Abstract
Advanced glycation end products (AGEs) are implicated in diabetic complications. However, their role in β-cell dysfunction is less clear. In this study we examined the effects of AGEs on islet function in mice and in isolated islets. AGE-BSA or BSA was administered ip to normal mice twice a day for 2 wk. We showed that AGE-BSA-treated mice exhibited significantly higher glucose levels and lower insulin levels in response to glucose challenge than did BSA-treated mice, although there were no significant differences in insulin sensitivity and islet morphology between two groups. Glucose-stimulated insulin secretion by islets of the AGE-BSA-treated mice or AGE-BSA-treated normal islets was significantly lower than that by islets isolated from the BSA-treated mice or BSA-treated normal islets. Furthermore, AGE treatment of islet β-cells inhibited ATP production, and glimepiride, a sulfonylurea derivative, restored glucose-stimulated insulin secretion. Further investigation indicated that AGEs inhibited cytochrome c oxidase activity by inducing the expression of inducible nitric oxide synthase (iNOS). Blocking the formation of nitric oxide with an iNOS selective inhibitor aminoguanidine reversed the inhibitory effects of AGEs on ATP production and insulin secretion. We conclude that AGEs inhibit cytochrome c oxidase and ATP production, leading to the impairment of glucose-stimulated insulin secretion through iNOS-dependent nitric oxide production.
Advanced glycation end products impair the function of pancreatic β-cells through nitric oxide-dependent inhibition of cytochrome c oxidase and ATP production.
Type 2 diabetes is characterized by hyperglycemia due to insulin resistance in peripheral tissues and deficient insulin secretion by pancreatic islet β-cells (1). Prolonged hyperglycemia leads to diabetic complications, including vascular and renal disease (2). Hyperglycemia also fosters the endogenous nonenzymatic glycoxidation of proteins, lipids, and nucleic acids, and results in the accumulation of heterogeneous molecules known as advanced glycation end products (AGEs) (3). Compelling evidence implicates this accumulation of AGEs in the pathogenesis of diabetic complications (3,4). AGEs may exert their effects through altering protein function, causing abnormal interactions among matrix proteins, and interfering with cellular functions by increasing the expression of cytokines and the production of reactive oxidative species through their receptor, receptor for AGE (RAGE) (2).
Diet is also an important source of AGEs (5). An estimated 10% of ingested AGEs are absorbed into the body’s circulation by the intestinal epithelium, and two thirds of those absorbed are retained (5,6). Increased dietary AGE intake has been associated with elevated serum AGE levels in diabetes subjects (7), as well as with atherosclerosis (8,9), nephropathy (10,11), and impaired wound healing (12) in diabetic animal models. In contrast, reductions in AGE intake have prevented both type 1 and 2 diabetes as well as insulin resistance in experimental settings (13,14,15).
Islet β-cell dysfunction is a central component of diabetic pathogenesis (16). Increased oxidative and endoplasmic reticulum (ER) stress, accelerated glucolipotoxicity, and activation of uncoupling protein 2 and inflammatory pathways appear to contribute to β-cell dysfunction. Although AGEs have been shown to increase the production of reactive oxidative species and activate inflammatory pathways, their role in β-cell dysfunction remains to be elucidated. This is mostly because β-cell function has already deteriorated by the time hyperglycemia occurs (17,18) and because high glucose levels themselves impair β-cell function (19,20,21,22).
Early studies have indicated that aminoguanidine (AG), which inhibits the formation of AGEs, protects islet β-cell function in C57/BLKsJ diabetic mice (23) as well as in isolated rat islets (24) or cultured INS-1 cells (25). Because low-AGE diets significantly protect pancreatic islet morphology and function in mice susceptible to genetically, dietary fat-induced or aging-related diabetes (13,14,15), we hypothesize that AGEs may play a role in β-cell dysfunction. Here, we investigate the effect of ip administration of AGE-BSA on islet function in normal mice and in cultured islets. We found that AGE treatment impairs glucose-stimulated insulin secretion in the mice by inducing the expression of inducible nitric oxide synthase (iNOS), which leads to the subsequent inhibition of cytochrome c oxidase and ATP synthesis by nitric oxide (NO).
Materials and Methods
Materials
The Mouse Insulin ELISA Kit was purchased from Crystal Chem Inc. (Downers Grove, IL). ENLITEN rLuciferase/Luciferin Reagent for ATP measurement was from Promega Corp. (Madison, WI). The Nitrate Assay Kit was from R&D Systems, Inc. (Minneapolis, MN). The RNeasy Mini Kit was from QIAGEN, Inc. (Valencia, CA). The DC Protein Assay Kit was from Bio-Rad Laboratories, Inc. (Hercules, CA). Endotoxin-binding affinity column was from Pierce (Rockford, IL). iNOS polyclonal antibodies were from Cayman Chemical Co. (Chicago, IL). Fatty acid free BSA, AG, NG-nitro-l-arginine methyl ester (L-NAME), glimepiride (GP), and all chemicals were from Sigma Chemical Co. (St. Louis, MO) unless otherwise noted.
Preparation of AGE-modified BSA
AGE-BSA was prepared according to a standard protocol (26). Briefly, low lipopolysaccharide and fatty acid-free BSA (50 mg/ml) was incubated with 0.5 m glucose in 0.2 m sodium phosphate buffer (pH 7.4) at 37 C for 8 wk under sterile conditions. Nonglycated BSA was prepared by incubation of BSA under the same conditions without glucose. Unincorporated sugar was removed by exhaustive dialysis against PBS. All preparations were passed over an endotoxin-binding affinity column. Levels of AGEs were tested by ELISA using a well-characterized monoclonal antibody (4G9; Alteon, Northvale, NJ) against Nε-(carboxymethyl)lysine (CML) (26). CML units of AGEs are defined against a universally available standard, normal human serum, as described previously. One AGE unit is defined as the 50% inhibition that results from 1:5 diluted normal human serum in the competitive AGE-ELISA (26). AGE values of each preparation were determined from a linear regression of the standard curve and expressed as U/mg.
Animals and AGE-BSA administration
Six-month-old C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were randomly divided into control (BSA) and AGE groups (six males and six females per group). BSA (200 μg/g, body weight) or AGE-BSA with 12 U/g (body weight), 24 U/g, and 60 U/g was administered ip twice a day for 2 wk. This protocol was based on the AGE-BSA clearance curve after one injection of AGE-BSA (24 U/g). Peak AGE-BSA levels occurred within 1–3 h and about 50% remained after 5 h (supplemental Fig. 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals. org). All mice were housed under controlled conditions: 23 ± 1 C and a 12-h light, 12-h dark cycle. They were allowed free access to standard rodent chow and water. All animal studies were performed in compliance with policies outlined in the Guidelines of Institutional Animal Care and Use Committee of Mount Sinai School of Medicine.
