Abstract
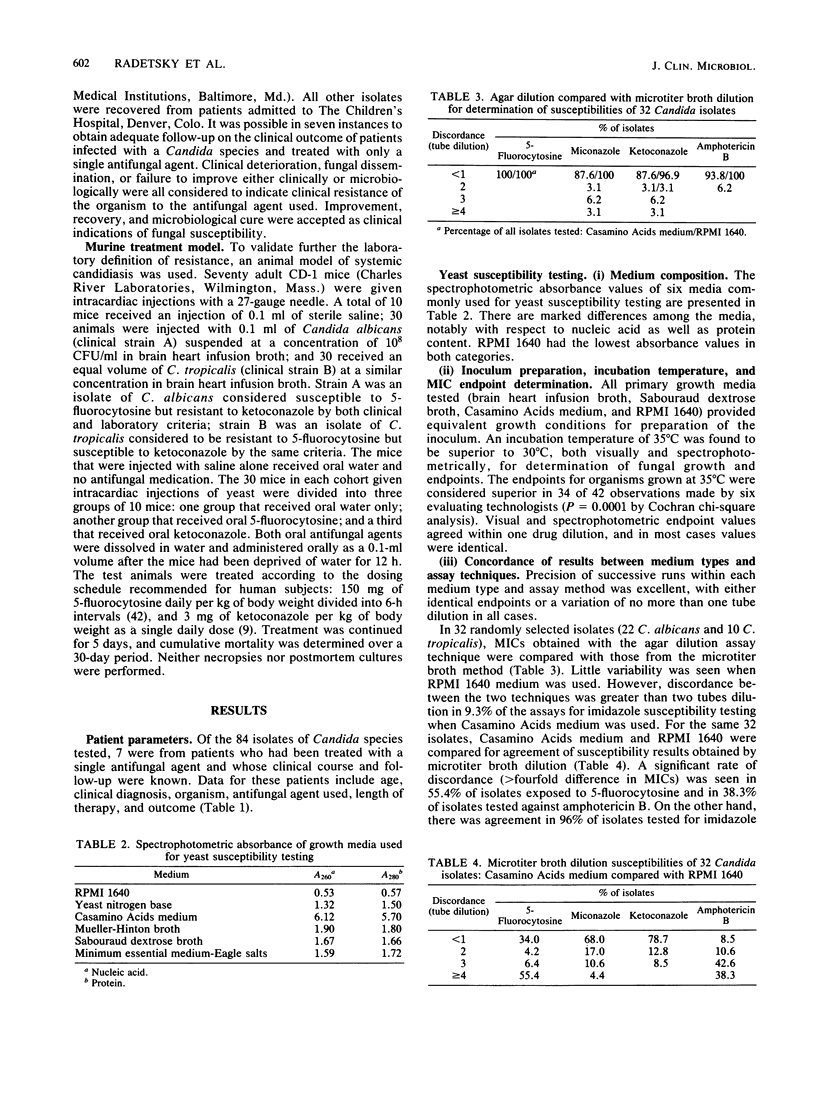
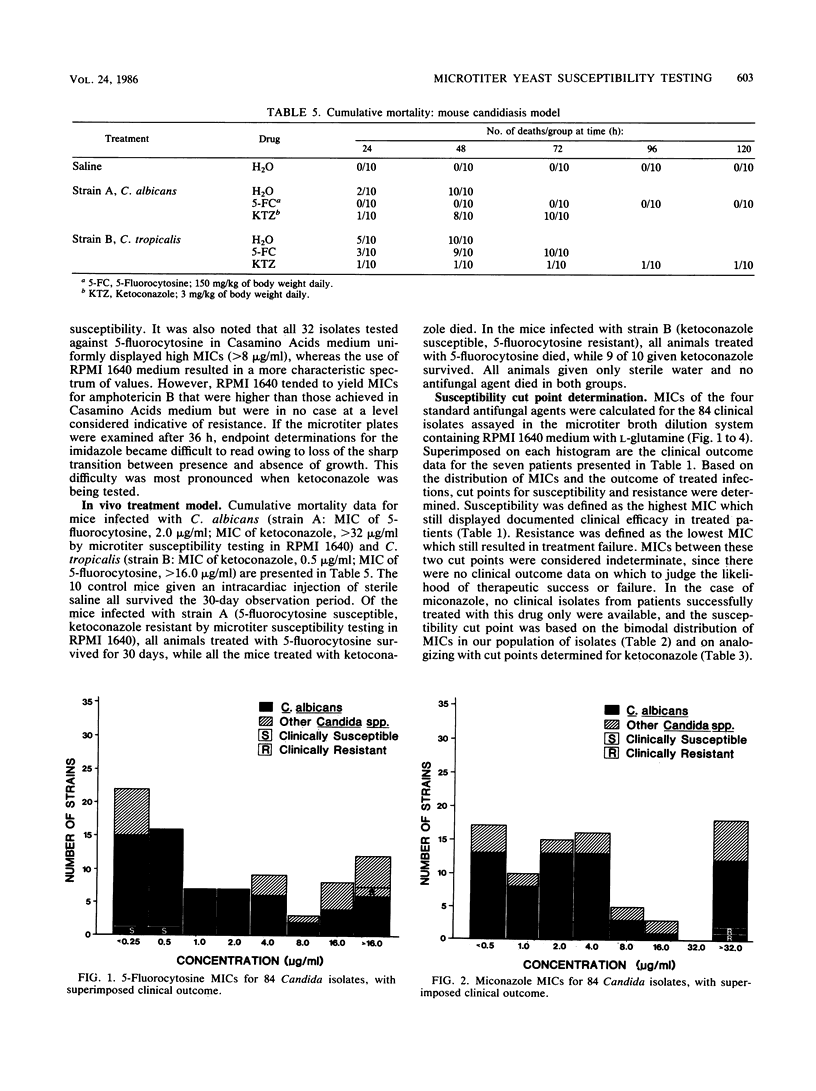
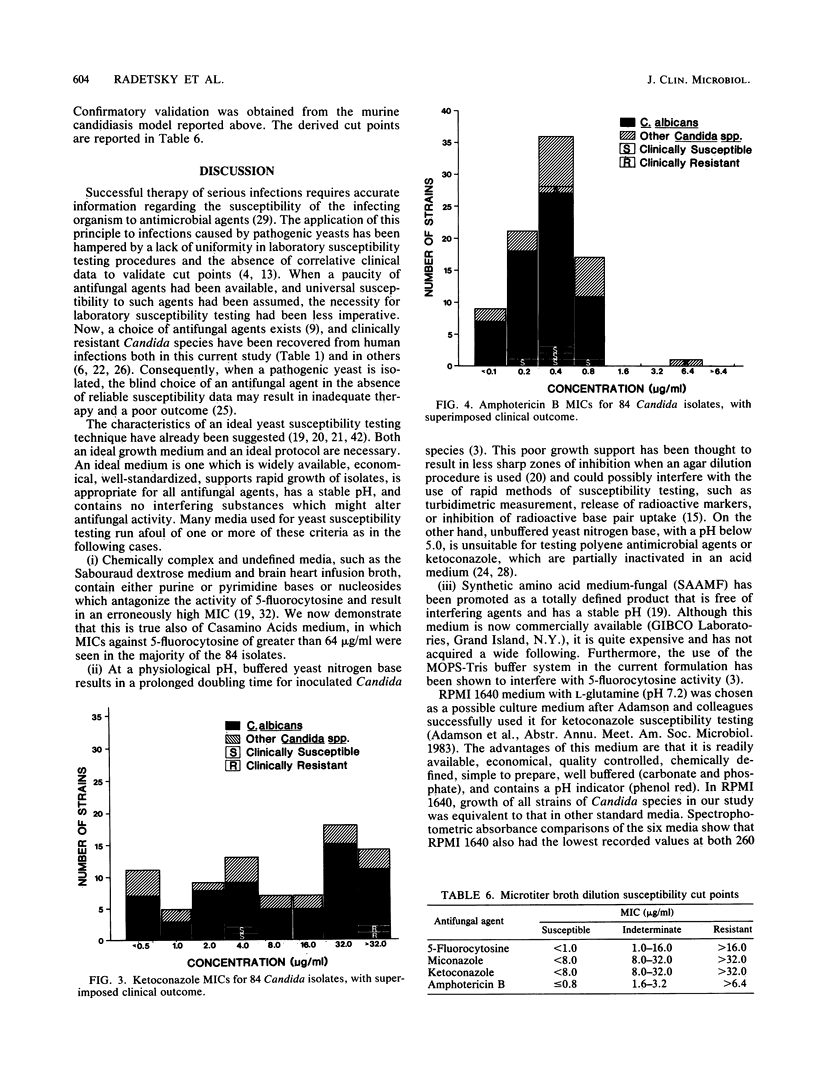
There is no ideal laboratory procedure or culture medium in current use for susceptibility testing of pathogenic yeasts. Six candidate growth media (RPMI 1640 with L-glutamine, yeast nitrogen base, Casamino Acids medium, Mueller-Hinton broth, Sabouraud dextrose broth, and minimum essential medium-Eagle salts) were screened by spectrophotometric absorbance for nucleic acid and protein. From these, two media were selected: a chemically defined growth medium (RPMI 1640 with L-glutamine) and a chemically complex medium (Casamino Acids). MICs of four antifungal agents (5-fluorocytosine, miconazole, ketoconazole, and amphotericin B) for 84 clinical isolates of various Candida species were then determined with both media in agar dilution and microtiter broth dilution systems. The resultant MICs were correlated with clinical outcome for those isolates obtained from patients treated with single antifungal agents, and susceptibility cut points were calculated. Derived MIC cut points for susceptibility were validated in a murine model of systemic candidiasis. RPMI 1640 with L-glutamine was found to have the lowest absorbance values for both nucleic acid and protein, while Casamino Acids medium was highest in both categories. We found that RPMI 1640 with L-glutamine was superior to Casamino Acids medium in the yield of MICs which correlated with actual clinical and animal outcome data. While there were no significant differences in MICs when RPMI 1640 medium was used, the microtiter broth dilution technique was superior to agar dilution in efficiency and ease of performance. We conclude that a microtiter broth system containing RPMI 1640 medium with L-glutamine is a simple, precise, and economical technique for susceptibility testing of pathogenic Candida species. We also suggest that the validation of susceptibility cut points with patient and animal outcome data make this microtiter broth system a preferential method for yeast susceptibility testing.