Abstract
Objective
To determine the frequency of the mutant pyruvate kinase (PK) allele, haematological parameters and AB blood types of Abyssinian and Somali cats in Australia.
Design
Complete blood cell and reticulocyte counts, DNA PK mutation testing and blood typing were performed in all cats.
Results
A total of 60 cats (36 Abyssinians, 24 Somalis) were included (37 females, 23 males). For the mutant PK allele, three female Somalis were homozygous (affected, 5%), 17 cats were heterozygous (carrier, 28%) and 40 cats tested negative (normal, 67%). Pedigree analysis revealed common ancestry of affected and many carrier cats. Of affected cats, two had regenerative anaemias and all had reticulocytosis (range 64-390 × 109/L; P < 0.001 compared with normal or carrier cats). The only consistent historical sign was lethargy. One affected cat was euthanased 18 months after testing, because of anaemia, neutropenia, anorexia and weight loss. The mutant allele frequency was 0.19 overall (0.29 in Somalis, 0.13 in Abyssinians). All cats had blood type A. The commercial blood typing card method incorrectly identified 12 cats as having type AB blood.
Conclusions
The frequency of the mutant PK allele is high in Australia. Screening for PK deficiency is indicated before mating and in individual cats of these breeds, even in the absence of anaemia and especially when there is reticulocytosis. Although all cats in the present study had blood type A, blood type B is common in these breeds worldwide. Retyping of any AB typed cats by a laboratory technique is recommended.
Keywords: cats, blood type, congenital, red blood cell abnormality, feline, haemolytic anaemia, neonatal isoerythrolysis, pyruvate kinase
Pyruvate kinase (PK) deficiency, a glycolytic erythroenzymopathy, was first documented in humans in the early 1960s,1 in basenji and beagle dogs in the 1970s2,3 and in Abyssinian and Somali cats in the early 1990s.4,5 Because erythrocytes lack mitochondria, they rely on anaerobic glycolysis for energy production. PK catalyses one of the two major steps of ATP production in red blood cells (RBC) through conversion of phosphoenolpyruvate to pyruvate. ATP is essential for RBC functions, including maintenance of shape and deformability, membrane transport and metabolic functions. PK deficiency thus results in ATP depletion, with reduced erythrocyte survival and haemolytic anaemia.6-9
PK deficiency in Abyssinian and Somali cats (also seen rarely in domestic shorthair cats) is caused by a splicing defect at the 3′ end of exon 6 of the R/L-PK gene, resulting in a 13-base-pair deletion.5 The mode of inheritance of PK deficiency is autosomal recessive. To date, reports on the prevalence of this disease in cats have been confined to the United States and Europe.7,10,11 PK deficiency has been previously reported in one Australian Abyssinian cat,12 but the extent of the problem in this country is unknown. The primary aim of the present study was to determine the prevalence of the mutant PK allele in a population of Abyssinian and Somali cats in Australia.
The AB blood group system is of major importance in cats because AB mismatched transfusions can result in life-threatening haemolytic transfusion reactions.13-15 Furthermore, in breeds of cats with type B blood, kittens from matings between type B queens and type A toms are at risk of neonatal isoerythrolysis (NI). NI results from the absorption of anti-A alloantibodies from the colostrum or milk of type B queens by their type A or AB offspring during the first day of life.13 The frequency of types A, B and AB varies geographically and among breeds.13,14 Although in some countries domestic crossbred or non-pedigree cats are exclusively type A, surveys of domestic crossbred cats in Brisbane in 198115 and Sydney in 200516 indicated a type B frequency of 27% and 36%, respectively. Type AB occurs rarely worldwide and only in breeds in which type B cats occur (< 1%). Based upon large surveys in the United States, the reported frequency of type B blood in Abyssinians and Somalis is approximately 16% and 18%, respectively.14 Published data on blood types are available for only 18 Abyssinian and no Somalis in Australia.16 A secondary aim of this study was therefore to determine the prevalence of the AB blood type in these breeds and thus the risk of haemolytic transfusion reactions and NI.
Materials and methods
Cats were recruited into the study from the Abyssinian Cat Club of Australasia. Approval for this study was granted by The University of Sydney Animal Care and Ethics Committee. Any cat was eligible unless it or both its parents had been previously tested for PK deficiency. All cats were scanned for the presence of a microchip and pedigrees (required minimum of four generations) were matched with the microchips. After physical examination, 1.5 to 2 mL of blood was collected by jugular venipuncture into EDTA tubes. Sampling was carried out at the Valentine Charlton Cat Centre, University of Sydney, New South Wales, Australia. If distance precluded this, EDTA blood samples were taken by primary care veterinarians and transported on ice to the Centre within 20 h of collection.