Measurement of serum AGEs
AGE concentrations in mouse serum were determined by competitive ELISA using monoclonal antibody that reacts with CML-like epitopes (4G9) as previously described (26,27). AGE-BSA was used as a competing antigen to generate an AGE-standard curve (0.1–100 AGE U/ml) for each experiment. AGE values were calculated from a linear regression of the standard curve.
Glucose tolerance test and serum insulin levels
Intraperitoneal glucose tolerance tests were performed after 2 wk AGE-BSA treatment. After an overnight fasting, mice were injected with 10% glucose (2 mg/g body weight) ip, and glucose levels were determined at 0, 30, 60, 90, and 120 min by a Glucometer Elite (Bayer Corp., Elkhart, IN) (28). For measuring serum insulin levels, after glucose loading, blood was collected from submandibular artery at 0, 30, and 60 min, and insulin levels were determined using the Ultra Sensitive Mouse Insulin ELISA Kit (Crystal Chem).
Insulin tolerance test (ITT)
ITTs were performed 2 wk after AGE-BSA treatment (13). After 6 h fasting, human regular insulin (0.75 U/kg) (Sigma Chemical) was ip administrated to mice that freely accessed water and chow. Blood samples were collected from tails before and after insulin administration every 15 min for 120 min, and serum glucose levels were determined.
Immunohistochemical analysis
Slides of 4-μm sections of three different pancreata from each group (BSA 0.2 mg/g, 12 U/g, and 24 U/g, body weight) were stained with standard hematoxylin/eosin (HE) or double stained with insulin/glucagon using rabbit anti-insulin antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and rabbit antiglucagon antibody (Dako Corp., Carpinteria, CA) as described (28). Slides were counterstained with hematoxylin mounted with Cytoseal xylene-based mounting medium (Stephens Scientific, Kalamazoo, MI) for visualization by light microscopy (magnification, ×100).
Culture and AGE treatment of isolated islets and INS-1 cells
Islets were isolated from the pancreas of C57BL/6J mice by Liberase (Roche Diagnostics, Indianapolis, IN) digestion, discontinuous Ficoll gradient separation, and hand pickup, according to the method of Lacy and Kostianovsky (29) with modifications as previously described (28). Freshly isolated islets from 6-month-old normal C57/BL6 mice were cultured in extracellular matrix-coated plates in RPMI 1640 media supplemented with 10% fetal bovine serum, 2 mm l-glutamine, 1% sodium pyruvate, 50 μm β-mercaptoethanol, 100 U/ml penicillin, and 100 μg/ml streptomycin. These conditions allow the islets to attach to the dishes and spread, and preserve their functional integrity (30). Two days after plating, after most islets had attached and begun to flatten, the medium was changed to RPMI 1640 media supplemented with 2 mm l-glutamine, 1% sodium pyruvate, and 100 U/ml penicillin and 100 μg/ml streptomycin, containing either BSA (0.5 mg/ml) or AGE-BSA at 60 and 120 U/ml that were corresponding to the serum AGE levels in the AGE-treated mice showing abnormal glucose tolerance tests and closely resembled the concentrations of AGEs observed in these either genetic or diet-induced diabetes mouse models (13,15). After 48 h the medium and islets were collected for further experiments. INS-1 cells were cultured as described (28,31) and treated with either BSA or AGE-BSA as described previously.
Determination of insulin secretion and contents
Isolated islets or INS-1 cells were incubated for 2 h in glass vials containing 500 μl Krebs-Ringer bicarbonate buffer supplemented with 10 mm HEPES, 2 mg/ml BSA in the presence of 1.7 mm glucose in humidified air containing 5% CO2 at 37 C, and then further incubated for 60 min at 37 C in the presence of 1.7 or 16.7 mm glucose. At the end of the incubation, the medium was removed and stored at −20 C for insulin assay. Islets were washed briefly in Hanks’ solution and homogenized by sonication for 30 sec in 200 μl acid ethanol (15 ml 12 mol/liter hydrochloride in 70% ethanol) and extracted overnight at 4 C. The extracts were stored at −20 C for insulin assay. Insulin concentrations in media and islet extracts were determined using the Ultra Sensitive Mouse Insulin ELISA Kit and normalized by total proteins.
Measurement of intracellular ATP content
INS-1 cells or islets were treated the same as that in insulin secretion. After incubation with Krebs-Ringer bicarbonate buffer containing stimulatory glucose concentration for 1 h, the INS-1 cells or islets were washed briefly in Hanks’ solution, suspended with 50 μl trichloroacetic acid (5%), and immediately homogenized with a plastic homogenizer. The suspension was spun at 2000 rpm and the supernatant stored at −80 C. Intracellular ATP content was measured using ENLITEN rLuciferase/Luciferin Reagent according to the manufacturer’s instructions. Briefly, samples were neutralized to pH 7.4 with 10 μl 4 m Tris, and 10 μl was aliquoted to a new tube with 90 μl ATP-free water. Luciferase reagent was added 1 sec before a 5-sec measurement in the 20/20n Luminometer (Turner BioSystems, Inc., Sunnyvale CA). ATP concentrations were determined by comparison to an ATP standard curve. Supernatant protein content was determined with a DC Protein Assay Kit and used to standardize ATP levels.
Measurement of NO and cytochrome c oxidase activity
Freshly isolated islets were treated with BSA or AGE-BSA in the presence or absence of 1 mm L-NAME or 2 mm AG for 48 h. Mitochondria were isolated with a Mitochondria/Cytosol Fractionation Kit (BioVision, Mountain View, CA). Released NO was measured using Nitrate Assay Kits according to the manufacturer’s instructions. Cytochrome c oxidase activity in isolated mitochondria was measured using a Cytochrome c Oxidase Assay Kit (Sigma Chemical), which monitors the oxidation of ferrocytochrome c at 550 nm. Protein concentrations in mitochondrial preparations were determined with a DC Protein Assay Kit. Cytochrome c oxidase activity was expressed as μmol/min/mg protein.