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahearn D. G., McGlohn M. S. In vitro susceptibilities of sucrose-negative Candida tropicalis, Candida lusitaniae, and Candida norvegensis to amphotericin B, 5-fluorocytosine, miconazole, and ketoconazole. J Clin Microbiol. 1984 Mar;19(3):412–416. doi: 10.1128/jcm.19.3.412-416.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baley J. E., Kliegman R. M., Fanaroff A. A. Disseminated fungal infections in very low-birth-weight infants: clinical manifestations and epidemiology. Pediatrics. 1984 Feb;73(2):144–152. [PubMed] [Google Scholar]
- Calhoun D. L., Roberts G. D., Galgiani J. N., Bennett J. E., Feingold D. S., Jorgensen J., Kobayashi G. S., Shadomy S. Results of a survey of antifungal susceptibility tests in the United States and interlaboratory comparison of broth dilution testing of flucytosine and amphotericin B. J Clin Microbiol. 1986 Feb;23(2):298–301. doi: 10.1128/jcm.23.2.298-301.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals J. B. Tablet sensitivity testing on pathogenic fungi. J Clin Pathol. 1979 Jul;32(7):719–722. doi: 10.1136/jcp.32.7.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick J. D., Rosengard B. R., Merz W. G., Stuart R. K., Hutchins G. M., Saral R. Fatal disseminated candidiasis due to amphotericin-B-resistant Candida guilliermondii. Ann Intern Med. 1985 Jan;102(1):67–68. doi: 10.7326/0003-4819-102-1-67. [DOI] [PubMed] [Google Scholar]
- Dismukes W. E., Stamm A. M., Graybill J. R., Craven P. C., Stevens D. A., Stiller R. L., Sarosi G. A., Medoff G., Gregg C. R., Gallis H. A. Treatment of systemic mycoses with ketoconazole: emphasis on toxicity and clinical response in 52 patients. National Institute of Allergy and Infectious Diseases collaborative antifungal study. Ann Intern Med. 1983 Jan;98(1):13–20. doi: 10.7326/0003-4819-98-1-13. [DOI] [PubMed] [Google Scholar]
- Drouhet E., Dupont B. Chronic mucocutaneous candidosis and other superficial and systemic mycoses successfully treated with ketoconazole. Rev Infect Dis. 1980 Jul-Aug;2(4):606–619. doi: 10.1093/clinids/2.4.606. [DOI] [PubMed] [Google Scholar]