PK deficiency testing
Samples were stored at -80°C prior to testing. Approximately 0.5 to 1 mL of EDTA blood from each cat was air-freighted at room temperature to the Josephine Deubler Genetics Laboratory at the University of Pennsylvania (PennGen) for PK DNA testing as previously described.5
Haematology and blood typing
A complete blood cell count using an automated haematology analyser (Sysmex K-4500 TOA Medical Electronics Co., Kobe, Japan), manual aggregate reticulocyte count of a brilliant cresylblue-stained blood smear and blood typing was performed on all samples within 24 h of collection. Blood typing was performed using commercially available card test kits (Rapid Vet-H [Feline], DMS and Agrolab products, Turino, Italy) according to the manufacturer's instructions and as previously described.17 In samples with marked autoagglutination, rouleaux formation or inconclusive results, blood typing was also performed by slide agglutination. Briefly, 0.2 mL of washed packed RBCs was resuspended in 4.8 mL of normal saline. One drop of the packed RBC suspension was mixed with one drop of type A plasma (pooled from two type A blood donor cats) on one slide and with one drop of type B plasma on another slide. Slides were examined macroscopically and microscopically for agglutination at 2 to 5 min. For each test, blood samples from the known type A and type B cats were used as positive controls. A third gel-based blood typing technique (DiaMed, Switzerland) was performed,14,17 using EDTA blood samples drawn at a later date in cats typed as type AB by the card typing kit. This novel method only became available in a commercial laboratory in Australia (Symbion Health, Sydney) after completion of the original typing study. A serum biochemical profile and feline leukaemia virus (FeLV) antigen and feline immunodeficiency virus (FIV) antibody testing (Simplify, AGEN) was performed in any cats testing positive for PK deficiency.
Statistical analysis
Descriptive statistics were calculated using a commercial statistics software package (GenStat for Windows Release 9, Lawes Agricultural Trust, 2006). Haematological data, apart from reticulocyte counts, were analysed using a linear mixed model implemented using residual maximum likelihood (REML) by ASReml-W (VSN International Ltd, UK, 2006). This method was selected because it allowed for the correction for signalment characteristics such as gender and age. The relationship between PK genotype and reticulocytosis was analysed by ordinal logistic regression, based on three genotypic classes (Genstat 9). The pedigree was drawn with the assistance of computer software, PEDRAW,18 and inspected manually for consistency with the observed PK genotypes. A chi-squared test was used to test whether the observed frequencies of genotypes were significantly different from those expected in a population in Hardy-Weinberg equilibrium, taking into account that the population was small and not randomly distributed.
Results
PK testing
A total of 63 cats were recruited into the study, including 40 cats examined at the Valentine Charlton Cat Centre and 23 other cats that had blood collected by primary care veterinarians. Three cats were excluded because of unsuccessful venipuncture (n = 1) and because pedigree analysis revealed both parents had already been recruited to the study (n = 2). The study population included cats from 9 of 24 breeding households in Australia registered with the Abyssinian Cat Club of Australasia, with a range of 2 to 11 cats per cattery and five pet households, with 1 to 3 cats per household. The 60 cats tested included 37 females (29 entire, 8 neutered) and 23 males (21 entire, 2 neutered). There were 32 cats from New South Wales (22 Abyssinians, 10 Somalis), 9 cats from the Australian Capital Territory (5 Abyssinians, 4 Somalis), 7 Somalis from Queensland, 6 cats from Victoria (3 Abyssinians, 3 Somalis) and 6 Abyssinians from Western Australia.
Overall, 5% of the cats were homozygous, 28% heterozygous and 67% were negative for the mutant PK allele (Table 1). The three affected cats were females (2 entire multiparous; Table 2, affected cats 1 and 2) and one non-bred, neutered cat (Table 2, affected cat 3) of young age (2-3 years old) that had originated from Queensland or NSW. Although all three affected cats were Somalis, there were nine heterozygous Abyssinian and eight Somali cats with representation from each Australian region tested except for the Australian Capital Territory (Table 1).
Table 1.