RT-PCR analysis
Total RNA was extracted from islets isolated from mice treated with or without AGE-BSA using the RNeasy Mini Kit, and RT-PCR was performed according to the manufacturer’s instructions. Integrated DNA Technologies (Coralville, IA) SciTools were used to design iNOS-specific primers: forward 5′-TCTTTGACGCTCGGAACTGTAGCA-3′, reverse 5′-TAGGTCGATGCACAACTGGGTGAA-3′. Band intensity was quantified by the Java-based image-processing program ImageJ (National Institutes of Health, Bethesda, MD).
Western blot analysis
Islets were treated with 0.5 mg/ml BSA, 60 U/ml, and 120 U/ml AGE-BSA, collected after 48 h and lysed in radioimmunoprecipitation assay buffer containing protease inhibitors (28). Aliquots containing 60 μg proteins were resolved on 8% SDS-PAGE. After electrophoresis, proteins were blotted onto nitrocellulose membranes, and probed with rabbit anti- iNOS polyclonal antibodies followed by incubation with antirabbit IgG conjugated with horseradish peroxidase. iNOS proteins were detected by enhanced chemiluminescence Western blotting detection reagents, and the intensity of each band was quantitated by ImageJ.
Statistical analysis
Results were expressed as a mean ± sem. The statistical significance of differences was analyzed with a two-tailed unpaired t test; P < 0.05 was considered statistically significant.
Results
Administration of AGE-BSA to mice results in an abnormal response to glucose
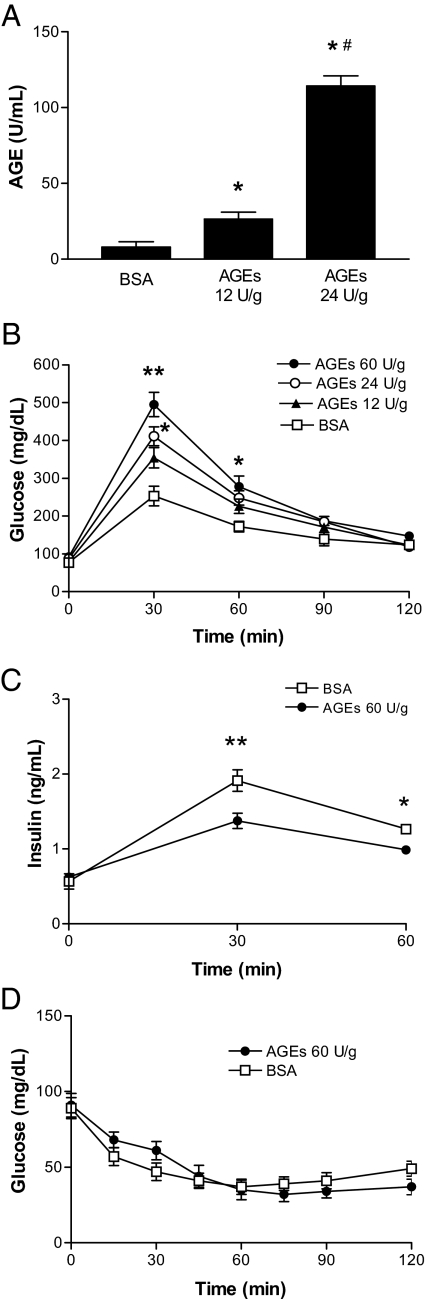
To examine the effects of AGEs on murine glucose metabolism, 6-month-old C57BL/6J mice were injected ip with AGE-BSA twice a day for 2 wk. As shown in Fig. 1A, AGE serum levels in mice treated with AGE-BSA were significantly elevated and exhibited a positive dose-response curve. As shown in Fig. 1B, after the start of the glucose tolerance test (2 mg/kg), glucose levels were significantly elevated in mice treated with AGE-BSA at either 24 (after 30 min) or 60 U/g (after both 30 and 60 min) compared with mice treated with BSA alone, and these elevated glucose levels returned to normal within 120 min, which were correlated with lower insulin levels at 30 and 60 min in AGE-BSA-treated mice than that in BSA-treated mice (Fig. 1C). This effect was not due to the development of insulin resistance because AGE-BSA treatment did not affect fasting glucose levels (Fig. 1B) and peripheral insulin sensitivity (Fig. 1D) in the mice compared with controls. Together, these results suggest that a 2-wk course of AGE-BSA administration in mice leads to elevated levels of serum AGEs and blunts the response of islet β-cells to glucose.
Figure 1.
Effects of AGEs on glucose metabolism in mice. A, After 2 wk ip administration of BSA (0.2 mg/g body weight) or AGE-BSA (12 U/g and 24 U/g body weight) to mice, the serum levels of AGEs were assessed. Data are expressed as means ± sem (n = 6). *, P < 0.01 compared with the control; #, P < 0.01 compared with the group with 12 U/g AGEs. Intraperitoneal glucose (2 mg/g body weight) tolerance tests (B) and serum insulin levels (C). D, Intraperitoneal ITTs. Data are expressed as means ± sem (n = 12). **, P < 0.01 and *, P < 0.05 compared with control at the same time point.
AGE-treatment decreases islet insulin secretion and islet insulin content
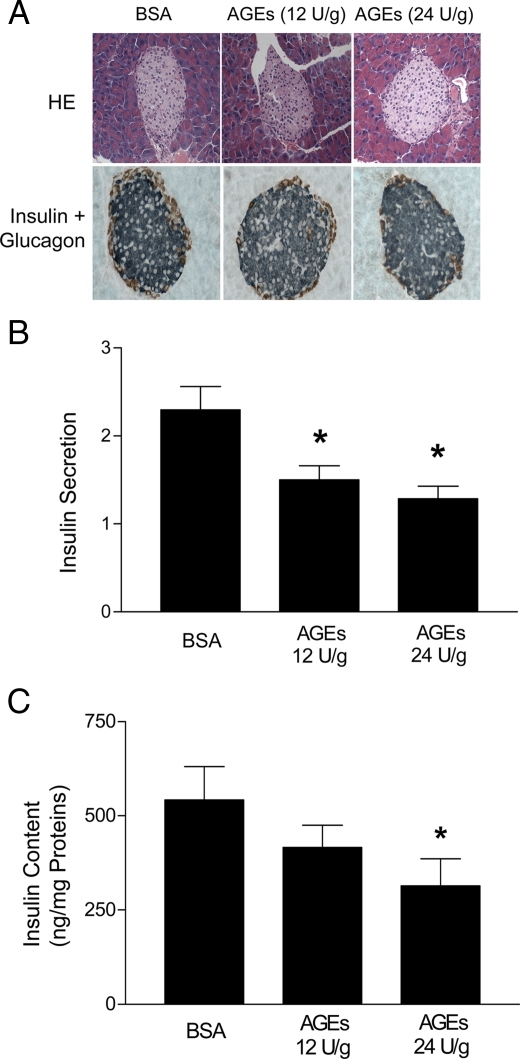
To determine whether administration of AGEs to mice affects pancreatic islet structure, we examined islet morphology. Immunohistochemical analysis showed that 2 wk AGE treatment caused few morphological changes in pancreatic islets (Fig. 2A). We next isolated islets from the mice treated with BSA or AGE-BSA and analyzed their ability to secret insulin, as well as their insulin content. As shown in Fig. 2B, islets isolated from mice treated with AGE-BSA at either 12 or 24 U/g secreted significantly less insulin in response to glucose (16.7 mm) than did islets from mice treated with BSA alone. In addition, the insulin content of the islets was inversely correlated to the dose of AGE-BSA administered to the mice (Fig. 2C).