- Edwards J. E., Jr, Lehrer R. I., Stiehm E. R., Fischer T. J., Young L. S. Severe candidal infections: clinical perspective, immune defense mechanisms, and current concepts of therapy. Ann Intern Med. 1978 Jul;89(1):91–106. doi: 10.7326/0003-4819-89-1-91. [DOI] [PubMed] [Google Scholar]
- Ellis N. S., Bartlett M. S., Smith J. W. Assay for yeast susceptibility to 5-fluorocytosine and amphotericin B in a frozen microtiter system. Am J Clin Pathol. 1979 Aug;72(2):194–198. doi: 10.1093/ajcp/72.2.194. [DOI] [PubMed] [Google Scholar]
- Epstein J. B., Truelove E. L., Izutzu K. T. Oral candidiasis: pathogenesis and host defense. Rev Infect Dis. 1984 Jan-Feb;6(1):96–106. doi: 10.1093/clinids/6.1.96. [DOI] [PubMed] [Google Scholar]
- Galgiani J. N., Stevens D. A. Antimicrobial susceptibility testing of yeasts: a turbidimetric technique independent of inoculum size. Antimicrob Agents Chemother. 1976 Oct;10(4):721–728. doi: 10.1128/aac.10.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgiant J. N., Stevens D. A. Turbidimetric studies of growth inhibition of yeasts with three drugs: inquiry into inoculum-dependent susceptibility testing, time of onset of drug effect, and implications for current and newer methods. Antimicrob Agents Chemother. 1978 Feb;13(2):249–254. doi: 10.1128/aac.13.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E., Hoeprich P. D. Problems in the diagnosis and treatment of systemic candidiasis. J Infect Dis. 1972 Feb;125(2):190–193. doi: 10.1093/infdis/125.2.190. [DOI] [PubMed] [Google Scholar]
- Henderson D. K., Edwards J. E., Jr, Montgomerie J. Z. Hematogenous candida endophthalmitis in patients receiving parenteral hyperalimentation fluids. J Infect Dis. 1981 May;143(5):655–661. doi: 10.1093/infdis/143.5.655. [DOI] [PubMed] [Google Scholar]
- Hiemenz J., Skelton J., Pizzo P. A. Perspective on the management of catheter-related infections in cancer patients. Pediatr Infect Dis. 1986 Jan-Feb;5(1):6–11. doi: 10.1097/00006454-198601000-00002. [DOI] [PubMed] [Google Scholar]