Pyruvate kinase mutation analysis of Abyssinian and Somali cats in Australia, including frequency of the normal (wild-type) allele p, and mutant (variant) allele q
| Breed | Homozygous (affected) |
Heterozygous (carriers) |
Normals (negative) |
Total | Allele frequency |
||||
|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | p | q | ||
| Abyssinian | 0 | 0 | 3 | 6 | 11 | 16 | 36 | 0.87 | 0.13 |
| Somali | 0 | 3 | 6 | 2 | 3 | 10 | 24 | 0.71 | 0.29 |
| Total/together | 3 (5%) | 17 (28%) | 40 (67%) | 60 | 0.81 | 0.19 | |||
Table 2.
Serial haematological findings in three PK-deficient Somali cats (cats 1 -3) and mean haematological data from affected, carrier and normal cats
| Affected cat 1 | Affected cat 2 | Affected cat 3 | Affected cats (n = 3) | Carrier cats (n = 17) | Negative cats (n = 40) | Reference range | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Months from diagnosis | 0 | 4 | 17 | 0 | 4 | 17 | 0 | 4 | 17 | ||||
| Hct L/L | 0.19 | 0.25 | 0.21 | 0.24 | 0.29 | 0.31 | 0.34 | 0.37 | 0.37 | 0.26* | 0.38 | 0.37 | 0.30-0.45 |
| Hb g/L | 61 | 74 | 69 | 81 | 91 | 87 | 113 | 113 | 111 | 85* | 131 | 131 | 80-140 |
| RBC × 1012/L | 3.0 | 3.9 | 35 | 5.3 | 5.3 | 5.4 | 6.7 | 6.8 | 6.5 | 5* | 8.6 | 8.4 | 6-10 |
| MCVfL | 63 | 64 | 60 | 46 | 55 | 57 | 51 | 55 | 57 | 53* | 45 | 45 | 40-45 |
| MCHpg | 20 | 19 | 20 | 15 | 17 | 16 | 17 | 17 | 17 | 17 | 15 | 15 | 13-17 |
| MCHC g/L | 321 | 296 | 329 | 338 | 314 | 281 | 332 | 302 | 299 | 330 | 343 | 346 | 310-350 |
| Aggregate Reticulocyte % | 13 | 3.5 | 0 | 1.8 | 1.2 | 2 | 5 | ND | 6 | 6.6 | 0.35 | 0.35 | |
| Aggregate Reticulocyte count × 109/L | 390 | 136 | 0 | 95 | 64 | 108 | 334 | ND | 325 | 273 | 28 | 30 | <60 |
| Platelets × 109/L | 400 | 341 | AD | AD | 629 | AD | 453 | 473 | 497 | AD | AD | AD | 300-700 |
| Leukocytes × 109/L | 20.1 | 10.9 | 7.3 | 29.5 | 10.4 | 8 | 8.8 | 7.9 | 7.6 | 19.5* | 16.9* | 13.9 | 8-14 |
| Neutrophils × 109/L | 5.6 | 4.1 | 1.2 | 14.5 | 4.1 | 4.5 | 6.3 | 4.8 | 3 | 8.8 | 10.9* | 9.1 | 3.76-10.8 |
| Lymphocytes × 109/L | 13.3 | 5.9 | 6.1 | 10.3 | 3 | 2 | 1.8 | 2.8 | 4.1 | 8.5 | 4.1 | 3.1 | 1.6-7 |
| Monocytes × 109/L | 0.4 | 0.3 | <0.1 | 0.9 | 0.4 | 0.2 | 0.1 | 0.1 | 0.2 | 0.5 | 0.6 | 0.5 | 0.08-0.56 |
| Eosinophils × 109/L | 0.8 | 0.5 | <0.1 | 3.83 | 2.8 | 0.7 | 0.62 | 0.2 | 0.4 | 1.75 | 1.28 | 1 | 0.16-1.4 |
| Total protein g/L | 89 | ND | ND | 79 | ND | ND | 82 | ND | ND | 83 | 79 | 79 | 59-78 |
| Bilirubin μmol/L | 5.8 | ND | ND | 6.9 | ND | ND | 7 | ND | ND | 6.6 | ND | ND | 1-3.5 |
| E | A | A | |||||||||||
Significant difference from normal genotypes for this parameter.
A, alive; AD, adequate; E, euthanased; Hb, haemoglobin; Hct, haematocrit; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; MCV, mean corpuscular volume; ND, not done; PK, pyruvate kinase; RBC, red blood cells.