Figure 2.
Effects of AGEs on islet function in vivo. A, Immunohistochemical analysis of pancreatic islets. Slides of 4-μm sections of three different pancreata from BSA or AGE-BSA-treated mice were stained by standard HE and anti-insulin plus glucagon antibodies as indicated (magnification, ×100). Islets were isolated from mice treated with BSA (0.2 mg/g, body weight) or AGE-BSA (12 and 24 U/g, body weight) for 2 wk and were then stimulated with 1.7 or 16.7 mm glucose for 1 h. Media were then collected for assays of insulin secretion (B), and the islets were collected for measurement of insulin content (C). Data are presented as means ± sem of three independent experiments. *, P < 0.05 compared with controls.
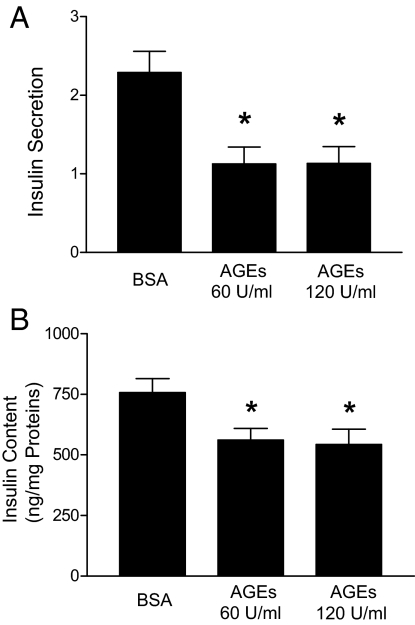
To verify these results in vitro, we treated freshly isolated islets from 6-month-old normal C57BL/6 mice with increasing concentrations of AGE-BSA for 48 h and assessed their ability to secrete insulin in response to glucose stimulation. As shown in Fig. 3A, both 60 and 120 U/ml AGE-BSA corresponding to the serum AGE levels of the AGE-treated mice showing abnormal glucose tolerance tests inhibited glucose-stimulated insulin secretion by these islets. Further analysis indicated that insulin levels in the AGE-BSA-treated islets were about 30% lower than those in the control islets (Fig. 3B). These results, which are consistent with what we observed in vivo, further indicate that elevated AGE levels in mice inhibit acute insulin secretion in response to glucose stimulation and decrease insulin production in β-cells.
Figure 3.
Effects of AGEs on islet function in vitro. Freshly isolated islets from normal C57/BL6 mice were cultured in the presence of BSA or AGE-BSA for 48 h. Islets were then stimulated with 1.7 or 16.7 mm glucose for 1 h. Media were then collected for use in an insulin secretion assay (A), and the islets were collected to measure insulin content (B). Data are presented as means ± sem of three independent experiments. *, P < 0.05 compared with the controls.
AGE treatment inhibits ATP production in islets
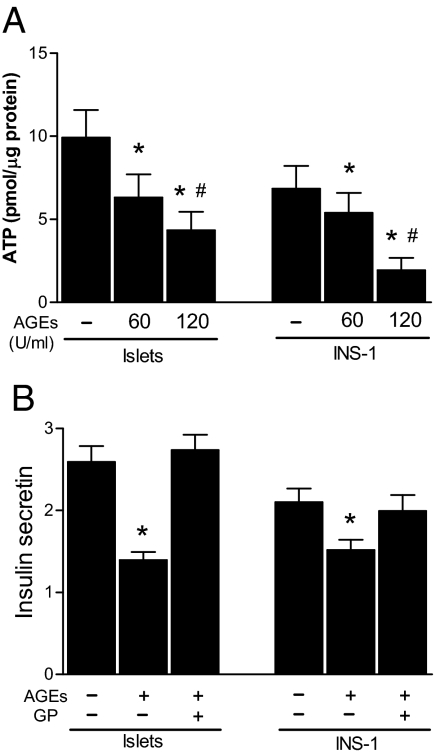
An increased ratio of ATP to ADP in response to glucose stimulation is required to close ATP-dependent K+ channels and depolarize cell membranes in preparation for insulin secretion (32). Because AGEs inhibit glucose-stimulated insulin secretion, we examined ATP levels in islets treated with BSA or AGE-BSA (60 and 120 U/ml). As shown in Fig. 4A, glucose stimulation resulted in significantly lower ATP levels in AGE-BSA-treated islets and INS-1 cells than in BSA-treated islets and INS-1 cells. Because reduction of ATP levels in AGE-treated cells may not effectively close ATP-dependent K+ channels leading to reduced insulin secretion in response to glucose stimulation, we examined whether GP, one of the sulfonylurea derivatives that inhibits ATP-dependent K+ channels, can overcome the inhibitory effect of AGEs on insulin secretion. As shown in Fig. 4B, addition of GP completely restored glucose-stimulated insulin secretion by AGE-treated islets and INS-1 cells. These findings indicate that AGE treatment inhibits the glucose-stimulated production of ATP needed for insulin secretion by islet β-cells.
Figure 4.