- Hoeprich P. D., Huston A. C. Effect of culture media on the antifungal activity of miconazole and amphotericin B methyl ester. J Infect Dis. 1976 Oct;134(4):336–341. doi: 10.1093/infdis/134.4.336. [DOI] [PubMed] [Google Scholar]
- Holt R. J., Azmi A. Miconazole-resistant Candida. Lancet. 1978 Jan 7;1(8054):50–51. doi: 10.1016/s0140-6736(78)90403-8. [DOI] [PubMed] [Google Scholar]
- Holt R. J. Laboratory tests of antifungal drugs. J Clin Pathol. 1975 Oct;28(10):767–774. doi: 10.1136/jcp.28.10.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes W. T. Systemic candidiasis: a study of 109 fatal cases. Pediatr Infect Dis. 1982 Jan-Feb;1(1):11–18. [PubMed] [Google Scholar]
- Johnson B., White R. J., Williamson G. M. Factors influencing the susceptibility of Candida albicans to the polyenoic antibiotics nystatin and amphotericin B. J Gen Microbiol. 1978 Feb;104(2):325–333. doi: 10.1099/00221287-104-2-325. [DOI] [PubMed] [Google Scholar]
- Maksymiuk A. W., Thongprasert S., Hopfer R., Luna M., Fainstein V., Bodey G. P. Systemic candidiasis in cancer patients. Am J Med. 1984 Oct 30;77(4D):20–27. [PubMed] [Google Scholar]
- McDougall P. N., Fleming P. J., Speller D. C., Daish P., Speidel B. D. Neonatal systemic candidiasis: a failure to respond to intravenous miconazole in two neonates. Arch Dis Child. 1982 Nov;57(11):884–886. doi: 10.1136/adc.57.11.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa H., Kitaura K., Nakamizo N. Effects of pH on the activity of ketoconazole against Candida albicans. Antimicrob Agents Chemother. 1983 Jan;23(1):105–107. doi: 10.1128/aac.23.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R., Durack D. T. Comparison of amphotericin B and N-D-ornithyl amphotericin B methyl ester in experimental cryptococcal meningitis and Candida albicans endocarditis with pyelonephritis. Antimicrob Agents Chemother. 1985 Dec;28(6):751–755. doi: 10.1128/aac.28.6.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson M., Winblad B., Anséhn S., Schönebeck J. Pathogenicity in mice of strains of Candida albicans with different in vitro sensitivity to 5-fluorocytosine. Scand J Infect Dis. 1978;10(1):87–90. doi: 10.3109/inf.1978.10.issue-1.19. [DOI] [PubMed] [Google Scholar]