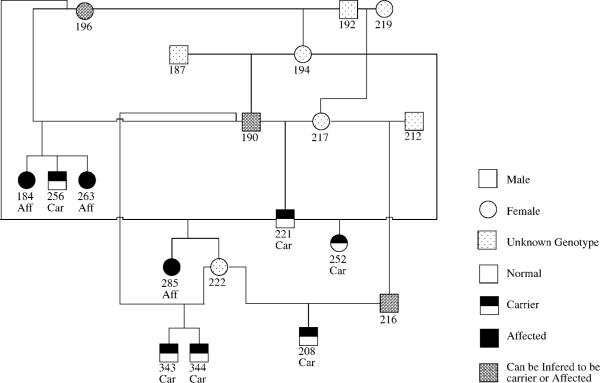
Pedigree analysis revealed common ancestry of all affected and carrier cats within five generations (Figure 1). Two PK-deficient Somalis were full siblings, and carriers were identified in 16 different litters from 15 queens and 14 toms. The observed distributions of affected, carrier and negative Somali and Abyssinian cats studied were not significantly different from the proportions expected of a population in Hardy-Weinberg equilibrium (chi-squared = 0.44; P = 0.51). The mutant allele frequency was 0.29 in the Somali group, 0.13 in the Abyssinian group and 0.19 overall (Table 1).
Figure 1.
Pedigree analysis showing common ancestry of affected and carrier Somali cats.
At the time of initial testing, two of the PK-deficient Somalis had regenerative anaemia and all three had reticulocytosis, but the mean haematological values for negative and carrier cats were within reference intervals (Table 2). For the haematological parameters (RBC count, haematocrit, mean corpuscular volume (MCV), reticulocyte count and haemoglobin concentration), there was a significant difference in the effect of PK genotype. RBC count (P < 0.001), haematocrit (P = 0.007) and haemoglobin concentration (P < 0.001) were significantly lower, whereas reticulocyte count (P = 0.002) and MCV (P < 0.001) were significantly higher in affected cats compared with normal and carrier cats. There were no significant differences between normal and carrier cats in the effect of PK genotype for the same RBC parameters (Table 2).
Both carrier and affected cats had significantly higher mean white blood cell counts than normal cats (P = 0.003), but there was no significant difference between carrier and affected cats. The neutrophil count of carriers was significantly higher than that of normal cats (P = 0.011), but not higher than PK-deficient cats. Affected cats did not have significantly greater neutrophil counts than normal cats. Two of the affected cats were documented to have had one episode of neutropenia (Table 2). For lymphocytes, monocytes and eosinophils, there were no significant differences between the PK genotypes.
Of the 60 cats in the study, 6 were anaemic with a haematocrit ranging from 0.24 to 0.29 L/L (reference range 0.30-0.45 L/L); 2 of the anaemic cats had PK deficiency and reticulocytosis, and the other 4 cats, including 3 carriers and a PK-negative cat, all had non-regenerative anaemia based on aggregate reticulocyte counts. Excluding cats with PK deficiency, aggregate reticulocyte counts were elevated (> 60 × 109/L) in 9 cats (7 Abyssinians, 2 Somalis) without anaemia (mean 91 × 109/L, median 78 × 109/L, range 62-118 × 109/L). The mean MCV in cats with reticulocytosis was 45.4 ± 3.3 fL (reference range 40-45 fL), and both the mean and median total plasma protein was 78 g/L, which was elevated (reference range 54-73 g/L), but was not significantly different to the mean and median of total plasma protein for the population as a whole (80 and 79 g/L, respectively). Abdominal palpation was performed at the time of blood sampling and mild splenomegaly was detected in one PK-negative cat, which had the highest reticulocyte count. Excluding cats with PK deficiency, there was no significant difference in the PK genotype (normal or carrier) between cats with reticulocytosis and cats without reticulocytosis (P = 0.5).
Physical examination of the three cats with PK deficiency was normal and splenomegaly was not detected on abdominal palpation. Serology for FIV antibody and for FeLV antigen was negative, and serum biochemical profiles were normal, except for mild hyperbilirubinaemia and hyperproteinaemia (Table 2). There was no significant difference in the heart rate, respiratory rate, rectal temperature, body weight or body condition score of affected cats compared with PK negative or carrier cats. The only consistent sign observed by owners before and after diagnosis of PK deficiency was lethargy. The two affected entire cats were spayed after diagnosis. Although two of the affected cats remained clinically normal for the entire observation period (2 years post diagnosis), cat 1 was euthanased at 4 years of age because of severe weight loss, anorexia and lethargy. The cat had had non-regenerative anaemia, neutropenia, monocytopenia and eosinopenia (Table 2). Clinical evaluations and treatments were limited. Postmortem was not performed.