Effects of AGEs on ATP production. A, Islets isolated from normal C57/BL6 mice and INS-1 cells were culture with RPMI 1640 in the presence of 0.5 mg/ml BSA (control) or AGE-BSA for 48 h. The islets or INS-1 cells were stimulated with 16.7 mm glucose for 1 h and then collected for ATP content determination. B, Insulin secretion assay. Islets and INS-1 cells were treated with 0.5 mg/ml BSA or AGE-BSA (120 U/ml) for 48 h. Islets and INS-1 cells were then stimulated with 1.7 or 16.7 mm glucose or GP as indicated for 1 h. Data were normalized by total proteins, and the ratio of insulin secretion (16.7:1.7 mm glucose) was presented as means ± sem of three independent experiments. *, P < 0.01 compared with control. #, P < 0.01 compared with groups treated with 60 U/ml AGEs.
AGE treatment stimulates the expression of iNOS and the production of NO in islets
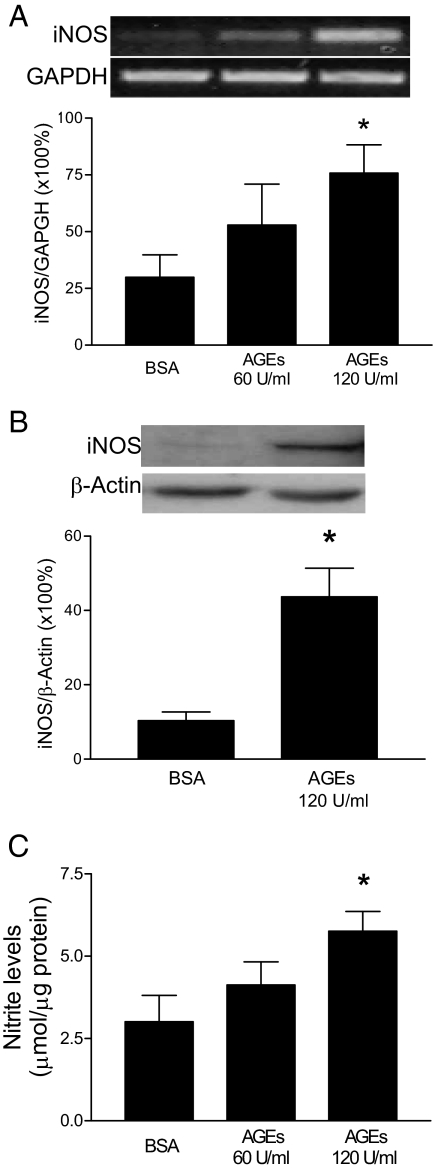
AGEs stimulate the expression of iNOS in endothelial cells and macrophages (33,34,35,36). Interestingly, iNOS expression has been implicated in the cytokine-induced dysfunction of islets (37,38). To investigate whether AGEs induce iNOS expression in mouse islets, we treated freshly isolated islets with BSA or AGE-BSA for 48 h and then analyzed iNOS levels in the islets. As shown in Fig. 5, A and B, AGEs stimulated the expression of iNOS mRNA and proteins in a concentration-dependent manner. Furthermore, this expression was accompanied by NO release from islets in an AGE-dosage dependent manner (Fig. 5C).
Figure 5.
Effect of AGE treatment on iNOS expression and NO production in islets. Isolated islets were treated with BSA or AGE-BSA for 48 h. A, Total RNA was isolated for determination of iNOS and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels by RT-PCR and 1% agarose electrophoresis, and the ratio of iNOS vs. glyceraldehyde-3-phosphate dehydrogenase was plotted. B, Islet cell lysates were prepared for Western blot analysis of iNOS and β-actin expression, and the ratio of iNOS vs. β-actin was plotted. C, Nitrite levels in islet-cultured media were measured using Griess reagent (modified). Data are presented as means ± sem of three independent experiments. *, P < 0.01 compared with control.
AGEs inhibit cytochrome c oxidase activity through NO production in islets
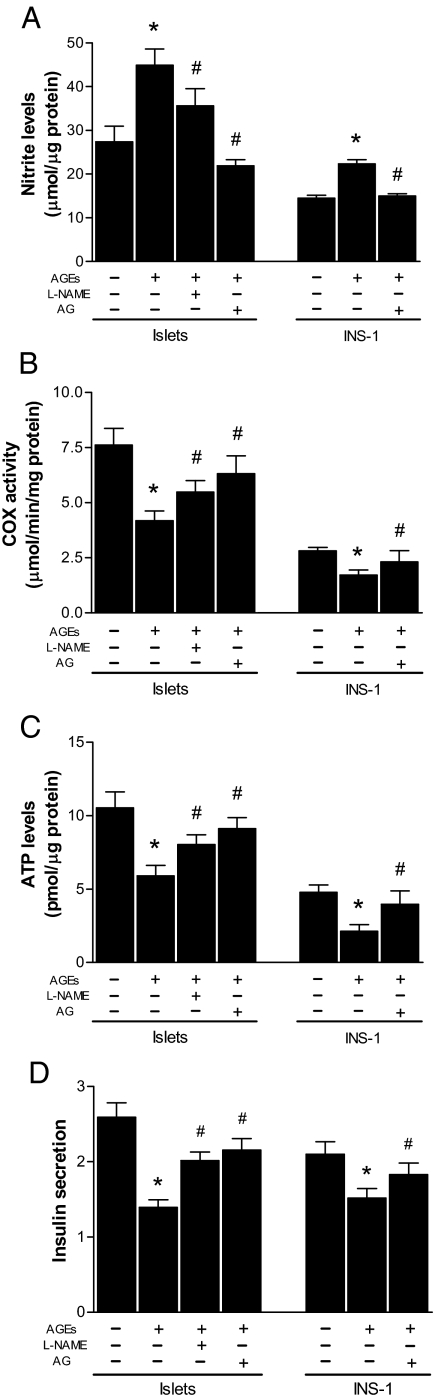
A growing body of evidence suggests that NO regulates mitochondrial oxygen consumption by inhibiting cytochrome c oxidase activity (39,40). We next determined whether AGE-induced NO production affects cytochrome c oxidase activity in islets. Islets were treated with AGE-BSA in the presence or absence of L-NAME, an inhibitor of nitric oxide synthase (41), or AG, a selective inhibitor of iNOS (42). As shown in Fig. 6A, the L-NAME partially inhibited NO production, but AG, an iNOS selective inhibitor, completely inhibited the AGE-induced NO production in islets and in INS-1 cells. Importantly, AGE-BSA significantly inhibited cytochrome c oxidase activity in both islets and INS-1 cells, and this activity was also fully restored by specific inhibition of iNOS (Fig. 6B), suggesting that AGEs negatively regulate cytochrome c oxidase through iNOS-dependent NO production in islet β-cells.