- Polak A., Scholer H. J. Fungistatic activity, uptake and incorporation of 5-fluorocytosine in Candida albicans, as influenced by pyrimidines and purines. I. Reversal experiments. Pathol Microbiol (Basel) 1973;39(2):148–159. doi: 10.1159/000162642. [DOI] [PubMed] [Google Scholar]
- Ryley J. F., Wilson R. G., Barrett-Bee K. J. Azole resistance in Candida albicans. Sabouraudia. 1984;22(1):53–63. [PubMed] [Google Scholar]
- Saubolle M. A., Hoeprich P. D. Disk agar diffusion susceptibility testing of yeasts. Antimicrob Agents Chemother. 1978 Oct;14(4):517–530. doi: 10.1128/aac.14.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smego R. A., Jr, Perfect J. R., Durack D. T. Combined therapy with amphotericin B and 5-fluorocytosine for Candida meningitis. Rev Infect Dis. 1984 Nov-Dec;6(6):791–801. doi: 10.1093/clinids/6.6.791. [DOI] [PubMed] [Google Scholar]
- Stiller R. L., Bennett J. E., Scholer H. J., Wall M., Polak A., Stevens D. A. Correlation of in vitro susceptibility test results with in vivo response: flucytosine therapy in a systemic candidiasis model. J Infect Dis. 1983 Jun;147(6):1070–1077. doi: 10.1093/infdis/147.6.1070. [DOI] [PubMed] [Google Scholar]
- Stiller R. L., Bennett J. E., Scholer H. J., Wall M., Polak A., Stevens D. A. Susceptibility to 5-fluorocytosine and prevalence of serotype in 402 Candida albicans isolates from the United States. Antimicrob Agents Chemother. 1982 Sep;22(3):482–487. doi: 10.1128/aac.22.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud I. J., Feingold D. S. Heterogeneity of action of mechanisms among antimycotic imidazoles. Antimicrob Agents Chemother. 1981 Jul;20(1):71–74. doi: 10.1128/aac.20.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno J., Shigematsu M. L., Arai T. Primary site of action of ketoconazole on Candida albicans. Antimicrob Agents Chemother. 1982 Jun;21(6):912–918. doi: 10.1128/aac.21.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Bossche H., Willemsens G., Van Cutsem J. M. The action of miconazole of the growth of Candida albicans. Sabouraudia. 1975 Mar;13(Pt 1):63–73. [PubMed] [Google Scholar]