Blood typing
All 60 cats were ultimately found to have blood type A, but difficulties were encountered when using the typing card kits; 16 cats (10 Abyssinians, 6 Somalis) were initially typed as AB and 44 as type A. However, microscopic rouleaux formation was detected in four cases of type AB. Rouleaux was abolished by saline dilution of the blood samples (1:4 ratio blood:saline) and all four cats were retyped as type A with the card kits. Blood type was also confirmed as type A using the slide agglutination method in these four cats. For the remaining 12 cats with type AB, saline dilution made no difference to the blood typing result with the card kit. However, when using the slide agglutination test, all 12 were type A. There were strong agglutination reactions with the anti-A reagent and none with the anti-B reagent. Blood was available for typing using a third method (the gel column technique) in 11 of the 12 cats, and all 11 were found to be type A; 11 of these 12 cats were negative for the mutant PK allele and 1 was a carrier.
Discussion
The frequency of the mutant PK allele in this survey of Australian Abyssinian and Somali cats in Australia was 0.19, which is high; three Somalis (5%) were PK deficient, and 25% of the Abyssinian and 33% of the Somali cats tested were carriers. This is similar to the prevalence of PK-deficient (7.4-8.5%) and carrier cats (23.2-30.8%) reported in two small European surveys10,11 and to the results of large unpublished surveys (> 3000 cats tested) in the USA and Europe (U Giger, personal communication).7-9 It was not unexpected to find a similar distribution of the mutant PK allele on a third continent, because the Australian population of Abyssinian and Somali cats arose from the importation of these breeds from the US and Europe.
The population sampled included cats from many major breeders in Australia, but was not randomly selected and thus may not reflect the true prevalence of the mutant PK allele in the wider population. Breeders who had previously detected carriers in their cattery may have been more likely to participate in the study, which may have biased this and the other reported surveys toward the mutant allele. Interestingly, the only other PK-deficient cat reported from Australia was also a Somali.12
The availability of a DNA test for the mutant PK allele permits early diagnosis of PK deficiency and thus informed breeding is possible. Screening of any Abyssinian and Somali cat for PK deficiency is recommended until the parents are known to be negative for the mutation. However, reducing the frequency of the mutant allele in a small breeding population, such as that present in Australia, should not be done abruptly or in isolation for one trait. Loss of genetic diversity could result in selection of other hereditary diseases. In order to reduce the frequency of the mutant allele, and because of the health risks to a PK-deficient queen or tom, affected cats should not be used for breeding. However, if only affected cats are excluded from the breeding pool, the high frequency of the mutant allele will only be slightly reduced. Affected cats would still be produced if carrier cats are allowed to breed. To both eliminate the expression of PK deficiency and to reduce the frequency of the mutant allele, all animals intended for breeding should be tested for PK deficiency.
Carrier cats should be permitted only to breed with cats known to have a normal genotype. This will prevent the birth of affected kittens, but will continue to produce carrier kittens. To reduce the frequency of the mutant allele in the population, cats negative for the mutant PK allele should be recruited into the breeding population in preference to carrier animals, where possible within the scope of other breeding objectives. Once the mutant allele frequency has fallen sufficiently to allow for the safe exclusion of all carrier animals from the population, the mutant allele can be eliminated within a generation by breeding from only PK-negative cats.
PK status should not be the sole selection criterion in a breeding program. Both Abyssinian and Somali breeds have been recognised as having predispositions to other diseases, including another inherited erythrocyte defect known as erythrocytic osmotic fragility (OF),19 progressive retinal atrophy,20 patella luxation,21,22 renal amyloidosis23 and a recently described glomerular disease.24
Clinical signs in cats with PK deficiency range from non-specific signs such as lethargy and inappetence to pallor, jaundice and collapse from severe episodic haemolytic anaemia.4,7-9 In the present study, the macrocytic normo- to hypochromic regenerative anaemia in the two anaemic affected cats, low normal haematocrit in the other affected cat and mild hyperbilirubinaemia in all three PK-deficient cats were typical for feline PK deficiency.7,8,11 Haemolytic anaemia has been reported in affected cats as young as a few months of age.7,8 It is likely that haemolytic crises are precipitated by concurrent infection or toxin exposure and only the most severe crises are recognised by owners. In contrast to increased OF, which is characterised by intermittent severe haemolytic anaemia and marked splenomegaly, cats with PK deficiency typically have no or only mild splenomegaly.9,19
Leucopenia has been reported occasionally in cats with PK deficiency.10,12 Two of the affected cats in the present study had neutropenia, including one that developed pancytopenia. The cause of the cytopenia was not identified by us or in the previous reports and may not be related to PK deficiency. Even in dogs, which are the only species with PK deficiency that reportedly develop myelofibrosis and osteosclerosis, leucocyte counts are generally normal or slightly increased with a mature neutrophilia.6,7 The reason for the higher leucocyte count in carrier and affected cats compared with normal cats is unclear, and in the present study could be spurious because of the small numbers of cats tested, or relate to higher stress levels during blood collection.