Figure 6.
Effects of inhibition of iNOS on NO formation and ATP synthesis in islets. Isolated islets and INS-1 cells were treated with BSA (0.5 mg/ml) or AGE-BSA (120 U/ml) in the presence or absence of AG or L-NAME for 48 h. Released NO in islet-cultured media (A), cytochrome c oxidase (COX) activity (B) and ATP levels in cells (C), and glucose-stimulated insulin secretion (D) were determined. Data are presented as means ± sem of three independent experiments. *, P < 0.01 compared with the control; #, P < 0.01 compared with the groups treated with AGEs alone.
Because blocking of the AGE-induced NO production restored cytochrome c oxidase activity, we further examined whether inhibition of iNOS also reverses AGE-induced inhibition of ATP production and insulin secretion. As shown in Fig. 6, C and D, specific inhibition of iNOS in both islets and INS-1 cells diminished the inhibition of ATP production and of insulin secretion by AGEs. Together, we conclude that AGEs inhibit glucose-stimulated insulin secretion by inhibiting cytochrome c oxidase and ATP synthesis through iNOS-dependent NO production in islets.
Discussion
We report here that the administration of AGEs to normal mice for 2 wk dramatically increased the animals’ serum AGE levels and resulted in abnormally high levels of blood glucose due to the low-serum levels of insulin in response to glucose challenge. Furthermore, islets isolated from AGE-treated mice secreted significantly less insulin in response to glucose stimulation than did islets from BSA-treated mice. We attribute these effects directly to the action of AGEs for several reasons: 1) the AGE-treated mice did not exhibit apparent signs of peripheral insulin resistance because their ITT was normal, and their fasting serum insulin levels were unchanged (indicating that the effect is not related to insulin resistance); 2) fasting glucose levels in the AGE-treated mice were unaffected (indicating that the effect is unlikely to be due to glucose toxicity); and 3) treatment with BSA alone had little effect on fasting glucose levels in mice or on glucose-stimulated insulin secretion by islets from these mice (indicating that the effect cannot be attributed to a nonspecific immune response in the mice). Therefore, our results clearly show that elevated levels of AGEs directly impair β-cell function in vivo.
AGE formation in vivo is greatly accelerated in response to the conditions of hyperglycemia and oxidative stress that occur in diabetic subjects (4). For example, the serum levels of AGEs in 4-wk-old female db/db were about 53 U/ml and increased to 93 U/ml after 20 wk with low-AGE diets and to 194 U/ml with high-AGE diets (13). Moreover, the serum levels of AGEs in diet-induced diabetic mice also reached 124 U/ml when diabetes has developed (15). Importantly, the doses of AGEs (60 and 120 U/ml) used in this study closely resemble the concentrations of AGEs observed in these genetic and diet-induced diabetic mouse models (13,15). It was reported that inhibition of AGE formation in db/db mice by AG partially protected islet β-cell function (23). Thus, our findings suggest that elevation of AGEs could be a factor contributing to the dysfunction of β-cells in type 2 diabetes, although elevated glucose levels have been clearly shown to impair β-cell function in rodent and human pancreatic islets (19,20,21,22) by affecting IL-1β production (43) and superoxide-dependent uncoupling protein 2 activation (44,45).
Our results clearly showed that elevation of AGEs alone is sufficient to initiate β-cell dysfunction in vivo, suggesting that any conditions that increase AGEs in circulation may affect the function of islet β-cells. Accumulated evidence suggests that diet is an important exogenous source of AGEs (46); a single meal high in AGEs significantly increases serum AGE levels in human subjects with or without diabetes (5). It has now become clear that a major source of preformed AGEs and AGE precursors is the Western heat-processed diet (46). Dietary AGEs include reactive AGE precursors (e.g. 1- or 3-deoxyglucosone, methylglyoxal, and pentosidine) and noncross-linking AGEs, such as pyrraline, CML, and their derivatives (4). In addition, amino lipids from dietary fats (e.g. 4-hydroxynonenal, CML, and their analogs) are major targets for lipid peroxidation (47). Thus, ingested glycoxidation and lipoxidation products could be more active in vivo. Indeed, it has recently been shown that diets low in AGEs significantly improve the morphology and function of pancreatic islets in nonobese diabetic mice (14), in db/db mice (13), and in insulin resistance and diabetes induced by a high-fat diet in rodents (15).
In this study we show for the first time that islets isolated from AGE-treated mice exhibit significantly lower levels of ATP than do islets from BSA-treated mice, and AGE-treated islets isolated from normal mice also exhibited decreased ATP levels. Furthermore, GP, one of the sulfonylurea derivatives that inhibits ATP-dependent K+ channels, can fully reverse the inhibitory effect of AGEs on ATP production and insulin secretion. Because ATP is required to close ATP-dependent K+ channels and depolarize β-cell membranes for glucose-stimulated insulin secretion (32), our finding implies that AGEs inhibit ATP synthesis, and reduced ATP levels cannot effectively close ATP-dependent K+ channels, leading to the impairment of glucose-stimulated insulin secretion.
How do AGEs inhibit ATP production in islet β-cells to impair glucose-stimulated insulin secretion? Previously, AGEs have stimulated NO release by inducing the expression of iNOS in endothelial cells and macrophages (33,34,35,36). NO is an important cellular signaling molecule. It controls blood flow and pressure by activating the heme enzyme soluble guanylate cyclase (48). A growing body of evidence suggests that nanomolar levels of NO block ATP production by rapidly and reversibly inhibiting cytochrome c oxidase in the mitochondrial respiratory chain (39,40,49,50).
To test this hypothesis, we examined whether AGEs also stimulate the accumulation of NO by inducing iNOS expression in islet β-cells. We found that AGE treatment significantly increases NO production in both islets and INS-1 cells by inducing iNOS expression, and the specific inhibition of iNOS completely blocked the NO production. It has been shown that AGEs induce iNOS expression by activation of nuclear factor-κB through a RAGE and its signaling cascade (35). Indeed, RAGE has been expressed in the islet β-cells of nonobese diabetic mice (51) and in INS-1 cells (52).
