zebrafish embryos. Environ Toxicol Chem. 2000;19:3024–3031. [Google Scholar]
- Das RC. Cadmium toxicity to gonads in a freshwater fish, Labeo bata (Hamilton) Arch Hydrobiol. 1988;112:467–474. [Google Scholar]
- Foran CM, Peterson BN, Benson H. Influence of parental and developmental cadmium exposure on endocrine and reproductive function in Japanese medaka (Oryzias latipes) Comp Biochem Physiol. 2002;133C:345–354. doi: 10.1016/s1532-0456(02)00128-x. [DOI] [PubMed] [Google Scholar]
- Hatakeyama S, Yasuno M. Chronic effects of Cd on the Reproduction of the guppy (Poecilia reticulata) through Cd-accumulated midge larvae (Chironomus yoshimatsui) Ecotoxicol Environ Safe. 1986;14:191–207. doi: 10.1016/0147-6513(87)90062-5. [DOI] [PubMed] [Google Scholar]
- Henson MC, Chedrese PJ. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp Biol Med. 2004;229:383–392. doi: 10.1177/153537020422900506. [DOI] [PubMed] [Google Scholar]
- Hwang PP, Lin SW, Lin HC. Different sensitivities to cadmium in tilapia larvae (Oreochromis mossambicus; Teleostei) Arch Environ Contam Toxicol. 1995;29:1–7. [Google Scholar]
- Keitt SK, Fagan TF, Marts SA. Understanding sex differences in environmental health: A thought leaders' roundtable. Environ Health Perspect. 2004;112:604–609. doi: 10.1289/ehp.6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kime DE, Nash JP. Gamete viability as an indicator of reproductive endocrine disruption in fish. Sci Total Environ. 1999;233:123–129. [Google Scholar]
- Laberge F, Hara TJ. Neurobiology of fish olfaction: a review. Brain Res Rev. 2001;36:46–59. doi: 10.1016/s0165-0173(01)00064-9. [DOI] [PubMed] [Google Scholar]
- Middaugh DP, Dean JM. Comparative sensitivity of eggs, larvae and adults of the estuarine teleosts, Fundulus heteroclitus and Menidia menidia to cadmium. Bull Environ Contam Toxicol. 1977;17:645–652. doi: 10.1007/BF01685947. [DOI] [PubMed] [Google Scholar]
- Migliarini B, Campisis AM, Maradonna F, Truzzi C, Annibaldi A, Scarponi G, Carnevali O. Effects of cadmium exposure on testis apoptosis in the marine teleost Gobius niger. Gen Comp Endocr. 2005;142:241–247. doi: 10.1016/j.ygcen.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Muramoto S. Vertebral column damage and decrease of calcium concentration in fish exposed experimentally to cadmium. Environ Pollut. 1981;24A:125–133. [Google Scholar]
- Nichols KM, Snyder EM, Snyder SA, Pierens SL, Miles-Richardson SR, Giesy JP. Effects of nonylphenol ethoxylate exposure on reproductive output and bioindicators of environmental estrogen exposure in fathead minnows, Pimephales promelas. Environ Toxicol Chem. 2001;20:510–522. doi: 10.1897/1551-5028(2001)020<0510:eoneeo>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Orlando EF, Kolok AS, Binzcik GA, Gates JL, Horton MK, Lam-bright CS, Earl Gray L, Jr, Soto AM, Guillette LJ., Jr Endocrine-disrupting effects of cattle feedlot effluent on an aquatic sentinel species, the fathead minnow. Environ Health Perspect. 2004;112:353–358. doi: 10.1289/ehp.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake EB, Locke JC, Tierney LL, Kolok AS. Copper tolerance in fathead minnows: II. Maternal transfer. Environ Toxicol Chem. 2004;23:208–211. doi: 10.1897/02-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roch M, Maly EJ. Relationship of cadmium-induced hypocalcemia with mortality in rainbow trout (Salmo gairdneri) and the influence of temperature on toxicity. J Fish Res Bd Can. 1979;36:1297–1303. [Google Scholar]
- Scott GR, Sloman KA, Rouleau C, Wood CM. Cadmium disrupts behavioural and physiological responses to alarm substance in juvenile rainbow trout (Oncorhynchus mykiss) J Exp Biol. 2003;206:1779–1790. doi: 10.1242/jeb.00353. [DOI] [PubMed] [Google Scholar]
- Sellin MK, Kolok AS. Cd exposures during early development: Do they lead to reproductive impairment in fathead minnows? Environ Toxicol Chem. 2006 doi: 10.1897/05-559r1.1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloman KA, Baker DW, Ho CG, McDonald DG, Wood CM. The effects of trace metal exposure on agonistic encounters in juvenile rainbow trout, Oncorhynchus mykiss. Aquat Toxicol. 2003a;63:187–196. doi: 10.1016/s0166-445x(02)00176-5. [DOI] [PubMed] [Google Scholar]
- Sloman KA, Scott GR, Diao Z, Rouleau C, Wood CM, McDonald DG. Cadmium affects the social behaviour of rainbow trout, Oncorhynchus mykiss. Aquat Toxicol. 2003b;65:171–185. doi: 10.1016/s0166-445x(03)00122-x. [DOI] [PubMed] [Google Scholar]
- Singhal RL, Vijayvargiya R, Shukla GS. Toxic effects of cadmium and lead on reproductive functions. In: Thomas JA, Korach KS, McLachlan JA, editors. Target organ toxicology series: Endocrine toxicology. Raven Press; New York: 1985. pp. 149–180. [Google Scholar]
- Thomas P. Effects of Aroclor 1254 and cadmium on reproductive endocrine function and ovarian growth in Atlantic croaker. Mar Environ Res. 1989;28:499–503. [Google Scholar]
- Tilton SC, Foran CM, Benson WH. Effects of cadmium on the reproductive axis of Japanese medaka (Oryzias latipes) Comp Biochem Phys. 2003;136C:265–276. doi: 10.1016/j.cca.2003.09.009. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (EPA) Methods for chemical analysis of water and wastes. Cincinnati, OH: 1983. EPA/600/4-79/20. [Google Scholar]
- Vetillard A, Bailhache T. Cadmium: an endocrine disruptor that affects gene expression in the liver and brain of juvenile rainbow trout. Biol Reprod. 2005;72:119–126. doi: 10.1095/biolreprod.104.029520. [DOI] [PubMed] [Google Scholar]
- Wendelaar Bonga SE, Lock RAC. Toxicants and osmoregulation in fish. Neth J Zool. 1992;42:478–493. [Google Scholar]