Bilirubin cholelithiasis, a consequence of chronic haemolysis, has been reported rarely in cats with PK deficiency, sometimes with concurrent cholangitis.12,25,26 Chronic iron overload in dogs and humans with PK deficiency can result in haemosiderosis (increased deposition of tissue iron), which most commonly manifests as a hepatopathy.6,27 Fibrotic organ damage is thought to be mediated by free radical formation in the presence of free iron, which occurs when the storage capacity of the liver is exceeded. Death from anaemia and liver failure before 8 years of age is typical in dogs with PK deficiency.9 Haemochromatosis was recently described in a cat with PK deficiency that died at 2.7 years of age.11 In affected cats in which the course of disease has been followed, owners consider the cats to be often asymptomatic. No liver biopsy or necropsy information was obtained from the three affected cats in this study. Age of death in affected cats has been reported from 1 to 13 years.8,11
Mild to moderate reticulocytosis was seen consistently in PK-deficient cats in this study and in other reports,4,7-11 although one of the cats in this survey developed a terminal cytopenia. The finding of an elevated reticulocyte count in Somali and Abyssinian cats, even in the absence of anaemia, should alert practitioners to the possibility of PK deficiency. However, mild reticulocytosis was also common in PK-negative and carrier cats in this study. The cause of the mild reticulocytosis in the nine cats that did not have PK deficiency was not identified. Pedigree analysis was not suggestive of a hereditary cause and these cats did not have typical features of increased OF. Abyssinian and Somali cats reported with increased OF were anaemic at the time of diagnosis.19 Also, the instrument-derived MCV in those cats was markedly elevated, likely in part because of agglutination. Moreover, hyperglobulinaemia, lymphocytosis and marked splenomegaly were commonly observed. In cats with increased OF, the lysis curve is markedly shifted toward higher saline concentrations, compared with the lysis curve for control cats. Although a specific OF test of erythrocytes was not performed in any cats in the present study, gross haemolysis of EDTA blood samples was not observed after overnight refrigeration, which is a typical finding in samples from OF cats.9,19
All Abyssinian and Somali cats in this study had type A blood, which is interesting because in a large prevalence study from the US 16% and 18% of Abyssinian and Somali cats, respectively, had type B blood, the remainder were almost exclusively type A and type AB was rare.13,14 When evaluating the results with the typing card and comparing them with either the slide agglutination or gel column technique, 20% (n = 12) of cats were falsely considered to have type AB blood. Autoagglutination was minimal on the slide, but could have resulted in false agglutination in the B-well even when the autoagglutination control well was negative. Rouleaux was not the cause, because the addition of saline to EDTA blood did not change the card result in these 12 samples. Moreover, repeat typing with either the slide or gel column method using RBC suspensions revealed unambiguously that these cats had type A blood. Finally, it would be unexpected to see any AB cats in the absence of type B cats, as expression of both antigens is recessive to the `a' allele.28 Interestingly, in one other study of 11 Abyssinian and Somali cats that were blood typed using the same card method, three were typed as AB,29 but those results were not confirmed. It has been recommended to retype any type AB cat of any breed using RBCs washed three times with saline and/or a laboratory method.7-9,14,28
Although no cats of blood type B were identified in the present study, the risk of NI for these two breed populations in Australia cannot be inferred to be negligible. In the only other study to record blood types in 18 Abyssinian cats in Australia, 16 had type A blood and 2 had type B blood.16 Therefore, it is prudent to type Abyssinian and Somali cats prior to breeding or transfusions to assure blood compatibility. After completion of the present study, some breeders volunteered information, including records from Abyssinian Cat Club of Australasia newsletters, that active selection against the type B genotype occurred in the early 1990s in an attempt to eliminate problems with NI, which had been reported in Abyssinians in the US in 1991.13
In conclusion, the mutant PK allele frequency is high in Abyssinian and Somali cats in Australia, similar to that observed in the United States and in Europe. Therefore, DNA screening of these breeds is indicated, especially when there is reticulocytosis, even in the absence of anaemia or clinical signs, and prior to breeding. The frequency of blood type B in Abyssinian and Somali cats may be lower in Australia because breeders may have selected against such cats; however, AB blood typing of all Abyssinian and Somali cats and retyping by a commercial laboratory gel column technique of any AB typed cats is recommended prior to transfusion and breeding to avoid acute haemolytic transfusion reactions and NI.