Importantly, we demonstrated that AGEs significantly inhibited the cytochrome c oxidase activity in islets and INS-1 cells, and this inhibition was fully reversed by iNOS selective inhibitor. We observed that AGEs stimulate the release of micromolar levels of NO much higher than the levels needed to inhibit cytochrome c oxidase activity and oxidative phosphorylation (39,40,49,50). Furthermore, blocking of NO formation by specific inhibition of iNOS completely diminished the inhibitory effect of AGEs on ATP production and glucose-stimulated insulin secretion. Our findings reveal a novel mechanism by which AGEs inhibit acute insulin secretion by impairing the mitochondrial respiratory chain and ATP synthesis through the iNOS-dependent formation of NO in islet β-cells.
In summary, we have established that elevated levels of AGEs, in addition to playing a role in the pathogenesis of diabetic complications, independently contribute to the dysfunction of pancreatic β-cells by stimulating the expression of iNOS and the generation of NO in the cells. Elevated levels of NO in turn inhibit cytochrome c oxidase activity and ATP production, leading to impairment of glucose-stimulated insulin secretion.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grants DK063076 and DK074805, and an American Diabetes Association Research Award 7-06RA-87.
Disclosure Summary: The authors have nothing to disclosure.
First Published Online February 26, 2009
Abbreviations: AG, Aminoguanidine; AGE, advanced glycation end product; CML, Nε-(carboxymethyl)lysine; HE, hematoxylin/eosin; GP, glimepiride; iNOS, inducible nitric oxide synthase; ITT, insulin tolerance test; L-NAME, NG-nitro-l-arginine methyl ester; RAGE, receptor for advanced glycation end product.
References
- Kasuga M 2006 Insulin resistance and pancreatic β cell failure. J Clin Invest 116:1756–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M 2001 Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820 [DOI] [PubMed] [Google Scholar]
- Vlassara H, Palace MR 2002 Diabetes and advanced glycation end products. J Intern Med 251:87–101 [DOI] [PubMed] [Google Scholar]
- Huebschmann AG, Regensteiner JG, Vlassara H, Reusch JE 2006 Diabetes and advanced glycoxidation end products. Diabetes Care 29:1420–1432 [DOI] [PubMed] [Google Scholar]
- Koschinsky T, He CJ, Mitsuhashi T, Bucala R, Liu C, Buenting C, Heitmann K, Vlassara H 1997 Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci USA 94:6474–6479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finot PA 2005 Historical perspective of the Maillard reaction in food science. Ann NY Acad Sci 1043:1–8 [DOI] [PubMed] [Google Scholar]
- Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, Peppa M, Rayfield EJ 2002 Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci USA 99:15596–15601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RY, Choudhury RP, Cai W, Lu M, Fallon JT, Fisher EA, Vlassara H 2003 Dietary glycotoxins promote diabetic atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 168:213–220 [DOI] [PubMed] [Google Scholar]
- Lin RY, Reis ED, Dore AT, Lu M, Ghodsi N, Fallon JT, Fisher EA, Vlassara H 2002 Lowering of dietary advanced glycation end products (AGE) reduces neointimal formation after arterial injury in genetically hypercholesterolemic mice. Atherosclerosis 163:303–311 [DOI] [PubMed] [Google Scholar]
- Zheng F, He C, Cai W, Hattori M, Steffes M, Vlassara H 2002 Prevention of diabetic nephropathy in mice by a diet low in glycoxidation products. Diabetes Metab Res Rev 18:224–237 [DOI] [PubMed] [Google Scholar]
- Sebekova K, Faist V, Hofmann T, Schinzel R, Heidland A 2003 Effects of a diet rich in advanced glycation end products in the rat remnant kidney model. Am J Kidney Dis 41(Suppl 1):S48–S51 [DOI] [PubMed] [Google Scholar]
- Peppa M, Brem H, Ehrlich P, Zhang JG, Cai W, Li Z, Croitoru A, Thung S, Vlassara H 2003 Adverse effects of dietary glycotoxins on wound healing in genetically diabetic mice. Diabetes 52:2805–2813 [DOI] [PubMed] [Google Scholar]
- Hofmann SM, Dong HJ, Li Z, Cai W, Altomonte J, Thung SN, Zeng F, Fisher EA, Vlassara H 2002 Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes 51:2082–2089 [DOI] [PubMed] [Google Scholar]
- Peppa M, He C, Hattori M, McEvoy R, Zheng F, Vlassara H 2003 Fetal or neonatal low-glycotoxin environment prevents autoimmune diabetes in NOD mice. Diabetes 52:1441–1448 [DOI] [PubMed] [Google Scholar]
- Sandu O, Song K, Cai W, Zheng F, Uribarri J, Vlassara H 2005 Insulin resistance and type 2 diabetes in high-fat-fed mice are linked to high glycotoxin intake. Diabetes 54:2314–2319 [DOI] [PubMed] [Google Scholar]
- Prentki M, Nolan CJ 2006 Islet β cell failure in type 2 diabetes. J Clin Invest 116:1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte Jr D, Kahn SE 2001 β-cell dysfunction and failure in type 2 diabetes: potential mechanisms. Diabetes 50(Suppl 1):S160–S163 [DOI] [PubMed] [Google Scholar]
- Kahn SE 2001 The importance of β-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab 86:4047–4058 [DOI] [PubMed] [Google Scholar]
- Unger RH, Grundy S 1985 Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia 28:119–121 [DOI] [PubMed] [Google Scholar]
- Leahy JL, Cooper HE, Deal DA, Weir GC 1986 Chronic hyperglycemia is associated with impaired glucose influence on insulin secretion. A study in normal rats using chronic in vivo glucose infusions. J Clin Invest 77:908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eizirik DL, Korbutt GS, Hellerström C 1992 Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the β-cell function. J Clin Invest 90:1263–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak S, Leibowitz G, Bertuzzi F, Socci C, Kaiser N, Gross DJ, Cerasi E, Melloul D 1999 Impaired β-cell functions induced by chronic exposure of cultured human pancreatic islets to high glucose. Diabetes 48:1230–1236 [DOI] [PubMed] [Google Scholar]
- Piercy V, Toseland CD, Turner NC 1998 Potential benefit of inhibitors of advanced glycation end products in the progression of type II diabetes: a study with aminoguanidine in C57/BLKsJ diabetic mice. Metabolism 47:1477–1480 [DOI] [PubMed] [Google Scholar]
- Tajiri Y, Möller C, Grill V 1997 Long-term effects of aminoguanidine on insulin release and biosynthesis: evidence that the formation of advanced glycosylation end products inhibits B cell function. Endocrinology 138:273–280 [DOI] [PubMed] [Google Scholar]