Acknowledgements
The authors wish to thank members of the Abyssinian Cat Club of Australasia for participating in this study, staff of the Valentine Charlton Cat Centre and Veterinary Pathology Diagnostic Services, David Snow from Symbion Health and veterinarians who were generous with their time and assistance, especially Drs Isabelle Resch and Stephen Rose. The study was funded by the Dorothy E Penny bequest of the Faculty of Veterinary Science, University of Sydney and by NIH RR02512.
Glossary
Abbreviations
- FeLV
feline leukaemia virus
- FIV
feline immunodeficiency virus
- MCV
mean corpuscular volume
- NI
neonatal isoerythrolysis
- OF
osmotic fragility
- PK
pyruvate kinase
References
- 1.Valentine WN, Tanaka KR, Miwa S. A specific erythrocyte glycolytic enzyme defect (pyruvate kinase) in three subjects with congenital non-spherocytic haemolytic anaemia. Trans Assoc Am Physicians. 1961;74:100–110. [PubMed] [Google Scholar]
- 2.Searcy GP, Miller DR, Tasker JB. Congenital haemolytic anaemia in the basenji dog due to erythrocyte pyruvate kinase deficiency. Can J Comp Med. 1971;35:67–70. [PMC free article] [PubMed] [Google Scholar]
- 3.Prasse KW, Crouser D, Beutler E, Walker M, Schall WD. Pyruvate kinase deficiency anemia with terminal myelofibrosis and osteosclerosis in a beagle. J Am Vet Med Assoc. 1975;166:1170–1175. [PubMed] [Google Scholar]
- 4.Ford S, Giger U, Duesberg C, Beutler E, Wang P. Inherited erythrocyte pyruvate kinase (PK) deficiency causing haemolytic anaemia in an Abyssinian cat (Abstract) J Vet Intern Med. 1992;6:123. [Google Scholar]
- 5.Giger U, Rajpurohit Y, Wang P, et al. Molecular basis of erythrocyte pyruvate kinase (R-PK) deficiency in cats (Abstract) Blood. 1997;90(Suppl):5b. [Google Scholar]
- 6.Harvey JW. Pathogenesis, laboratory diagnosis, and clinical implications of erythrocyte enzyme deficiencies in dogs, cats and horses. Vet Clin Pathol. 2006;35:144–156. doi: 10.1111/j.1939-165x.2006.tb00108.x. [DOI] [PubMed] [Google Scholar]
- 7.Giger U. Erythrocyte phosphofructokinase and pyruvate kinase deficiencies. In: Feldman BF, Zinkl JG, Jain NC, editors. Schalm's veterinary hematology. 5th edn. Lippincott Williams & Wilkins; Philadelphia: 2000. pp. 1020–1025. [Google Scholar]
- 8.Giger U. Hereditary erythrocyte disorders. In: August JR, editor. Consultations in feline medicine. 4th edn. WB Saunders; Philadelphia: 2001. pp. 484–489. [Google Scholar]
- 9.Giger U. Regenerative anemias caused by blood loss. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary internal medicine. 6th edn Vol 2. Saunders Elsevier; Philadelphia: 2005. pp. 1886–1907. [Google Scholar]
- 10.Kohn B, Fumi C, Seng A, Giger U. Anaemia due to erythrocytic pyruvate kinase deficiency in Somali and Abyssinian cats in Germany. Kleintierpraxis. 2005;50:305–312. [Google Scholar]
- 11.Kohn B, Fumi C. Clinical course of pyruvate kinase deficiency in Abyssinian and Somali cats. J Feline Med Surg. 2008;10:145–153. doi: 10.1016/j.jfms.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansfield CS, Clark P. Pyruvate kinase deficiency in a Somali cat in Australia. Aust Vet J. 2005;83:483–485. doi: 10.1111/j.1751-0813.2005.tb13298.x. [DOI] [PubMed] [Google Scholar]
- 13.Giger U, Bucheler J, Patterson DF. Frequency and inheritance of A and B blood types in feline breeds of the United States. J Hered. 1991;82:15–20. doi: 10.1093/jhered/82.1.15. [DOI] [PubMed] [Google Scholar]
- 14.Giger U. Blood-typing and crossmatching. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XIV. Saunders Elsevier; Missouri: 2009. pp. 260–265. [Google Scholar]
- 15.Auer L, Bell K. The AB blood group system of cats. Anim Blood Groups Biochem Genet. 1981;12:287–297. doi: 10.1111/j.1365-2052.1981.tb01561.x. [DOI] [PubMed] [Google Scholar]
- 16.Malik R, Griffin DL, White JD, et al. The prevalence of feline A/B blood types in the Sydney Region. Aust Vet J. 2005;83:38–44. doi: 10.1111/j.1751-0813.2005.tb12190.x. [DOI] [PubMed] [Google Scholar]
- 17.Stieger K, Palos H, Giger U. Comparison of various blood-typing methods for the feline AB blood group system. Am J Vet Res. 2005;66:1393–1399. doi: 10.2460/ajvr.2005.66.1393. [DOI] [PubMed] [Google Scholar]
- 18.Curtis D. A program to draw pedigrees using LINKAGE or LINKSYS data files. Ann Hum Genet. 1990;54:365–367. doi: 10.1111/j.1469-1809.1990.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 19.Kohn B, Goldschmidt M, Hohenhaus A, Giger U. Anaemia, splenomegaly, and increased OF of erythrocytes in Abyssinian and Somali cats. J Am Vet Med Assoc. 2000;217:1483–1491. doi: 10.2460/javma.2000.217.1483. [DOI] [PubMed] [Google Scholar]
- 20.Menotti-Raymond M, David VA, Schäffer AA, et al. Mutation in CEP290 discovered for cat model of human retinal degeneration. J Hered. 2007;98:211–220. doi: 10.1093/jhered/esm019. [DOI] [PubMed] [Google Scholar]
- 21.Engvall E, Bushnell N. Patellar luxation in Abyssinian cats. Feline Pract. 1990;18:20–22. [Google Scholar]
- 22.Smith GK, Langenbach A, Green PA, et al. Evaluation of the association between medial patellar luxation and hip dysplasia in cats. J Am Vet Med Assoc. 1999;215:40–45. [PubMed] [Google Scholar]
- 23.Boyce JT, DiBartola SP, Chew DJ, Gasper PW. Familial renal amyloidosis in Abyssinian cats. Vet Pathol. 1984;21:33–8. doi: 10.1177/030098588402100106. [DOI] [PubMed] [Google Scholar]
- 24.White JD, Norris JM, Bosward KL, et al. Persistent haematuria and proteinuria in related Abyssinian cats. J Fel Med Surg. 2008;10:219–229. doi: 10.1016/j.jfms.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey AM, Holt PE, Barr FJ, Rizzo F, Tasker S. Treatment and long-term follow-up of extrahepatic biliary obstruction with bilirubin cholelithiasis in a Somali cat with pyruvate kinase deficiency. J Fel Med Surg. 2007;9:424–431. doi: 10.1016/j.jfms.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Geffen C, Savary-Bataille K, Chiers K, Giger U, Daminet S. Bilirubin cholelithiasis and haemosiderosis in an anaemic pyruvate kinase-deficient Somali cat. J Sm An Pract. 2008;49:479–482. doi: 10.1111/j.1748-5827.2008.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaer M, Harvey JW, Calrderwood-Mays M, Giger U. Pyruvate kinase deficiency causing haemolytic anemia with secondary hemochromatosis in a Cairn Terrier. J Am An Hosp Vet Assos. 1992;28:233–239. [Google Scholar]
- 28.Griot-Wenk ME, Callan MB, Casal ML, et al. Blood type AB in the feline AB blood group system. Am J Vet Res. 1996;57:1438–1442. [PubMed] [Google Scholar]
- 29.Knottenbelt CM, Addie DD, Day MJ, Mackin AJ. Determination of the prevalence of feline blood types in the UK. J Small Anim Pract. 1999;40:115–118. doi: 10.1111/j.1748-5827.1999.tb03051.x. [DOI] [PubMed] [Google Scholar]