- Tajiri Y, Grill V 2000 Aminoguanidine exerts a β-cell function-preserving effect in high glucose-cultured β-cells (INS-1). Int J Exp Diabetes Res 1:111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi T, Vlassara H, Founds HW, Li YM 1997 Standardizing the immunological measurement of advanced glycation endproducts using normal human serum. J Immunol Methods 207:79–88 [DOI] [PubMed] [Google Scholar]
- Cai W, Gao QD, Zhu L, Peppa M, He C, Vlassara H 2002 Oxidative stress-inducing carbonyl compounds from common foods: novel mediators of cellular dysfunction. Mol Med 8:337–346 [PMC free article] [PubMed] [Google Scholar]
- Song K, Zhang X, Zhao C, Ang NT, Ma ZA 2005 Inhibition of Ca2+-independent phospholipase A2 results in insufficient insulin secretion and impaired glucose tolerance. Mol Endocrinol 19:504–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy PE, Kostianovsky M 1967 Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 16:35–39 [DOI] [PubMed] [Google Scholar]
- Kaiser N, Corcos AP, Sarel I, Cerasi E 1991 Monolayer culture of adult rat pancreatic islets on extracellular matrix: modulation of B-cell function by chronic exposure to high glucose. Endocrinology 129:2067–2076 [DOI] [PubMed] [Google Scholar]
- Seleznev K, Zhao C, Zhang XH, Song K, Ma ZA 2006 Calcium-independent phospholipase A2 localizes in and protects mitochondria during apoptotic induction by staurosporine. J Biol Chem 281:22275–22288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschinsky FM 1996 Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes 45:223–241 [DOI] [PubMed] [Google Scholar]
- Amore A, Cirina P, Mitola S, Peruzzi L, Gianoglio B, Rabbone I, Sacchetti C, Cerutti F, Grillo C, Coppo R 1997 Nonenzymatically glycated albumin (Amadori adducts) enhances nitric oxide synthase activity and gene expression in endothelial cells. Kidney Int 51:27–35 [DOI] [PubMed] [Google Scholar]
- Rojas A, Caveda L, Romay C, López E, Valdés S, Padrón J, Glaría L, Martínez O, Delgado R 1996 Effect of advanced glycosylation end products on the induction of nitric oxide synthase in murine macrophages. Biochem Biophys Res Commun 225:358–362 [DOI] [PubMed] [Google Scholar]
- Wu CH, Huang CM, Lin CH, Ho YS, Chen CM, Lee HM 2002 Advanced glycosylation end products induce NF-κB dependent iNOS expression in RAW 264.7 cells. Mol Cell Endocrinol 194:9–17 [DOI] [PubMed] [Google Scholar]
- Sumi D, Ignarro LJ 2004 Regulation of inducible nitric oxide synthase expression in advanced glycation end product-stimulated raw 264.7 cells: the role of heme oxygenase-1 and endogenous nitric oxide. Diabetes 53:1841–1850 [DOI] [PubMed] [Google Scholar]
- Corbett JA, McDaniel ML 1995 Intraislet release of interleukin 1 inhibits β cell function by inducing β cell expression of inducible nitric oxide synthase. J Exp Med 181:559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett JA, Wang JL, Hughes JH, Wolf BA, Sweetland MA, Lancaster Jr JR, McDaniel ML 1992 Nitric oxide and cyclic GMP formation induced by interleukin 1 β in islets of Langerhans. Evidence for an effector role of nitric oxide in islet dysfunction. Biochem J 287(Pt 1):229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulivi C, Kato K, Cooper CE 2006 Nitric oxide regulation of mitochondrial oxygen consumption I: cellular physiology. Am J Physiol Cell Physiol 291:C1225–C1231 [DOI] [PubMed] [Google Scholar]
- Cooper CE, Giulivi C 2007 Nitric oxide regulation of mitochondrial oxygen consumption II: molecular mechanism and tissue physiology. Am J Physiol Cell Physiol 292:C1993–C2003 [DOI] [PubMed] [Google Scholar]
- Sakuma I, Stuehr DJ, Gross SS, Nathan C, Levi R 1988 Identification of arginine as a precursor of endothelium-derived relaxing factor. Proc Natl Acad Sci USA 85:8664–8667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett JA, McDaniel ML 1996 The use of aminoguanidine, a selective iNOS inhibitor, to evaluate the role of nitric oxide in the development of autoimmune diabetes. Methods 10:21–30 [DOI] [PubMed] [Google Scholar]
- Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY 2002 Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J Clin Invest 110:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patanè G, Anello M, Piro S, Vigneri R, Purrello F, Rabuazzo AM 2002 Role of ATP production and uncoupling protein-2 in the insulin secretory defect induced by chronic exposure to high glucose or free fatty acids and effects of peroxisome proliferator-activated receptor-γ inhibition. Diabetes 51:2749–2756 [DOI] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Scorrano L, Dalgaard LT, St-Pierre J, Grey ST, Lowell BB 2003 Superoxide-mediated activation of uncoupling protein 2 causes pancreatic {β} cell dysfunction. J Clin Invest 112:1831–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H, Uribarri J 2004 Glycoxidation and diabetic complications: modern lessons and a warning? Rev Endocr Metab Disord 5:181–188 [DOI] [PubMed] [Google Scholar]
- Bucala R, Makita Z, Koschinsky T, Cerami A, Vlassara H 1993 Lipid advanced glycosylation: pathway for lipid oxidation in vivo. Proc Natl Acad Sci USA 90:6434–6438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro LJ 1990 Haem-dependent activation of guanylate cyclase and cyclic GMP formation by endogenous nitric oxide: a unique transduction mechanism for transcellular signaling. Pharmacol Toxicol 67:1–7 [DOI] [PubMed] [Google Scholar]
- Brown GC, Cooper CE 1994 Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett 356:295–298 [DOI] [PubMed] [Google Scholar]
- Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH 1994 Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett 345:50–54 [DOI] [PubMed] [Google Scholar]
- Chen Y, Yan SS, Colgan J, Zhang HP, Luban J, Schmidt AM, Stern D, Herold KC 2004 Blockade of late stages of autoimmune diabetes by inhibition of the receptor for advanced glycation end products. J Immunol 173:1399–1405 [DOI] [PubMed] [Google Scholar]
- Lim M, Park L, Shin G, Hong H, Kang I, Park Y 2008 Induction of apoptosis of β cells of the pancreas by advanced glycation end-products, important mediators of chronic complications of diabetes mellitus. Ann NY Acad Sci 1150:311–315 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.